Quality Evaluation of Crude and Salt-Processed Cuscutae Semen through Qualitative and Quantitative Analysis of Multiple Components Using HPLC Combined with Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Salt-Processed Cuscutae Semen
2.3. Sample Preparation and Standard Solutions
2.4. Chromatographic Fingerprinting Conditions and Quantitative Analysis
2.5. Method Validation
2.6. Data Analysis
3. Results and Discussion
3.1. Optimization of Chromatographic Conditions
3.2. Method Validation
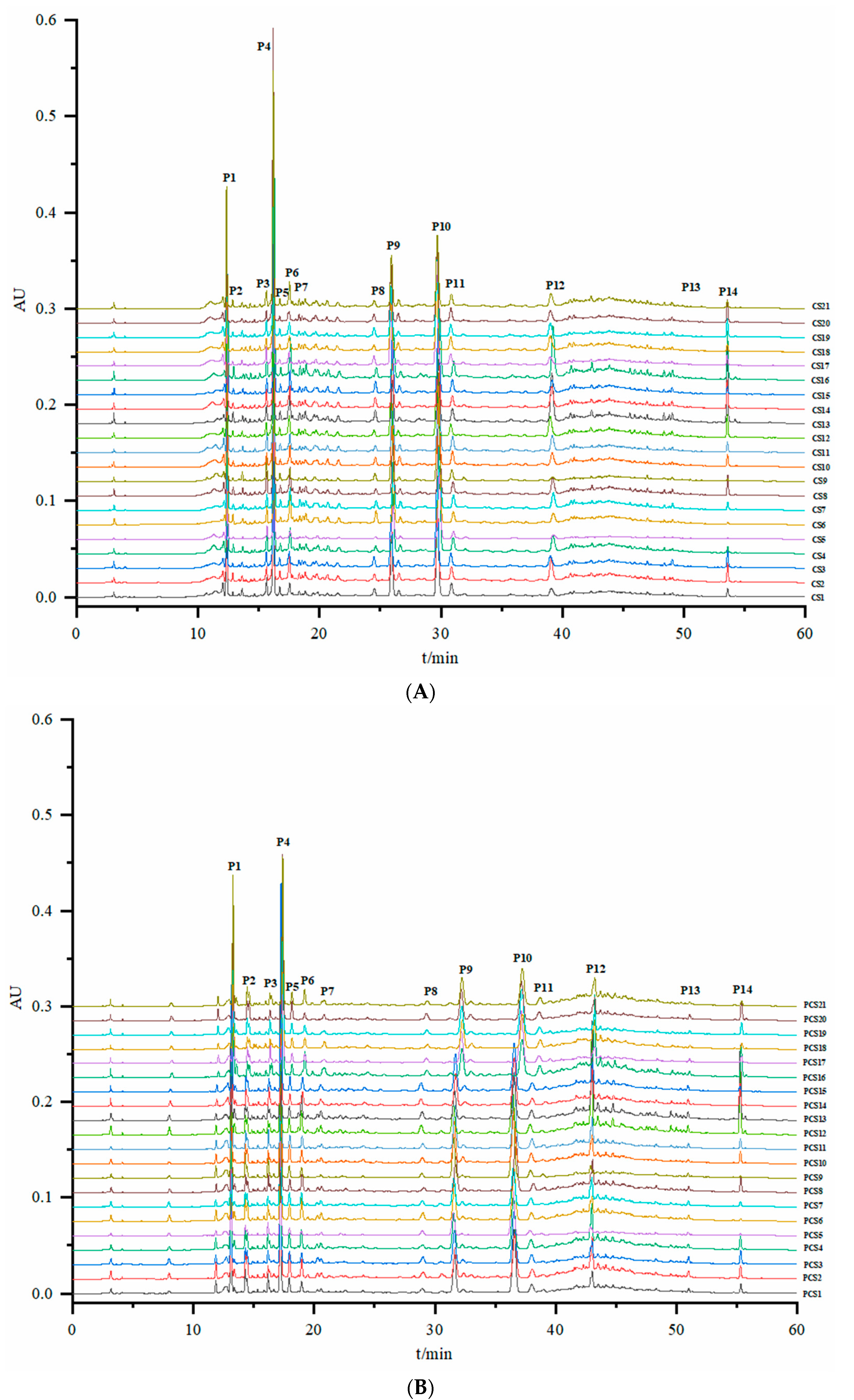
3.3. Establishment and Similarity Evaluation of the HPLC Fingerprints of CS and PCS
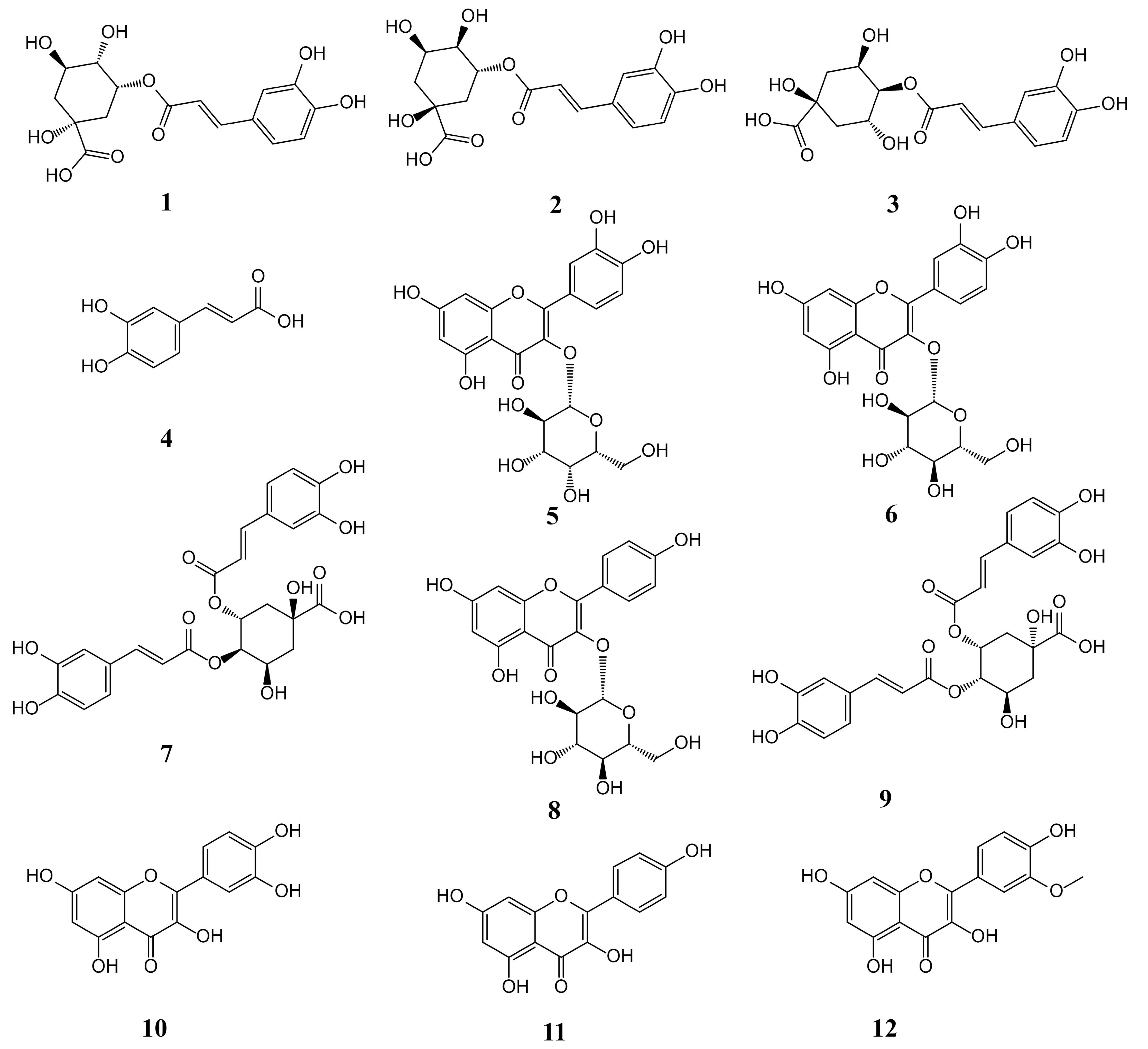
3.4. Quantitative Analysis of the 12 Components in CS and PCS Samples
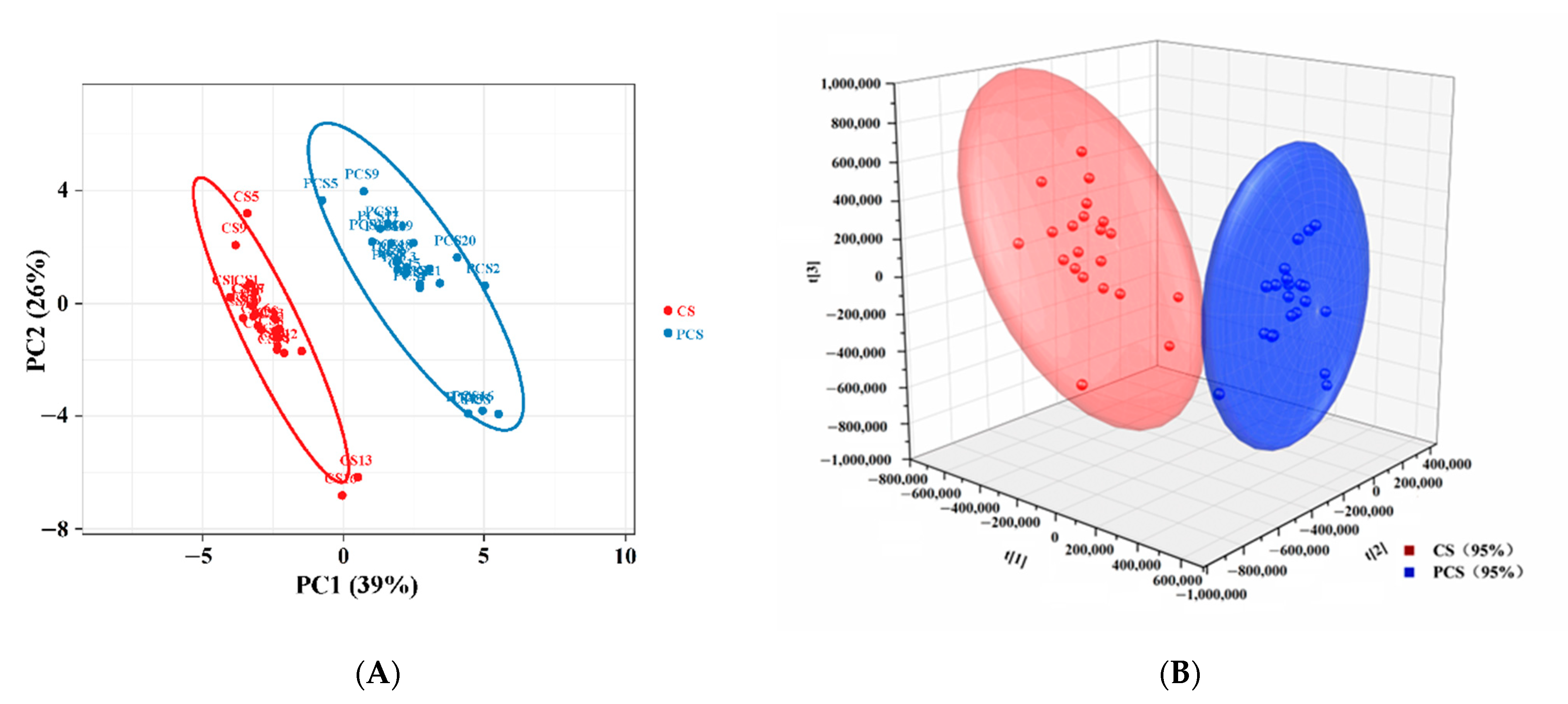
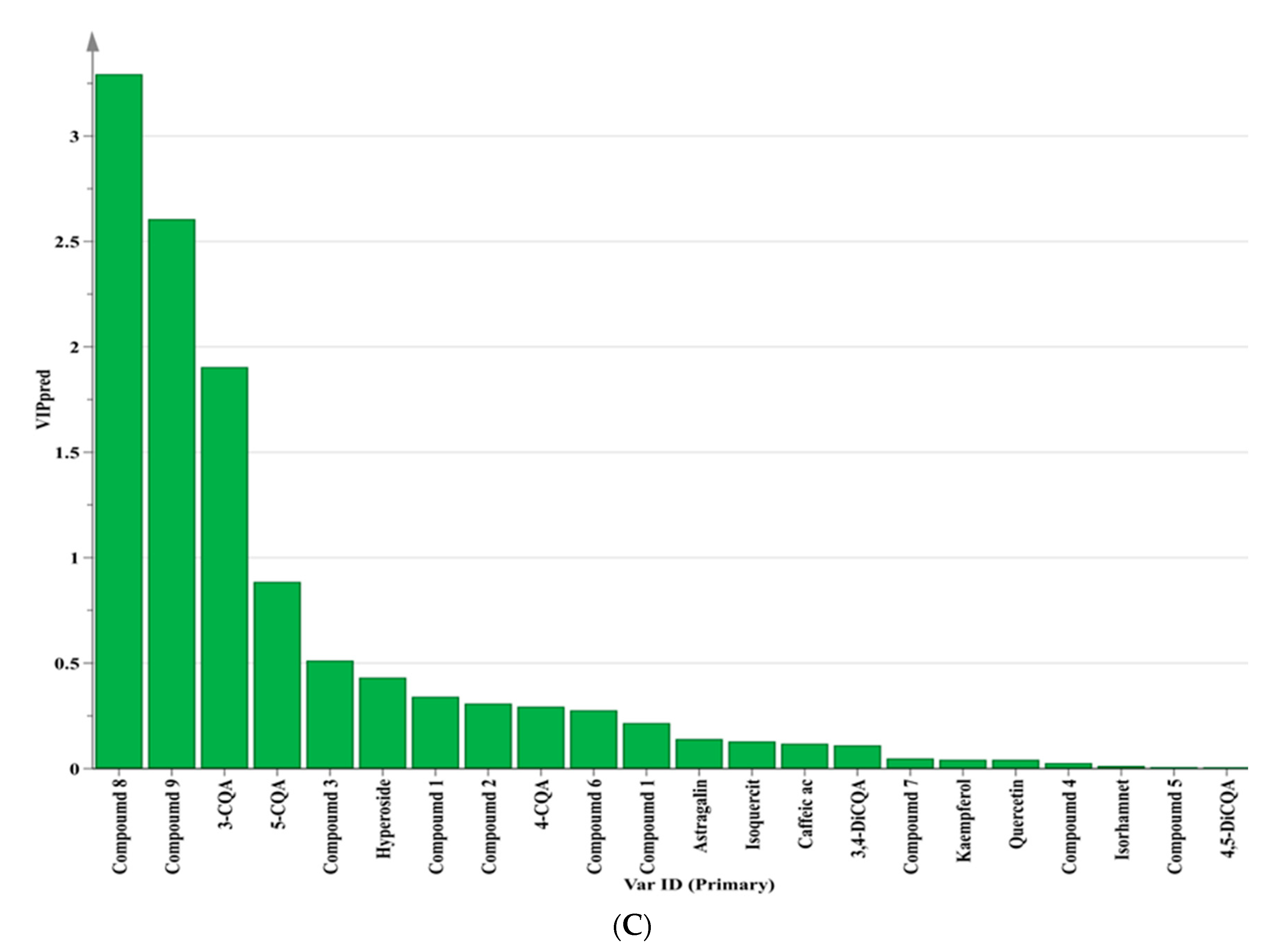
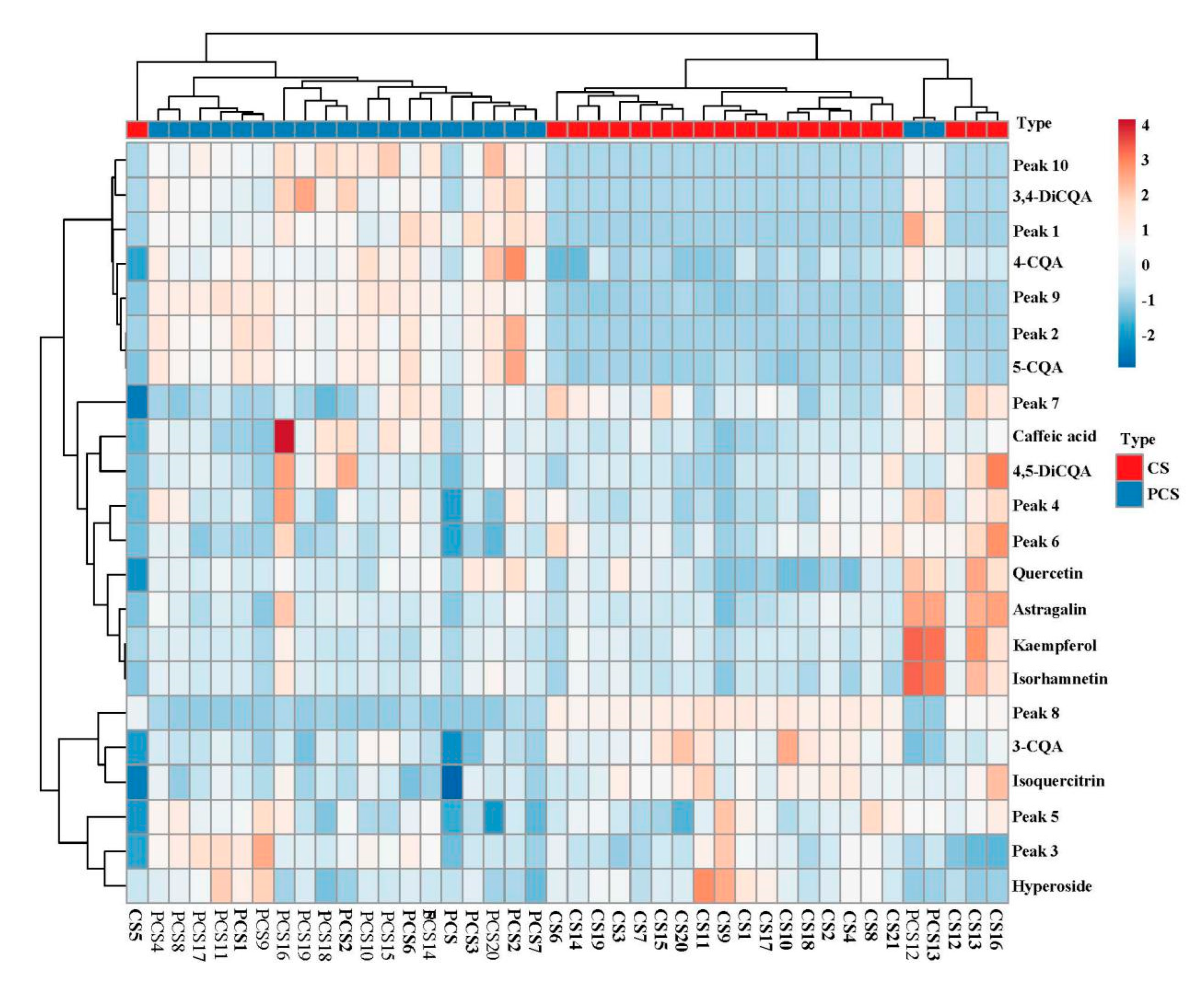
3.5. Chemometric Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Xu, X.; Xu, H.; Xu, S.; Lin, Q.; Jia, Z.; Han, T.; Zhang, H.; Zhang, Y.; Liu, H.; et al. Purification, characterization and biological effect of reversing the kidney-yang deficiency of polysaccharides from Semen cuscutae. Carbohyd. Polym. 2017, 175, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen, D.; Chu, C.L. Kaempferol from Semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Bao, Y.; Guo, J. The total flavones from Semen cuscutae reverse the reduction of testosterone level and the expression of androgen receptor gene in kidney-yang deficient mice. J. Ethnopharmacol. 2008, 119, 166–171. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Gao, L. Study on extraction process of tannins from Semen cuscutae and their anti-papilloma activity. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, E.K.; Lee, H.S.; Sohn, Y.; Soh, Y.; Jung, H.S.; Sohn, N.W. Protective effects of Cuscutae semen against dimethylnitrosamine-induced acute liver injury in Sprague-Dawley rats. Biol. Pharm. Bull. 2007, 30, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, H.; Zhao, B.; Li, S.; Li, T.; Xu, X.; Zhang, T.; Lin, R.; Li, J.; Li, X. The difference of chemical components and biological activities of the crude products and the salt-processed product from Semen cuscutae. Evid.-Based Compl. Alt. Med. 2016, 2016, 8656740. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Q.; Wang, F.; Zhang, G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. J. Ethnopharmacol. 2011, 135, 553–560. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in Semen Cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Z.T.; Xie, Y.; Duan, Z.W.; Wang, Y.; Fan, R.H. High resolution UPLC-MS/MS method for simultaneous separation and determination of six flavonoids from Semen cuscutae extract in rat plasma: Application to comparative pharmacokinetic studies in normal and kidney-deficient rats. Nat. Prod. Res. 2020, 34, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhou, C.; Qiu, W.; Wu, H.; Li, J.; Peng, J.; Qiu, M.; Liang, C.; Gao, J.; Luo, S. Total flavonoids from Semen cuscutae target MMP9 and promote invasion of EVT cells via Notch/AKT/MAPK signaling pathways. Sci. Rep. 2018, 8, 17342. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.Y.; Jung, H.W.; Lee, M.Y.; Lee, H.W.; Chae, S.W.; Park, Y.K. Effect of the semen extract of Cuscuta chinensis on inflammatory responses in LPS-stimulated BV-2 microglia. Chin. J. Nat. Med. 2014, 12, 573–581. [Google Scholar] [CrossRef]
- Han, S.X.; Yao, H.L.; Li, Y.Y.; Wang, T.S. Protective effects of Cuscuta chinensis lam. extract on myocardial ischemia/reperfusion injury in rats. Chin. Pharm. Bull. 2011, 27, 533–536. [Google Scholar]
- Ye, M.; Li, Y.; Yan, Y.N.; Liu, H.W.; Ji, X.H. Determination of flavonoids in Semen cuscutae by RP-HPLC. J. Pharm. Biomed. Anal. 2002, 28, 621–628. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Guo, D.A. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical fingerprinting profile and targeted quantitative analysis of phenolic compounds from Rooibos Tea (Aspalathus linearis) and dietary supplements using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, Y.; Shi, D.; Ji, J.; Qin, K. Quantitative and chemical fingerprint analysis for the quality evaluation of Fructus arctii by HPLC coupled with hierarchical clustering analysis. Lat. Am. J. Pharm. 2019, 38, 643–650. [Google Scholar]
- Zhou, X.; Tang, L.; Wu, H.; Zhou, G.; Wang, T.; Kou, Z.; Li, S.; Wang, Z. Chemometric analyses for the characterization of raw and processed seeds of Descurainia sophia (L.) based on HPLC fingerprints. J. Pharm. Biomed. Anal. 2015, 111, 1–6. [Google Scholar]
- Yang, B.; Hu, J.; Zhu, X.; Zhuang, Y.; Yin, F.; Qin, K.; Cai, B. Qualitative analysis of multiple compounds in raw and prepared Semen cassiae coupled with multiple statistical strategies. J. Sep. Sci. 2017, 40, 4718–4729. [Google Scholar]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.H.; Donkor, P.O.; Gao, X.M.; Chang, Y.X. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef]
- Stpnik, K.; Kukula-Koch, W. In silico studies on triterpenoid saponins permeation through the blood-brain barrier combined with postmortem research on the brain tissues of mice affected by astragaloside IV administration. Int. J. Mol. Sci. 2020, 21, 2534. [Google Scholar] [CrossRef]
- Hamazaki, Y.; Kato, M.; Karasawa, K. Methylnigakinone content determination and geographical origin discrimination for P. quassioides via fluorescence fingerprint and principal component analyses. J. Pharm. Biomed. Anal. 2022, 219, 114932. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Sun, L.; Li, D.; You, G.; Wang, M.; Ren, X. Quality evaluation of Phellodendri Chinensis Cortex by fingerprint-chemical pattern recognition. Molecules 2018, 23, 2307. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Su, X.; Huang, Z.; Su, L.; Li, L.; Lu, T. Analysis of chemical variations between crude and salt-processed anemarrhenae rhizoma using ultra-high-performance liquid chromatography-mass spectrometry methods. Molecules 2017, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Oshima, R.; Kotani, A.; Kuroda, M.; Yamamoto, K.; Mimaki, Y.; Hakamata, H. Discrimination of Schisandrae Chinensis Fructus and Schisandrae Sphenantherae Fructus based on fingerprint profiles of hydrophilic components by high-performance liquid chromatography with ultraviolet detection. J. Nat. Med. 2018, 72, 399–408. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): Advances in new perspectives. Chin. Med. 2018, 13, 4–12. [Google Scholar] [CrossRef]


| No. | Origin | Batch Number |
|---|---|---|
| S1 | Inner Mongolia | 20,210,301 |
| S2 | Inner Mongolia | 20,201,201 |
| S3 | Liaoning | 201,101 |
| S4 | Ningxia | 20,121,001 |
| S5 | Gansu | 20,210,524 |
| S6 | Inner Mongolia | 210,301 |
| S7 | Ningxia | 20,210,514 |
| S8 | Inner Mongolia | 200,726 |
| S9 | Inner Mongolia | 210,103 |
| S10 | Heibei | 200,901 |
| S11 | Shandong | 201,222 |
| S12 | Yunnan | 20,210,606 |
| S13 | Henan | 202,009 |
| S14 | Guangxi | 202,007 |
| S15 | Inner Mongolia | 200,915 |
| S16 | Hebei | 202,106 |
| S17 | Jiangsu | 202,008 |
| S18 | Anhui | 20,210,610 |
| S19 | Ningxia | 202,010 |
| S20 | Inner Mongolia | 200,401 |
| S21 | Shandong | 210,200,181 |
| No. | Analyte | Calibration Curve Equation | r | Linear Range (mg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | 5-CQA | Y = 24,908,773X − 2450 | 1.0000 | 0.0002~0.0860 | 0.21 | 0.43 |
| 2 | 3-CQA | Y = 25,509,829X + 15,086 | 0.9990 | 0.0006~0.2238 | 0.56 | 1.12 |
| 3 | 4-CQA | Y = 20,357,556X − 1490 | 1.0000 | 0.0002~0.0816 | 0.20 | 0.41 |
| 4 | caffeic acid | Y = 47,472,731X + 17,007 | 0.9994 | 0.0002~0.0903 | 0.23 | 0.45 |
| 5 | hyperoside | Y = 13,723,924X + 13,326 | 0.9994 | 0.0006~0.2442 | 0.61 | 1.22 |
| 6 | isoquercitrin | Y = 13,711,789X + 3450 | 0.9996 | 0.0002~0.0865 | 0.22 | 0.43 |
| 7 | 3,4-DiCQA | Y = 30,307,263X − 23,528 | 0.9998 | 0.0002~0.0865 | 0.22 | 0.43 |
| 8 | astragalin | Y = 17,030,336X + 45,986 | 0.9994 | 0.0015~0.6079 | 1.52 | 3.04 |
| 9 | 4,5-DiCQA | Y = 33,839,153X − 13,637 | 0.9991 | 0.0002~0.0815 | 0.20 | 0.41 |
| 10 | quercetin | Y = 17,558,745X + 1850 | 0.9995 | 0.0002~0.0812 | 0.20 | 0.41 |
| 11 | kaempferol | Y = 21,261,390X + 12,682 | 0.9994 | 0.0006~0.2448 | 0.61 | 1.22 |
| 12 | isorhamnetin | Y = 16,630,604X + 5704 | 0.9994 | 0.0003~0.1044 | 0.26 | 0.52 |
| No. | Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | CS | 0.12 ± 0.00 | 3.41 ± 0.00 | 0.19 ± 0.00 | 0.04 ± 0.00 | 5.49 ± 0.01 | 0.72 ± 0.00 | ND | 0.36 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.00 | 0.14 ± 0.00 | DUL | 10.59 ± 0.01 |
| PCS | 0.58 ± 0.04 | 2.55 ± 0.11 | 0.35 ± 0.03 | 0.03 ± 0.00 | 4.91 ± 0.13 | 0.59 ± 0.01 | 0.06 ± 0.00 | 0.49 ± 0.00 | 0.05 ± 0.01 | 0.11 ± 0.01 | 0.21 ± 0.01 | DUL | 9.93 ± 0.18 | |
| S2 | CS | 0.11 ± 0.00 | 3.79 ± 0.00 | 0.20 ± 0.00 | 0.05 ± 0.00 | 4.17 ± 0.00 | 0.77 ± 0.00 | ND | 1.15 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.35 ± 0.00 | 0.03 ± 0.00 | 10.78 ± 0.01 |
| PCS | 0.72 ± 0.11 | 2.60 ± 0.21 | 0.46 ± 0.07 | 0.06 ± 0.00 | 3.44 ± 0.17 | 0.60 ± 0.02 | 0.10 ± 0.01 | 1.09 ± 0.04 | 0.07 ± 0.00 | 0.16 ± 0.04 | 0.30 ± 0.01 | 0.03 ± 0.00 | 9.63 ± 0.01 | |
| S3 | CS | 0.09 ± 0.00 | 3.17 ± 0.01 | 0.14 ± 0.00 | 0.05 ± 0.00 | 4.72 ± 0.01 | 0.75 ± 0.00 | ND | 0.52 ± 0.00 | 0.06 ± 0.00 | 0.17 ± 0.00 | 0.34 ± 0.00 | 0.03 ± 0.00 | 10.04 ± 0.01 |
| PCS | 0.45 ± 0.03 | 2.22 ± 0.15 | 0.29 ± 0.02 | 0.06 ± 0.00 | 4.01 ± 0.21 | 0.62 ± 0.01 | 0.07 ± 0.00 | 0.55 ± 0.01 | 0.05 ± 0.00 | 0.17 ± 0.01 | 0.30 ± 0.02 | 0.03 ± 0.00 | 8.82 ± 0.25 | |
| S4 | CS | 0.09 ± 0.00 | 3.76 ± 0.01 | 0.16 ± 0.00 | 0.06 ± 0.00 | 4.77 ± 0.00 | 0.77 ± 0.00 | ND | 0.83 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.09 ± 0.00 | DUL | 10.66 ± 0.00 |
| PCS | 0.54 ± 0.00 | 2.77 ± 0.06 | 0.33 ± 0.00 | 0.07 ± 0.00 | 4.09 ± 0.07 | 0.65 ± 0.01 | 0.08 ± 0.00 | 1.15 ± 0.08 | 0.07 ± 0.01 | 0.12 ± 0.00 | 0.29 ± 0.03 | DUL | 10.16 ± 0.17 | |
| S5 | CS | 0.03 ± 0.00 | 1.76 ± 0.01 | 0.05 ± 0.00 | DUL | 3.81 ± 0.01 | 0.42 ± 0.00 | ND | DUL | DUL | 0.02 ± 0.00 | DUL | ND | 6.09 ± 0.01 |
| PCS | 0.24 ± 0.01 | 1.52 ± 0.05 | 0.16 ± 0.00 | 0.03 ± 0.00 | 3.53 ± 0.06 | 0.35 ± 0.01 | DUL | DUL | 0.02 ± 0.00 | 0.08 ± 0.00 | DUL | DUL | 5.93 ± 0.07 | |
| S6 | CS | 0.11 ± 0.00 | 3.67 ± 0.02 | 0.09 ± 0.00 | 0.05 ± 0.00 | 4.39 ± 0.01 | 0.60 ± 0.01 | ND | 0.40 ± 0.00 | 0.04 ± 0.00 | 0.09 ± 0.01 | DUL | ND | 9.44 ± 0.03 |
| PCS | 0.57 ± 0.05 | 2.84 ± 0.05 | 0.33 ± 0.02 | 0.09 ± 0.00 | 3.97 ± 0.03 | 0.50 ± 0.01 | 0.08 ± 0.00 | 0.56 ± 0.00 | 0.05 ± 0.00 | 0.13 ± 0.01 | DUL | DUL | 9.12 ± 0.04 | |
| S7 | CS | 0.08 ± 0.01 | 3.36 ± 0.01 | 0.15 ± 0.00 | 0.08 ± 0.00 | 3.66 ± 0.00 | 0.72 ± 0.00 | ND | 0.72 ± 0.00 | 0.06 ± 0.00 | 0.13 ± 0.00 | 0.11 ± 0.00 | DUL | 9.07 ± 0.03 |
| PCS | 0.35 ± 0.07 | 2.44 ± 0.17 | 0.25 ± 0.04 | 0.07 ± 0.01 | 3.00 ± 0.11 | 0.54 ± 0.02 | 0.07 ± 0.00 | 0.71 ± 0.05 | 0.05 ± 0.00 | 0.12 ± 0.01 | 0.13 ± 0.02 | DUL | 7.73 ± 0.18 | |
| S8 | CS | 0.11 ± 0.01 | 3.30 ± 0.01 | 0.15 ± 0.02 | 0.06 ± 0.00 | 4.86 ± 0.01 | 0.67 ± 0.00 | ND | 0.79 ± 0.00 | 0.06 ± 0.03 | 0.11 ± 0.00 | 0.38 ± 0.00 | 0.03 ± 0.00 | 10.52 ± 0.06 |
| PCS | 0.37 ± 0.05 | 2.62 ± 0.12 | 0.22 ± 0.03 | 0.07 ± 0.00 | 4.33 ± 0.23 | 0.56 ± 0.04 | 0.07 ± 0.00 | 0.76 ± 0.05 | 0.06 ± 0.01 | 0.11 ± 0.01 | 0.34 ± 0.02 | DUL | 9.51 ± 0.32 | |
| S9 | CS | 0.11 ± 0.01 | 2.98 ± 0.02 | 0.12 ± 0.00 | 0.03 ± 0.00 | 6.45 ± 0.01 | 0.60 ± 0.03 | ND | DUL | 0.03 ± 0.00 | 0.07 ± 0.00 | DUL | ND | 10.39 ± 0.07 |
| PCS | 0.48 ± 0.02 | 2.32 ± 0.10 | 0.25 ± 0.01 | 0.03 ± 0.01 | 5.75 ± 0.18 | 0.55 ± 0.02 | 0.05 ± 0.00 | DUL | 0.03 ± 0.00 | 0.10 ± 0.01 | DUL | DUL | 9.56 ± 0.19 | |
| S10 | CS | 0.05 ± 0.00 | 4.70 ± 0.04 | 0.13 ± 0.04 | 0.04 ± 0.01 | 4.38 ± 0.00 | 0.76 ± 0.00 | ND | 0.56 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.21 ± 0.00 | DUL | 10.94 ± 0.08 |
| PCS | 0.46 ± 0.04 | 3.42 ± 0.05 | 0.36 ± 0.03 | 0.05 ± 0.01 | 3.81 ± 0.11 | 0.63 ± 0.02 | 0.07 ± 0.00 | 0.66 ± 0.04 | 0.06 ± 0.00 | 0.08 ± 0.01 | 0.26 ± 0.02 | DUL | 9.86 ± 0.20 | |
| S11 | CS | 0.08 ± 0.00 | 4.00 ± 0.00 | 0.12 ± 0.00 | 0.04 ± 0.00 | 6.78 ± 0.00 | 0.83 ± 0.00 | ND | 0.77 ± 0.00 | 0.04 ± 0.00 | 0.09 ± 0.00 | 0.16 ± 0.00 | DUL | 12.91 ± 0.01 |
| PCS | 0.41 ± 0.01 | 3.05 ± 0.09 | 0.28 ± 0.01 | 0.04 ± 0.01 | 5.83 ± 0.15 | 0.64 ± 0.01 | 0.07 ± 0.00 | 0.76 ± 0.03 | 0.06 ± 0.01 | 0.13 ± 0.01 | 0.19 ± 0.01 | DUL | 11.46 ± 0.14 | |
| S12 | CS | 0.10 ± 0.00 | 3.06 ± 0.01 | 0.22 ± 0.00 | 0.07 ± 0.00 | 3.69 ± 0.01 | 0.66 ± 0.00 | ND | 1.08 ± 0.00 | 0.10 ± 0.00 | 0.12 ± 0.00 | 0.44 ± 0.00 | 0.04 ± 0.00 | 9.58 ± 0.01 |
| PCS | 0.47 ± 0.06 | 2.24 ± 0.32 | 0.30 ± 0.03 | 0.09 ± 0.01 | 3.28 ± 0.26 | 0.60 ± 0.03 | 0.08 ± 0.01 | 2.00 ± 0.84 | 0.05 ± 0.00 | 0.17 ± 0.05 | 1.21 ± 0.67 | 0.08 ± 0.03 | 10.57 ± 1.41 | |
| S13 | CS | 0.10 ± 0.01 | 2.67 ± 0.01 | 0.20 ± 0.00 | 0.07 ± 0.00 | 3.36 ± 0.01 | 0.72 ± 0.00 | ND | 2.52 ± 0.00 | 0.13 ± 0.00 | 0.24 ± 0.00 | 1.51 ± 0.00 | 0.09 ± 0.00 | 11.61 ± 0.01 |
| PCS | 0.42 ± 0.01 | 2.33 ± 0.12 | 0.26 ± 0.01 | 0.09 ± 0.02 | 3.32 ± 0.06 | 0.61 ± 0.04 | 0.08 ± 0.01 | 2.07 ± 0.83 | 0.06 ± 0.01 | 0.17 ± 0.04 | 1.24 ± 0.66 | 0.09 ± 0.03 | 10.74 ± 1.55 | |
| S14 | CS | 0.07 ± 0.01 | 3.09 ± 0.01 | 0.09 ± 0.00 | 0.06 ± 0.00 | 4.21 ± 0.02 | 0.62 ± 0.00 | ND | 1.06 ± 0.00 | 0.06 ± 0.00 | 0.14 ± 0.01 | 0.55 ± 0.00 | 0.04 ± 0.00 | 9.99 ± 0.05 |
| PCS | 0.37 ± 0.01 | 2.51 ± 0.03 | 0.25 ± 0.01 | 0.11 ± 0.00 | 3.69 ± 0.08 | 0.52 ± 0.02 | 0.07 ± 0.00 | 0.95 ± 0.05 | 0.05 ± 0.01 | 0.14 ± 0.01 | 0.46 ± 0.04 | 0.03 ± 0.00 | 9.15 ± 0.11 | |
| S15 | CS | 0.08 ± 0.00 | 4.08 ± 0.02 | 0.17 ± 0.02 | 0.05 ± 0.00 | 4.22 ± 0.00 | 0.71 ± 0.00 | ND | 0.59 ± 0.00 | 0.06 ± 0.00 | 0.12 ± 0.00 | 0.13 ± 0.00 | DUL | 10.21 ± 0.04 |
| PCS | 0.40 ± 0.03 | 3.38 ± 0.07 | 0.32 ± 0.02 | 0.11 ± 0.00 | 3.70 ± 0.03 | 0.60 ± 0.01 | 0.07 ± 0.00 | 0.67 ± 0.02 | 0.06 ± 0.01 | 0.15 ± 0.00 | 0.13 ± 0.01 | DUL | 9.59 ± 0.05 | |
| S16 | CS | 0.09 ± 0.01 | 3.28 ± 0.01 | 0.19 ± 0.00 | 0.09 ± 0.00 | 3.43 ± 0.01 | 0.87 ± 0.01 | ND | 2.67 ± 0.00 | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.95 ± 0.00 | 0.06 ± 0.00 | 12.01 ± 0.03 |
| PCS | 0.39 ± 0.02 | 2.70 ± 0.05 | 0.26 ± 0.01 | 0.21 ± 0.00 | 3.28 ± 0.09 | 0.71 ± 0.03 | 0.10 ± 0.01 | 2.21 ± 0.02 | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.72 ± 0.02 | 0.06 ± 0.00 | 10.93 ± 0.16 | |
| S17 | CS | 0.08 ± 0.00 | 3.16 ± 0.00 | 0.14 ± 0.00 | 0.04 ± 0.00 | 5.11 ± 0.00 | 0.65 ± 0.00 | ND | 0.40 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.19 ± 0.00 | DUL | 9.90 ± 0.01 |
| PCS | 0.38 ± 0.01 | 2.57 ± 0.05 | 0.24 ± 0.01 | 0.06 ± 0.00 | 4.61 ± 0.07 | 0.57 ± 0.01 | 0.07 ± 0.00 | 0.38 ± 0.02 | 0.07 ± 0.00 | 0.11 ± 0.00 | 0.17 ± 0.02 | DUL | 9.23 ± 0.12 | |
| S18 | CS | 0.08 ± 0.00 | 3.89 ± 0.02 | 0.14 ± 0.00 | 0.05 ± 0.00 | 3.70 ± 0.02 | 0.73 ± 0.00 | ND | 0.74 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.12 ± 0.00 | DUL | 9.57 ± 0.06 |
| PCS | 0.41 ± 0.14 | 2.74 ± 0.27 | 0.30 ± 0.09 | 0.11 ± 0.02 | 3.03 ± 0.11 | 0.59 ± 0.01 | 0.08 ± 0.01 | 0.75 ± 0.02 | 0.11 ± 0.00 | 0.11 ± 0.03 | 0.15 ± 0.01 | DUL | 8.38 ± 0.11 | |
| S19 | CS | 0.09 ± 0.00 | 3.04 ± 0.01 | 0.20 ± 0.00 | 0.05 ± 0.00 | 4.65 ± 0.01 | 0.66 ± 0.00 | ND | 0.66 ± 0.00 | 0.06 ± 0.00 | 0.11 ± 0.00 | 0.34 ± 0.00 | DUL | 9.86 ± 0.01 |
| PCS | 0.42 ± 0.04 | 2.05 ± 0.11 | 0.26 ± 0.02 | 0.07 ± 0.01 | 3.81 ± 0.14 | 0.52 ± 0.02 | 0.09 ± 0.03 | 0.57 ± 0.02 | 0.07 ± 0.00 | 0.11 ± 0.01 | 0.28 ± 0.01 | DUL | 8.25 ± 0.22 | |
| S20 | CS | 0.07 ± 0.00 | 4.38 ± 0.01 | 0.10 ± 0.01 | 0.06 ± 0.00 | 4.11 ± 0.01 | 0.74 ± 0.00 | ND | 0.54 ± 0.00 | 0.03 ± 0.01 | 0.12 ± 0.00 | 0.42 ± 0.00 | DUL | 10.57 ± 0.02 |
| PCS | 0.54 ± 0.06 | 2.92 ± 0.15 | 0.41 ± 0.04 | 0.09 ± 0.01 | 3.38 ± 0.10 | 0.60 ± 0.02 | 0.09 ± 0.01 | 0.61 ± 0.09 | 0.09 ± 0.00 | 0.15 ± 0.02 | 0.44 ± 0.02 | 0.05 ± 0.00 | 9.37 ± 0.12 | |
| S21 | CS | 0.09 ± 0.01 | 3.63 ± 0.00 | 0.19 ± 0.00 | 0.06 ± 0.00 | 3.94 ± 0.00 | 0.66 ± 0.00 | ND | 0.66 ± 0.00 | 0.12 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | DUL | 9.55 ± 0.01 |
| PCS | 0.45 ± 0.07 | 2.61 ± 0.03 | 0.28 ± 0.04 | 0.12 ± 0.01 | 3.35 ± 0.03 | 0.55 ± 0.01 | 0.10 ± 0.01 | 0.87 ± 0.11 | 0.15 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.00 | DUL | 8.71 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Xu, C.-L.; Zhu, Y.-J.; Tian, Y.-W.; Liu, X.; Gao, X.; Qin, K.-M.; Li, W.-D. Quality Evaluation of Crude and Salt-Processed Cuscutae Semen through Qualitative and Quantitative Analysis of Multiple Components Using HPLC Combined with Chemometrics. Separations 2022, 9, 231. https://doi.org/10.3390/separations9090231
Shi Y, Xu C-L, Zhu Y-J, Tian Y-W, Liu X, Gao X, Qin K-M, Li W-D. Quality Evaluation of Crude and Salt-Processed Cuscutae Semen through Qualitative and Quantitative Analysis of Multiple Components Using HPLC Combined with Chemometrics. Separations. 2022; 9(9):231. https://doi.org/10.3390/separations9090231
Chicago/Turabian StyleShi, Yun, Chang-Li Xu, Yu-Jie Zhu, Yi-Wen Tian, Xiao Liu, Xun Gao, Kun-Ming Qin, and Wei-Dong Li. 2022. "Quality Evaluation of Crude and Salt-Processed Cuscutae Semen through Qualitative and Quantitative Analysis of Multiple Components Using HPLC Combined with Chemometrics" Separations 9, no. 9: 231. https://doi.org/10.3390/separations9090231
APA StyleShi, Y., Xu, C.-L., Zhu, Y.-J., Tian, Y.-W., Liu, X., Gao, X., Qin, K.-M., & Li, W.-D. (2022). Quality Evaluation of Crude and Salt-Processed Cuscutae Semen through Qualitative and Quantitative Analysis of Multiple Components Using HPLC Combined with Chemometrics. Separations, 9(9), 231. https://doi.org/10.3390/separations9090231





