Abstract
A review of the investigations devoted to the solvent extraction processes of metal ions with a chelating ligand thenoyltrifluoroacetone (HTTA) is presented herein. It seems that this molecule has been preferred in the field for more than half a century, and that it is used very often as an extractant for almost all metals. The main objective of the present review is also to provide an overview of the synergistic solvent extraction of lanthanoids, particularly with the use of a β-diketone−neutral mixture. Based on the previous published results in the open literature, the extraction efficiency has been examined in detail and discussed further mainly in terms of the corresponding equilibrium constants among other outlined, so-important parameters. Major conclusions on the role of ligating groups of extractants towards the mechanism, an improved extraction enhancement, and selectivity are additionally provided. The fact that ionic liquids (ILs) appear to be replacing volatile diluents in the field of the liquid–liquid extraction of metals, again with the participation of this β-diketone, is not surprising. As is well known, a very efficient and simple way to determine the stoichiometry of the extracted species in the organic phase is by the simple use of the slope analysis method; however, it is sometimes difficult to perform, either because it somehow requests good solubility of the ligand or because obtained slopes are quite often far from integer values in ILs.
1. Introduction
As a matter of fact, solvent extraction is perhaps obviously the most-common chemical technique used for the separation and purification of almost all metals presented in the periodic table, including various valuable transition d-cations as well as f-elements. In the liquid–liquid extraction processes of metallic species, the two phases should be immiscible, as they can be mixed together in all proportions to form two separate appearances at ambient temperature and pressure. However, the solvent extraction chemistry of metallic cations such as f-ions needs the use of suitable organic ligands to coordinate the targeted cation easily, to make it soluble in an organic phase, and then to allow the phase transfer from the aqueous phase to the organic one. Therefore, molecular design is likely the holy grail of scientific research. It has enjoyed a long history in the chemist’s agenda, providing fascinating molecules with unique architectures to affect the complexation and solvent extraction performance, as well as immense selectivity.
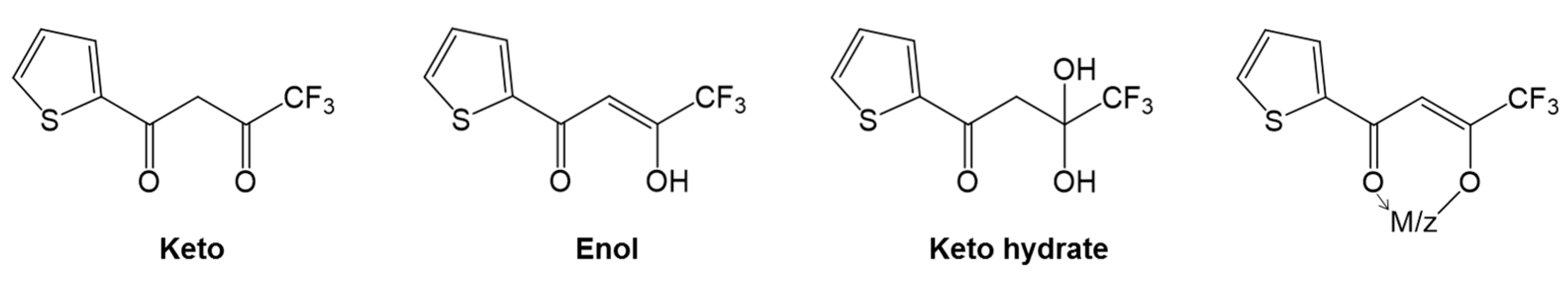
In general, the β-diketones are organic compounds bearing two carbonyl groups that are separated by one carbon atom and usually exhibit keto–enol tautomerism [1]; see Figure 1. In most β-diketones, the substituents on the α-carbon are hydrogen atoms, while the substituent connected to the carbonyl functions can be an alkyl or a fluorinated alkyl group, or an aromatic or a heteroaromatic group. The simplest molecule of this family is acetylacetone, in which both end substituents neighboring the carbonyls are methyl groups. For instance, thenoyltrifluoroacetone (HTTA) was introduced as an analytical reagent by Calvin and Reid in late 1947, and since then, it has been extensively exploited as an extractant for lanthanoid elements. It is a white crystalline compound with an acid dissociation constant of 6.7 × 10−7 at 25 °C. In fact, due to the presence of trifluoromethyl group in its chemical structure, it has high acidity in the enol form, which, in turn, is very useful in the solvent extraction process of various metals at low pH [2].
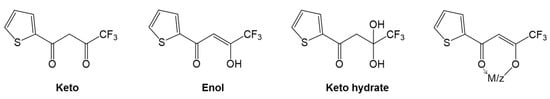
Figure 1.
Structural formulas of the chelating extractant thenoyltrifluoroacetone (HTTA).
The presence of electron-withdrawing groups such as −CF3 de facto favors the enol form. The choice of HTTA as a chelating agent in the preference to other β-dicarbonyl compounds is based on the following reasons [1]: (i) first of all, HTTA forms complexes with many metal ions more readily that acetylacetone (pKa = 9.7). In fact, HTTA (pKa = 6.2) is less basic and, hence, yields more stable chelates; (ii) secondly, HTTA chelates are, of course, insoluble in neutral and to some extent in acidic aqueous solutions, but are soluble in neutral aromatic hydrocarbons or similar non-polar organic diluents; (iii) thirdly, HTTA chelates are stable in highly acidic media and are non-volatile at room temperature. Therefore, this attractive chelating compound has been widely used over the years for the solvent extraction of s- [3], p- [4], d-ions [5], lanthanoids [6,7,8], and actinoids [9,10]. In general, the scientific investigations in the field have been carried out using a variety of organic diluents and different ionic media and ionic strengths in the aqueous phase. Nevertheless, this molecule continues to play a major role in the studies of metal complexations, although later on, the scenario changed over time. Therefore, this review deals with the basics of solvent extraction techniques implementing HTTA molecules and discusses in detail their applications in the field, focusing on the recovery of important or valuable metals. For instance, greater emphasis will be placed on interesting details, taking into account newer science implementing ionic liquids (ILs) being considered for advanced applications. This work covers the literature until the end of April 2022, and to the best of the author’s knowledge, no reviews highlighting this specific concept overview of a chelating extractant vis á vis various metals, and particularly 4f-ions in solvent extraction chemistry, have been previously published.
2. Presentation of Equilibrium Data and Evaluation Criteria
The example presented here, to illustrate a scheme of the solvent extraction process of trivalent lanthanoid ions (Ln3+) with chelating ligand (HL) alone and involving an additional ligand, i.e., synergist (S), can be represented by the following equilibria:
where the subscripts “aq” and “o” denote aqueous and organic phases, respectively.
Ln3+(aq) + 3HL(o) ⇌ LnL3(o) + 3H+(aq)
Ln3+(aq) + 4HL(o) ⇌ LnL3·HL(o) + 3H+(aq)
Ln3+(aq) + 3HL(o) + nS(o) ⇌ LnL3∙nS(o) + 3H+ (aq)
Thus, the formation of mixed complexes in the organic phase can be described by the following two equations:
LnL3(o) + nS(o) ⇌ LnL3∙nS(o)
LnL3·HL(o) + nS(o) ⇌ LnL3·nS(o) + HL
The corresponding equilibrium constants KL, KL,S, and βL,S are usually given by the expressions below. The square brackets denote the molar concentration of the corresponding species. Actually, the equilibrium constants KL, KL,S, and βL,S are concentration constants and, therefore, are based on the assumption that the activity coefficients of the species do not change significantly under the experimental conditions employed. This means that the activity coefficients were held (approximately) constant during measurements. Thus, such constants are strictly valid only in the chosen electrolyte at the stated ionic strength.
Quantitatively speaking, the distribution ratio of metallic species (M) at equilibrium can be defined as
where Vaq and Vo are the volumes of the two liquid phases. This equation gives the ratio of the total (analytical) concentration of a solute in the extract (regardless of its chemical form) to its total (analytical) concentration in the other phase (usually aqueous). Of course, with a phase ratio of unity, the completeness of extraction should be 99.9%, at an error of less than 0.1%: this means a distribution ratio of ≥103 is required [11].
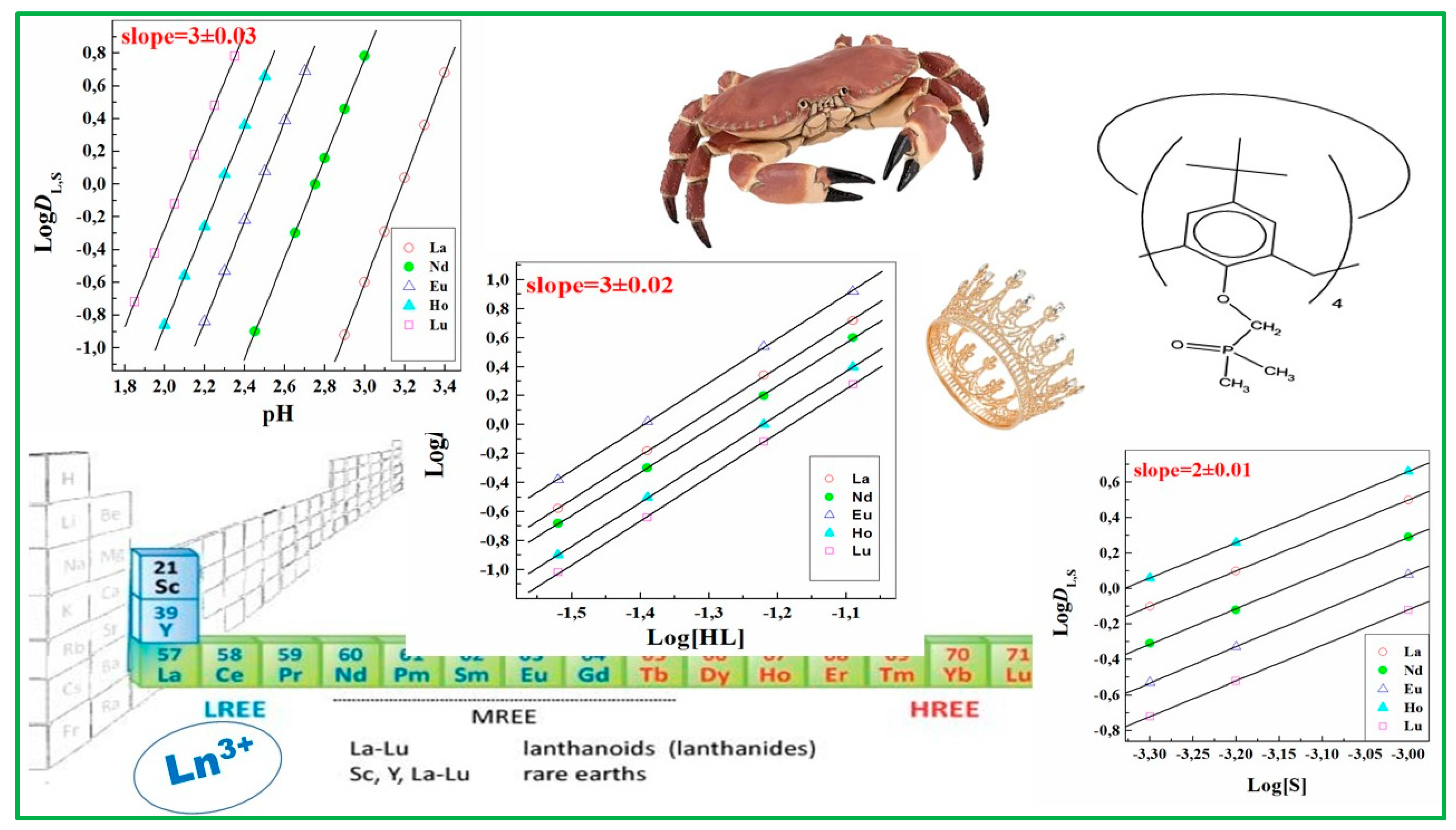
As an illustration, the synergistic solvent extraction of lanthanoids, i.e., with a combination of two ligands (HL−S), was usually studied using a traditional and effective means, called “slope analysis”, to obtain both stoichiometric and equilibrium constant information about the chemical extraction process. It is based on an examination of the variation of DL,S (the distribution ratio due to the synergistic effect) as a function of the relevant experimental variables. As lanthanoid extraction with the second ligand (S) alone is often negligible under experimental conditions, the values of D obtained experimentally are the sum of DL,S and DL (DL is the distribution ratio due to the lanthanoid extraction with HL alone under the same experimental conditions) [12]. So, the values of DL,S were calculated as D–DL. A log-log plot of DL,S vs. one of the variables [H+], [HL] and [S], keeping the other two constant, indicates the stoichiometry of the extractable complex, and thus leads to the derivation of a suitable equilibrium expression and then to the calculation of the equilibrium constant [12]. One example concerning the slope analysis application for lanthanoids solvent extraction with a mixed system combining HTTA and a synergistic agent, a calixarene molecule, is presented in Figure 2.
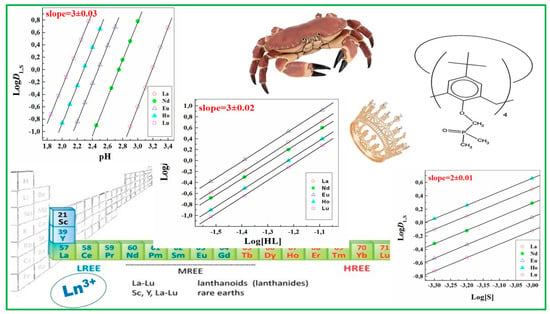
Figure 2.
LogDL,S vs. pH for the extraction of lanthanoid ions with mixtures of HL(HTTA)−S at [HL] = 6 × 10−2 mol/dm3 and [S] = 1 × 10−3 mol/dm3. LogDL,S vs. [HL] for the extraction of lanthanoid ions with mixtures of HL−S at [S] = 1 × 10−3 mol/dm3; La, pH = 3.30; Nd, pH = 2.75; Eu, pH = 2.65; Ho, pH = 2.35; Lu, pH = 2.05. LogDL,S vs. [S] for the extraction of lanthanoid ions with mixtures of HL−S at [HL] = 6 × 10−2 mol/dm3; La, pH = 3.35; Nd, pH = 2.85; Eu, pH = 2.50; Ho, pH = 2.50; Lu, pH = 2.05. Adaptation from Ref. [13] with permission.
If the extractant concentration is constant and hydrolysis in the aqueous phase and polymerization in the organic phase are both negligible, then the plots will be straight lines and their slopes will give the number of the ligands in the adducts formed. Indeed, the presence of anomalous points or outliers in experimental data can modify the plotting of these straight lines and therefore the values of the stoichiometry and stability constant obtained. Some other analytical methods used to establish formulas of species include IR, UV-Vis, and NMR spectra, together with the production and analysis of solids that might be produced [14]. The different methods are not equally sensitive to the concentrations of various species, so the adequacy and precision of the experimental work are somehow essential for the reliability of equilibrium constants, as the source of the greatest error is often the inadequacy of the experimental method used. Indeed, however, even an ideal experimental method will provide, of course, only poor data if the experimental work is not performed satisfactorily [15].
Some limitations of slope analysis arise from possible side reactions in the aqueous or organic phases as well as due to the interaction between extractants [16]. In point of fact, almost always, the aqueous phase contains inorganic (ClO4−, Cl−, NO3−, SO42−) or other anions, which to a certain degree complex with metal ions. However, both selectivity in extraction and efficiency in process design often rely on chemical reactions occurring in or moderated by the aqueous medium. By virtue of their high charge/radius ratios, the f-elements are strongly hydrated in aqueous solutions. Consequently, the nature of the extracted species depends on the acid being used (HCl, HNO3, HClO4, H2SO4) and its concentration in the aqueous phase [17]. It is common for highly charged metal ions to undergo strong interaction with anions in aqueous solution and give many kinds of metal complexes. Generally, the slope analysis method will also not give good results in solvent extraction chemistry if some impurities are presented in the organic phase that are likely to form addition compounds with extractants. As it is a well-known fact that the chelating agents can be regarded as weak acids, while neutral donors are bases, there is a possibility for interaction between them in the organic phase, which will reduce the concentrations of free ligands and particularly result in an antagonistic effect in some cases [18,19]. It can be assumed that this type of association between the reagents should decrease the concentration of both of them in the organic phase, making them available for the chelate extraction and further for adduct formation, and this should not decrease the synergistic enhancement at all [20].
For instance, the reliability of published equilibrium constants in the open literature for the formation of complexes by various ligand combinations with metal ions in solution has been evaluated using the criteria adopted in previous IUPAC publications in this area [21,22,23,24,25,26,27]. It may be worthwhile to mention several points again. A clear definition of reported constants and an unambiguous specification of the metal complex stoichiometry relies on:
- ✓
- The extent to which essential reaction conditions have been specified. These include the purity of the ligand(s), temperature, ionic strength, nature of the supporting electrolyte, reaction kinetics, ligand:metal ratios, etc. The extractant concentration range must be specified absolutely as well. Attention must be paid to the achievement of a clean separation, so that neither of the liquid phases are contaminated by the other, i.e., to prevent entrainment of less than 0.1% of the wrong liquid phase [11];
- ✓
- The soundness of apparatus calibration and the specification of whether concentration or mixed constants were calculated;
- ✓
- The maintenance of constant temperature and ionic strength during the experimental work-up;
- ✓
- Reliable mathematical treatment of the experimental data;
- ✓
- Correct selection of auxiliary data from the literature;
- ✓
- Details of the calculation method applied. As a whole, the experimental method applied and the numerical analysis chosen by authors are considered to have minimal systematic errors.
It is advisable for the reader to use extraction constants obtained under well-identified experimental conditions because, in spite of the numerous studies, recommended values can be offered in only a few cases, taking into account the diversity of ligands and experimental conditions for a given metal. Another obvious reason for this is associated with an inadequate description of chemical equilibria in a particular solvent extraction system, especially one implementing IL compounds.
For each solvent extraction system, information is provided for the chemical reaction, the equilibrium constant, the temperature, the composition of the aqueous and the organic phases, the homogeneous equilibria involving the acid dissociation of the main extractant in the aqueous phase, and, of course, the reference to the original published source. The organic diluent used must be also specified [28]. Generally, concentrations are expressed in mol dm−3, mol/dm3 (M). It is important that the total concentrations of the metal ion(s), ligand(s), and hydrogen ion should be within ranges that are relevant to the particular equilibria. In other words, beyond certain concentration ranges, additional equilibria may need to be taken into consideration [15]. All stability constants were usually reported in terms of concentrations.
However, this concept is not always sufficient when it comes to describing the chemical extraction mechanisms fully. Sometimes, a complete description of the organization of the species in the two solutions (a/o) is required, especially when the solvent systems are very complicated. More and more recent work calls for a multi-scale approach, which consists not only in simply determining the stoichiometry of the extracted complexes in the organic phase, but also in probing the interactions between the ligands, as well as investigating in detail the supramolecular organization of the metal complexes. Obviously, a spectra-assisted interpretation is necessary and highly desirable to cope with synergistic systems in which association can occur and several product species may form, but identifying each one can be a difficult task [29].
3. Liquid–Liquid Extraction of Metal Ions with 1-(2-Thienyl)-4,4,4-Trifluoro-1,3-Butanedione (HTTA) Alone
A summary of HTTA extraction coefficients has been published by Poskanzer and Foreman in the distant year 1961, in which a compilation of pH values of 50 percent extraction by 0.2 mol dm−3 HTTA in benzene was presented for around 48 elements in the form of a periodic table [6]. A graph is given, by which one may quickly estimate the percent extraction as a function of pH. Another interesting and useful aspect observed and not explained at the time that seems to warrant further investigation according to the authors is the fact that an increase in acidity leads to an increase in the solvent extraction process.
First, it must be stressed that the separation of Eu3+ and Am3+ ions has been studied by solvent extraction and the following reaction was proposed by Sekine and Dyrssen in late 1964 [30]:
M3+(aq) + (3+n)HA(o) ⇌ MA3(HA)n (o) + 3H+(aq)
The distribution of the chelate complexes of these metal ions of between 0.1 mol dm−3 NaClO4 and chloroform or methyl isobutyl ketone, with approximately nineteen chelating acids, has been determined. Even though no information on the hydrolysis of the tracer concentrations of lanthanoid or actinoid ions is available, it may be concluded from studies at higher metal concentrations that no hydrolysis occurs below a pH range of ca. 5–6. All experiments were, therefore, carried out at a pH below 5.5. The net distribution ratio, D, was determined between 0.01 and 100. Among the studied acids, it was observed that some extract Eu3+ into the organic phase better than Am3+, some extract Am3+ better, and some extract them equally well. Further, the separation factor SF = DEu/DAm was determined for twelve of these organic acids, covering the spectrum of available materials; see Table 1. Some important conclusions have been reached by the authors on the basis of this detail investigation: chelating acids with the same reacting groups seem to give separation factors close to each other. Examples of such groups are = POOH (in dialkylphosphates), -COCHC(OH)- (in acetylpyrazolone and HTTA molecule), -N(OH)NO and -N(OH)CO- (in cupferron, neocupferron and the benzoylhydroxylamines), and -COOH (in the hydroxynaphthoic acids) [30]. The solvent extraction is generally poor if the chelating acid is too weak, and a substitution which increases the acid strength of a reagent will usually improve the extraction properties. Thus, dichloroxine extracts better than oxine, HTTA better than acetylacetone, and 2-hydroxy-1-naphthoic acid better than 1-naphthoic acid. The basic strength of the ligand can only be one of the factors that influence the separation of a lanthanoid and an actinoid ion with the same position in each f-series. Qualitatively, however, the results up until 1960 suggest that an increase in the basicity and chelating power (stability constant) of the ligand will increase the net distribution ratio (chloroform/water) [31]. This relationship may facilitate the choice of a suitable chelating agent for the separation of lanthanoid and actinoid ions by solvent extraction process. The extraction may also be poor because of the formation of MA3 complexes in the aqueous phase, if the hydrophobic part of the chelating acid is too small in relation to the hydrophilic groups. In point of fact, the extraction of actinium (III) with the four chelating reagents (HTTA, dibutylphosphate, β-isopropyltropolone or 5,7-dichloroxine) was found to be always inferior to that of lanthanum (III) in CHCl3 [32].

Table 1.
Europium/americium separation factors for a series of acidic extractants. Adapted with permission form Ref. [30].
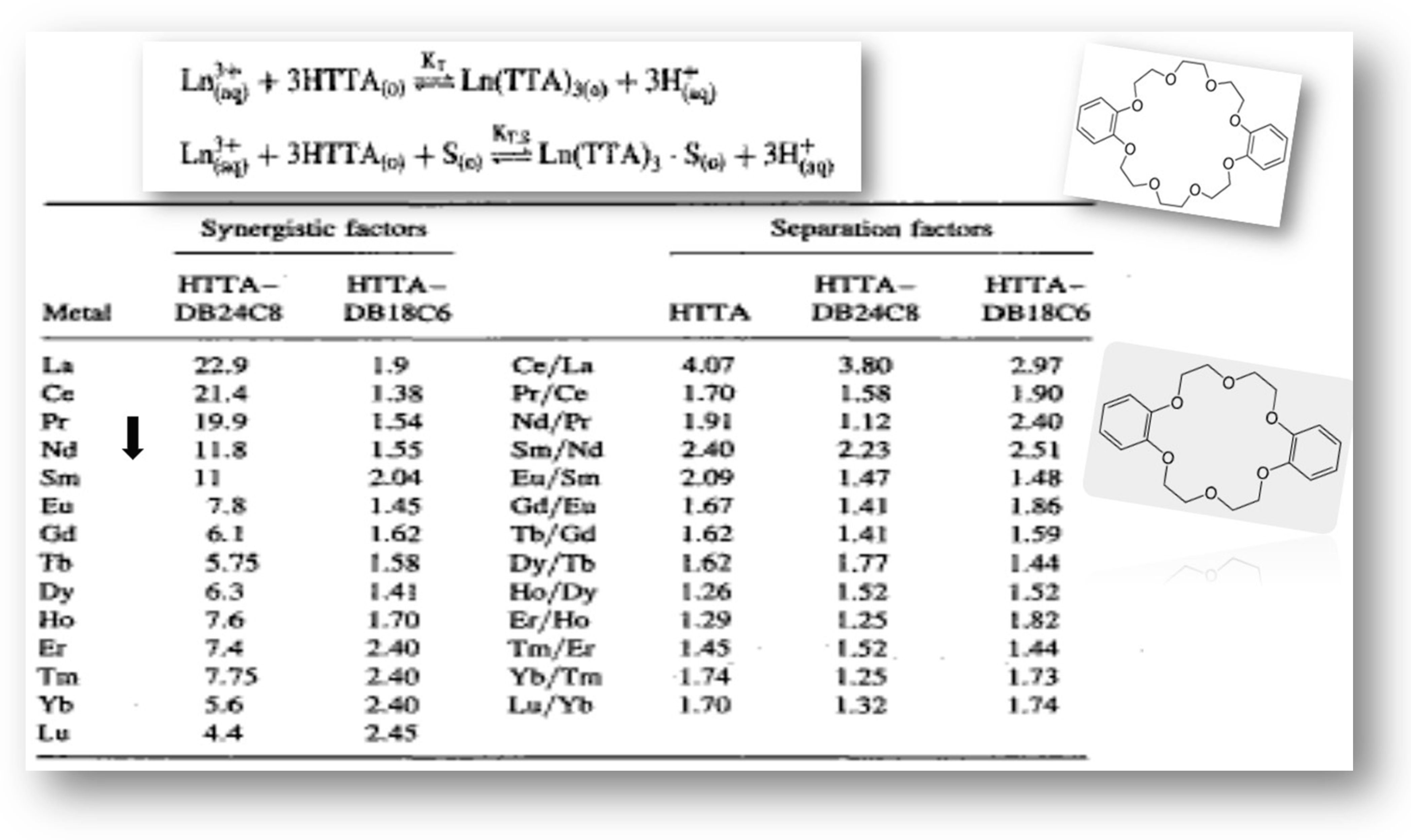
On the other hand, Albinsson has decided that in C6H6 medium at least two complexes exist, LnAa3 and the self-adduct LnAa3·HAa, while for the lower lanthanoids (especially La3+), a second self-adduct, LnAa3∙(HAa)2, was found to be extracted with acetylacetone [33,34]. Generally, these species have higher extraction as compared to LnL3 [16]. Such type of solvent extraction reaction was noted for the first time by Dyrssen for Sr2+ ion when 8-hydroxyquinoline was applied in CHCl3 [35]. However, Newman and Klotz have reported the formation of auto-synergistic species Am (TTA)3∙HTTA (autosynergism) in benzene with the calculated equilibrium constant ca. 0.75 ± 0.05 [36]. Furthermore, the corresponding equilibrium constants for the extraction of some 4fs and 5f-ions with several chelating extractants calculated on the basis of Equations (1) and (2) are summarized in Table 2.

Table 2.
LogK values of f-ions using different chelating agents, µ = 0.1/Cl̶ medium/CHCl3.
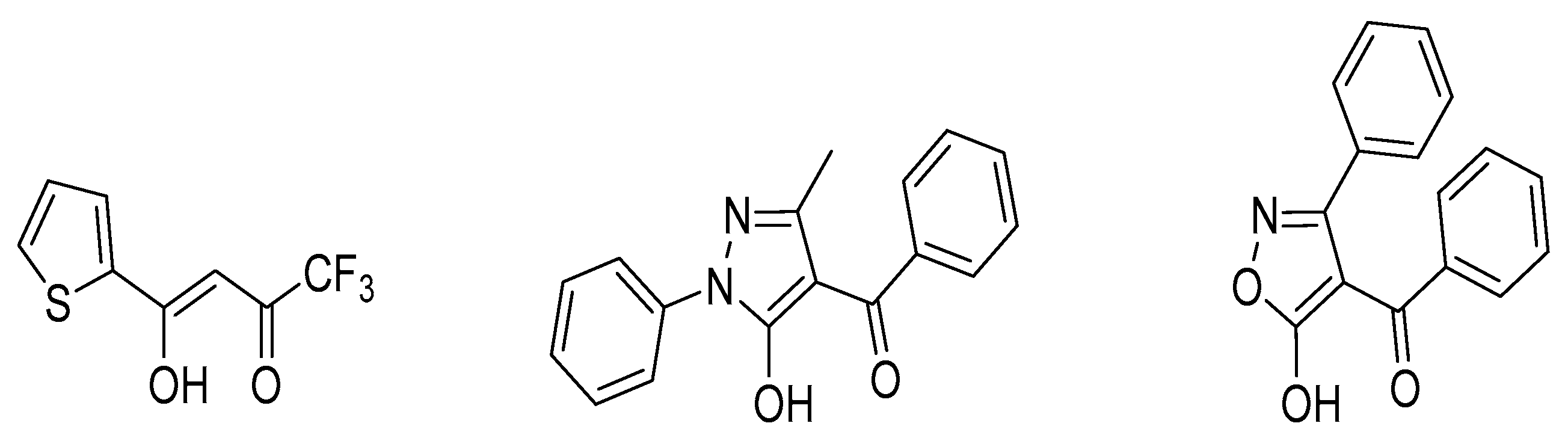
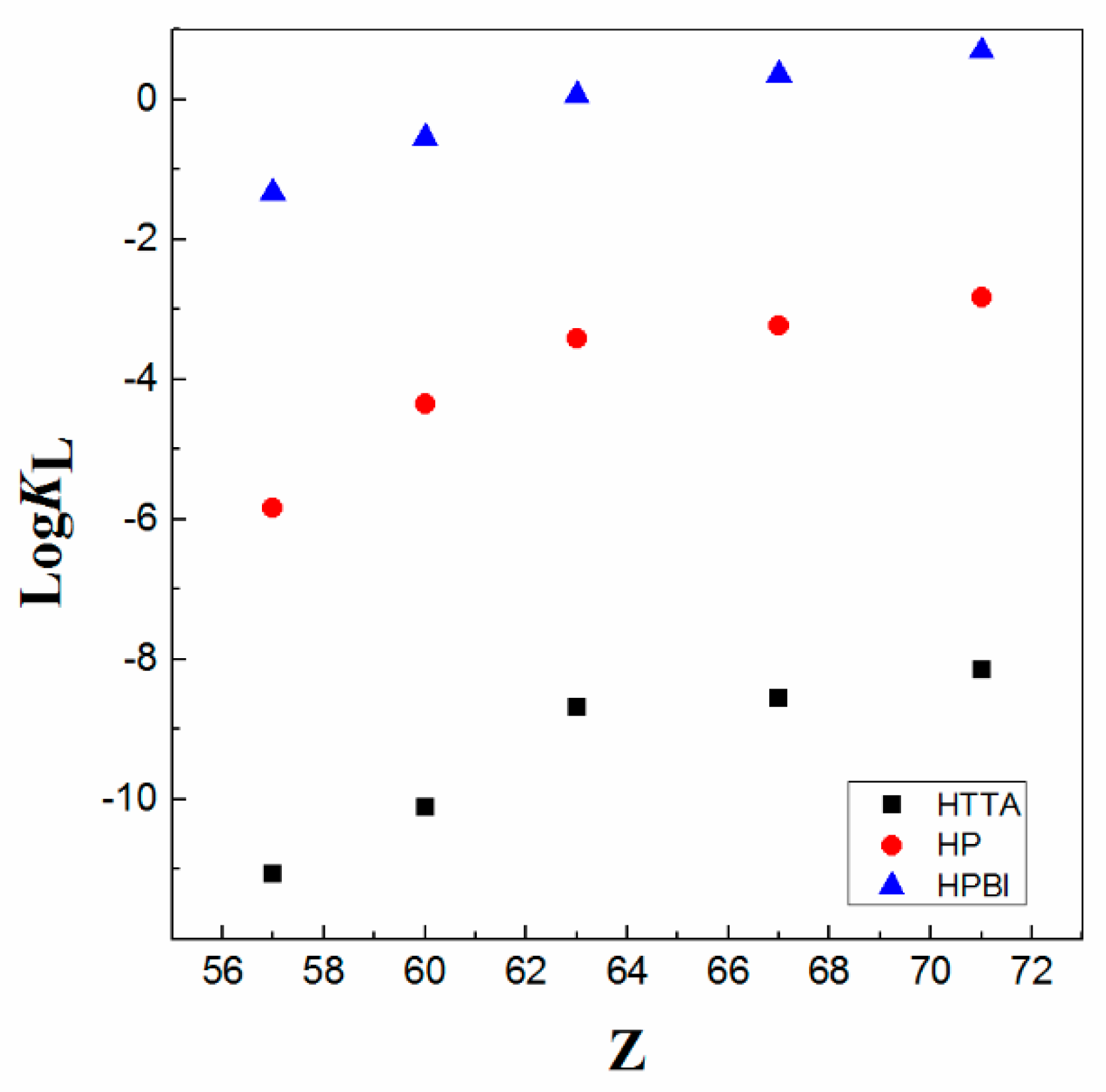
Moreover, the presented data show that the values of the logK of 4-benzoyl-3-phenyl-5-isoxazolone (HPBI) ligand (Figure 3) for 4f-ions are higher (up to eight times) than those obtained for the extraction of metals of the 4f-series with the typical β-diketone, HTTA reagent, as well as approximately four times higher as compared with 4-benzoyl-3-methyl-1-phenyl-5-pyrazolone (HP) as a chelating extractant in chloroform medium; see Figure 4.
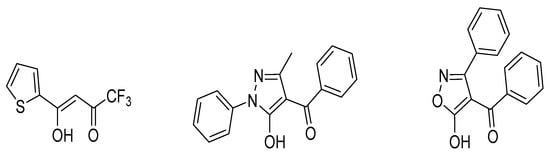
Figure 3.
Structural formulas of the chelating extractants (HTTA, HP, and HPBI, respectively).
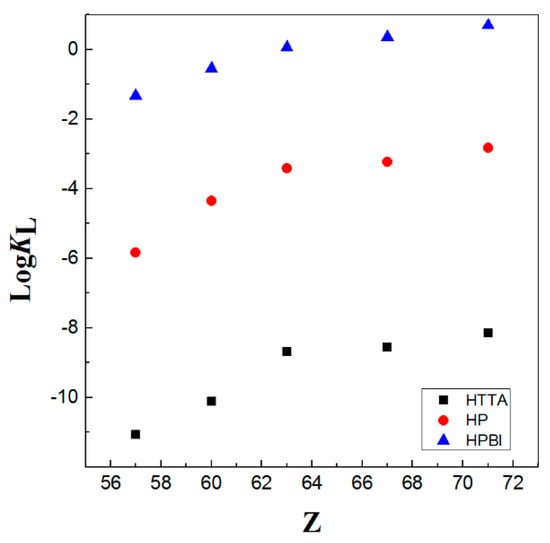
Figure 4.
LogKL of various chelating ligands (diluent CHCl3) vs. atomic number Z of La, Nd, Eu, Ho, and Lu. Adaptation from data Refs. [8,12].
As can be seen from the data presented in Table 2, the equilibrium constant values increase as the pKa values decrease. In other words, the inverse linear correlation of the extraction constant with respect to the pKa exists. The difference in the obtained extraction enhancement is seen when changing the type of the compound, i.e., typical β-diketone and β-diketones, in which the heterocyclic ring contained one of the ketonic groups (acylpyrazolone and isoxazolone). On other hand, apropos of the chemical structure in a β-diketone family (HBA)/HTTA), the substituent effect of the −CF3 group, which is very well recognized in solvent extraction chemistry [1], is somehow clearly demonstrated; see Table 2. Moreover, it is established that the substituted pyrazolones in para-position cause only quantitative changes in the synergistic extraction of lanthanoids with CHCl3 [38]. Furthermore, the obtained logK values with the 4-fluorophenyl terminal group (an electron withdrawing substituent) are larger than the case when electron-donating (−CH3) groups are introduced in the benzoyl moieties. Therefore, acid dissociation constants are essential parameters for a quantitative understanding of complex equilibria in solution and are of fundamental significance for how to predict the effectiveness of a substance. Hence, in liquid–liquid extractions of lanthanoids (Lns) with molecular diluents, great attention has been paid to the exact pKa value of the applied ligand. Of course, this physicochemical parameter has been carefully adjusted by introduction of various substituents.
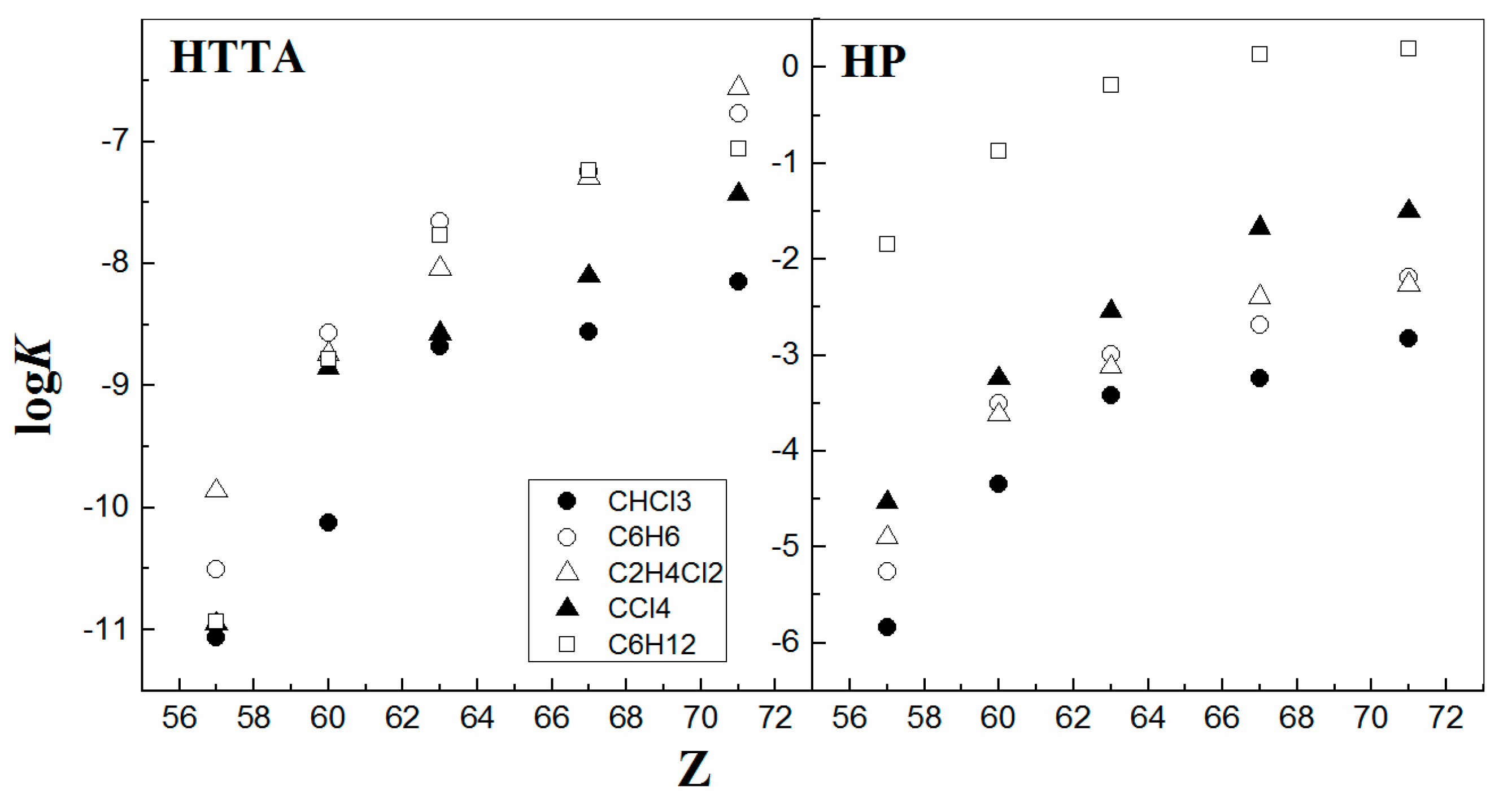
On the other hand, the type of organic diluent used has only a pronounced qualitative effect on the extraction efficiency of 4f-ions, without a noticeable influence on the reaction mechanism to such a degree as the data delineated in Figure 5, on a clear view. It is seen that, for a specific organic diluent, the values of the equilibrium constant logKL usually increase with the increasing atomic number of the metals, as expected from their decreasing ionic radii. It was found that for a given metal, the values of logKT (HTTA) and logKP (HP) for both solvent systems increase in the following order: CHCl3 < C6H6 < C2H4Cl2 < CCl4 < C6H12 [12,39,40]. For instance, a spectrophotometry study shows that the coordination of the metal chelate is invariant from one diluent to another. As a starting assumption for reflection, the organic molecular diluent is usually considered as inert (even it is chloroform), since it does not appear as an active participant in the solvent extraction equilibrium expression. However, the impact of the diluent has already been demonstrated on multiple occasions for various solvent systems in the field. Consequently, it may be concluded that, as a whole, the increase in the diluent’s solvating ability hinders the extraction process [41]. Probably, understanding that detail, it seems that the diluent effect on the solvent extraction process follows the general schematic behavior described in the solvation process.
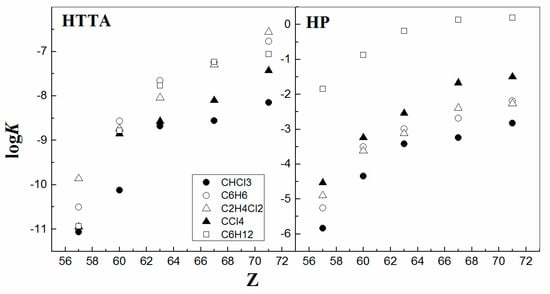
Figure 5.
LogK vs. Z. Data from Refs. [6,7,8,12].
Further, the calculated values of the separation factors (SFs) for adjacent light lanthanoid ions using different chelating ligands alone diluted in C6H6 are presented in Table 3. It should be noted that a significant loss of selectivity is observed after the first pair (Ce/La) in all studied extraction systems, with likely one exception. In addition, the values of the obtained SFs remain almost the same for the pairs of the light 4f-series when applying the HPBI ligand with, evidently, extraordinarily high extractability. In fact, good selectivity, to some extent, was found with the 4,4,4-trifluoro-1-(biphenyl-4-yl) butane-1,3-dione compound [42], almost similar to those shown with the HPMFBP compound. However, it is not so easy to make a general conclusion concerning the selectivity between light adjacent Ln (III) ions from the data collected in Table 3. Nevertheless, as a whole, based on our previous investigations and other research studies, the lanthanoid extraction increases in the order β-diketone ˂ 4-acylpyrazolone ˂ 4-acyl-3-phenyl-5-isoxazolone, while the selectivity decreases, regrettably. This survey also shows that the ligand number, and ipso facto the Kex, are both strongly dependent of the diluent used for the solvent extraction process. For example, the calculated SFs for a Lu/La pair are 6.0 × 102 with HPBI, 1.2 × 103 when applying an HP molecule and 5.4 × 103 with HTTA, and 1.7 × 105 with benzoylacetone (HBA) [8]. It is generally accepted in solvent extraction chemistry that the separation becomes poorer as the extractability increases, and the selectivity decreases with increasing extractant acidity, i.e., pKa. Additionally, Okamura et al. [43] have reported recently that this tendency, which is well recognized in conventional organic solvent systems, is quite similar in an IL medium ([C1C4im+] [Tf2N−]) as well. For instance, the order of the selectivity of Ln (III) ions is consistent with that of the extractant’s pKa values: HBA (benzoylacetone, 8.96) >> HNTA (2-naphthoyltrifluoroacetone, 6.35) ≈ HTTA (6.23) [43].

Table 3.
Values of the separation factors of the light lanthanoid pairs obtained using chelating extractants alone in C6H6.
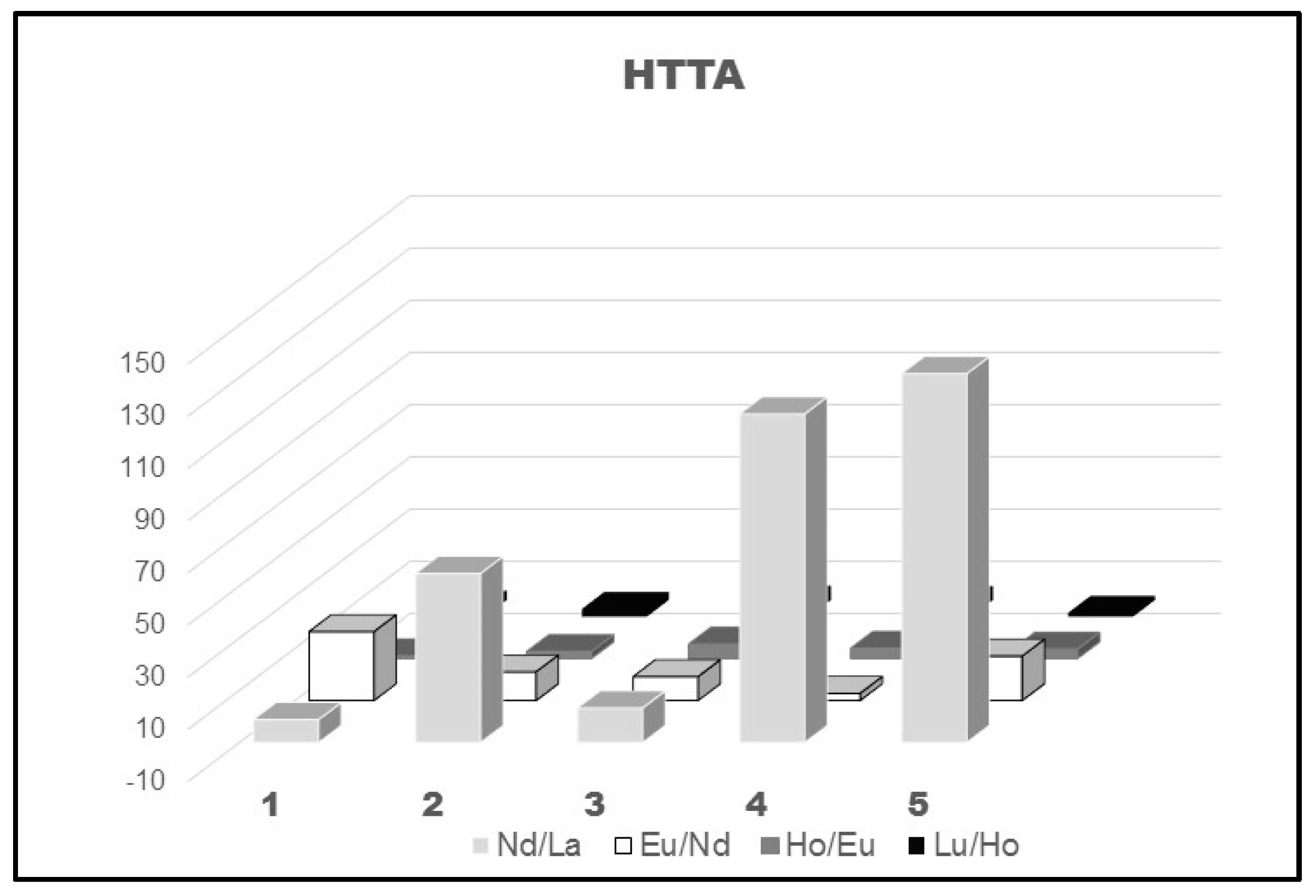
Moreover, the exposed data in Figure 6 confirm the well-known fact that the separation factors of the lighter lanthanoids are higher than those calculated for the heavier representatives of 4f-ions. On the other hand, the change of the diluent CHCl3 and, to some extent, C2H4Cl2 also, with C6H6, CCl4, and C6H12, caused a rather large increase in the SFs with HTTA ligand of the first investigated pair Nd/La, but such a tendency was not observed for the other lanthanoid pair, Figure 6.
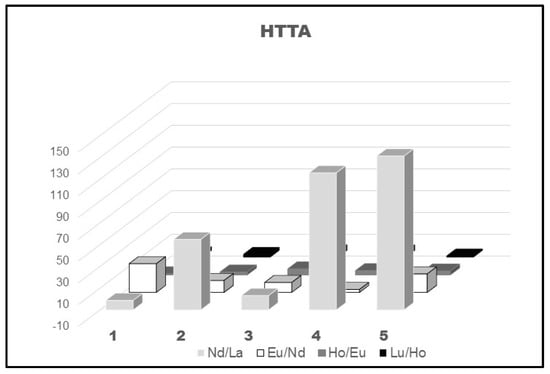
Figure 6.
SFs of a few lanthanoid pairs using HTTA diluted in molecular diluents: 1-CHCl3; 2-C6H6; 3-C2H4Cl2; 4-CCl4; 5-C6H12, [8,46].
In order to establish the influence of the extraction of lanthanoid ions with different chelating reagents on their separation, the SFs of three pairs with representatives of light, intermediate, and heavy lanthanoids are given in Table 4. It is seen that the SFs decrease in the order HTTA > HP > HPBI, while the lanthanoid extraction increases in the opposite order: HTTA < HP < HPBI. It is interesting to note that the HTTA ligand, which is a much poorer extractant for lanthanoids than HPBI, exhibits a separation for the three studied pairs, Lu/La, Eu/La, and Lu/Eu, which is 8.9, 13.8, and 1.77 times higher than those obtained when HPBI is used as a chelating agent in C6H6 organic environment. Moreover, a similar selectivity trend was observed by Pavithran and Reddy [47,48,49] among the lanthanoids, and by Brunette et al. [50] for alkaline earth extraction, too. As a whole, the separation becomes poorer as the extractability increases [47], and the selectivity decreases with increasing extractant acidity [50]. Furthermore, the log𝐾 value generally decreases as the diluent gains the ability to enter into hydrogen bonding with the extractant or the extracted metal complex, in accordance with the following trend described by Kolarik [51]: inert diluents > n-donor > π-donor > acceptor.

Table 4.
Tendencies in the separation of the lanthanoids in their solvent extraction, implementing C6H6 [45].
4. Synergism in Liquid–Liquid Extraction of Metal Ions with a Chelating Ligand HTTA
The term “synergism” is utilized in the field of metal solvent extraction to describe cases where the extractive capability of a mixture of extractants is greater than the sum of their individual extractive capabilities [16]. The adjective recommended in the IUPAC Gold Book is “synergic”, but most scientists prefer “synergistic”. Synergistic extraction is an attractive research topic in the field of the liquid–liquid extraction of metal species because the possible combinations of various ligands possessing dissimilar chemical properties are really enormous. As a matter of fact, the synergistic effect was observed by Cunningham et al. [52] for the extraction of Pr(III) and Nd(III) ions from nitric acid solutions by mixtures of HTTA and tributylphosphate (arguably, the most important reagent for actinoid separations is TBP) in kerosene, but synergism acquired its name in 1958 when a group of scientists from Oak Ridge Laboratory (USA) investigated the extraction of U(VI) by combinations of organophosphorus-extracting agents [53]. The extraordinary surge of interest has shown that, historically, this phenomenon was noticed as early as in the year 1879 by Vogel in the extraction of Co (II) thiocyanate by an equal mixture of ether and amylalcohol [54]. Consequently, the phenomenon of synergism obtained from HTTA compounds is very important, both historically, since the first synergism described was obtained using a HTTA + TBP mixture, and by the quantity of published papers: about 80 publications on the subject have appeared since 1972 [55]. In point of fact, Irving and Edgington [56], for the first time, and further, Healy and co-workers [57], have employed HTTA molecules in combination with neutral organophosphates (S) using C6H6, C6H12, CHCl3, and CCl4 as diluents to demonstrate a strong enhancement (up to 108 times the partition ratio for HTTA or S alone) of the extraction of metal species such as alkaline earth, trivalent lanthanoids and actinoids, thorium(IV), as well as uranyl ions. For historical reasons, when the adduct is a molecule or molecules of the chelating acid itself or similar acid species, this has not been classified as synergism, although it is, in fact, a similar phenomenon [58]. The synergistic reactions described in these pioneering works have produced extracting species with the general formula M(TTA)xSy, where x is the valence of the metal ion and y varies between 1 and 3. The existence of this type of mixed adducts Ln (L)3Sy (y = 1–3) has been observed usually when 4f-ions were extracted with synergistic acidic/neutral mixtures [13,42,59,60,61,62,63,64,65]. In the majority of solvent systems investigated, the nature of the diluent used and the composition of the aqueous phase do not affect the stoichiometry of the synergistic adduct [66]. Though successful solvent systems do exhibit greater extraction strength, unfortunately, synergistic systems rarely achieve much enhancement in selectivity for a given group of metals such as lanthanoids, or in the mutual separation of members of the groups [67]. Generally, in synergistic solvent systems, the acidic (chelating) compound (HL) typically deprotonates to form an anionic ligand L− that can chelate with the metal ion Mn+, while the neutral ligand or, in other words, the synergistic agent S replaces any remaining water molecules from the coordination shell of the neutral complex, enhancing its solubility in the organic liquid phase. This process can be expressed as:
Mn+ + nHL + xS ⇌ MLn·Sx + nH+
No standard method for the quantification of the phenomenon—synergism—has been agreed on, and any approach should be clearly defined in a given situation (IUPAC Gold Book). Actually, the synergistic effect can be represented by the synergistic coefficient (SC) defined by Taube and Siekierski as SC = log [(D1,2/D1 + D2)], where D1, D2, and D1,2 denote the distribution ratios of a metal ion between the organic and aqueous phase, containing one of the extractants and their mixture [68]. In other words, the solvent extraction is synergistic when SC > 0, and antagonistic when SC < 0, i.e., negative synergism [18,69]. For instance, antisynergism was first reported as a phenomenon, of course, by Blake et al. [53], and later for some separation procedures concerning certain ions, e.g., Np(IV), Np(VI), U(VI), U(VI), Pu(III), Y(III), and Ln (III), by Peppard et al. [70] in the organophosphorus acid–phosphorus ester series as a result of interaction between the two ligands mono(2-ethylhexyl) orthophosphoric acid and TBP (tri-n-butyl phosphate) in toluene. Some researchers have used the following expression for the synergistic coefficient (SC) too:
Or, as defined by Tagushi and Goto [71]:
where D1,2 denotes the distribution ratio of the species for the mixture of extractants.
Further, except by the coefficient of synergism, it can be also proved if the following equality is fulfilled [66]:
where the increase ∆D is the magnitude of the synergistic enhancement of extraction. In the presence of a large synergistic effect, D1 and D2 remain negligibly small compared to ∆D, and then D1,2 ≈ ∆D. In analytical chemistry, for an extraction system based on a pH-dependent chemical reaction, as in the case of acidic extractants, the value of SC has been estimated by the following equation as well: SC = n∆pH50. This is supposable where n is the charge of the metal ion (Ln3+: n = 3) and ∆pH50 is the difference of pH corresponding to 50 % extraction (D = 1) when the total concentration of the extraction system is the same for the single solvent system (e.g., HL) and for the mixtures with the neutral ligand. As a whole, this will give good results only in cases when the extraction with a synergistic agent used alone is almost negligible under the applied experimental conditions. Per contra, good results can be obtained if the following three assumptions are fulfilled [54]: (1) the metal concentration is negligibly small when compared with the concentration of the chelating acid; (2) the distribution of HL and S remains constant in the pH range investigated; (3) the formation of hydroxocomplexes can be ignored in the same pH range.
D1,2 = D1 + D2 + ∆D,
The chemistry of synergistic systems generally, and also of those making use of chelating ligands, is highly diverse, as the variety of extracting agents and their possible combinations are so numerous [72,73,74,75,76]. Nonetheless, the enhancement using HTTA is exhibited with various metallic species from the periodic table, and is much larger—up to 108—than the cases with dialkyl phosphoric acids, and up to 102 larger when changing the S from the neutral alkyl phosphates, through phosphonates, to the phosphine oxides [57]. As a matter of fact, the synergic enhancement of metal ion extraction has been studied most extensively using thenoyltrifluoroacetone (HTTA) ligand and various organic phosphate esters. Some of the findings valid for molecular diluents can be summarized as follows [77,78]:
- (i)
- HTTA metal chelate extraction systems show a much larger synergistic effect than most metal chelate extraction systems;
- (ii)
- The synergistic effect is larger when the neutral adduct-forming ligand is an organic phosphate ester than when it is any other neutral ligand;
- (iii)
- The synergistic effect is strongly dependent on the central metal ion; HTTA chelates of trivalent lanthanoids and actinoids or uranyl ions show larger synergistic effects than many other metals’ HTTA chelates;
- (iv)
- The synergistic effect also depends on the molecular organic diluents and it usually increases when water has a smaller solubility in the diluent.
It was also reported long ago that the synergistic effect is different in different inert organic diluents applied, and is usually larger when water has a smaller solubility in the used organic diluent [77]. It has been concluded that (i) the β-diketones which have one trifluoromethyl group extract Eu (III) extract better than those without a trifluoromethyl group. For instance, the pH of 50% extraction increases in the following order: TTA ≈ FTA < BFA < TAA < BZA < AA. Therefore, acetylacetone showed the least extraction of Eu (III): less than 10%. The difference in the metal extraction with acetylacetone or trifluoroacetylacetone and the metal extraction with the homologues of these β-diketones seems to be mainly due to the aqueous chelate complex formation. The substitution of a methyl group in acetylacetone or trifluoroacetone with a benzoyl, furoyl, or thenoyl group increases the possibility of metal extraction because the substitution increases the organophilic tendency of the molecules and prevents the formation of metal chelates in the aqueous phase. Moreover, the β-diketones with one trifluoromethyl group extract the metal ion from aqueous solutions at a lower pH than those without a trifluoromethyl group. (ii) Another thing to underline is that a metal chelate with a β-diketone with a trifluoromethyl group in its chemical structure is a much better acceptor of tributylphosphate to form further adduct complexes (MA3∙(TBP)) than a β-diketone with no trifluoromethyl group. In fact, the metal chelates with the CH3-group β-diketones do not form the second adduct, MA3∙(TBP)2, while the metal chelates with the CF3-group β-diketones form the second adducts. (iii>) The ability of the acetylacetone chelate or the trifluoroacetylacetone chelate to de facto form an adduct is not changed significantly by the replacement of the methyl group with groups such as benzoyl, furoyl, or thenoyl [77]. A study of the extraction of the thenoyltrifluoroacetone chelates of calcium and strontium has been described by Sekine and Dyrssen in the hope that a better understanding of these systems will lead to a wider use of this reagent in the solvent extraction and separation of these ions [79]. As a CCl4 diluent generally gives both rapid and satisfactory liquid phase separation, it was chosen for the investigation with TOPO, TBP, and methyl isobutyl ketone (hexone) as adduct-forming ligands. The separation of calcium and strontium can be effected successfully by mixture of 0.1 mol dm−3 TTA + 0.01 mol dm−3 TBP at an extraction pH of 4, removing most other metal ions, but it leaves Ca and Sr (probably also group 1 metals and Mg2+, Ba2+ and Ra2+) in the aqueous layer. When the pH is raised to 6.6, almost 99% of Ca was extracted, while in order to remove most of the remaining 30% of Sr, which is extracted simultaneously, the organic layer is washed three times at pH 6.6 with water buffered with p-nitrophenol or veronal. Further, the solvent extraction of In (III) and Eu (III) ions has been also studied by the same research team with mixtures of thenoyltrifluoroacetone and β-isopropyltropolone (IPT) in chloroform [80]. The distribution of In (III) was explained by the extraction of InAaB3-a, (0 ≤ a ≤ 3) complexes (type A). The mixed complexes of Eu (III) were considered to be EuAaBbHa + b − 3 (type B), but the addition of 1 mol dm−3 TBP (S) changes the species to EuAaB3-aL2 complexes (type C). The calculated equilibrium constants seem to indicate somehow that IPT in the undissociated form, HB, is a rather strong donor to the EuA3 chelate. Furthermore, adduct formation by the Eu (III) and Th (IV) chelate complexes of 2-thenoyltrifluoroacetone and β-isopropyltropolone with TBP and methyl isobutyl ketone (hexone) has been studied by the distribution method using CHCl3 and CCl4 as organic diluents also [81]. The formation of an adduct with the undissociated chelating acid was observed at high ligand concentrations, MA3∙HA. In most cases, an increase in the metal extraction was observed when TBP or hexone was added to the organic phase, and this effect, which is especially large for the TTA chelates, could be explained by the formation of mixed adducts, MAxSy. As a whole, the formation constants were larger for the Eu (III) chelates than for the Th (IV) chelates and larger for TBP used as a second ligand than hexone; they were also larger in CCl4 medium than in CHCl3. Moreover, the adduct formation of the tris−TTA complexes of In3+, Sc3+, La3+, Eu3+, Lu3+, and Am3+ ions have been studied by liquid–liquid distribution, applying CCl4 diluent and the aqueous phase of 1 mol dm−3 (H, Na) CIO4 [82]. This leads to MA3 and MA3Sn species’ formation in the organic phase, where the chelating acid, HA, is 2-thenoyltrifluoroacetone, and the neutral ligands, S, are TBP, dibutylsulfoxide (DBSO), and methyl isobutyl ketone (hexone) [82]. In fact, InA3 does not form adducts, ScA3 adds one TBP but no hexone, and LaA3, EuA3, LuA3, and AmA3 add two molecules of TBP ligand. In addition, the formation constants of the MA3∙(TBP)2 adducts showed the following order of stability: LaA3 > EuA3 ≈ LuA3. The ligand DBSO gives similar results to TBP, but the adducts with hexone are less stable than those with TBP.
The distribution of Eu (III) ions has been studied with various mixtures of ligands, including different chelating acids such as HTTA, 1-naphthoic acid, β-isopropyltropolone, 5,7-dichloro-oxine, N-benzoylphenylhydroxylamine, dioctylphosphate, and N-2,4-dichlorobenzoylphenylhydroxylamine [83]. The synergistic solvent extraction of the Eu (III)−TTA chelate into two diluents was found to increase by the addition of the following ligands: trioctylphosphine oxide > tributylphosphate ~ quinoline > coumarin > hexone > α-naphthol. As a matter of fact, two adducts, EuA3S and in some cases EuA3S2, have been obtained in the organic phase. In the case of the TTA−chelates, the PO group forms likely stronger adduct complexes than the more basic nitrogen atom in quinoline. On the other hand, the length of the hydrocarbon chain is not so important in future adduct formation processes in the organic phase, provided that the distribution constant of the donor ligand is not so low that most of it is concentrated in the aqueous layer, thus diminishing [S]o [83]. This is demonstrated, of course, by the results obtained with tributyl and trioctyl phosphine oxides. Further, it was found that the formation constants are larger when CCl4 is the diluent in use, rather than in CHCl3 medium.
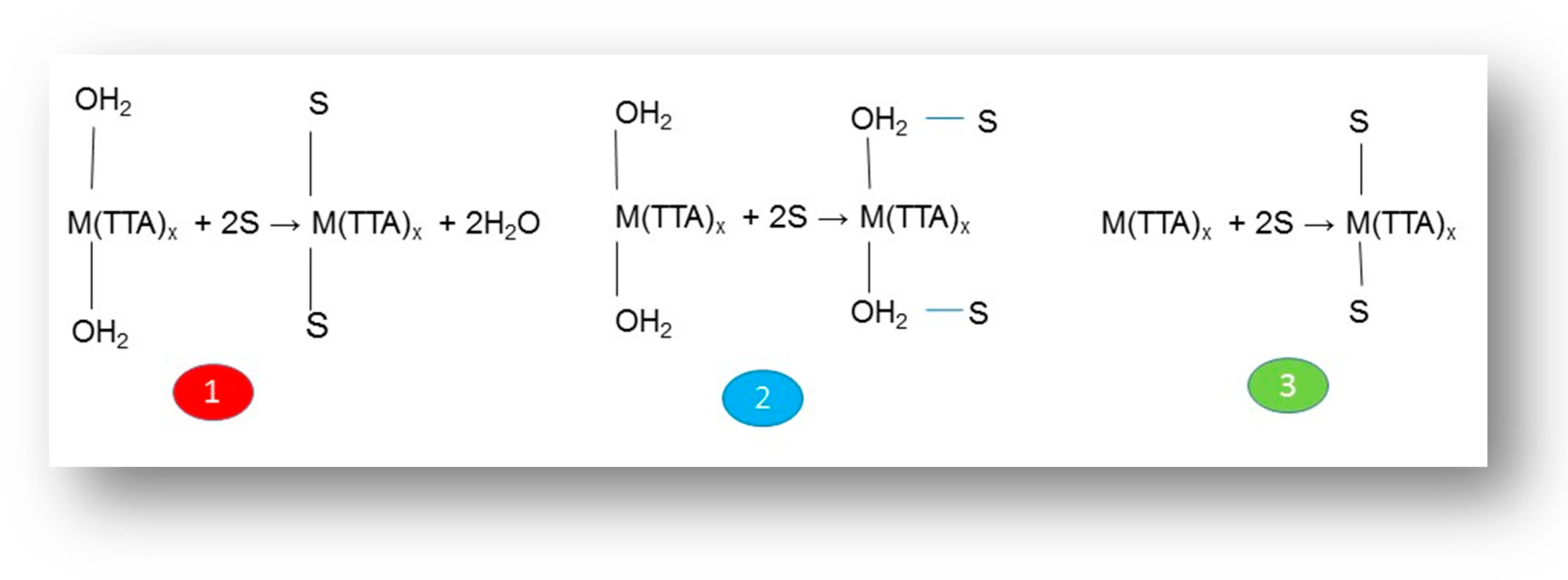
Furthermore, Marcus and Kertes [66] have proposed three types of possible reaction mechanisms to explain the data of the synergistic solvent extraction of metal ions by the combined action of two specific solutes in the organic phase (Scheme 1): (1) the neutral adduct replaces the residual water of hydration in the first coordination sphere of the metal ion; (2) the neutral adduct attaches the metal chelate, by means of a hydrogen bond, to the coordinated waters of the first hydration sphere; and (3) the coordination number of the non-hydrated chelate expands from 6 to 8 to accommodate the direct coordination of the adduct to metal. Actually, the geometry of the formed complex can play a significant role in the strength of many complexes [84].
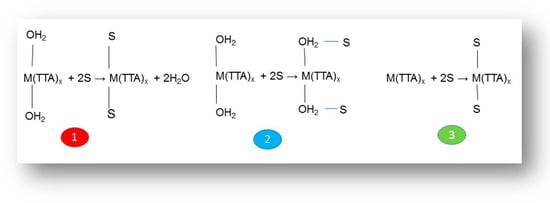
Scheme 1.
Possible reaction mechanism during synergistic solvent extraction.
For instance, Upor has made an attempt at generalizing the reaction mechanism with synergistic and antagonistic effects in 1970. Actually, however, this was not successful for any of the following components: (i) metal ions (Zr4+, Hf4+, Ti4+, Sc3+, Y3+, Ln3+, Th4+, UO22+, Am3+); (ii) active chelating agents (HTTA, dialkylphosphoric acid); (iii) neutral phosphorus compounds (TBP, TOPO), alcohols, aliphatic amines [85]. Nevertheless, the extraction of Th4+, UO22+, and Ln3+ ions was associated with a synergistic phenomenon in systems containing β-diketone and phosphoric ester in petroleum ether, such as an organic medium. In spite of this, it seems obvious that, in the case of antagonistic extraction, a single compound formed between the two complexing agents, resulting in the decreased activity of the chelating agent which, in turn, suppresses metal ion extraction, is not enough. In other words, the extent of antagonism is not at all independent of the chemical nature of the diluent either.
The potential use of the combination of the β-diketone HTTA with TOPO in n-dodecane for the recovery of a Sc3+, such as Sc (TTA)3∙TOPO, complex from a sulfuric acid solution containing transition metals such as Al3+, Fe3+, Mn2+, Co2+, Ni2+, and Zn2+ in a refining process was demonstrated by Zhao et al. [86]. Adduct formation constants have been determined also in the thirteen diluents for two types of synergistic adducts, namely, Eu (TTA)3·S and Eu (TTA)3·2S (S = TOPO), by Akiba et al. [87]. Moreover, Irving and Edington have established the mixed-adduct complexes of the type M(TTA)2(NO3)·2TBPO (M = Am or Eu) [88]. The optimum conditions were established when Ln3+ were synergistically extracted with hexafluoroacetylacetone and TOPO as mixed ligand complexes with the general formula LnL3·2TOPO [89]. Considering the interaction reaction between the extractants in cyclohexane media, Atalka and Favaro have identified, in the organic phase, the following species, Ln (TTA)3·TOPO and Ln (TTA)3·2TOPO, for La and Yb ions [90]. An extensive study on the extraction of the whole lanthanoid series, except for two ions (Pm and Lu) with HTTA and triphenylphosphine oxide (TPPO) mixture in benzene from an aqueous perchlorate medium at constant ionic strength of 0.2 mol dm−3 was carried out by Aly et al. [91]. It was found that the main extracted adduct contains the lanthanoid chelates together with two TPPO molecules. In the solvent extraction of metal β-diketonates, it is well known that the presence of a Lewis base such as TOPO enhances the extractability because of adduct formation between the metal chelates and the Lewis base. Further, Aly et al. have found that almost all lanthanoids are synergistically extracted by HTTA−TOPO mixtures in benzene under comparable conditions, and it was reported that the separation factors as well as the synergistic coefficients change periodically in a manner directly related to the tetrad effect, the double-double effect, and the so-called “inclined” W effect [91]. This contribution is an extensive study on the extraction of the whole 4f-series. In this work, the lanthanoid chelate, Ln (TTA)3, gave an adduct of a general formula Ln (TTA)3·2TPPO all over the whole series [91]:
Ln3+(aq) + 3HTTA(o) + 2TPPO(o) ⇌ Ln (TTA)3·2TPPO(o) + 3H+(aq)
So far, regrettably, no investigations are available concerning the coordination of water molecules to the chelate or the mixed adduct. In many cases, it was suggested that the lighter members, i.e., La–Nd, are mainly 9-coordinated, whereby the heavier representatives of 4f-series, Gd–Lu, are 8-coordinated in solutions. In fact, such synergistic occurrence has been widely investigated, but steric hindrance by the terminal groups in the β-diketones has hardly been recognized [92].
A survey of the synergistic data is given in Table 5 and Table 6. Data in the tables are first ordered according to the target 4f-ion, following by the type of the extracted metal complex in the organic phase; within each category, the data are sequenced in line with applied diluents.

Table 5.
Summary of synergistic systems of thenoyltrifluoroacetone with an organophosphorus ligand for the solvent extraction of trivalent lanthanoids.

Table 6.
Summary of synergistic systems containing β-diketone and organophosphorus ligands used for the solvent extraction of trivalent lanthanoids.
In view of a quantitative description of the synergism and antagonism as a phenomenon, [97] reports Eu3+ and Tm3+ extraction and separation with benzoyltrifluoroacetone and TBP, and on the interaction of the both these reagents with water in benzene. It was shown that the antagonistic effect can be accounted for quantitatively on the basis of the formation constants of the hydrated complexes detected in the organic phase: (HBTA)n(TBP)m(H2O)r. This behavior is attributed to an increase in the water content of the organic liquid phase, and can be fully accounted for by the decrease in the activity of both compounds due to the formation of several hydrated species in the organic phase also.
With regard to several essential factors affecting the stability of the formed synergistic metal species, the following four generalizations can be summarized [101]: (i) first of all, with organophosphorus compounds in the role of a neutral ligand, the order of synergistic enhancement obtained is that of increasing base strength. With considerable restrictions apropos of steric factors, the stability usually increases with increased donor properties of the molecule; (ii) though the composition of the adduct fortunately remains unaffected, the change of the organic diluent employed frequently affects the synergistic enhancement at the same time quite significantly. In addition, in some extreme cases reported, the diluent may have an effect on synergism that is greater than the initial synergistic effect observed already; (iii) thirdly, it should be emphasized that there is a small but finite decrease in the synergistic enhancement as a rule with decreasing ionic radius of the metal, regularly of course. This appearance can be attributed, at least partially, to a lower energy needed to accommodate the neutral ligand with increased ionic radius of the central metal atom; (iv) finally, we must note that stronger chelating agents form strong metal complexes and, as a consequence, have a lower tendency to facilitate the binding of the neutral ligand to the metal than a weaker one does [101].
By its nature, the effect of diluent may be a reflection of the effective concentrations of the extractant and of the adduct due to the interaction between them and the diluent in use [76]. The magnitude of the adduct formation constants in different diluents, such as cyclohexane, carbon tetrachloride, benzene, chloroform, pentachloroethane, and tetrachloroethane, was found to be correlated with the dimerization constants of benzoic acid (HB) used as a second extractant with HTTA in a mixture for Eu (III) extraction: Eu (TTA)3∙HB or Eu (TTA)3∙2HB. Synergism has also been observed when two β-diketones are used in a solvent system. For example, the solvent extraction of lanthanoids (La–Gd) with mixtures of a chelating extractants (HL), either 4-(4-fluorobenzoyl)-3-methyl-1-phenyl-pyrazol-5-one (HPMFBP) or 3-methyl-4-(4-methylbenzoyl)-1-phenyl-pyrazol-5-one (HPMMBP), in combination with a HTTA reagent has been studied in benzene [102]. The observed extraction mechanism in the two used systems is identical, indicating that the lanthanoid extraction behavior was not influenced by the 4-acylpyrazolone substituents. It was found that, in the presence of a thenoyltrifluoroacetone, the lanthanoids were extracted as LnL3·HTTA species. The small extraction enhancement upon the addition of HTTA in the solvent systems could be explained by the fact that probably the self-adduct LnL3·HL is anhydrous, as autosynergism must be accompanied by the displacement of coordinated water molecules. So, the transformation of the self-adduct (LnL3∙HL) into the mixed adduct LnL3·HTTA does not cause the formation of a more hydrophobic complex, as happens in most cases of synergistic solvent extraction. The statement of Hala, that in the mixed adducts formed in the organic phase during a solvent extraction process using a combination of two β-diketones, the weaker acid takes part as a neutral molecule in the extracted metallic species, is in accordance with the present finding, i.e., the LnL3·HTTA arrangement [103]. Honestly, similar results have been established by Woo et al. for the solvent extraction of Eu (III) ions with mixtures of HTTA and acetylacetone (HAcAc) [104]. On the basis of the chemical analyses conducted as well as infrared investigations, the researchers have found that the weaker acid is involved in the complex formation Eu (TTA)3·HAcAc as a neutral molecule.
Additionally, the introduction of a second neutral ligand, a crown ether molecule, resulted in an increased extraction efficiency of the 4f-ions in the following order: DB24C8 > DB18C6 (dibenzo-24-crown-8 and dibenzo-18-crown-6), established in the presence of the chelating reagent HTTA dissolved in 1,2-dichloroethane: Ln (TTA)3∙S, [105]. A greater stability for the formed mixed complexes in coordinating the DB24C8 molecule has also been established. Nevertheless, the calculated separation factors show a fairly uniform selectivity for the three HTTA, HTTA−DB24C8, or HTTA−DB18C6 solvent systems; see Figure 7. For example, the selectivity of the Sm/Nd metal ion pair is calculated to be 2.40, 2.23, and 2.51, respectively.
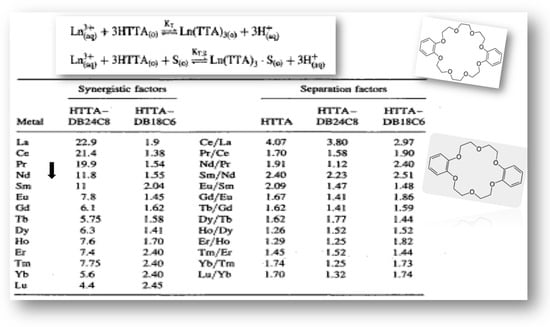
Figure 7.
Values of the synergistic and separation factors in the 4f-series produced by HTTA, HTTA−DB24C8, and HTTA−DB18C6 solvent systems in 1,2-dichloroethane, ([HTTA] = 6 × 10−2 mol/dm3, [DB24C8(DB18C6)] = 2.5 × 10−3 mol/dm3, pH = 3.80). Adaptation from Ref. [105]. ©2005 Taylor&Francis.
In the same way, the synergistic factors, mixed extraction constants, and formation constants of the different adducts, Ln (TTA)3∙2S (S: 15-crown-5), established in the organic phase (chloroform) were found to take the sequence Eu3+ > Tm3+ > Yb3+ [63]. However, using 12-crown-4, no enhancement in the 4f synergistic extraction was observed, which could be explained in terms of the small cavity size of the 12C4 compound relative to 15C5. On the other hand, characteristic ion-pair extraction of the lighter Ln (III) ions was observed with 1,2-dichloroethane extract containing HTTA and 18-crown-6 or dicychlohexano-18-crown-6 (CE), in which the cationic complex, Ln (TTA)2CE+, was formed and extracted [106]. Remarkable increases in extractability and selectivity were attained in the synergistic ion-pair extraction of the lighter 4f-ions, which could be elucidated on the basis of a size-fitting effect in the complex formation of the lighter ions with crown ether molecules. In addition, the interaction of the UOzz+, Eu3+, La3+, and Th4+ complexes of HTTA with several crown ethers have been investigated in two molecular diluents, namely, benzene and chloroform [107]. The thermodynamic parameters were found and the hydration of the complexes were determined by Karl Fischer and fluorescence techniques. Several lH and 13C NMR spectra used to further clarify the nature of the complexes indicated that the metal ion more often does not interact equally with all the ether oxygens, and the “fit” of the cation to the crown ether cavity is a not-so-significant characteristic. It was also found that the binding to the simple crowns by Ln (TTA)3 and UOz(TTA)2 chelates follows the basicity sequence, but with DC18C6 and DB18C6, steric effects become somehow more important. Moreover, such effects are very decisive for Th (TTA)4 interactions, even in the interactions with the simple crown ether.
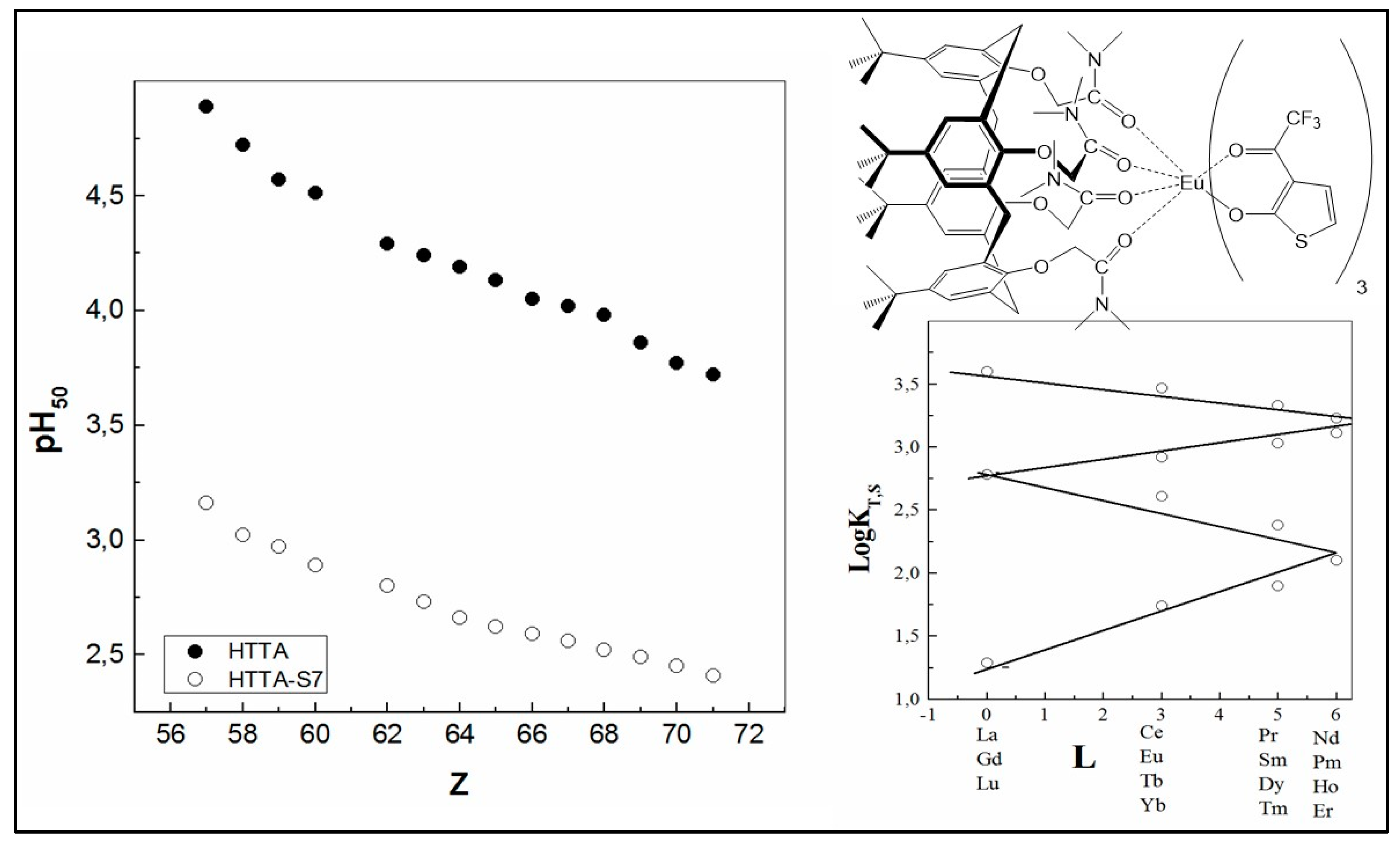
Furthermore, the great increase in the equilibrium constant value (approximately 11 orders of magnitude) was found by the addition of another interesting synergistic agent, the macrocyclic calixarene ligand, to the system Ln3+–HTTA, forming as well Eu (TTA)3·2S species [61]. Moreover, the data found in the study show that the linearity of the four segments to form “inclined W” is preserved in order to address the effect of L (the total angular momentum) [108] (Figure 8). For instance, the addition of a calixarene tert-butylcalix[4]arene tetrakis(N,N-dimethylacetamide) (S) to the chelating extractant HTTA improves the extraction efficiency of the all Ln (III) ions in 4f-series and produces large synergistic effects, up to 106. The very-high synergistic enhancement shows that the possible interaction between the extractants in the organic phase can be neglected; otherwise, an antagonistic effect would occur [109,110]. The SC values obtained in this case of acidic/neutral coupling are approximately two times higher than the case when HPBI (4-benzoyl-3-phenyl-5-isoxazolone) was used as a chelating extractant instead of HTTA in combination with the same synergist [60], as well as in comparison with the combination of HTTA and another calixarene, 5,11,17,23-tetra-tert-butyl-25,26,27,28-tetrakis-(dimethylphosphinoylmethoxy)calix[4]arene [13] (see Figure 2).
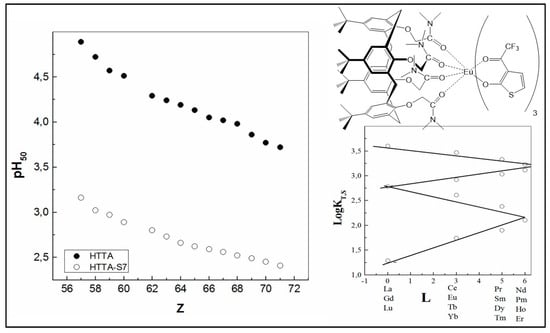
Figure 8.
Comparison of the pH50 values for the extraction of Ln (III) ions with HTTA in the absence and in the presence of tert-butylcalix[4]arene tetrakis(N,N-dimethylacetamide) (S) in CCl4. Proposed structure of the isolated solid complex Eu (TTA)3·S. Adaptation from Ref. [61] with permission.
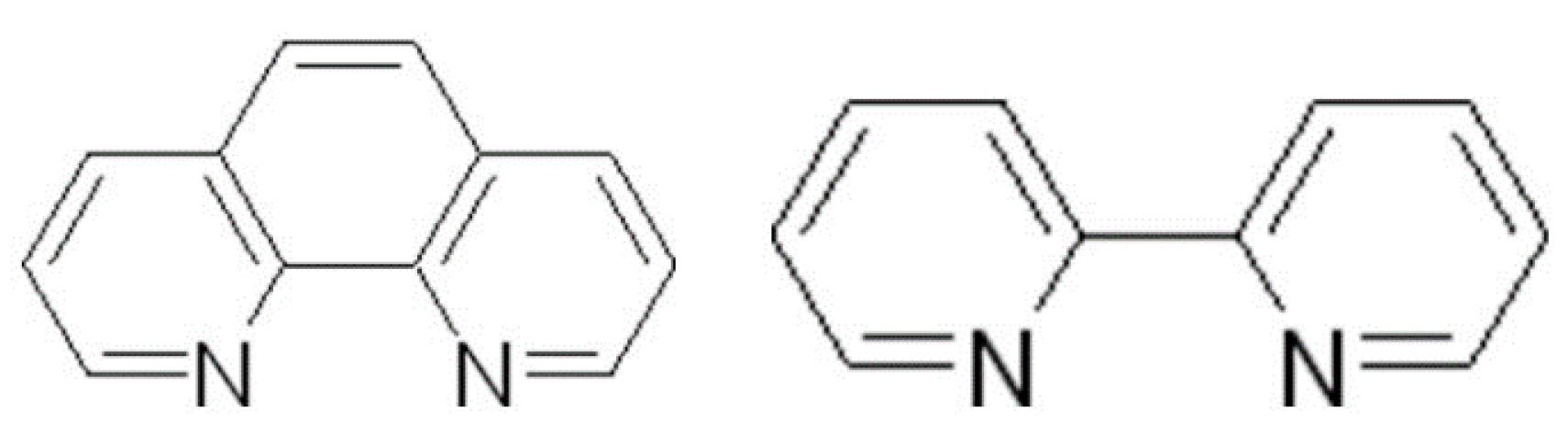
In mixed-ligand extraction systems of targeting lanthanoids, the Ln ion, to which three molecules of chelating reagent have already been coordinated, has space for the adduct-forming reagent in exact proportion to its ionic radius. Thus, steric hindrance for adduct formation somehow increases with atomic number. It is clearly seen from the obtained results herein that the synergistic enhancement is almost always higher for a particular lanthanoid ion when the HTTA compound is used as a chelating extractant. This is in accordance with the observation of Pai et al. [111], that the weaker the chelate, the higher the established synergism in solvent extraction processes. In addition, about 10- to 40-fold enhancement has been observed in the extraction of the trivalent La, Eu, Lu, and Am ions with HP ligand and bis-2-ethylhexylsulphoxide [112], whereas with mixtures of HPBI and sulphoxides, an about 2- to 4-fold enhancement was observed in the extraction of trivalent Nd, Tb, and Tm ions [113]. Therefore, many studies have been carried out using a neutral unidentate ligand as the synergistic agent, such as crown ethers [105,114], organophosphorus compounds, sulfoxides [115,116], or alkylammonium salts. Compared with these available literature sources, there are likely a limited number of scientific investigations devoted to the solvent extraction of metals with mixtures involving a neutral bidentate ligand. The formation of synergistic adducts containing a β-diketone as the acidic component (acetylacetone, benzoylacetone [117,118,119], pivaloyltrifluoroacetone [120], thenoyltrifluoroacetone [121,122,123,124,125,126,127], LIX 54 (a β-diketone derivative) [128] and 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone [129] and bidentate chelating agents, 1,10-phenanthroline and 2,2′-bipyridyl, have been reported for many metals under a variety of conditions. For example, synergistic effects of seven bidentate amines, S: 2,2′-bipyridyl, and 1,10-phenanthroline and its derivatives, on the extraction of zinc (II) with 2-thenoyltrifluoroacetone, HTTA, in benzene were investigated with established dominant extracted species Zn (TTA)2∙S in every system [130]; see Figure 9. The addition of the amines, even at a low concentration (<10−5 mol dm−3), caused a remarkable improvement in D, while at higher concentrations, a lowering of the D was observed because of the masking effect of amines in the aqueous phase. In fact, a very high, up to 106, synergistic effect was achieved by Kassierer and Kertes using a combination of two bidentate chelating agents, the β-diketone with a nitrogen base [121,122,124,126]. The synergistic extraction of lanthanoidsn (III), i.e., La, Sm, Tb, Tm, and Lu, using HTTA and nitrogen-containing neutral bidentate ligands (S), i.e., three ethylenediamine derivatives, such as N,N′-dimethylethylenediamine, N,N′-diethylethylenediamine, and cis-l,2-cyclohexanediamine, was studied in benzene: Ln (TTA)3∙S [131].
Figure 9.
Structural formulas of 1,10-phenantroline and 2,2′-bipyridine.
The stoichiometry Ln (TTA)3∙S [132], which includes one molecule of the synergist, was also established for the solvent extraction of some lanthanoid ions with the combination of HTTA–bipy in C6H6 [126]. Further, Duyckaerts et al. [123] have also reported the formation of mixed complexes in four molecular diluents, e.g., Eu (TTA)3∙S, where the synergistic agent, S, is 2,2′-dipy, 1,10-phen, and its 2,9-dimethyl derivative. Supplementarily, the comparison of calculated equilibrium constant KL,S values obtained for the HTTA–1,10-phen and HTTA–2,2′-bipy combinations shows that the stability of the complexes involving 1,10–phen used as a synergistic agent is higher than those involving 2,2′–bipy compound, probably because of the higher basicity of the former; see Table 7.

Table 7.
Values of the equilibrium constants KT, KT,S, and βT,S, and pH50 for the extraction of lanthanoid ions with HTTA and HTTA–S systems in CHCl3. Reprinted with permission from Ref. [132].
Moreover, it can be seen that the SC changes approximately from 102 to 106. So, the combination of two bidentate ligands, the acidic chelating agent and the Lewis base, i.e., phenanthroline or bipyridyl, produces a very strong synergistic effect in the solvent extraction process of lanthanoids. These published data show that the SC obtained for the mixtures including 1,10-phenantroline as a synergistic agent are approximately two and one order of magnitude higher than that when 2,2′-bipyridyl is used in combination with HTTA. This observation confirms previous investigations in which the synergistic effect decreases in the order phen > bipy with differences up to two orders of magnitude [121]. As a whole, the larger synergistic effect with phenanthroline is probably due to its stronger donor properties [128]. Moreover, the obtained SC values in the cited study (Table 8) are much higher than those in the cases when diphenylsulfoxide [115], crown ethers [110], or calix[4]arenes [13,60,133] were used as a second extractant for the synergistic extraction of Ln3+ with a β-diketone. In conclusion, the stability of these mixed-ligand adducts in organic diluents (as a rule, they are insoluble in water) depends on many factors, including the terminal groups of the β-diketone, its acidity and steric hindrance, the basicity of the donor atom(s) in the organic base and its structure, and the size of the metal atom too [134]. Usually, a comparison of the formation constants reveals that the greater the basicity of the donor atom(s), the greater the stability of the adduct formed; however, the greater the stability of the metal diketonate, the weaker the adduct that is formed.

Table 8.
Values of the synergistic coefficients ([HTTA] = 4 × 10−2 mol/dm3 and [S] = 5 × 10−3 mol/dm3, phen: pH = 2.50; bipy: pH = 3.20) and separation factors for the lanthanoid extraction with HTTA alone and HTTA–S systems in CHCl3. Reprinted with permission from Ref. [132].
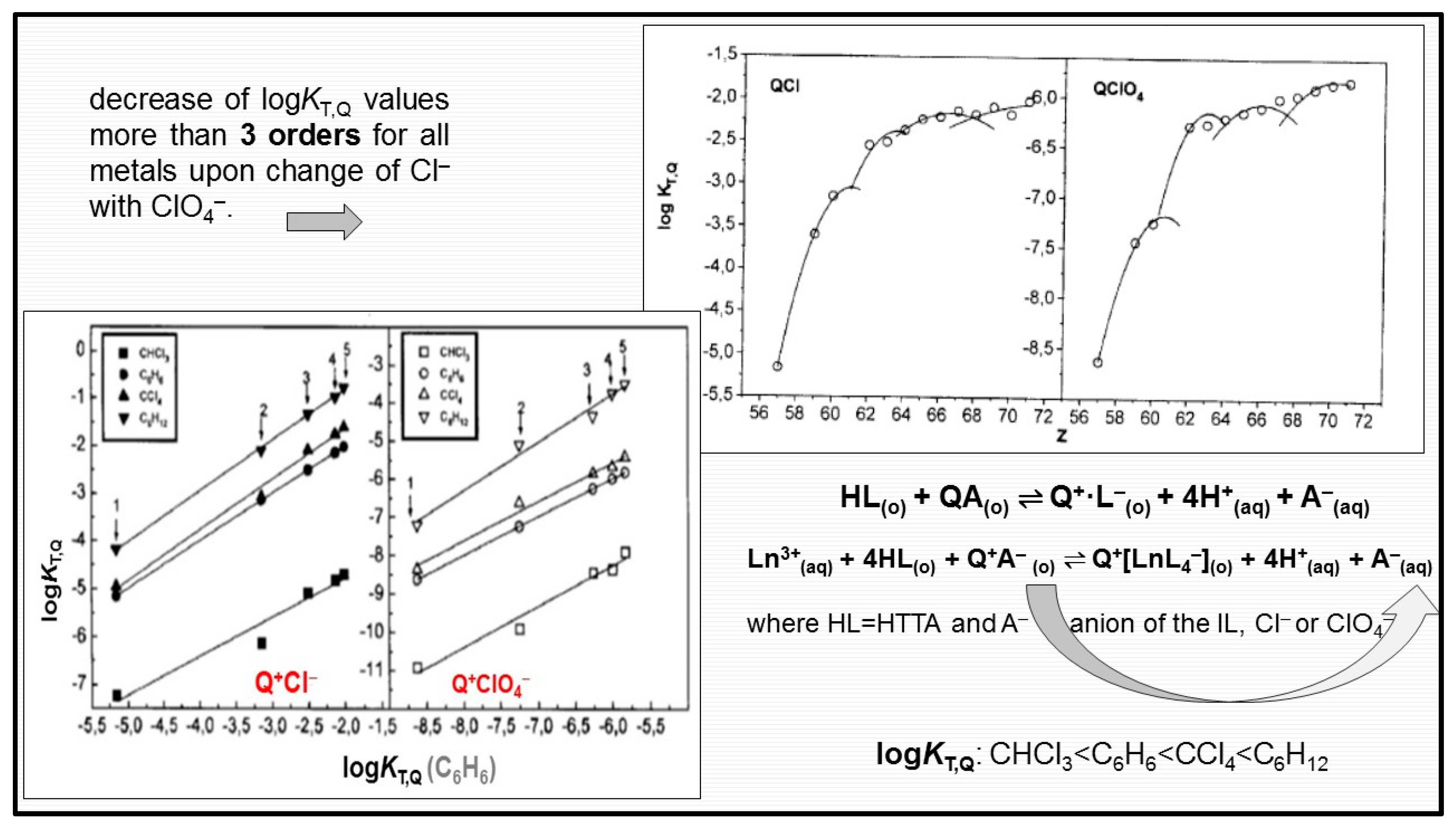
It is maybe worth mentioning that a large number of papers is devoted to the synergistic extraction with mixtures of HTTA in combination with amines or quaternary ammonium salts [12,45,135]. A novel family of engineering-purpose ILs derived from the quaternary ammonium salt Aliquat 336 (methyltrialkyl(C8–C10 chloride) have also been used for a long time for the synergistic solvent extraction of countless metals. One ideal representative of ILs is the quaternary ammonium salt, having the industrial nickname Aliquat 336 (trialkyl(C8–C10)methylammonium chloride, m.p.: −20 °C; viscosity: 1500 mPa·s). However, due to high viscosity, this IL is mostly used after dissolving it in organic diluents. Additionally, the extraction mechanism is based on anion exchange and, hence, strongly depends on the composition of the applied aqueous phase. The formation of anionic mixed complex Q+[Eu (TTA)4]− (where TTA− stands for the thenoyltrifluoroacetone anion) was firstly established in 1977 by Genov and Dukov [136] with the combination of an acidic chelating compound, β-diketone, and quaternary ammonium salts, Aliquat 336, in molecular diluents, and later confirmed not only for lanthanoids [46,137,138] but for the extraction of other metals too [139,140]. The four anions of the chelating extractant (L−) form the inner coordination sphere of the complex, satisfying the coordination abilities of the lanthanoid ion (CN = 8), while the cation of the liquid salt Aliquat 336, Q+, occupies the outer sphere of the formed anionic metal complex in the organic molecular phase. The variations of the calculated equilibrium constants logKL,Q with the atomic number of the lanthanoids are given in Figure 11. Because of gradation filling of electrons into the 4f shell, the properties of many lanthanoid compounds have shown changing gradations with an increase in the atomic number. An interesting property for Lns was firstly reported by Peppard et al. [141], i.e., logK values vs. atomic number Z formed four tetrads. It is seen that the logKL,Q values change in almost the same manner with increasing atomic number for both studied HL–QCl and HL–QClO4 combinations. Therefore, the change of the quaternary ammonium salt anion (Cl− with ClO4−) produces only quantitative differences during lanthanoid solvent extraction; see Figure 10. The considerable influence of the anion on the synergistic process can be explained by the fact that the bond in QClO4 is stronger than that in QCl, and as consequence, the formation of the complexes Q+[LnL4–] is more difficult in the presence of QClO4.
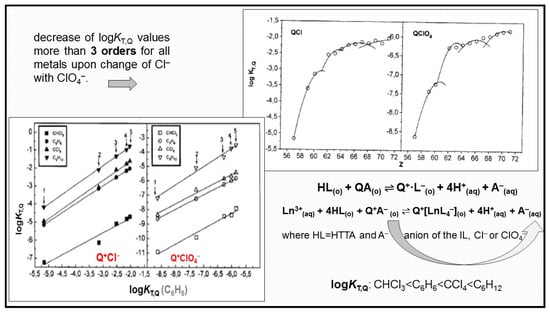
Figure 10.
Variation of logKT,Q with atomic number (Z) for lanthanoid extraction with HTTA−QCl(QClO4) mixtures in C6H6. Comparison of logKT,Q for CHCl3, C6H6, CCl4, and C6H12 with logKT,Q for C6H6 (1−La, 2−Nd, 3−Eu, 4−Ho and 5−Lu). Adapted from Ref. [46] and Ref. [137] with permission.
Taking into account the last consideration, it could be concluded that when metal ions are extracted with mixtures incorporating alkylammonium salt, two competitive reactions can occur in the organic phase, i.e., the formation of mixed metal complexes [12,18], leading to synergism or interaction between the two extractants producing ion-pair formation as a parallel reaction: Q+·L−. In other words, according to the Le Chatelier’s principle, the equilibrium connected with the ion-pairs’ Q+·L− formation will be shifted to the left, destroying it in the process. In the opposite case, antagonism will probably occur [137,142,143].
Furthermore, the solvent extraction of europium (III) in aqueous 0.1 mol dm−3 sodium chloride or sodium nitrate solutions with HTTA into chloroform was measured in the absence and presence of tetraethylammonium, tetrapropylammonium, tetrapentylammonium, or tetradecyldimethylbenzylammonium (base+) [144]. It was found that the formation equilibrium of the ternary complex, the Eu (tta)4−base+, by association of the neutral complex, Eu (TTA)3, with the ion-pair, TTA−base+, in the organic phase is essentially not affected by the size of the base+. The extraction of anions with a certain quaternary ammonium is better in the order Cl− < NO3− < TTA−, while that of a certain anion with the quaternary ammoniums is better in the order tea+ < tppa+ < tba+ < tpta+ < tddmba+. This order agrees with the general tendency regarding the solvent extraction of various ion-pairs; that is, extraction process is better when the ions are larger [144].
5. Ionic Liquid Media for Solvent Extraction of Metal Ions with HTTA Alone as Well as in Mixed-Solvent Systems
ILs are merely “liquid solids” that are, presently, considered to have some green characteristics, not omitting that, for engineering purposes, they should be also affordable. It is estimated that 1018, an ever-growing amount, of cation–anion combinations potentially exist, and the properties of ILs strongly depend on the choice of the cation (typically organic) and anion (usually inorganic with a -1 charge)—but some features are common [145]. However, in some cases, ILs do not offer advantages compared to organic diluents; there are several examples of high reactivity and selectivity [146]. A number of hydrophobic and potentially environmentally friendly ILs have been employed as substituents for volatile organic diluents (VOC) in liquid–liquid extraction due to their low vapor pressure and non-flammability, but likely only 1-alkyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imides ([C1Cnim+][Tf2N−]) were effectively chosen as unique media and could stand out [147,148].
Recognizably, the only common β-diketone ligand so intensively studied in biphasic aqueous/IL systems in the last 15 years is undoubtedly the compound 2-thenoyltrifluoroacetone [149,150,151,152,153,154,155,156]. The establishment of anionic lanthanoid complexes of the type [C1Cnim+][Ln (TTA)4−] in the organic “oil” phase is made possible likely by the anion exchange pathway reaction due to the loss of [Tf2N−] anion to the aqueous phase [157]. Nevertheless, the coordination environment of Eu3+ ions in the neutral Eu (TTA)3 chelate in [C1C4im+][Tf2N−] medium was investigated by time-resolved laser-induced fluorescence and infrared absorption spectroscopies, indicating that the complex is completely dehydrated in an IL saturated with water [156]. On the other hand, in molecular diluents, two or three water molecules are introduced in the first coordination sphere, i.e., Eu (TTA)3(H2O)3, since the coordination number of Eu3+ is generally eight or nine in solutions [155]. Despite all efforts made till now, little is likely known about the reaction mechanism in ionic organic media; nevertheless, it is worth developing the solvent extraction process in ionic diluents further.
5.1. IL’s Anion and Cation Solubility in the Upper Aqueous Phase
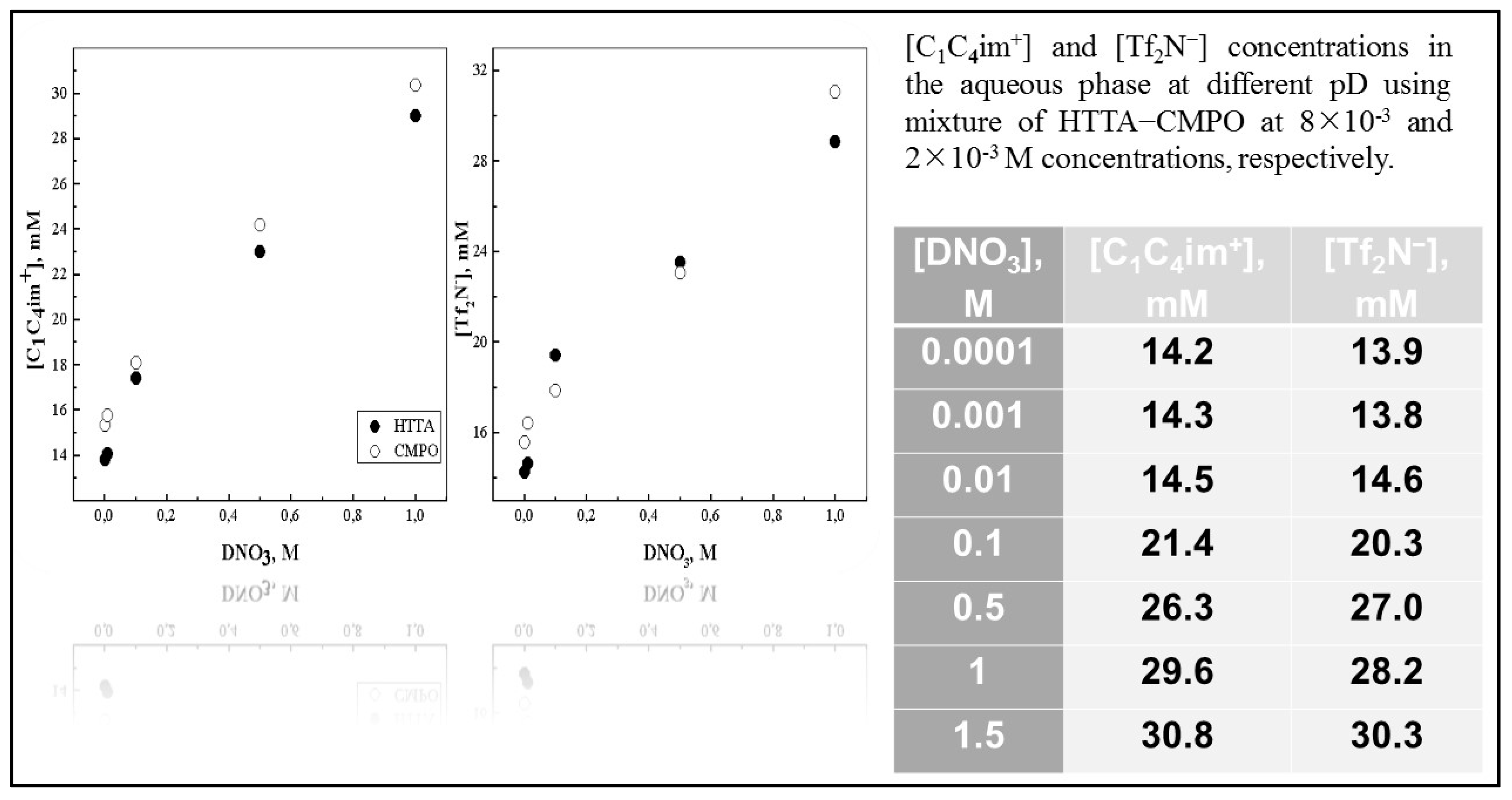
Definitely, the knowledge of the mutual solubility of water and ILs is very important as regards their further applications as a liquid organic media in the chemical engineering field [158]. Unfortunately, a limited number of analytical techniques are sensitive enough or are not quite efficient for the direct determination of trace components of ILs transferred in aqueous phase. In fact, as the reader can see, the concentration of cation [C1C4im+] in the aqueous phase was determined spectrophotometrically at 210 nm, a method proposed by Jensen and co-researchers [157], while that of anion [Tf2N−] as colored ion bis[(trifluoromethyl)sulfonyl)]imide bis(2,9-dimethyl-1,10-phenantroline)copper(I) in ethyl acetate was determined at 456 nm. As one would expect, the solubility of ILs in water is strongly influenced by their anions, e.g., salts of [Tf2N−] are less soluble than those containing [PF6−] [148]. In order to investigate the presence of IL’s ions in the upper phase, several experiments were carried out at low aqueous acidity (1 × 10−4 − 1.5 mol dm−3 DNO3) including the presence of a corresponding acidic/neutral ligand alone (Figure 11), or their mixture in the lower oil phase.
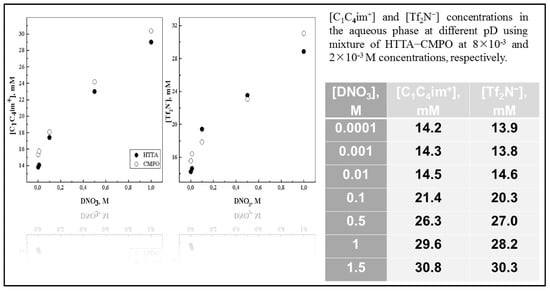
Figure 11.
[C1C4im+] or [Tf2N−] vs. [DNO3] of the aqueous phase using [C1C4im+][Tf2N−] as organic medium in the presence of ligands [HTTA] = 8 × 10−3 M and [CMPO] = 2 × 10−3 M. Adaptation from Ref. [78] with permission.
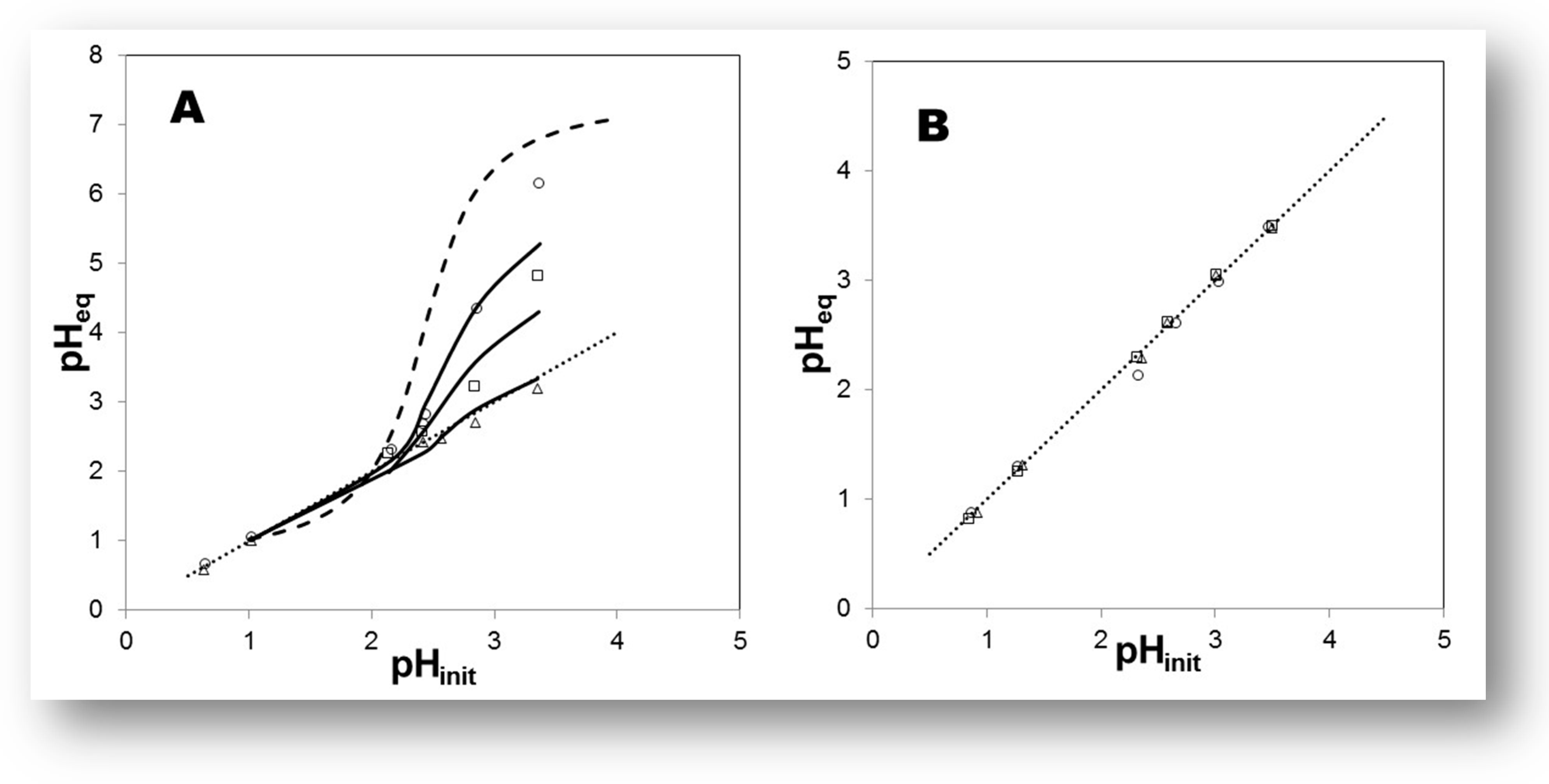
The quantity (mM) of the IL’s entities transferred from the “oil” to the water-rich solution (less-dense phase) were measured in the 1H and 19F NMR spectra (Q−NMR) of the samples in D2O, and plotted against the concentration values of DNO3 [78,159,160]. In all systems, generally speaking, the solubility of the cationic and anionic component of the IL increases slightly as a function of [DNO3], i.e., with decreasing pH values; see Figure 11. This finding is consistent with previous determinations of ca. 15 mM in D2O and 90 mM for 6 mol dm−3 DNO3 for [Me3BuN+][Tf2N−] IL [161]. Depuydt et al. also reported a higher solubility of the IL cation ([P444C1COOH+], tri-n-butyl(carboxylmethyl)-phosphonium) in water-rich phase at lower pH [162]. A very small difference between the IL cation and anion concentrations in the upper phase was noted previously, when biphasic mixtures of deuterated acids of various concentrations (DCl, DNO3, DClO4 from 10−2 to 10−4 mol dm−3 mainly) were examined [159]. Moreover, it was found that IL’s anion solubility, (CF3SO2)2N− in the water-rich phase, decreases in the order [C1C4im+][Tf2N−] > [C1C6im+][Tf2N−] > [C1C8im+][Tf2N−] > [C1C10im+][Tf2N−]. Therefore, using [C1C4im+][Tf2N−] samples, without the presence of sodium perchlorate in the upper phase, displays similar ionic solubility at ca. 17 mM, a value in agreement with results in pure H2O for the same IL phase: ca. 15 mM. In the presence of the neutral ligand octyl(phenyl)-N,N-diisobutylcarbaoylmethyl phosphine oxide (CMPO), the transfer of ILs’ components in the aqueous phase increases a little bit, around 1.3–2.2 mM, in comparison with the case when the chelating ligand (HTTA) was dissolved in [C1C4im+][Tf2N−], but practically no difference can be registered between cation and anion solubility, and it can be classified as equal under certain experimental conditions; see Figure 11. On the basis of the obtained data via NMR measurements, one may conclude that, under the applied experimental conditions, the solubility of ILs’ components in the aqueous phase does not depend on the presence of the organic molecule used as an extractant for 4f-ions, their concentration, nor its chemical nature (acidic, neutral or acidic/neutral combination) [160]. However, it is a generally accepted statement that, depending on the type of extracted complex, being anionic or cationic, anions or cations of the IL diffuse to the aqueous phase in exchange for the extracted metal complex. On the other hand, Fu et al. pointed out that, due to the formation of a metal neutral complex in the extraction process, losses of IL components can be avoided [149]. For instance, the extraction experiments of 4f-ions were carried out at low acidity (pH in the range 1–4), and the conclusion based on the findings is that the quantity (mM) of the IL’s entities ([C1C4im+], [Tf2N−]) transferred from “oil” to the water-rich solution is independent of the chemical composition of the aqueous phase when DNO3, DCl, or DClO4 mineral acids were exploited, including the examination at constant ionic strength µ = 0.1 (NaCl or NaNO3): ~14–17 mM [159]. ILs play a more active role not only in the partitioning process as a receiving phase, but also represent an important mode of metal ion extraction taking part in the formation of metallic species. Although they are often said to be “immiscible” with water, because of the existence of a meniscus, the water transfer to IL phases with which they are in contact with is known to be a key factor [158]. The average amount of water dissolved in [C1Cnim+][Tf2N−] strongly depends on the IL’s nature, but usually decreases with increasing length of the IL cation, from 12 200 ppm down to 8 350 ppm (n = 4 to 10), with a trend following that of the hydrophobicity the ILs’ cations. The addition of an acidic ligand to the IL phase has no significant effect onto the water solubility in the more hydrophobic ILs. For example, the average water content in [C1C10im+][Tf2N−] in the presence of HTTA is 8500 ppm, to be compared to an average value, in the absence of ligand, equal to 8350 ppm. Nevertheless, the situation is a little bit different in [C1C4im+][Tf2N−] media, where the H2O uptake can reach 14 000 ppm upon HTTA addition in the lower oil phase. On the other hand, the effect of adding chelating compounds to the IL phase (n = 4) onto the H+ distribution (pH at equilibrium as a function of pHin) was examined in the pH range of 1–3.5 too. The obtained results are presented in Figure 12 for the HTTA ligand [163]. The addition of any of the three investigated chelating ligands (HTTA, HP, HPBI) has a large impact on the pHeq values, which decrease so that the pHeq vs. pHin plot lies below the curve obtained in the absence of ligand: the more ligands introduced, the more acidic the aqueous phase becomes, at any initial pH value.
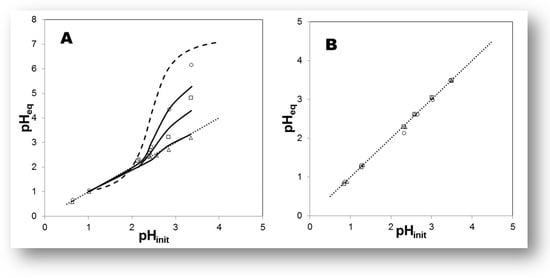
Figure 12.
(A) Effect of increasing concentrations of HTTA dissolved in [C1C4im+][Tf2N−] on the H+ concentration at equilibrium. ○: [HTTA] = 7 × 10−3 mol dm−3; □: [HTTA] = 1.5 × 10−2 mol dm−3; Δ: [HTTA] = 3 × 10−2 mol dm−3. Dotted line: y = x. Dashed line: in the absence of ligand. (B) Effect of increasing concentrations of HTTA dissolved in [C1C10im+][Tf2N−] on the H+ concentration at equilibrium. ○: [HTTA] = 7 × 10−3 mol dm−3; □: [HTTA] = 1.5 × 10−2 mol dm−3; Δ: [HTTA] = 3 × 10−2 mol dm−3. Dotted line: y = x. Adaptation from Ref. [163] with permission.
In this context, the investigation was performed using HTTA in the [C1C10im+][Tf2N−] phase, and the results are displayed in Figure 12 as well. Thus, using the latter IL, all data points, either with or without the HTTA compound, lie on the same y = x line. Clearly, such changes (from S-shaped curve to linear variation) occur abruptly when changing from one IL to the other, as these liquid [Tf2N−] media display different alkyl chain lengths. Further, one can safely assume that H+ solubility in [C1C10im+][Tf2N−] media is very low, although water still migrates to this IL.
Moreover, the pKaIL values of ligands with different-acidity HTTA, HP, and HPBI (see Figure 3) have been measured in [C1C4im+][Tf2N−] (pH range 1–3.5) by Atanassova and Billard [163]. The obtained three values of pKaIL corresponding to the ligand dissociation in a water-saturated [C1C4im+][Tf2N−] phase (12 200 ppm average H2O amount) are very similar, 2.0–3.5, in comparison with the large difference observed for their pKaW values of 1.12–6.23 [164]. Consequently, in the liquid–liquid extraction of lanthanoids with molecular diluents, great attention has been paid to the exact pKaw value of the used ligand, and this parameter has been carefully adjusted by the introduction of various substituents. [165].
5.2. Solvent Extraction of Ln (III) Ion with Thenoyltrifluoroacetone in an IL Media, [C1Cnim+][Tf2N−]
The chemistry of complexation reactions of β-diketone compounds with trivalent lanthanoid cations are very intensively studied in solution, aqueous, organic, or biphasic aqueous–organic liquid systems [166]. It was found that two types of neutral complexes are commonly formed in organic diluents during solvent extraction according to the following equilibria [12,13,59,61,157]:
Ln3+(aq) + 3 HTTA(o) ⇌ Ln (TTA)3(H2O)n(o) + 3H+(aq); n = 2, 3
Ln3+(aq) + 4 HTTA(o) ⇌ Ln (TTA)3(HTTA)(o) + 3H+(aq)
Even so, the coordination numbers (CN) of mononuclear β-diketone lanthanoid complexes are generally high, varying from 6 to 12, and depend on the ionic radii of ions, the ligand, the reaction medium, the diluent, and the experimental conditions, such as temperature or ligand to metal ratio, with 8 and 9 being rather common. In any case, it has been shown that, in ILs, the 4f-ions maintain the usual CNs that they display in molecular diluents [167]. When considering the mechanism of lanthanoid (III) extraction towards an IL or molecular diluent phases by the use of β-dicarbonyl compounds, two very different situations can be found: in molecular diluents, the extracted metal species are always neutral, while in ILs, extracted species are predominantly charged, owing to the ionic nature of this liquid compound. In particular, the solvent extraction of anionic complexes by HTTA has been shown to proceed by the exchange of the [Tf2N−] anions into the aqueous phase:
Ln3+(aq) + 4HTTA(o) + [C1C4im+][Tf2N−](o) ⇌ [C1C4im+][Ln (TTA)4−](o) + 4H+(aq) + [Tf2N−](aq)
However, other mechanisms are possible, such as cationic exchange or the extraction of neutral species, in a way rather similar to that occurring in molecular diluents. Generally, in addition to the formation of a neutral complex, cation (Ln (L2)+, L represents the anion of the chelating compound) and anion (Ln (L4)−) exchange extraction mechanisms are also proposed in the IL-based solvent extraction systems. When the reaction in the organic phase is via ion-exchange mode, the extraction of metal ions is more efficient in the system of a shorter alkyl IL’s chain [149].
Although good linear dependencies were obtained during the investigation of the fundamental stoichiometry of the complex in the Eu3+/HCl/HTTA/[C1Cnim+][Tf2N−] system, they have not led to an integer value, and as a consequence, it is not possible to determine the exact number of chelating ligands involved in the solvent extraction process [78]. Despite the fact that a simple slope analysis could not identify a single metal-extractant species in the oil phase, the limiting slopes clearly demonstrate that anionic complexes (1:4, Eu3+: TTA), i.e., Eu (TTA)4−, are not extracted in the cited study. However, it was observed for the [C1C4im+][Tf2N−]/HTTA/HClO4/1 mol dm−3 NaClO4 solvent system [157] and, probably, the self-adduct formation is not an important reaction in Equations (2) and (16) as well [150]. Complementary physicochemical methods have been used by Jensen and co-workers [157], namely, equilibrium thermodynamics, optical absorption, and luminescence spectroscopies, high-energy X-ray scattering, EXAFS, and molecular dynamics simulations, to support the formation of anionic Nd(TTA)4− or Eu (TTA)4− complexes without water coordinated to the metal center in [C1C4im+][Tf2N−] medium. Over the previous years, since 2003 [157], several extraction mechanisms have been proposed by different research groups to explain the extraction of various metals, including 4f-ions, by HTTA in 1-alkyl-3-methylimidazolium-based ILs:
- ⮚
- neutral species, n = Z+ [156]:
MZ+ + nHTTA ⇌ MZ+(TTA)n + nH+
A neutral complex extraction mechanism of Th(TTA)4 and stripping with sc-CO2 was reported in all four ILs, [C1Cnim+][Tf2N−], n = 2,4,6,8, without the direct participation of the ILs’ cation or anion in the extraction process, i.e., losses of IL can be avoided [149,168].
- ⮚
- anionic species, n > Z+ [78,169]:
MZ+ + nHTTA + (m-z)[Tf2N−](o) ⇌ MZ+(TTA)n− + nH+ + (m-z)[Tf2N−](aq)
IL [C1C4im+][NfO−] (nonafluoro-1-butanesulfonate anion) was used as the organic phase for europium and neodymium extraction from 1 mol dm−3 NaClO4/HClO4 aqueous solutions (1.74 ≤ pcH ≤ 2.92 and the HTTA concentration 0.0 mol dm−3 ≤ HTTA ≤ 0.625 mol dm−3 ) when metal:HTTA stoichiometries of 1:0 (Eu (H2O)93+), 1:2, and 1:3 were detected with different numbers of inner-sphere water molecules (2 or 0), which depend on the ligand concentration and pH [151]. At low extractant concentrations or low pcH, lanthanoids are extracted into the organic oil phase as the fully hydrated aqua cations, for example, Nd(H2O)3+9 or Eu (H2O)3+9. As the extractant concentration and pcH are increased, two other lanthanoid-containing complexes with different numbers of coordinated TTA− (2 or 3) and different numbers of inner-sphere water molecules (2 or 0) are also extracted. In other words, this suggests the presence of multiple extraction equilibria that vary in importance as the experimental solution conditions change.
- ⮚
- cationic species, n < Z+:
MZ+ + nHTTA + (z-m)[C1Cnim+](o) ⇌ MZ+(TTA)n+ + nH+ + (z-m)[C1Cnim+](aq)
For example, Kumar et al. have established the complex [UO2(TTA)22+(Tf2N−)2] in the oil phase [170].
The great variety of possible lanthanoid−TTA complexes, however, affords the chemical ionic systems considerable freedom in selecting between solvent extraction mechanisms involving neutral, cationic, or anionic species, depending on the experiment [96,171,172,173,174]. However, the presence of multiple extraction equilibria that vary in importance as the different extraction parameters change over a wide range (extraction conditions, the chemical composition of the biphasic system, acid concentration in the aqueous phase, the ionic strength, the ligand concentration in the organic phase, and of course the significant solubility of IL ions in the aqueous phase) are not commonly adopted, and an explicit extraction model when ionic diluent is proposed. Further, the special role of the [Tf2N−] anions in the IL–water distribution of Eu3+−TTA chelates was elucidated recently by Okamura et al. [175]. Almost all research groups have attempted to confirm the one speciation derived from modeling the distribution ratio experiments, UV-Vis spectroscopy, and EXAFS measurements, but the existence of few extracted species at the same time was not evoked. On the basis of the experimental results, three complexes can be proposed Eu (TTA)2+, Eu (TTA)2+, and Eu (TTA)3, which can contain other anions (Cl−, Tf2N−) or water molecules in order to satisfy the high CNs desired by lanthanoid cations and to complete the inner coordination spheres of the complexes. Therefore, in many cases, writing the composition of the complex-like simple chelate LnL3, instead of [LnL3(Tf2N−)x, (H2O)y, (C1C4im+)z](z − x), is only a shortcut used for the sake of convenience. However, in dry ILs or ones with a low water content, the [Tf2N–] ion can be a coordinating anion and may display several denticities and coordination modes [176]. Comparing the four extraction systems in ref. [78], one can see that the extraction efficiency or performance is influenced by the lengthening of the alkyl chain in the imidazolium cation, from C4 to C10. Generally, the HTTA reagent shows better extraction ability towards Eu3+ ions in the [C1C10im+][Tf2N−] solvent system. According to the results, the logD values decrease as a function of n, i.e., with a trend following that of the hydrophobicity of ILs’ cations: [C1C10im+][Tf2N−] > [C1C8im+][Tf2N−] > [C1C6im+][Tf2N−] > [C1C4im+][Tf2N−]. It is very interesting that the established order by Hirayama et al. [169] of logK values for the conventional chelate extraction is [C1C4im+][PF6−] > [C1C6im+][PF6−] > [C1C8im+][PF6−], whereas for anionic species it is [C1C4im+][PF6−] < [C1C6im+][PF6−] < [C1C8im+][PF6−]. In other words, M2+(TTA)2 species seem to favor a hydrophilic nature, while M2+(TTA)3− favour a hydrophobic one, according to the authors. Furthermore, the extraction behavior of several divalent metal cations (M2+) in IL chelate extraction systems was investigated using several ILs as an extraction diluent and thenoyltrifluoroacetone as an extractant [177] as well. Additionally, the behavior was compared with that using less hydrophobic media ([C1Cnim+][PF6−]). The extracted species in the [C1Cnim+][Tf2N−] solvent systems were neutral M(TTA)2 for M = Cu and anionic M(TTA)3– for M = Mn, Co, Ni, Zn, and Cd. The conversion of the IL anion from [PF6−] to more the hydrophobic [Tf2N−] resulted in changing the species extracted for Ni2+ from hydrated neutral complex (Ni(TTA)2(H2O)2) to hydrophobic anionic complex (Ni(TTA)3–). Furthermore, the extractability for these metals was governed, obviously, by the hydrophobicity of the IL ions. Thus, in the IL chelate extraction system, the selection of a suitable ionic liquid as the extraction phase seems to be an important factor for the enhancement of extraction selectivity. In all investigated [C1Cnim+][Tf2N−] systems, the order of logK values was found to be [C1C4im+] < [C1C6im+] < [C1C8im+], which is the same as the order in [C1Cnim+][PF6−]. These facts suggest that the 1-alkyl chain length of [C1Cnim+] of the IL phase affects the extractability of these metals as anionic species M(TTA)3–. Additionally, the chelate extraction of divalent transition metal cations into a [C1C4im+][PF6−] environment with β-diketones having a trifluoromethyl group such as benzoyltrifluoroacetone, 2-naphtoyltrifluoroacetone, and trifluoroacetylacetone was investigated by Hirayama et al. [178]. As a whole, the obtained results suggest that the important part of these β-diketone ligands in the stabilization of some anionic ML3− complexes (Mn, Co, Zn, Cd) or ML2 (Ni, Cu, Pb) in [C1C4im+][PF6−] media is not the hydrophobicity of ring structures such as 2-thienyl, phenyl, and 2-naphthyl groups, but the electronegativity of the trifluoromethyl group and, more strictly, that of the fluorine atoms in the group. Moreover, to underline again, hydrated ML2 chelate prefers a more hydrophilic regime, whereas ML3− prefers a more hydrophobic one.
Because of their favorable physico-chemical properties and superior remarkable extraction behavior in comparison with conventional diluents, ILs have been evaluated as innovative, perspective organic media for metal solvent extraction [179].
5.3. Synergistic Solvent Extraction of Ln (III) Ions with Mixtures including Thenoyltrifluoroacetone
The distribution ratios produced by a mixture of two or more compounds may be unfamiliar to the sum of their individual contributions generating the synergistic effect. When it is lower, contrarily, the synergism is negative. Significant effort has been devoted to the study of this phenomenon in solvent extraction over the past decades, including with the HTTA molecule as a principle extractant in duo with varied synergistic agents for f-block elements in molecular diluents, as was already seen in Section 4. Although synergism in traditional molecular liquids has been well investigated, so far only a very limited number of studies have shown that synergism as a phenomenon produced by two ligands also occurs in IL−based solvent systems until the end of 2013. As a matter of fact, only a handful of publications on synergism in ILs is available for that period since the first trial in 2005 [180]. In truth, the IL’s constituents can pass into the aqueous phase in the absence of Mn+ as well, i.e., losses of [C1Cnim+] and [Tf2N−] entities cannot be avoided at all [158,159]. If a large number of species are formed successively when the concentration of one of the extractants is varied, the equilibria are so numerous that even the best curve-fitting with a large number of experimental data may not lead to a clear interpretation of the synergistic effect. The stoichiometry of the adducts was unresolved and only trends in the relative solvating power of the extractants were deduced from the slope analysis [54]. For an example, the synergistic extractions concerning Ln3+/HCl/HTTA/CMPO/[C1Cnim+][Tf2N−] solvent systems have been published recently, and in reality, only a handful of studies are available at the moment in the literature that apply HTTA in IL based synergistic systems. Nevertheless, more detailed research investigation is necessary to establish the exact extraction mechanism in ionic diluents; the cationic mixed complexes of the type Eu (TTA)(CMPO)y2+, Eu (TTA)2(CMPO)y+, or Eu (TTA)3(CMPO)y seem to emerge as follows:
Eu3+(aq) + xHTTA(o) + yCMPO(o) + (3-z)[C1Cnim+](o) ⇌ [Eu3+(TTA)x(CMPO)y]+(o) + xH+(aq) + (3-z)[C1Cnim+](aq)
The formation of cationic ternary complexes of 4f-ions into ILs was already reported. Hirayama et al. have reported the La3+ extraction with a cation-exchange process such as La (TTA)2(18C6)+ and La (TTA)(18C6)2+ species formed in the organic phase, (18-crown-6) [150]. Moreover, the dicationic ternary complex Eu (BA)(TOPO)32+ (benzoylacetone, HBA) extracted via ion exchange was suggested by Okamura and co-researchers, too [43].
Adduct-formation constants have been determined in the thirteen molecular diluents for the two types of synergistic adducts, Eu (TTA)3·S and Eu (TTA)3·2S (S = TOPO), by Akiba et al. [87] The complexes of the type LnL3·2TOPO were also established when hexafluoroacetone was applied [89]. The simultaneous extraction of two synergistic species having one or two oxo-donor molecules (Eu (TTA)3·2S and Eu (TTA)3·S, S = DPSO(diphenyl sulphoxide), TBP and TOPO) in benzene was also observed by Mathur and co-researchers [181]. So, the addition of a neutral organophosphorous extractants to the Ln3+̶ chelate systems in molecular diluents improves the extraction efficiency and the donor ability of the phosphoryl oxygen, which is the key parameter that determines the increase in extraction [42,182].
In truth, the addition of a CMPO molecule to a Eu3+/HTTA/[C1C10im+][Tf2N−] mixture causes a relatively small synergism, i.e., the values are below one order of magnitude. Moreover, the Eu (III) ion is not extracted synergistically (SC < 0) in the cases when rather high extractant concentrations are used (1.5 × 10−2 mol dm−3). Almost no synergistic effect was observed either for all Ln (III) ions, except Lu (III), using a combination of HTTA and TOPO diluted in [C1C10im+][Tf2N−] media, as reported recently by Okamura et al. [96]. Healy reported, many years ago, that a synergistic enhancement occurs up to a molar concentration of additives between 10−2 and 10−1, and above this concentration a reverse effect is evidenced when molecular diluents were used [57]. So, taking into account that, in ILs, the lower extractant concentrations are necessary, this antisynergistic region probably exists below 10−2 mol dm−3. The authors used diverse classes of molecular diluents, such as aromatic, alicyclic, and aliphatic diluents, and concluded that the synergism was not influenced by the class-wise behavior. Despite the fact that different synergistic agents are employed, the lowering of SCs in an IL media is clearly distinguishable in comparison with the systems implemented the molecular diluents, in which up to 106 synergistic enhancement was usually observed [12,14,69]. This tendency was already observed in similar synergistic mixtures (acidic/neutral duo) by Atanassova et al. [165,183]
The slope analysis method was also used to assess the synergistic extraction system for a combination of HTTA and tri-n-octyl phosphine oxide (TOPO), and the IL itself was found to be directly involved in the complex formation of the metal extraction system [184]. The synergistic effect was strongly influenced by the diluent in which the extractants were dissolved. Furthermore, extraction efficiencies for all the rare-earth metal ions were enhanced by synergism when a conventional organic diluent was used (n-dodecane). However, the synergistic effect in the IL preferentially improved the scandium extraction, leading to higher selectivity for Sc than for other rare-earth metals. This suggests that the extracted species in the synergistic system had a stoichiometry of 3:2:1 (HTTA:TOPO:M, M = Y, La, Nd, Eu, and Dy) and 2:2:1 for Sc3+. The synergistic extraction reaction in the n-dodecane system can, therefore, be expressed as
while the stoichiometry of the synergistic extraction in the IL system was found to be 2:3:1 for Eu3+ and 1:3:1 for Sc3+ with the release of an imidazolium cation into the aqueous phase, which accelerated the partitioning of the metal complexes, expressed as
M3+(aq) + mHTTA(o) + nTOPO(o) + xNO3−(aq) ⇌ M(TTA)m(TOPO)n(NO3−)x(o) + mH+(aq)
M3+(aq) + mHTTA(o) + nTOPO(o) + xNO3−(aq) + (3-m-x)[C1C4im+][Tf2N−](o) ⇌ M(TTA)m(TOPO)n(NO3−)x(3-m-x) [Tf2N−](3-m-x) (o) + (3-m-x)[C1C4im+](aq) + mH+(aq)
On the other hand, the selective synergistic effect for heavier Ln (III) ions was found also using a combination of HTTA and TOPO in [C1C4im+][Tf2N−], leading to enhanced separability among Ln (III) ions [96]. The extracted Ln (III) species and the corresponding extraction constants obtained in the HTTA–TOPO system were determined by three-dimensional extraction equilibrium analysis. In this synergistic system, the neutral adduct, Ln (TTA)3(TOPO)2, and the cationic charged adducts, Ln (TTA)2(TOPO)2+, Ln (TTA)2(TOPO)3+, as well as Ln (TTA)(TOPO)32+, were extracted into the IL phase. Moreover, highly selective synergism was found for lanthanoid extraction with β-diketones such as 2-thenoyltrifluoroacetone, 2-naphthoyltrifluoroacetone (HNTA), and benzoylacetone (HBA) in the presence of TOPO in the same IL, [C1C4im+][Tf2N−] [43]. The formation of dicationic ternary complex Eu (BA)(TOPO)32+, extracted via an ion exchange, was suggested by Okamura and co-researchers in this IL, too. The synergistic enhancement for each of the three studied Ln3+ ions (La, Eu, Lu) in the HTTA−TOPO and HNTA−TOPO solvent systems was reported to be similar, while being quite different from the HBA−TOPO mixture due to the absence of a CF3 group in the β-diketone chemical structure. Additionally, the synergistic effect for Eu3+ and Lu3+ ions in HBA−TOPO systems was found to be considerably larger than that in the HNTA−TOPO case. It is already known that benzoylacetone is a poor extractant for 4f-ions due to its higher pKa value (8.96), as compared to thenoyltrifluoroacetone (6.2), and its quality of being more competitive was not ameliorated in the ionic organic environment.
Another synergistic extraction system was investigated for the possible selective separation of light lanthanoids using an IL, [C1C4im+][Tf2N−], as an extraction medium for HTTA and 18-crown-6 extractants [150]. Trivalent lanthanum was efficiently extracted as a cationic ternary complex by the cation-exchange process of La (TTA)2(18C6)+ and La (TTA)(18C6)2+, whereas europium and lutetium showed relatively low extractability without forming respective ternary complexes. This result is thought to originate in a size-fitting effect of 18-crown-6 to lanthanum (18C6, cavity size = 0.268–0.286 nm and La3+, ionic diameter = 0.243 nm at the coordination number 9) and the unique nature of the IL as a diluent. Nevertheless, characteristic ion-pair extraction of the lighter Ln (III) was observed with 1,2-dichloroethane, i.e., identified in conventional organic molecular diluent containing HTTA and 18-crown-6 or dicychlohexano-18-crown-6 as synergistic agents, in which the cationic complex, Ln(TTA)2CE+, was formed and extracted [106]. Remarkable increases in extractability and selectivity were attained in the synergistic ion-pair extraction of the lighter Ln ions (from La to Gd), which could be elucidated on the basis of the size-fitting effect in the complex formation of the lighter Ln ions with crown ether as well. The synergistic effect of 18-crown-6 derivatives was also reported by the same group, including cis-dicyclohexano-18-crown-6 (DC18C6), dibenzo-18-crown-6 (DB18C6), and 15-crown-5 (15C5), in combination with HTTA as a main chelating agent in the [C1C4im+][Tf2N−] organic phase for all 14 lanthanoids [185]. For instance, the extractability of lighter Ln3+ ions was enhanced upon the addition of a crown ether in the established order, DC18C6 > 18C6 >> DB18C6 = 15C5, whereas the extraction behavior of heavier ones was hardly changed.
Moreover, room-temperature ILs containing β-diketonate anions have been prepared and tested for the solvent extraction of 5f-ions such as 239Pu (IV), 233U(VI), and 241Am (III) from aqueous nitric acid medium [155]. Several new ionic liquid compounds such as alkylquaternaryammonium thenoyltrifluoroacetonate (R4NTTA) and 1-alkyl-3-methylimidazolium thenoyltrifluoroacetonate (amimTTA), with methyl, butyl, hexyl, heptyl, and octyl moieties, have been designed [155]. Further, the distribution ratio of Pu (IV) in a solution of tri-n-octylmethylammonium thenoyltrifluoroacetonate (TOMATTA) present in tri-n-octylmethylammonium bis(trifluoromethylsulfonyl)imide (TOMA[Tf2N−]) and amimTTA in amim[Tf2N−] was investigated as a function of the various experimental parameters applied. The unique property of these newly synthesized β-diketonate ILs, namely, the miscibility in molecular diluents, was exploited in order to elucidate the reaction mechanism during Pu (IV) extraction. Furthermore, a series of ammonium-bifunctionalized ionic liquid extractants combined with a number of anions, including carboxylic acids and 1,3-diketonates, have been prepared and studied toward rare-earth ions [186]. The results demonstrate that these compounds have a synergistic effect between the cation [A336+] and 1,3-diketonate anions in the separation of Res (III) ions, the so-called inner-synergistic effects. By comparison, the so-synthesized ammonium-ILs containing 1,3-diketonates (HTTA, 1,1,1-trifluoro-2,4-pentanedione (HTFA) and benzoyltrifluoroacetone (HBTA)) have obviously more potential applications concerning La (III)/RE(III) selectivity than compounds with carboxylic acids (sec-octylphenoxyacetic acid and sec-nonylphenoxyacetic acid).
6. Conclusions and Remarks on Metal Solvent Extraction: Yesterday–Today–Tomorrow
In the same manner, apropos of the formation of synergism in solvent extraction chemistry, water-like molecules would once again have a preponderant influence; however, this influence will be on the establishment of the antagonistic phenomenon this time. The important role that water plays in the heterogeneous equilibria of synergistic metal extraction was recognized as early as was the synergistic phenomenon itself [187]. In the years after its discovery, the topic was interpreted by several authors, emphasizing the phenomenon that the formation of synergistic adducts is characterized by a substantial decrease in the water content in the organic phase. The consensus of opinion is that the competition between water and the neutral synergistic component is responsible for the formation of an anhydrous, or almost anhydrous, synergistic mixed adduct in the organic solution. Even the effect of a diluent on the stability of the synergistic adduct formed is explained through the activity of water in the diluent itself. Therefore, the principle action of typical organic, molecular diluents on setting up the antagonistic effect during a solvent extraction process is related historically to their ability to dissolve water in their volume [137]. This would explain the greater synergistic effect observed frequently with more hydrophobic molecular diluents. Healy showed that the very large effects produced on Am(TTA)3(TBP)2 adduct extraction by so-called inert diluents were in the reverse order of increasing water solubility in these diluents [58]. In general, the extraction of a particular metal ion in different diluents follow the order cyclohexane > hexane > CCl4 > C6H6 > CHCl3, which is the reverse of the order of the solubility of water [188]. As a rule, lower extraction is achieved in diluents capable of hydrogen bonding, either by their donor or acceptor properties. For an example, UV work indicates that, on changing the diluent from cyclohexane to benzene, the HTTA compound tends to produce more ketohydrate forms and less enol forms. In fact, the kinetics of the keto ⇌ enol reaction are not fast, although they seem to be catalyzed by the presence of an adduct such as TBP or TOPO ligands. They react with the enol form in drier molecular diluents, but cannot compete with water in wetter ones [189]. For example, a water-saturated hexane solution consisting of 0.1 mol dm−3 HTTA and 0.05 mol dm−3 TOPO contained 0.035 mol dm−3 water, but the addition of 0.05 mol dm−3 uranyl ion caused the water content to decrease by a factor of nearly 10 to 0.004 mol dm−3, approximately the figure of water-saturated hexane alone (7 × 10−2 g∙dm3). It is interesting to see that the order of bringing about synergism in the molecular diluents used is also the same order as the solubility of water in these diluents [190]. As an illustration, cyclohexane, which has the poorest antisynergistic effect and lowest water solubility, is followed by hexane (7 × 10−2 g∙dm−3) and TBP (60 g∙dm−3). In this way, one can assess the amount of enolate and ketohydrate forms that are formed from the initial 90–100 percent enol form of HTTA normally found in organic diluents. This almost-complete water removal, concomitant with a metal ion solution, has been established for uranium, thorium, and neodymium ions during solvent extraction [57]. Thus, one is led to the assumption that the presence of water in the organic phase has, somehow, an appreciable effect on the breakdown of the formed anhydrous synergistic complex M(TTA)xSy to the creation of M(TTA)x chelate, again presumably hydrated. In other words, the positive entropy change observed usually in many complexation reactions has been more related to the release of a large number of water molecules already coordinated than to the number of new bound ligands. As a result, the total degrees of freedom of the system are increased by complexation and results in a positive value of the entropy change [85]. Since the bonds between the metal and water are generally quite strong, a considerable amount of energy must be expended in the process of removing the two water molecules from the LnL3∙2H2O chelates [134]. The release of water molecules plays an important role in the entropy change of reaction in spite of the fact that no charged species are involved. Therefore, the phenomenon of antisynergism observed in molecular solvent systems is shown to be connected with the water content of the organic phase, and further, to the destruction of the anhydrous synergistic species M(TTA)xSy [72]. In fact, in aqueous equilibrated hexane solutions of the HTTA−TOPO system, the formation of ketohydrate and TOPO hydrate would be greater than the case of TBP, due to the strong electron-donor properties of the TOPO ligand. In fact, the competition of TBP with water was also shown for the solvent system UO2(TTA)2 by applying hexane as a diluent, which produces the UO2(TTA)2∙TBP complex as well as for thorium, Th(TTA)4∙TBP. Further, Karl Fischer water content measurements have shown that, as one adds UO2, Th, or Nd ions to HTTA−TBP mixtures in hexane, the water content of the phase gradually decreases until reaching the stoichiometric quantity of metal to HTTA and TBP, and the hexane solution is practically free of water. Nevertheless, the addition of excess TBP molecules causes the water content of the hexane phase to somehow rise again [72]. However, the further addition of some excess TBP compounds causes this complex to be broken down automatically to UO2(TTA)2, i.e., synergism was followed likely by antisynergism. The addition of a greater amount of a donor solvent will simply cause an increase in the activity of [S], and so the only way to cause antisynergism must be decreasing the enol [TTA] activity. Unfortunately, the reduction in [TTA] enol activity is obviously bound up with the organic water content—and do not forget that water is necessary for the formation of the ketohydrate species. This new structure probably contains attached water molecules, but in any case, it is very much influenced by the presence of water; it may or may not contain an additional ligand, S [72].
In practice, in the solvent extraction process, the molecular organic phase is always saturated with water. The effect of the diluent may be a reflection of the effective concentrations of the extractant and of the adduct due to interaction between them and the diluent [72]. Accordingly, all species in the organic phase can be hydrated. In fact, the extraction of chelate Eu (TTA)3 generally decreases as the solubility of water in the organic molecular phase increases; see Table 9. This is understandable if the water solubility is a measure of the ability of the diluent to interact also with the extractant solute, in this case the HTTA molecule. Further, the adduct-formation reaction of Eu (TTA)3 with a synergistic agent would be in competition with the H2O molecules.

Table 9.
Values of water solubility and the extraction constant for Eu3+−TTA in different diluents [72].©1983 Taylor&Francis.
A final point of interest is that there is an inverse relationship between the amount of water extracted in the organic phase and the basicity of the used donor [181]. The influence of water appears to be largely unresolved because it was noted also that the water content of an organic phase may drastically vary as well, as with the nature of the metal involved. A direct comparison of the relative effects of cyclohexane and benzene on the extraction of metals as synergistic adducts shows the effect to be very great for the tetra and trivalent, and much less for the di- and monovalent, metals [58].
Regardless, the ionic compound [C1C4im+][Tf2N−] does tolerate large amounts of water in its bulk, and highly or fully hydrated lanthanoid ions could easily be dissolved as a consequence in this IL [165]. In an IL solution, the possible completion of the coordination sphere of extracted species can be achieved either through binding with the [Tf2N–] anions or by water molecules that are known to transfer to the IL phase: ca. 12,000 ppm under such chemical conditions. Consequently, the presence of a co-extractant, i.e., a synergistic agent, does not appear to be as mandatory as it is in molecular diluents in order to improve extraction efficiency significantly. Thus, there is, in fact, no absolute need for a water-free coordination sphere to obtain high-extraction D values when using an IL medium. Nevertheless, this obligation of the synergistic agent seems to fall away because almost-completely dehydrated chelate complexes exist likely in an IL environment, as already mentioned [156,175]. Generally, a synergistic effect less than one order of magnitude occurs in the solvent extraction of Ln (III) ions with mixtures of chelating ligand (HTTA) in combination with various synergistic agents in an IL medium. In other words, one can say that the limited synergistic effect observed in an IL medium is in line with the observation made in several molecular diluents—that an increase in water solubility leads to a decrease in a synergism [57,60,137]. As a result, the whole story of the “destruction of synergism” (the observed slight increase) in an IL medium is not yet finalized; today, even more, it could produce much more complicated extraction mechanisms in comparison to molecular diluents that are likely to vary from solvent system to solvent system in use. Turning to the synergistic couples employed, from the obtained results within mainly the IL phase [C1C4im+][Tf2N−], again, lanthanoid extraction is always more efficient than in typical organic diluents. Further, the implementation of an acidic−neutral mixture of extracting molecules leads to more effective processes for the extraction of various metal by, applying VOCs, and owing to recently published studies, this tendency was confirmed to be true in ILs at present as well. Nevertheless, the investigations of synergistic enhancement with mixtures of two neutral ligands in nature that usually exhibit only donor properties [191] or two chelating ligands [102] in ILs have clearly shown a slight synergistic effect, but little information is available on such solvent systems in the open literature, and implementing both type of diluents and the mechanism of the synergistic enhancement is only somewhat understood. Actually, the continued development and adaptation of synergistic solvent extraction processes indicate the general utility of this concept [192]. In spite of the recent, very numerous innovative advances and advantages in the field of solvent extraction chemistry that have been achieved, there are plentiful constant drawbacks and shortcomings to be overcome in the near future.
Funding
This research was funded by the Research and Development Sector at the UCTM, grant number 12206/2022.
Conflicts of Interest
The author declare no conflict of interest.
References
- Binnemans, K. Rare Earth beta-diketones. ch. 225. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Gschneider, K.A., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 35. [Google Scholar]
- De, A.K.; Khopkar, S. Analytical application of thenoyltrifluoroacetone. J. Sci. Indust. Des. 1962, 21A, 131–135. [Google Scholar]
- Komatsu, Y.; Fujiki, Y.; Michine, Y.; Yajima, Y.; Sasaki, T. Solvent extraction separation of alkaline earth metal ions with thenoyltrifluoroacetone and trioctylphosphine oxide in carbon tetrachloride. Solvent Extr. Ion Exch. 1991, 9, 471–479. [Google Scholar] [CrossRef]
- Akki, S.B.; Khopkar, J.M. Solvent extraction of selenium(IV) with 2-thenoyltrifluoroacetone. Sep. Sci. Technol. 1971, 6, 455–460. [Google Scholar] [CrossRef]
- De, A.; Rahaman, M. Extraction and spectroscopic determination of platinum(IV) and palladium(II) with 2-thenoyltrifluoroacetone. Analyst 1964, 89, 795–799. [Google Scholar] [CrossRef]
- Poskanzer, A.M.; Foreman, B.M. A summary of TTA extraction coefficients. J. Inorg. Nucl. Chem. 1961, 16, 323–336. [Google Scholar] [CrossRef]
- Alstad, J.; Augustson, L.H.; Farbu, L.H. Solvent extraction of rare earth metal ions with thenoyltrifluoroacetone in carbon tetrachloride. J. Inorg. Nucl. Chem. 1974, 36, 899–903. [Google Scholar] [CrossRef]
- Stary, J.; Freiser, H. Equilibrium Constants of Liquid Distribution Reactions: Part IV. Chelating Extractants; IUPAC Chemical Data Series, 18; Pergamon: Oxford, UK, 1975; pp. 131–178. [Google Scholar]
- Ensor, D.D.; Nicks, M.; Pruett, D. Extraction of uranyl ions by synergistic mixtures of thenoyltrifluoroacetone and macrocyclic donors. Solvent Extr. Ion Exch. 1988, 6, 621–629. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, L.K. Solvent extraction separation of some actinides and lanthanides with 2-thenoyltrifluoroacetone. Sep. Sci. Technol. 1980, 15, 1263–1269. [Google Scholar] [CrossRef]
- Marcus, Y. Development and publication of solvent extraction methods. Talanta 1976, 23, 203–209. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. High molecular weight amines and quaternary ammonium salts as synergistic agents in the solvent extraction of metal ions with chelating extractants. chap. 7. In Handbook of Inorganic Chemistry Researc; Morrison, D.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 245–267. [Google Scholar]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Shivachev, B.; Varbanov, S.; Dukov, I. Effect of p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms as synergistic agent in the solvent extraction of trivalent lanthanoids with 4,4,4-trifluoro-1-(2-thienyl)1,3-butanedione and structural study of solid complexes by IR, NMR and X-ray. Polyhedron 2008, 27, 3306–3312. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Some aspects of synergistic metal extractions with chelating extractants and amines or quaternary ammonium salts. J. Univ. Chem. Technol. Met. 2003, 38, 7–22. [Google Scholar]
- Beck, M.T. Critical evaluation of equilibrium constants in solution. Stability constants of metal complexes. Pure Appl. Chem. 1977, 49, 127–135. [Google Scholar] [CrossRef]
- Mathur, J. Synergism of trivalent actinides and lanthanides. Solvent Extr. Ion Exch. 1983, 1, 349–412. [Google Scholar] [CrossRef]
- Nash, K. Aqueous complexes in separations of f-elements: Options and strategies for future development. Sep. Sci. Technol. 1999, 34, 911–929. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Dukov, I. The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction? RSC Adv. 2016, 6, 81250–81265. [Google Scholar] [CrossRef]
- Quinn, J.; Soldenhoff, K.; Stevens, G.; Lengkeek, N. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298–305. [Google Scholar] [CrossRef]
- Hokura, A.; Sekine, T. Gradual destruction of synergistic enhancement of copper(II) extraction into carbon tetrachloride with 2-thenoyltrifluoroacetone and trioctylphosphine oxide. Solvent Extr. Res. Dev. Jpn. 1995, 2, 102–111. [Google Scholar]
- Kiss, T.; Sovago, I.; Gergely, A. Critical survey of stability constants of complexes of glycine. Pure Appl. Chem. 1991, 63, 597–638. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Chen, Y. Critical evaluation of stability constants for nucleotide complexes with protons and metal ions and the accompanying enthalpy changes. Pure Appl. Chem. 1991, 63, 1015–1080. [Google Scholar] [CrossRef]
- Yamauchi, O.; Odani, A. Stability constants of metal complexes of amino acids with charged side chains—Part I: Positively charged side chains (Technical Report). Pure Appl. Chem. 1996, 68, 469–496. [Google Scholar] [CrossRef]
- Lajunen, L.; Portanova, R.; Piispanen, J.; Tolazzi, M. Critical evaluation of stability constants for alpha-hydroxycarboxylic acid complexes with protons and metal ions and the accompanying enthalpy changes. Part I: Aromatic ortho-hydroxycarboxylic acids (Technical Report). Pure Appl. Chem. 1997, 69, 329–382. [Google Scholar] [CrossRef]
- Popov, K.I.; Rönkkömäki, H.; Lajunen, L.H.J. Critical evaluation of stability constants of phosphonic acids (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Delgado, R.; Chaves, S. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 71–102. [Google Scholar] [CrossRef]
- Marcus, Y. Diluent effects in solvent extraction. Solvent Extr. Ion Exch. 1989, 7, 567–575. [Google Scholar] [CrossRef]
- Baes, C.F., Jr.; McDowell, W.J.; Byan, S.A. The interpretation of equilibrium data from synergistic solvent extraction systems. Solvent Extr. Ion Exch. 1987, 5, 1–28. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Separation of europium(III) and americium(III) by solvent extraction of their metal chelate complexes. Talanta 1964, 11, 864–873. [Google Scholar] [CrossRef]
- Dyrssen, D.; Heffez, M.; Sekine, T. Influence of the chelating agent on the separation of Eu3+ and Am3+ ions by solvent extraction. J. Inog. Nucl. Chem. 1961, 16, 367–370. [Google Scholar] [CrossRef]
- Sekine, T.; Koike, Y.; Hasegawa, Y. Studies of actinium (III) in various solutions. II. Distribution behavior of lanthanum (III) and actinium (III) in some chelate extraction systems. Bull. Chem. Soc. Jpn. 1969, 42, 432–436. [Google Scholar] [CrossRef]
- Albinsson, Y. Solvent extraction studies of lanthanide acetylacetonates. Part III. Complexes formed by Tb, Ho, Tm and Lu. Acta Chim. Scand. 1989, 43, 919–925. [Google Scholar] [CrossRef][Green Version]
- Albinsson, Y.; Mahmood, A.; Majdan, M.; Rydberg, J. Solvent extraction studies of lanthanide acetylacetonates. Complexes formed by La, Nd, Sm and Eu. Radiochim. Acta 1989, 48, 49–57. [Google Scholar] [CrossRef]
- Dyrssen, D. On the complex formation of strontium with oxine. Sven. Kem. Tidsk. 1955, 67, 311. [Google Scholar]
- Newman, L.; Klotz, P. Synergistic effect of tri-n-octylamine on the solvent extraction of americium by thenoyltrifluoroacetone. Inorg. Chem. 1966, 5, 461–466. [Google Scholar] [CrossRef]
- Moyer, B.A. Ion Exchange and Solvent Extraction: A Series of Advances; CRC Press: Boca Raton, FL, USA; Taylor & Francis group: Tokyo, Japan, 2010; Volume 19, pp. 1–673. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Dukov, I. Behavior of mixed systems based on para-substituted 4-aroyl-5-pyrazolones in the presence of phosphorus containing calix[4]arene towards lanthanoids: Synergistic solvent extraction and separation. Sep. Purif. Technol. 2012, 95, 58–63. [Google Scholar] [CrossRef]
- Foust, A.; Wenzel, L.; Maus, L.; Clump, C.; Andesen, L. Solvent Extraction; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Atanassova, M.; Dukov, I. Synergistic solvent extraction of trivalent lanthanoids with mixtures of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and crown ethers. Acta Chim. Slov. 2006, 53, 457–463. [Google Scholar]
- Schmidt, V.S. Amine Extraction; Kerter Press: Jerusalem, Israel, 1971. [Google Scholar]
- Atanassova, M.; Kaloyanova, S.; Deligeorgiev, T. Application of 4,4,4-trifluoro-1-(biphenyl-4-yl)butane-1,3-dione as a chelating extractant in the solvent extraction and separation of light lanthanoids in combination with phosphine oxides. Acta Chim. Slov. 2010, 57, 821–827. [Google Scholar] [PubMed]
- Okamura, H.; Takagi, H.; Isomura, T.; Morita, K.; Nagatani, H.; Imura, H. Highly selective synergism for the extraction of lanthanoid (III) ions with β-diketones and trioctylphosphine oxide in an ionic liquid. Anal. Sci. 2014, 30, 323–325. [Google Scholar] [CrossRef]
- Jordanov, V.; Atanassova, M.; Dukov, I. Solvent extraction of lanthanides with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. Sep. Sci. Technol. 2002, 37, 3349–3356. [Google Scholar] [CrossRef]
- Atanassova, M. Synergistic solvent extraction and separation of lanthanide (III) ions with 4-benzoyl-3-phenyl-5-isoxazolone and the quaternary ammonium salt. Solvent Extr. Ion Exch. 2009, 27, 159–171. [Google Scholar] [CrossRef]
- Atanassova, M.; Jordanov, V.; Dukov, I. Effect of the quaternary ammonium salt Aliquat 336 on the solvent extraction of lanthanoid(III) ions with thenoyltrifluoroacetone. Hydrometallurgy 2002, 63, 41–47. [Google Scholar] [CrossRef]
- Pavithran, R.; Reddy, M.L.P. Crown ethers as synergists in the extraction of trivalent lanthanoids with 3-phenyl-4-(4-fluorobenzoyl)-5-isoxazolone. Radiochim. Acta 2004, 92, 31–38. [Google Scholar] [CrossRef]
- Reddy, K.; Kumar, A.; Reddy, M. Synergistic extraction of zirconium(IV) and hafnium(IV) with 4-acylbis(1-phenyl-3-methyl-5-pyrazolones) in the presence of neutral organophosphorus extractants. Solvent Extr. Ion Exch. 2006, 24, 419–432. [Google Scholar] [CrossRef]
- Remya, P.; Raj, D.; Reddy, M.L.P. Para-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones as selective extractants for vanadium (V) from acidic chloride solutions. Solvent Extr. Ion Exch. 2006, 24, 877–892. [Google Scholar] [CrossRef]
- Messaodi, A.; Torkestani, K.; Goetz-Grandmont, G.; Brunette, J. Synergistic extraction of alkaline earth cations with 3-phenyl-4-benzoyl-isoxazol-5-one and tri-n-octylphosphine oxide in toluene. J. Radioanal. Nucl. Chem. 1996, 208, 123–132. [Google Scholar] [CrossRef]
- Kolarik, Z. The effect of diluent on the solvent extraction of Tb(III) and Eu(III) by di-n-octyl phosphoric acid. In Solvent Extraction Chemistry; Dyrssen, D., Liljenzin, J.-O., Rydberg, J., Eds.; John Wiley: Amsterdam, The Netherlands, 1967; pp. 250–255. [Google Scholar]
- Cunningham, J.G.; Scargill, D.; Willis, H. The Solvent Extraction of Praseodymium and Neodymium; (AERE-C/M 215); UKAEA-Research Group: Harwell, UK, 1954. [Google Scholar]
- Blake, C.A.; Baes, C.F.; Brown, K.B.; Coleman, C.F.; White, C. Proceedings of the Second United Nations International Conference on the Peaceful Uses of Atomic Energy, Geneva, Switzerland, 1–13 September 1958; IAEA: Vienna, Austria, 1958; Volume 28, p. 289. [Google Scholar]
- Duyckaerts, G.; Desreux, J. Recent developments and new combinations of extractants in synergic processes. In Proceedings of the International Solvent Extraction Conference (ISEC’77), Toronto, ON, Canada, 9–16 September 1977; CIM, special, Lucas, B., Ritcey, G., Smith, H., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montrea, QC, Canada, 1979; Volume 21, pp. 73–85. [Google Scholar]
- Madic, C. Phénomènes de synergismes lors de l’extraction par solvant. Chim. Anal. 1972, 54, 102–113. [Google Scholar]
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides I-uranium(VI). J. Inorg. Nucl. Chem. 1960, 15, 158–170. [Google Scholar] [CrossRef]
- Healy, T.V. Synergism with thenoyltrifluoroacetone in the solvent extraction of metallic species. Nucl. Sci. Eng. 1963, 16, 413–420. [Google Scholar] [CrossRef]
- Healy, T. Synergistic adducts as coordination compounds. In Proceedings of the Fifth International Solvent Extraction Conference, Jerusalem, Israel, 16–18 September 1969; Kerters, A.S., Marcus, Y., Eds.; Wiley Interscience: New York, NY, USA, 1969; pp. 257–277. [Google Scholar]
- Atanassova, M. Lanthanoid complexes with thenoyltrifluoroacetone and 4-tert-butylcalix[4]arene-tetraacetic acid tetraethyl ester: Synergistic extraction and separation. Microchim. Acta 2011, 174, 175–181. [Google Scholar] [CrossRef]
- Petrova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S. Effect of the diluents on the synergistic solvent extraction and separation of trivalent lanthanoids with 4-benzoyl-3-phenyl-5-isoxazolone and tert-butylcalix[4]arene tetrakis(N,N-dimethyl acetamide) and structural study of Gd(III) solid complex by IR and NMR. Ind. Eng. Chem. Res. 2010, 49, 6189–6195. [Google Scholar]
- Atanassova, M.; Vassilev, N.; Dukov, I. p-tert-Butylcalix[4]arene tetrakis(N,N-dimethylacetamide) as a second ligand in the complexation of trivalent lanthanoids with thenoyltrifluoroacetone in solution and investigation of a solid Eu(III) complex. Sep. Purif. Technol. 2011, 78, 214–219. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Baulin, D.; Baulin, V.; Tsivadze, A. Extraction of rare earth elements(III) with mixtures of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and 2-phosphorylphenoxyacetamides. Russion J. Inorg. Chem. 2019, 64, 407–413. [Google Scholar] [CrossRef]
- Aly, H.F.; Khalifa, S.M.; Navratil, J.D.; Saba, M.T. Extraction of some lanthanides and actinides by 15-crown-5 thenoyltrifluroacetone mixture in chloroform. Solvent Extr. Ion Exch. 1985, 3, 623–636. [Google Scholar] [CrossRef]
- Caceci, M.; Choppin, G.; Liu, Q. Calorimetric studies of the synergistic effect. The reaction of UO22+, Th4+ and Nd3+ TTA complexes with TBP and TOPO in benzene. Solvent Extr. Ion Exch. 1985, 3, 60–621. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Baulin, V.; Baulin, D.; Tsivadze, A. Synergistic solvent extraction of lanthanides(III) with mixtures of 4-benzoyl-3-methyl-1-phenyl-5-pyrazolone and phosphoryl-containing podands. Acta Chim. Slov. 2020, 67, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Kertes, A. Ion Exchange and Solvent Extraction of Metal Complexes; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Nash, K. A review of the basic chemistry and recent developments in trivalent f-elements separation. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Taube, M.; Siekierski, S. General remarks on synergic effects in the extraction of uranium and plutonium compounds. Nucleonica 1961, 6, 489–501. [Google Scholar]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303–11324. [Google Scholar] [CrossRef]
- Peparrd, D.; Mason, G.; Sironen, R. Isolation of neptunium as a mono octyl phosphoric acid complex by liquid-liquid extraction. J. Inorg. Nucl. Chem. 1959, 10, 117–127. [Google Scholar] [CrossRef]
- Tagushi, S.; Goto, K. Pre-Concentration Techniques for Trace Elements; Alfassi, Z.B., Wai, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1989; p. 31. [Google Scholar]
- Hasegawa, Y.; Nakako, T.; Choppin, G. Synergistic extraction of europium(III) with benzoic acid and thenoyltrifluoroacetone. Solvent Extr. Ion Exch. 1983, 1, 745–753. [Google Scholar] [CrossRef]
- El-Reefy, J.A.; Dessouky, N.A.; Aly, H.F. Extraction of Am(III) and Eu(III) from nitrate medium by thenoyltrifluoroacetone mixed with some soft donor ligands. Solvent Extr. Ion Exch. 1993, 11, 19–32. [Google Scholar] [CrossRef]
- Ensor, D.D.; Shah, A.H. Extraction of trivalent lanthanides and actinides by a synergistic mixture of thenoyltrifluoroacetone and a linear polyether. Solvent Extr. Ion Exch. 1984, 2, 591–605. [Google Scholar] [CrossRef]
- Khopkar, P.; Mathur, J. Synergistic extraction of trivalent actinides by mixtures of thenoyltrifluoroacetone and neutral oxo donors. Sep. Sci. Technol. 1981, 16, 957–969. [Google Scholar] [CrossRef]
- Ramaknishna, V.; Patil, S.; Hara Prakas, B. Solvent extraction and spectrophotometric studies on synergistic extraction of plutonium(IV) by mixtures of thenoyltrifluoroacetone (HTTA) and tri-n-butylphosphate (TBP) in benzene. Sep. Sci. Technol. 1979, 14, 571–589. [Google Scholar] [CrossRef]
- Sekine, T.; Ono, M. Studies of the liquid-liquid partition systems. IV. The solvent extraction study of europium(III) adduct chelate complexes with six acetylacetone derivatives and tributylphosphate. Bull. Chem. Soc. Jpn. 1965, 38, 2087–2094. [Google Scholar] [CrossRef]
- Atanasova, M.; Kurteva, V. Synergism in the solvent extraction of europium(III) with thenoyltrifluoroacetone and CMPO in methylimidazolium ionic liquids. J. Solution Chem. 2019, 48, 15–30. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. Part VII. Extration and separation of calcium and stroncium with TTA and TBP in carbone tetrachloride. Anal. Chim. Acta 1967, 37, 217–226. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands.-VI Extraction of In(III) and Eu(III) with mixtures of thenoyltrifluoroacetone and β-isopropyltropolone. J. Inorg. Nucl. Chem. 1967, 29, 1489–1498. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands-III. Adduct formation of Eu(III) and Th(IV) chelates with TTA and IPR. J. Inorg. Nucl. Chem. 1967, 29, 1457–1473. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. V. Adduct formations of some tris-TTA complexes. J. Inorg. Nucl. Chem. 1967, 29, 1481–1487. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. IV Extraction of Eu(III) with chelating acids and neutral adduct-forming ligands. J. Inorg. Nucl. Chem. 1967, 29, 1475–1480. [Google Scholar] [CrossRef]
- Choppin, G.; Morgenstern, A. Thermodynamics of solvent extraction. Solvent Extr. Ion Exch. 2000, 18, 1029–1049. [Google Scholar] [CrossRef]
- Upor, E. Synergistic and antagonistic effects in solvent extraction processes. In Proceedings of the 3rd Symp. “Coordination Chemistry” Debrecen, Hungary, 1970; Akádémiai. Kiado: Budapest, Hungary, 1970; Volume 1, pp. 143–155. [Google Scholar]
- Zhao, Z.; Kubota, F.; Kamiya, N.; Goto, M. Selective extraction of scandium from transition metals by synergistic extraction with 2-thenoyltrifluroacetone and tri-n-octylphosphine oxide. Solv. Extr. Res. Dev. Jpn. 2016, 23, 137–143. [Google Scholar]
- Akiba, K.; Wada, M.; Kanno, T. Effect of diluent on synergic extraction of europium by thenoyltrifluoroacetone and tri-n-octyl phosphine oxide. J. Inorg. Nucl. Chem. 1981, 43, 1031–1034. [Google Scholar] [CrossRef]
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides—IV: Trivalent plutonium, americium and europium. J. Inorg. Nucl. Chem. 1961, 21, 169–180. [Google Scholar] [CrossRef]
- Murthy, K.S.R.; Krupadam, R.J.; Aujaneyuly, Y. Studies on the solvent extraction of trivalent lanthanides with hexafluoroacetylacetone (HFAA) and tri-n-octylphosphineoxide (TOPO). Proc. Indian Acad. Sci. Chem. Sci. 1998, 110, 83–92. [Google Scholar] [CrossRef]
- Favaro, D.; Atalla, L. Interaction effect between thenoyltrifluorocetone and tri-n-octylphosphine oxide in the synergistic extraction of trivalent lanthanides. Determination of the composition of the extracted species. J. Radioanal. Nucl. Chem. Art 1987, 111, 81–94. [Google Scholar] [CrossRef]
- Aly, H.F.; Khalifa, S.M.; Zakareia, N. Periodic variations of lanthanides synergistic extraction by thenoyltrifluoroacetone and triphenylphosphine oxide mixture. Solvent Extr. Ion Exch. 1984, 2, 887–898. [Google Scholar] [CrossRef]
- Sasayama, K.; Umetani, S.; Matsui, M. The substituent effect on the synergic extraction of europium and scandium with 1-phenyl-3-methyl-4-acylpyrazol-5-one and tri and tri-n-octylphosphine oxide. Anal. Chim. Acta 1983, 149, 253–258. [Google Scholar] [CrossRef]
- Akiba, K. Some regularities in the formation constants of TBP adducts of europium tris-TTA chelate. J. Inorg. Nucl. Chem. 1973, 35, 2525–2535. [Google Scholar] [CrossRef]
- Farbu, L.; Alstad, J.; Augustson, J.H. Synergistic solvent extraction of rare-earth metal ions with thenoyltrifluoroacetone admixed with tributylphosphate. J. Inorg. Nucl. Chem. 1974, 36, 2091–2095. [Google Scholar] [CrossRef]
- Sekine, T.; Koizumi, A.; Sakairi, M. Studies of scandium in various solutions. III. The solvent extraction and complex formation of scandium (III) with acetylacetone and thenoyltrifluoroacetone. Bull. Chem. Soc. Jpn. 1966, 39, 2681–2684. [Google Scholar] [CrossRef]
- Okamura, H.; Mizuno, M.; Hirayama, N.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic enhancement of the extraction and separation efficiencies of lanthanoid(III) ions by the formation of charged adducts in an ionic liquid. Ind. Eng. Chem. Res. 2020, 59, 329–340. [Google Scholar] [CrossRef]
- D’Olieslager, W.; Sannen, L. Extraction of Lanthanide ions with Benzoyltrifluroacetone-tri-n-Butylphosphate: Synergism and Antagonism. 967–972. ISEC’90, Solvent Extraction, Kyoto, Japan, 16–21 July 1990; Sekine, T., Ed.; Elsevier Science Publ. B.V.: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Shigematsu, T.; Tabushi, M.; Matsui, M.; Honjyo, T. The synergistic effect in solvent extraction-The correlation of the ionic radius of rare earth elements with the stability constants of rare earth benzoyltrifluoroacetonate adducts with n-hexyl alcohol, TBP and TOPO. Bull. Chem. Soc. Jpn. 1967, 40, 2807–2812. [Google Scholar] [CrossRef]
- Umetani, S.; Kawase, Y.; Le, Q.; Matsui, M. The synergistic extraction of lanthanides with trifluoroacetylcycloalkanones and trioctylphosphine oxide. Chem. Lett. 1997, 26, 771–772. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Nishiyama, Y.; Nagatani, H.; Okamura, H.; Imura, H. Synergistic extraction equilibrium of lanthanide(III) ions with benzoylacetone and a neutral ligand in an ionic liquid. Solvent Extr. Res. Dev. Jpn. 2018, 25, 79–89. [Google Scholar] [CrossRef]
- Kertes, A. The chemistry of solvent extraction. In Recent Advances in Liquid-Liquid Extraction, 1st ed.; Hadson, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1971; Chapter 2; pp. 15–92. [Google Scholar]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Solvent extraction and separation of light lanthanoids with mixtures of two chelating extractants: Benzene vs. ionic liquid. Sep. Sci. Technol. 2016, 51, 290–299. [Google Scholar] [CrossRef]
- Hala, J. Some aspects of synergistic extraction with chelating extractants. J. Radioanal. Chem. 1979, 51, 15–25. [Google Scholar] [CrossRef]
- Woo, C.; Wagner, W.E.; Sands, D.F. Acetylacetone adducts of rare earth trithenoyltrifluoroacetonates in synergistic solvent extraction. J. Inorg. Nucl. Chem. 1972, 34, 307–312. [Google Scholar] [CrossRef]
- Atanasova, M.; Dukov, I. Crown ethers as synergistic agents in the solvent extraction of trivalent lanthanoids with thenoyltrifluoroacetone. Sep. Sci. Technol. 2005, 40, 1104–1113. [Google Scholar] [CrossRef]
- Kitatsuji, Y.; Meguro, Y.; Yoshida, Z.; Yamamoto, T.; Nishizawa, K. Synergistic ion-pair extraction of lanthanide(III) with thenoyltrifluoroacetone and crown ether into 1,2-dichloroethane. Solvent Extr. Ion Exch. 1995, 13, 289–300. [Google Scholar] [CrossRef]
- Mathur, J.; Choppin, G. The interaction of crown ethers with β−diketonate complexes of f-elements. Solvent Extr. Ion Exch. 1993, 111, 1–18. [Google Scholar] [CrossRef]
- Shyama, P. Sinha Structure and Bonding 30. Rare Earths; Springer: Berlin/Heidelberg, Germany, 1976; pp. 1–64. [Google Scholar]
- Zhang, A. Study of the antagonistic extraction of palladium (II) with 1-phenyl-3-methyl-4-propionylpyrazolone-5-one and an organic amine. Solvent Extr. Ion Exch. 2001, 19, 925–938. [Google Scholar]
- Zhang, A.; Wanyan, G.; Kumagai, M. Association behavior of 4-acylpyrazolone derivative and tertiary amine of high molecular weight in antagonistic synergistic extraction of palladium. J. Solut. Chem. 2004, 33, 1017–1028. [Google Scholar] [CrossRef]
- Pai, S.A.; Lohithakshan, K.V.; Mithapara, P.D.; Aggarwal, S.K. Solvent extraction of uranium (VI), plutonium (VI) and americium (III) with HTTA/HPMBP using mono-and bi-functional neutral donors: Synergism and thermodynamics. J. Radioanal. Nucl. Chem. 2000, 245, 623–628. [Google Scholar] [CrossRef]
- Santhi, P.B.; Reddy, M.L.P.; Ramamohan, T.R.; Damodaran, A.D. Radiochemical extraction of lanthanide thiocyanate complexes with bis-2-ethylhexyl sulphoxide. Radiochim. Acta 1994, 64, 121–125. [Google Scholar] [CrossRef]
- Sahu, S.K.; Chakravortty, V.; Reddy, M.L.P.; Ramamohan, T.R. Synergistic extraction of trivalent lanthanoids with 3-phenyl-4-benzoyl-5-isoxazolone and various sulphoxides. Radiochim. Acta 1999, 85, 107–111. [Google Scholar] [CrossRef]
- Bond, A.H.; Dietz, M.L.; Chiarizia, R. Incorporating size selectivity into synergistic solvent extraction: A review of crown ether-containing systems. Ind. Eng. Chem. Res. 2000, 39, 3442–3464. [Google Scholar] [CrossRef]
- Atanassova, M.; Dukov, I. A comparative study of the solvent extraction of the trivalent elements of the lanthanoid series with thenoyltrifluoroacetone and 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one using diphenylsulphoxide as synergistic agent. J. Solut. Chem. 2009, 38, 289–301. [Google Scholar] [CrossRef]
- Ramamohan, T.R.; Prasada Rao, T.; Iyer, C.S.P.; Damodaran, A.D.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Mixed ligand chelate extraction of trivalente lanthanides and actinides with 3-phenyl-4-benzoyl-5-isoxazolone and neutral oxo-donors. Radiochim. Acta 1995, 69, 55–60. [Google Scholar]
- Nakamura, S.; Suzuki, N. Adduct formation constant in the synergic extraction of several rare earth elements with acetylacetone and 1,10-phenanthroline in nonpolar organic solvents. J. Radional. Nucl. Chem. Artic. 1986, 99, 145–152. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. Effect of an acidic chelating agent in the synergic extraction systems of rare earths(III)-β-diketones-2,2’-bipyridine. Inorg. Chim. Acta 1986, 114, 101–107. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. A new trend in the synergistic extraction of rare-earth(III) complexes with various β-diketones and 1,10-phenanthroline. Polyhedron 1986, 5, 1805–1813. [Google Scholar] [CrossRef]
- Matsubayshi, I.; Hasegawa, Y. Thermodynamic consideration for solvent effects in the synergistic extraction of europium(III) with pivaloyltrifluoroacetone and 1,10-phenanthrolone. Anal. Sci. 2001, 17, 221–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kassierer, E.F.; Kertes, A.S. Synergic extraction of some lanthanides and transition metals by two bidentate chelating agents. J. Inorg. Nucl. Chem. 1972, 34, 3221–3231. [Google Scholar] [CrossRef]
- Kassierer, E.F.; Kertes, A.S. Thermodynamics of solvent extraction processes. Synergic adducts of copper and zinc diketonates with bidentate nitrogen bases. J. Inorg. Nucl. Chem. 1972, 34, 3209–3214. [Google Scholar] [CrossRef]
- Bhatti, M.S.; Desreux, J.F.; Duyckaerts, G. Behavior of bidentate amines in the synergic extraction of lanthanides by thenoyltrifluoroacetone. J. Inorg. Nucl. Chem. 1980, 42, 767–770. [Google Scholar] [CrossRef]
- Xia, Y.-X.; Chen, J.-F.; Choppin, G. Solubility, dissociation and complexation with Nd(III) and Th(IV) of oxine, thenoyltrifluoroacetone and 1,10-phanantroline in 5.0 m NaCl. Talanta 1996, 43, 2073–2081. [Google Scholar] [CrossRef]
- Nakamura, S.; Imura, H.; Suzuki, N. Synergic extraction of thulium(III) with thenoyltrifluoroacetone and neutral unidentate and bidentate heterocyclic amines. Inorg. Chim. Acta 1985, 110, 101–105. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzuki, N. Synergic extraction of lanthanide(III) ions with 2-thenoyltrifluoroacetone in the presence of 2,2’-bipyridine or pyridine. Polyhedron 1988, 7, 155–159. [Google Scholar] [CrossRef]
- Ishimori, K.; Imura, H.; Ohashi, K. Effect of 1,10-phenantroline on the extraction and separation of lithium(I), sodium(I) and potassium with thenoyltrifluoroacetone. Anal. Chim. Acta 2002, 454, 241–247. [Google Scholar] [CrossRef]
- Nakamura, S.; Takai, S.; Akiba, K. Synergic extraction of lanthanoids by mixtures of LIX 54 (High molecular weight β-diketone) and bidentate neutral amines. Anal. Sci. 2002, 18, 319–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roy, A.; Nag, K. Investigation on the lanthanoid chelates of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone. An examination of the phenomenon of hypersensitivity. Bull. Chem. Soc. Jpn. 1978, 51, 1525–1529. [Google Scholar] [CrossRef]
- Kikuta, Y.; Watarai, H.; Suzuki, N. Synergic effect of some heterocyclic bidentate amines on the extraction of zinc with 2-thenoyltrifluoroacetone. Correlation between formation constants of amine-complex in organic and aqueous phases. Polyhedron 1982, 4, 387–392. [Google Scholar] [CrossRef]
- Satake, S.; Tsukahara, S.; Suzuki, N. Synergistic extraction equilibrium of lanthanoids(III) with 2-thenoyltrifluoroacetone and nitrogen-containing bidentate ligands, ethylenediamine derivatives. Solvent Extr. Ion Exch. 1999, 17, 259–275. [Google Scholar] [CrossRef]
- Atanassova, M. Adduct formation constants in the synergistic solvent extraction of several lanthanoids with two chelating extractants: β-Diketone and bidentate nitrogen bases. J. Chem. Eng. Data 2010, 55, 5791–5796. [Google Scholar]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S.; Dukov, I. Complexation of trivalent lanthanoid ions with 4-benzoyl-3-phenyl-5-isoxazolone and p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms in solution during synergistic solvent extraction and structural study of solid complexes by IR and NMR. Polyhedron 2010, 29, 655–663. [Google Scholar] [CrossRef]
- Kertes, A.; Kassierer, E. Thermochemistry of lanthanide complexes in the thenoyltrifluoroacetone-bipyridyl system. Inorg. Chem. 1972, 11, 2108–2111. [Google Scholar] [CrossRef]
- Saved, S.A.; Sami, T.M.; Abd El Tawab, A.A. Synergistic extraction of some rare earths(III) from nitrate media by thenoyltrifluoroacetone and tri-n-octylamine. Sep. Sci. Technol. 1996, 31, 2579–2587. [Google Scholar] [CrossRef]
- Dukov, I.; Genov, L. On the mechanism of the synergistic extraction of lanthanides with thenoyltrifluoroacetone and Aliquat-336S. Acta Chim. Acad. Sci. Hung. 1980, 104, 329–335. [Google Scholar]
- Dukov, I.; Atanassova, M. Effect of diluents on the synergistic solvent extraction of some lanthanides with thenoyltrifluoroacetone and quaternary ammonium salt. Hydrometallurgy 2003, 68, 89–96. [Google Scholar] [CrossRef]
- Atanassova, M.; Dukov, I. Synergistic solvent extraction and separation of trivalent lanthanide metals with mixtures of 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one and Aliquat 336. Sep. Purif. Technol. 2004, 40, 171–176. [Google Scholar] [CrossRef]
- Brunette, J.P.; Lakkis, Z.; Lakkis, M.; Leroy, M.J.F. Effect of inorganic aqueous anions on the synergic extraction of cadmium and zinc with 1-phenyl—3-methyl-4-benzoyl-pyrazol-5-one (HPMBP) and lipophilic ammonium salts. Polyhedron 1985, 4, 577–582. [Google Scholar] [CrossRef]
- Sekine, T.; Murai, R.; Konno, H. Solvent extraction equilibria of tris(benzoyl-trifluroacetonato)complex of cobalt(II) and nickel(II) as ion-pairs with tetrabutylammonium. Solvent Extr. Ion Exch. 1984, 2, 213–225. [Google Scholar] [CrossRef]
- Peppard, D.F.; Mason, G.W.; Lewey, S. A tetrad effect in the liquid-liquid extraction ordering of lanthanides. J. Inorg. Nucl. Chem. 1969, 31, 2271–2272. [Google Scholar] [CrossRef]
- Dukov, I.; Jordanov, V.; Atanassova, M. Stoichiometry of the praseodymium complex extracted by a mixture of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and Aliquat 336. Solv. Extr. Ion Exch. 2001, 19, 619–628. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Synergistic solvent extraction and separation of lanthanides using mixtures of 1-phenyl-3-methyl-4-benzoyl-pyrazol-5-one and Aliquat 336: Influence of the ammonium salt anion. Sep. Sci. Technol. 2004, 39, 227–239. [Google Scholar] [CrossRef]
- Noro, J.; Sekine, T. Effect of several quarternary ammoniums on the solvent extraction of europium (III) 2-thenoyltrifluoroacetonate anionic complex into chloroform. Bull. Chem. Soc. Jpn. 1993, 66, 804–809. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Virtanen, P.; Sjöholm, R. Aliquat 336-a versatile and affordable cation source for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanides and actinides in ionic liquids. In Comprehensive Inorganic Chemistry II; Reedijk, J., Peoppelmeir, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 12, pp. 641–673. [Google Scholar]
- Billard, I. In Ionic liquids: New hopes for efficient lanthanide/actinide extraction and separation. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.C.G., Pecharsky, V., Eds.; Elsevier Science Publ. B. V.: Amsterdam, The Netherlands, 2013; Volume 43, Chapter 256; pp. 213–273. [Google Scholar]
- Kolaric, Z. Ionic liquids: How far do they extend the potential of solvent extraction of f-elements? Solvent. Extr. Ion Exch. 2013, 31, 24–60. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Q.; Sun, T.; Shen, Y. Extraction of Th(IV) from aqueous solution by room temperature ionic liquids and coupled with supercritical carbon dioxide. Sep. Purif. Technol. 2013, 119, 66–71. [Google Scholar] [CrossRef]
- Hirayama, N.; Okamura, H.; Kidani, K.; Imura, H. Ionic liquid synergistic cation-exchange system for the selective extraction of lanthanum(III) using 2-thenoyltrifluoroacetone and 18-crown-6. Anal. Sci. 2008, 24, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Borkowski, M.; Laszak, I.; Beitz, J.; Rickert, P.; Dietz, M. Anion effects in the extraction of lanthanide 2-thenoyltrifluoroacetone complexes into an ionic liquid. Sep. Sci. Technol. 2012, 47, 233–243. [Google Scholar] [CrossRef]
- Ranjbar, L.; Yamini, Y.; Saleh, A.; Seidi, J.; Faraji, M. Ionic liquid based dispersive liquid-liquid microextraction combined with ICP-OES for the determination of trace quantities of cobalt, copper, manganese, nickel and zinc in environmental water samples. Microchim. Acta 2012, 177, 119–127. [Google Scholar] [CrossRef]
- Pandey, S.; Baker, G.; Sze, L.; Pandey, S.; Kamath, G.; Zhao, H.; Baker, S. Ionic liquids containing fluorinated β-diketonate anions: Synthesis, characterization and potential applications. New J. Chem. 2013, 37, 909–919. [Google Scholar] [CrossRef]
- Kidani, K.; Imura, H. Solvent effect of ionic liquids on the distribution constants of 2-thenoyltrifluoroacetone and its nickel(II) and copper(II) chelates and the evaluation of the solvent properties based on the regular solution theory. Talanta 2010, 83, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.; Venkatesan, K.; Srinivasan, T.; Vasudeva Rao, P. Ionic liquid extractants in molecular diluents: Extraction behavior of plutonium(IV) in 1,3-diketone ionic liquids. Solvent Extr. Ion Exch. 2011, 29, 602–618. [Google Scholar] [CrossRef]
- Okamura, H.; Sakae, H.; Kidani, K.; Hirayama, N.; Aoyagi, N.; Saito, T.; Shimojo, K.; Naganawa, H.; Imura, H. Laser-induced fluorescence and infrared spectroscopic studies on the specific solvation of tris(1-(2-thienyl)-4,4,4-trifluoro-1,3-butanedionato)europium(III) in an ionic liquid. Polyhedron 2012, 31, 748–753. [Google Scholar] [CrossRef]
- Jensen, M.; Neuefeind, J.; Beitz, J.; Skanthakumar, S.; Soderholm, L. Mechanism of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef]
- Petrova, M. The Crucial Performance of Mutual Solubility among Water and Ionic Liquids in the Time of Liquid-Liquid Extraction of Metallic Species; Academic Solutions: Sofia, Bulgaria, 2020; pp. 1–159. ISBN 978-954-2940-23-4. [Google Scholar]
- Atanassova, M.; Mazan, V.; Billard, I. Modulating the solubilities of ionic liquid components in aqueous-ionic liquid biphasic systems: A Q-NMR investigation. Chem. Phys. Chem. 2015, 16, 1703–1711. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Peculiar synergistic extraction behavior of Eu(III) in ionic liquids: Benzoylacetone and CMPO fusion. Sep. Purif. Technol. 2017, 183, 226–236. [Google Scholar] [CrossRef]
- Mazan, V.; Billard, I.; Papaiconomou, N. Experimental connections between aqueous aqueous and aqueous ionic biphasic systems. RSC Adv. 2014, 4, 13371–13384. [Google Scholar] [CrossRef]
- Depuydt, D.; Dehaen, W.; Binnemans, K. Solvent extraction of scandium(III) by an aqueous biphasic system with a nonfluorinated functionalized ionic liquid. Ind. Eng. Chem. Res. 2015, 54, 8988–8996. [Google Scholar] [CrossRef]
- Atanassova, M.; Billard, I. Determination of pKaIL values of three chelating extractants in ILs. Consequences on extraction process of 4f-elements. J. Solut. Chem. 2015, 44, 606–620. [Google Scholar] [CrossRef]
- Bouby, M.; Billard, I.; Duplâtre, G.; Simonin, J.P.; Bernard, O.; Brunette, J.P.; Goetz-Grandmont, G. Determination of the thermodynamic acidity constant of 3-phenyl-4-benzoylisoxazol-5-one (HPBI) using the binding mean spherical approximation model. Phys. Chem. Chem. Phys. 1999, 1, 3765–3769. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Comparing extraction, synergism and separation of lanthanoids using acidic and neutral compounds in chloroform and one ionic liquid: Is the latter always “better”? RSC Adv. 2014, 4, 38820–38829. [Google Scholar] [CrossRef]
- Kurteva, V.; Atanassova, M.; Billard, I. NMR study on the possible interaction between imidazolium based ionic liquids and extractants widely applied in solvent extraction and separation of f-ions. J. Solut. Chem. 2015, 44, 2416–2430. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Billard, I. Coordination chemistry of europium(III) ion towards acylpyrazolone ligands. Anal. Sci. 2015, 31, 917–922. [Google Scholar] [CrossRef]
- Hirayama, N. Chelate extraction of metals into ionic liquids. Solvent Extr. Res. Dev. Jpn. 2011, 18, 1–14. [Google Scholar] [CrossRef]
- Hirayama, N.; Deguchi, M.; Kawasumi, H.; Honjo, T. Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta 2005, 65, 255–260. [Google Scholar] [CrossRef]
- Kumar, P.; Vincent, T.; Khanna, A. Extraction of UO22+ into ionic liquids using TTA and TBP as extractants. Sep. Sci. Technol. 2015, 50, 2668–2675. [Google Scholar] [CrossRef]
- Dietz, M.; Jensen, M.; Beitz, J.; Dzielawa, J. Room Temperature Ionic Liquids as Diluents for the Liquid-Liquid Extraction of Metal Ions: Promise and limitations. Hydrometallurgy 2003-Fifth International Conference in Honor of Prof. I. Ritchie; TMS (The Minerals, Metals&Materials Socieaty): Warrendale, PA, USA, 2003; Volume 1, pp. 929–939. [Google Scholar]
- Dietz, M.; Dzielawa, J.; Jensen, M.; Beitz, J.; Borkowski, M. The Road to Partition。 The Road to Partition. Mechanism of Metal Ion Transfer into Ionic Liquids and Their Implications for the Application of Ionic Liquids as Extraction Solvents. ACS Symposium Series, Ionic Liquids IIIB: Fundamentals, Progress, Challenges and Opportunities. 2005, Volume 902, Chapter 1. pp. 2–18. Available online: https://global.oup.com/academic/product/ionic-liquids-iiib-fundamentals-progress-challenges-and-opportunities-9780841238947?cc=us&lang=en&# (accessed on 28 April 2022).
- Okamura, H.; Hirayama, N. Recent progress in ionic liquid extraction for the separation of rare earth elements. Anal. Sci. 2021, 37, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.; Marcias-Ruvalcaba, N.; Aguilar-Martinez, M.; Kobrak, M. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Revs. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyagi, N.; Shimojo, K.; Naganawa, H.; Imura, H. Role of Tf2N- anions in the ionic liquid-water distribution of europium(III) chelates. RSC Adv. 2017, 7, 7610–7618. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Alcali cation extraction by calix[4]crown-6 to room-temperature ionic liquids. The effect of solvent anion and humidity investigation by molecular dynamics simulations. J. Phys. Chem. A 2006, 110, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Kidani, K.; Hirayama, N.; Imura, H. Extraction behavior of divalent metal cations in ionic liquid chelate extraction system using 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides and thenoyltrifluoroacetone. Anal. Sci. 2008, 24, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, N.; Deguchi, M.; Honjo, T. The behavior of divalent transition metal cations on extraction into 1-bytul-3-methylimidazolium hexafluorophosphate room temperature ionic liquid with β-diketones having a trifluoromethyl group. Solvent Extr. Res. Dev. Jpn. 2006, 13, 83–88. [Google Scholar]
- Matsumiya, M.; Yamada, T.; Muramaki, S.; Kohno, Y.; Tsunashima, K. Evaluation of the extraction properties and stability of extracted rare earth complexes in ionic liquid extraction system using β-diketone. Solvent Extr. Ion Exch. 2016, 34, 454–468. [Google Scholar] [CrossRef]
- Stepinski, D.; Jensen, M.; Dzielawa, J.; Dietz, M. Synergistic effects in the facilitated transfer of metal ions into room-temperature ionic liquids. Green Chem. 2005, 7, 151–158. [Google Scholar] [CrossRef]
- Mathur, J.; Pai, S.; Khopkar, P.; Sumramanian, M. Thermodynamics of synergistic extraction of europium(III) by mixtures of thenoyltrifluoroacetone and some neutral oxo-donors. J. Inorg. Nucl. Chem. 1977, 39, 653–657. [Google Scholar] [CrossRef]
- Petrova, M.; Kurteva, V.; Lubenov, L. Synergistic effect in the solvent extraction and separation of lanthanoids by 4-(4-fluorobenoyl)-3-methyl-1-phenyl-pyrazol-5-one in the presence of monofunctional neutral organophosphorus extractants. Ind. Eng. Chem. Res. 2011, 50, 12170–12176. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Billard, I. Are fancy acidic or neutral ligands really needed for synergism in ionic liquids? A comparative study of lanthanoid extraction in CHCl3 and an ionic liquid. New J. Chem. 2015, 39, 7932–7941. [Google Scholar] [CrossRef]
- Zhao, Z.; Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Synergistic extraction of rare earth metals and separation of scandium using 2-thenoyltrifluoroacetone and tri-n-octylphosphine oxide in an ionic liquid system. J. Chem. Eng. Jpn. 2014, 47, 656–662. [Google Scholar] [CrossRef]
- Okamura, H.; Hirayama, N.; Morita, K.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic effect of 18-crown-6 derivatives on chelate extraction of lanthanoids (III) into an ionic liquid with 2-thenoyltrifluoroacetone. Anal. Sci. 2010, 26, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, J.; Wang, W.; Cui, H.M.; Liu, W.G.; Liu, Y. Extraction mechanism of rare earths from chloride acidic solution with ammonium-bifunctionalized ionic liquid extractants. Sci. China Chem. 2016, 59, 532–537. [Google Scholar] [CrossRef]
- Kassierer, E.; Kertes, A. Displacement of water in synergic extraction systems. J. Inorg. Nucl. Chem. 1972, 34, 778–780. [Google Scholar] [CrossRef]
- Healy, T. Synergism in the solvent extraction of di-, tri-and tetravalent metal ions—II: Synergic effects in so-called inert diluents. J. Inorg. Nucl. Chem. 1961, 19, 328–339. [Google Scholar] [CrossRef]
- Choppin, G. Studies of the synergistic effect. Sep. Sci. Technol. 1981, 16, 1113–1126. [Google Scholar] [CrossRef]
- Healy, T.; Peppard, D.; Mason, G. Synergism in the solvent extraction of di, tri and tetravalent metal ions—III: Antisynergism with thenoyltrifluoracetone. J. Inorg. Nucl. Chem. 1962, 24, 1429–1448. [Google Scholar] [CrossRef]
- Atanassova, M. Investigation of synergism and selectivity using mixture of two neutral extractants in IL media for lanthanoids extraction. Sep. Purif. Technol. 2016, 169, 253–261. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent extraction chemistry in ionic liquids: An overview of f-ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).