Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Samples
2.2. Preparation of Standard Solutions
2.3. Sample Preparation
2.4. Validation Procedure
2.5. Instrumentation and Experimental Conditions
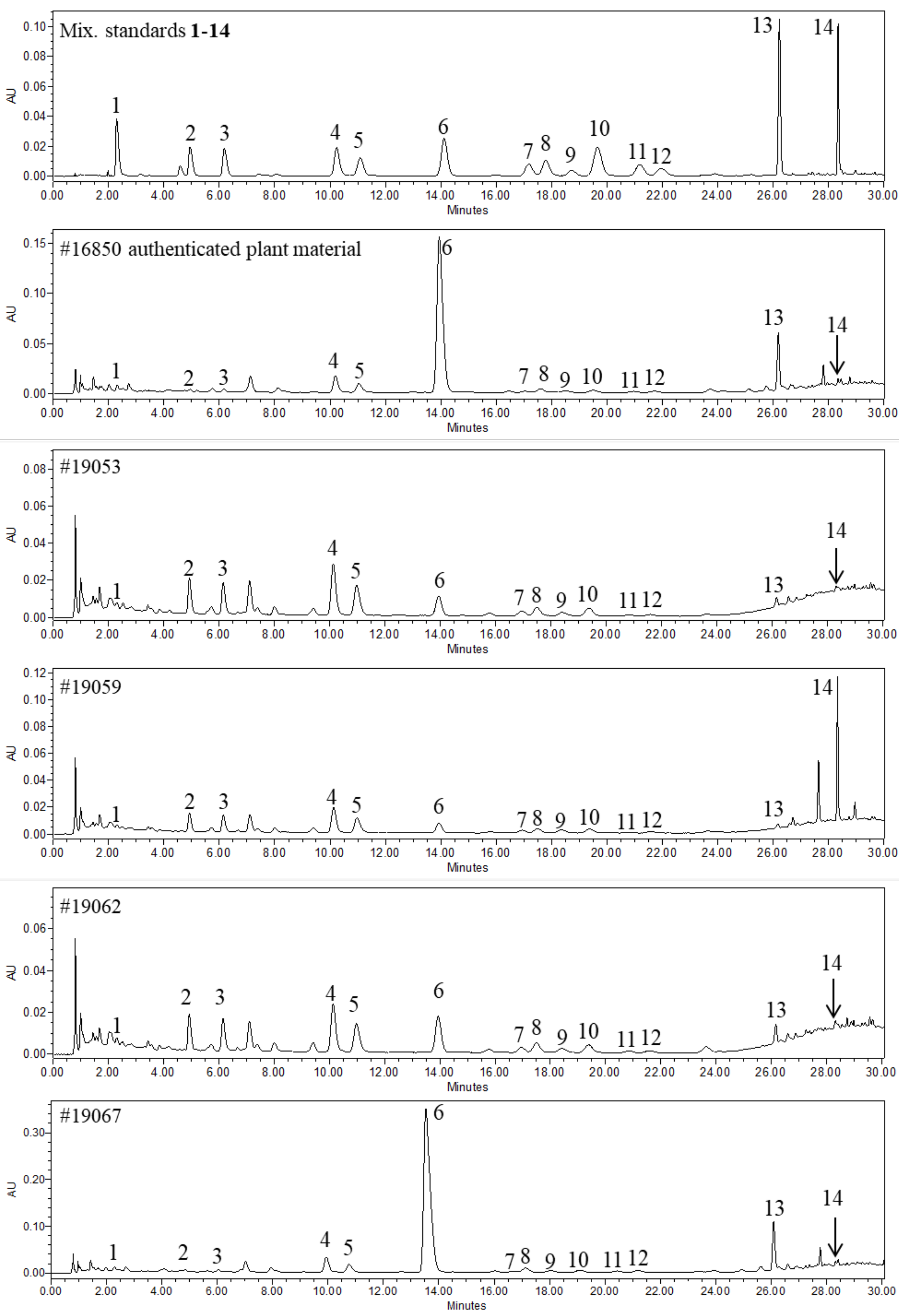
3. Results
3.1. Sample Preparation
3.2. Method Development and Optimization
3.3. Method Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Gorelik, B. The history and ethnobotany of Cape herbal teas. S. Afr. J. Bot. 2017, 110, 18–38. [Google Scholar] [CrossRef]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Khan, A.U.; Ghayur, M.N.; Ali, S.F.; Herzig, J.W. Antispasmodic effects of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+ -channel activation. Basic Clin. Pharmacol. Toxicol. 2006, 99, 365–373. [Google Scholar] [CrossRef]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of South African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Johnson, R.; Beer, D.; Dludla, P.V.; Ferreira, D.; Muller, C.J.F.; Joubert, E. Aspalathin from Rooibos (Aspalathus linearis): A Bioactive C-glucosyl Dihydrochalcone with Potential to Target the Metabolic Syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef]
- Krafczyk, N.; Heinrich, T.; Porzel, A.; Glomb, M.A. Oxidation of the dihydrochalcone aspalathin leads to dimerization. J. Agric. Food Chem. 2009, 57, 6838–6843. [Google Scholar] [CrossRef]
- Marais, C.; van Rensburg, W.J.; Ferreira, D.; Steenkamp, J.A. (S)- and (R)-eriodictyol-6-C-beta-D-glucopyranoside, novel keys to the fermentation of rooibos (Aspalathus linearis). Phytochemistry 2000, 55, 43–49. [Google Scholar] [CrossRef]
- Ajuwon, O.R.; Ayeleso, A.O.; Adefolaju, G.A. The Potential of South African Herbal Tisanes, Rooibos and Honeybush in the Management of Type 2 Diabetes Mellitus. Molecules 2018, 23, 3207. [Google Scholar] [CrossRef]
- Schulz, H.; Joubert, E.; Schütze, W. Quantification of quality parameters for reliable evaluation of green rooibos (Aspalathus linearis). Eur. Food Res. Technol. 2003, 216, 539–543. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D. Phenolic content and antioxidant activity of rooibos food ingredient extracts. J. Food Compos. Anal. 2012, 27, 45–51. [Google Scholar] [CrossRef]
- Waridel, P.; Wolfender, J.-L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef]
- Philbin, C.S.; Schwartz, S.J. Resolution of diastereomeric flavonoid (1S)-(-)-camphanic acid esters via reversed-phase HPLC. Phytochemistry 2007, 68, 1206–1211. [Google Scholar] [CrossRef]
- Iswaldi, I.; Arraez-Roman, D.; Rodriguez-Medina, I.; Beltran-Debon, R.; Joven, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Identification of phenolic compounds in aqueous and ethanolic rooibos extracts (Aspalathus linearis) by HPLC-ESI-MS (TOF/IT). Anal. Bioanal. Chem. 2011, 400, 3643–3654. [Google Scholar] [CrossRef]
- Bramati, L.; Minoggio, M.; Gardana, C.; Simonetti, P.; Mauri, P.; Pietta, P. Unfermented Rooibos Tea: Quantitative Characterization of Flavonoids by HPLC−UV and Determination of the Total Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 7472–7474. [Google Scholar] [CrossRef]
- Bramati, L.; Minoggio, M.; Gardana, C.; Simonetti, P.; Mauri, P.; Pietta, P. Quantitative characterization of flavonoid compounds in Rooibos tea (Aspalathus linearis) by LC-UV/DAD. J. Agric. Food Chem. 2002, 50, 5513–5519. [Google Scholar] [CrossRef]
- Beelders, T.; Kalili, K.M.; Joubert, E.; de Beer, D.; de Villiers, A. Comprehensive two-dimensional liquid chromatographic analysis of rooibos (Aspalathus linearis) phenolics. J. Sep. Sci. 2012, 35, 1808–1820. [Google Scholar] [CrossRef]
- Walters, N.A.; de Villiers, A.; Joubert, E.; de Beer, D. Phenolic profiling of rooibos using off-line comprehensive normal phase countercurrent chromatographyxreversed phase liquid chromatography. J. Chromatogr. A 2017, 1490, 102–114. [Google Scholar] [CrossRef]
- Arries, W.J.; Tredoux, A.G.; de Beer, D.; Joubert, E.; de Villiers, A. Evaluation of capillary electrophoresis for the analysis of rooibos and honeybush tea phenolics. Electrophoresis 2017, 38, 897–905. [Google Scholar] [CrossRef]
- Stander, M.A.; Van Wyk, B.E.; Taylor, M.J.C.; Long, H.S. Analysis of Phenolic Compounds in Rooibos Tea (Aspalathus linearis) with a Comparison of Flavonoid-Based Compounds in Natural Populations of Plants from Different Regions. J. Agric. Food Chem. 2017, 65, 10270–10281. [Google Scholar] [CrossRef]
- Walters, N.A.; de Villiers, A.; Joubert, E.; de Beer, D. Improved HPLC method for rooibos phenolics targeting changes due to fermentation. J. Food Compos. Anal. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Beelders, T.; Sigge, G.O.; Joubert, E.; de Beer, D.; de Villiers, A. Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation. J. Chromatogr. A 2012, 1219, 128–139. [Google Scholar] [CrossRef]
- Fantoukh, O.I.; Dale, O.R.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Manda, V.K.; Khan, S.I.; Chittiboyina, A.G.; Viljoen, A.; Khan, I.A. Safety Assessment of Phytochemicals Derived from the Globalized South African Rooibos Tea (Aspalathus linearis) through Interaction with CYP, PXR, and P-gp. J. Agric. Food Chem. 2019, 67, 4967–4975. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH). ICH harmonised tripartite guideline—Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 10 November 2005; pp. 11–12. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 18 February 2019).
- Zeng, P.; Zhang, Y.; Pan, C.; Jia, Q.; Guo, F.; Li, Y.; Zhu, W.; Chen, K. Advances in studying of the pharmacological activities and structure–activity relationships of natural C-glycosylflavonoids. Acta Pharm. Sin. B 2013, 3, 154–162. [Google Scholar] [CrossRef][Green Version]
- Muller, C.J.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.C.; Ding, Y.; Green, I.R.; Gelderblom, W.C. Antioxidant activity of the dihydrochalcones Aspalathin and Nothofagin and their corresponding flavones in relation to other Rooibos (Aspalathus linearis) Flavonoids, Epigallocatechin Gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Avula, B.; Bae, J.Y.; Raman, V.; Fantoukh, O.I.; Wang, Y.H.; Osman, A.G.; Wang, M.; Ali, Z.; Khan, I.A. Quantification of Phenolic Compounds from Fadogia agrestis and Dietary Supplements using UHPLC-PDA-MS. Planta Med. 2019, 85, 145–153. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D. Chapter 14—Antioxidants of Rooibos Beverages: Role of Plant Composition and Processing. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 131–144. [Google Scholar] [CrossRef]
- De Beer, D.; Miller, N.; Joubert, E. Production of dihydrochalcone-rich green rooibos (Aspalathus linearis) extract taking into account seasonal and batch-to-batch variation in phenolic composition of plant material. S. Afr. J. Bot. 2017, 110, 138–143. [Google Scholar] [CrossRef]

| NCNPR Code | Dosage Form | Serving Size | Red/Green | Additional Notes | Plant Part |
|---|---|---|---|---|---|
| 20750 | Tea Bag | N/A | Red | N/A | leaves |
| 19070 | Tea Bag | 1 tea bag | N/A | USDA organic | N/A |
| 19069 | Powder | N/A | N/A | N/A | N/A |
| 19068 | Powder | 1 teaspoon | Red | USDA organic | N/A |
| 19067 | Tea Bag | N/A | green | USDA organic | N/A |
| 19066 | Powder | N/A | Red | N/A | N/A |
| 19065 | Powder | 1 teaspoon | N/A | USDA organic | N/A |
| 19064 | Powder | N/A | Red | N/A | leaves |
| 19063 | Powder | 2 g | N/A | USDA organic | leaves |
| 19062 | Powder | N/A | N/A | N/A | N/A |
| 19061 | Tea Bag | 1 tea bag | N/A | USDA organic—contains other herbs | leaves |
| 19060 | Tea Bag | N/A | Red | N/A | N/A |
| 19059 | Powder | 1.5 teaspoon | N/A | Contains other herbs | N/A |
| 19058 | Tea Bag | N/A | N/A | USDA organic | N/A |
| 19057 | Tea Bag | N/A | N/A | Contains other herbs | N/A |
| 19056 | Powder | N/A | Red | N/A | N/A |
| 19055 | Tea Bag | 1 tea bag (2 g) | N/A | USDA organic | leaves |
| 19054 | Tea Bag | N/A | N/A | USDA organic | leaves |
| 19053 | Tea Bag | N/A | Red | N/A | N/A |
| 19052 | Powder | N/A | N/A | N/A | leaves |
| 19051 | Powder | N/A | N/A | USDA organic—contains other herbs | N/A |
| Sample # | Compound Name | tR (min) | LOD (μg/mL) | LOD (mg/100 mg) | LOQ (μg/mL) | LOQ (mg/100 mg) | Molecular Weight | Molecular Formula | UV (nm) | m/z [M + H]+/[M + Na]+ | m/z [M − H]−/[M − H+ HCO2H]− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Syringin | 2.295 | 0.5 | 0.003 | 2 | 0.012 | 372.37 | C17H24O9 | 220,264,383 | 395.22 [M + Na]+ | 417.21 [M − H+ HCO2H]− |
| 2 | (S)-eriodictyol-6-C-β-d-glucopyranoside | 4.944 | 1 | 0.006 | 5 | 0.031 | 450.12 | C21H22O11 | 215,288,384 | 451.05 (frag., 433.17, 415.29, 331.01) | 449.26 (frag., 329.05) |
| 3 | (R)-eriodictyol-6-C-β-d-glucopyranoside | 6.187 | 1 | 0.006 | 5 | 0.031 | 450.12 | C21H22O11 | 215,288,384 | 451.11 (frag., 432.98, 415.16, 331.26) | 449.26 (frag., 329.07) |
| 4 | Isoorientin | 10.248 | 0.5 | 0.004 | 2 | 0.018 | 448.10 | C21H20O11 | 211,269,349 | 449.18 (frag., 329.13) | 447.24 |
| 5 | Orientin | 11.106 | 1 | 0.010 | 5 | 0.050 | 448.10 | C21H20O11 | 211,269,349 | 449.24 (frag., 329.47) | 447.35 |
| 6 | Aspalathin | 13.980 | 0.5 | 0.002 | 2 | 0.007 | 452.13 | C21H24O11 | 227,288 | 452.92 (frag., 434.98, 333.32) | 451.17 (frag., 330.94) |
| 7 | Vitexin | 17.164 | 1 | 0.010 | 5 | 0.050 | 432.10 | C21H20O10 | 214,268,337 | 433.17 (frag., 415.29, 313.01) | 431.13 |
| 8 | Bioquercetin | 17.768 | 1 | 0.004 | 5 | 0.020 | 610.15 | C27H30O16 | 255,353 | 611.20 (frag., 465.05, 303.12) | 609.34 |
| 9 | Hyperoside | 18.701 | 1 | 0.004 | 5 | 0.019 | 464.10 | C21H20O12 | 255,355 | 465.19 (frag., 303.04) | 463.11 |
| 10 | Isovitexin | 19.630 | 0.5 | 0.006 | 2 | 0.025 | 432.10 | C21H20O10 | 214,268,337 | 433.17 (frag., 415.10, 312.87) | 431.19 |
| 11 | Rutin | 21.156 | 1 | 0.007 | 5 | 0.030 | 610.15 | C27H30O16 | 255,353 | 611.11 (frag., 465.25, 303.11) | 609.36 |
| 12 | Isoquercitrin | 21.916 | 1 | 0.004 | 5 | 0.019 | 464.10 | C21H20O12 | 255,350 | 465.31 (frag., 303.11) | 463.25 |
| 13 | Nothofagin | 26.218 | 0.2 | 0.001 | 1 | 0.005 | 436.14 | C21H24O10 | 226,287 | 437.11 (frag., 419.29, 383.22, 341.14, 317.13) | 435.28 (frag., 315.11) |
| 14 | Thermopsoside | 28.338 | 0.5 | 0.005 | 2 | 0.020 | 462.12 | C22H22O11 | 252,348 | 463.23(frag., 301.09) | 461.28 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code # | |||||||||||||||
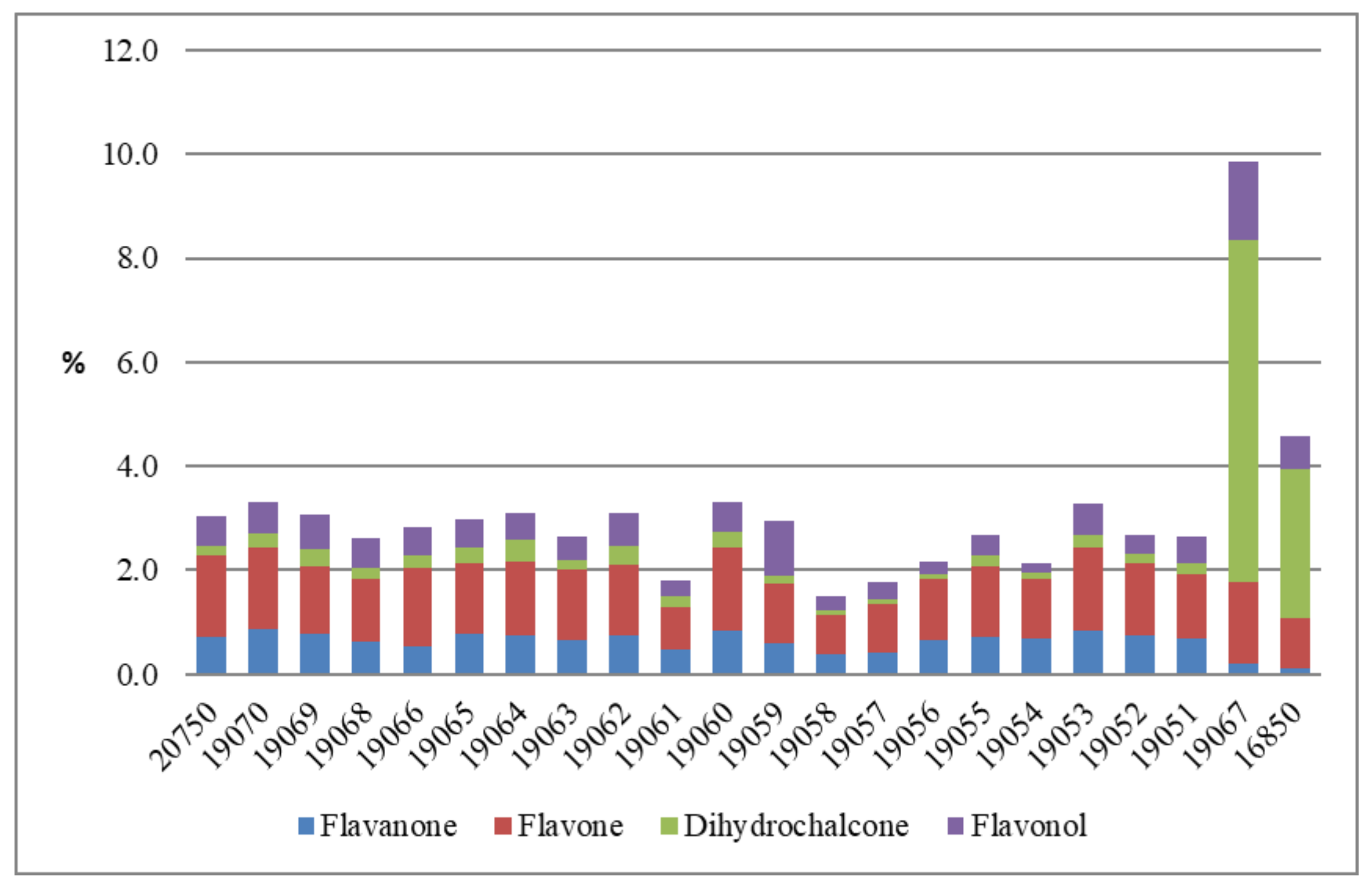
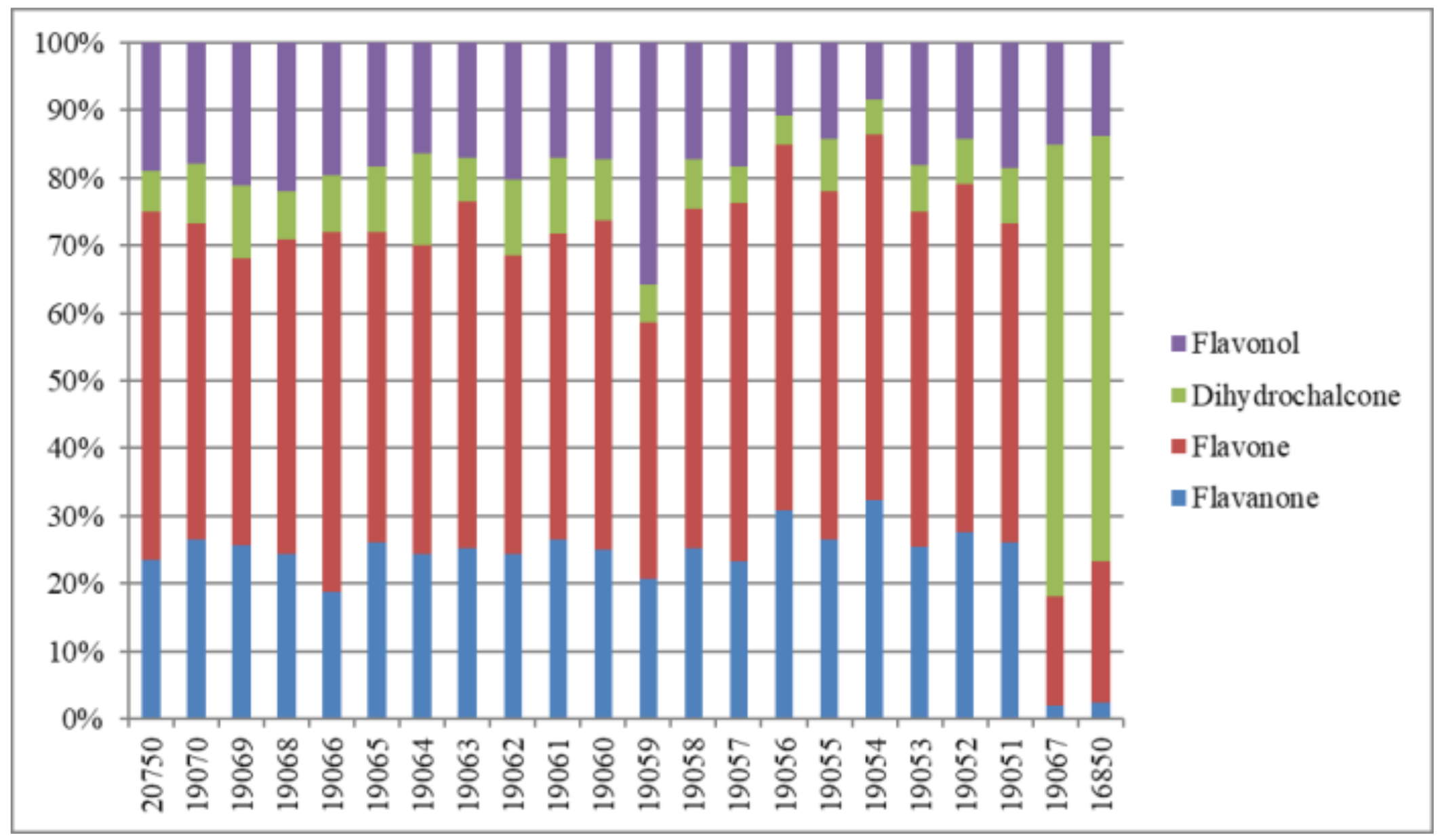
| 16850 | 0.05 | 0.04 | 0.07 | 0.42 | 0.41 | 2.58 | 0.07 | 0.16 | 0.21 | 0.06 | 0.08 | 0.14 | 0.30 | 0.04 | 4.58 |
| 20750 | 0.02 | 0.36 | 0.36 | 0.67 | 0.65 | 0.16 | 0.15 | 0.20 | 0.22 | 0.10 | 0.04 | 0.08 | 0.03 | 0.03 | 3.05 |
| 19070 | 0.03 | 0.44 | 0.43 | 0.69 | 0.64 | 0.25 | 0.12 | 0.16 | 0.23 | 0.11 | 0.06 | 0.12 | 0.04 | 0.02 | 3.31 |
| 19069 | 0.02 | 0.40 | 0.38 | 0.56 | 0.56 | 0.30 | 0.08 | 0.16 | 0.35 | 0.10 | 0.03 | 0.08 | 0.03 | 0.02 | 3.06 |
| 19068 | 0.01 | 0.32 | 0.31 | 0.52 | 0.53 | 0.17 | 0.08 | 0.23 | 0.24 | 0.08 | 0.03 | 0.06 | 0.02 | 0.02 | 2.61 |
| 19067 | 0.09 | 0.08 | 0.11 | 0.74 | 0.65 | 6.05 | 0.08 | 0.45 | 0.47 | 0.12 | 0.18 | 0.34 | 0.52 | 0.05 | 9.85 |
| 19066 | 0.01 | 0.26 | 0.27 | 0.63 | 0.64 | 0.21 | 0.14 | 0.19 | 0.25 | 0.10 | DUL | 0.08 | 0.03 | 0.03 | 2.81 |
| 19065 | 0.02 | 0.39 | 0.39 | 0.58 | 0.57 | 0.25 | 0.12 | 0.17 | 0.23 | 0.09 | 0.05 | 0.07 | 0.04 | DUL | 2.96 |
| 19064 | 0.03 | 0.38 | 0.37 | 0.61 | 0.59 | 0.37 | 0.12 | 0.14 | 0.21 | 0.09 | 0.05 | 0.09 | 0.05 | 0.03 | 3.10 |
| 19063 | 0.02 | 0.34 | 0.33 | 0.59 | 0.57 | 0.15 | 0.12 | 0.13 | 0.20 | 0.09 | DUL | 0.07 | 0.02 | 0.02 | 2.63 |
| 19062 | 0.02 | 0.38 | 0.38 | 0.58 | 0.57 | 0.30 | 0.12 | 0.18 | 0.27 | 0.09 | 0.05 | 0.09 | 0.05 | 0.02 | 3.09 |
| 19061 | 0.03 | 0.21 | 0.27 | 0.34 | 0.33 | 0.18 | 0.07 | 0.10 | 0.13 | 0.07 | 0.03 | 0.04 | 0.02 | DUL | 1.79 |
| 19060 | 0.02 | 0.42 | 0.41 | 0.70 | 0.67 | 0.26 | 0.14 | 0.18 | 0.24 | 0.11 | 0.05 | 0.09 | 0.04 | 0.02 | 3.32 |
| 19059 | 0.02 | 0.31 | 0.30 | 0.47 | 0.48 | 0.14 | 0.11 | 0.13 | 0.30 | 0.08 | 0.03 | 0.11 | 0.02 | 0.50 | 2.96 |
| 19058 | 0.02 | 0.17 | 0.21 | 0.30 | 0.30 | 0.10 | 0.08 | 0.07 | 0.12 | 0.06 | DUL | 0.05 | 0.01 | 0.02 | 1.50 |
| 19057 | 0.05 | 0.21 | 0.21 | 0.39 | 0.39 | 0.08 | 0.10 | 0.09 | 0.15 | 0.06 | DUL | 0.05 | 0.01 | 0.02 | 1.78 |
| 19056 | 0.01 | 0.34 | 0.33 | 0.49 | 0.49 | 0.08 | 0.11 | 0.05 | 0.13 | 0.07 | DUL | 0.03 | 0.02 | 0.02 | 2.16 |
| 19055 | 0.01 | 0.36 | 0.35 | 0.58 | 0.57 | 0.18 | 0.13 | 0.10 | 0.20 | 0.09 | DUL | 0.05 | 0.03 | 0.02 | 2.67 |
| 19054 | 0.01 | 0.35 | 0.34 | 0.47 | 0.50 | 0.09 | 0.11 | DUL | 0.11 | 0.07 | DUL | 0.03 | 0.02 | DUL | 2.10 |
| 19053 | 0.02 | 0.42 | 0.41 | 0.70 | 0.67 | 0.20 | 0.14 | 0.18 | 0.27 | 0.11 | 0.04 | 0.09 | 0.03 | 0.02 | 3.27 |
| 19052 | 0.01 | 0.37 | 0.37 | 0.58 | 0.58 | 0.16 | 0.13 | 0.10 | 0.19 | 0.09 | DUL | 0.06 | 0.02 | 0.02 | 2.69 |
| 19051 | 0.03 | 0.33 | 0.36 | 0.52 | 0.50 | 0.18 | 0.13 | 0.15 | 0.19 | 0.10 | 0.04 | 0.10 | 0.03 | 0.02 | 2.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. https://doi.org/10.3390/separations9070159
Fantoukh OI, Wang Y-H, Parveen A, Hawwal MF, Ali Z, Al-Hamoud GA, Chittiboyina AG, Joubert E, Viljoen A, Khan IA. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations. 2022; 9(7):159. https://doi.org/10.3390/separations9070159
Chicago/Turabian StyleFantoukh, Omer I., Yan-Hong Wang, Abidah Parveen, Mohammed F. Hawwal, Zulfiqar Ali, Gadah A. Al-Hamoud, Amar G. Chittiboyina, Elizabeth Joubert, Alvaro Viljoen, and Ikhlas A. Khan. 2022. "Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS" Separations 9, no. 7: 159. https://doi.org/10.3390/separations9070159
APA StyleFantoukh, O. I., Wang, Y.-H., Parveen, A., Hawwal, M. F., Ali, Z., Al-Hamoud, G. A., Chittiboyina, A. G., Joubert, E., Viljoen, A., & Khan, I. A. (2022). Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations, 9(7), 159. https://doi.org/10.3390/separations9070159









