Investigation of Selective Ribavirin Extraction from Serum Samples Using a Monolithic Silica Disk-Packed Spin Column
Abstract
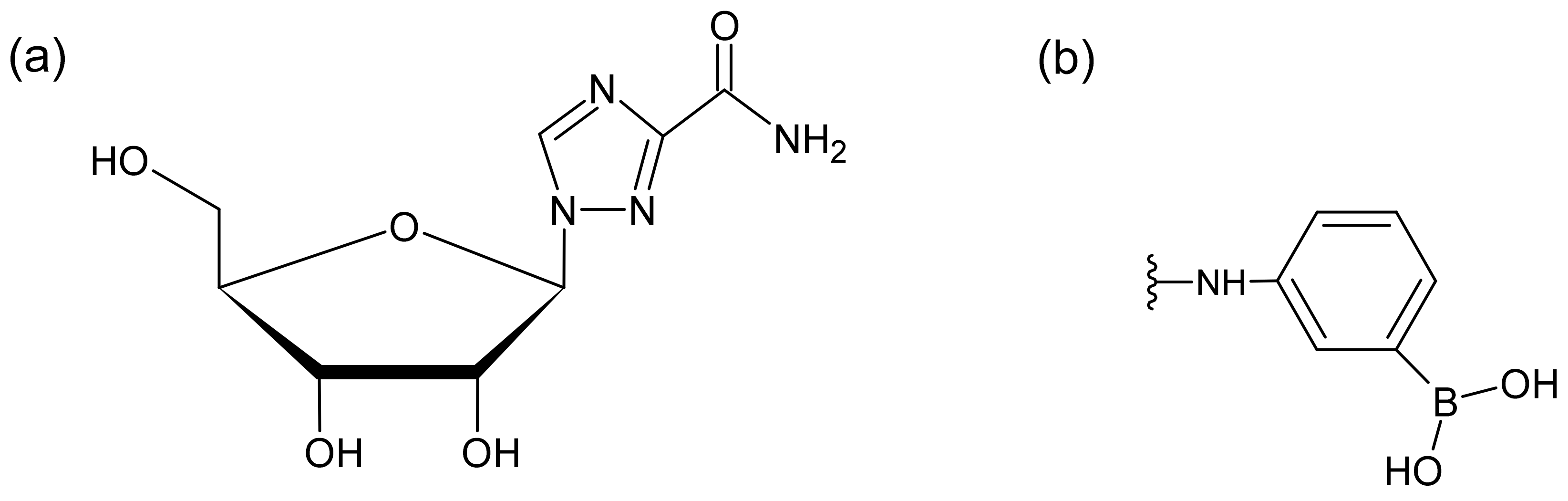
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus and Chromatographic Conditions
2.3. Preparation of Standards
2.4. Sample Preparation and Extraction
3. Results
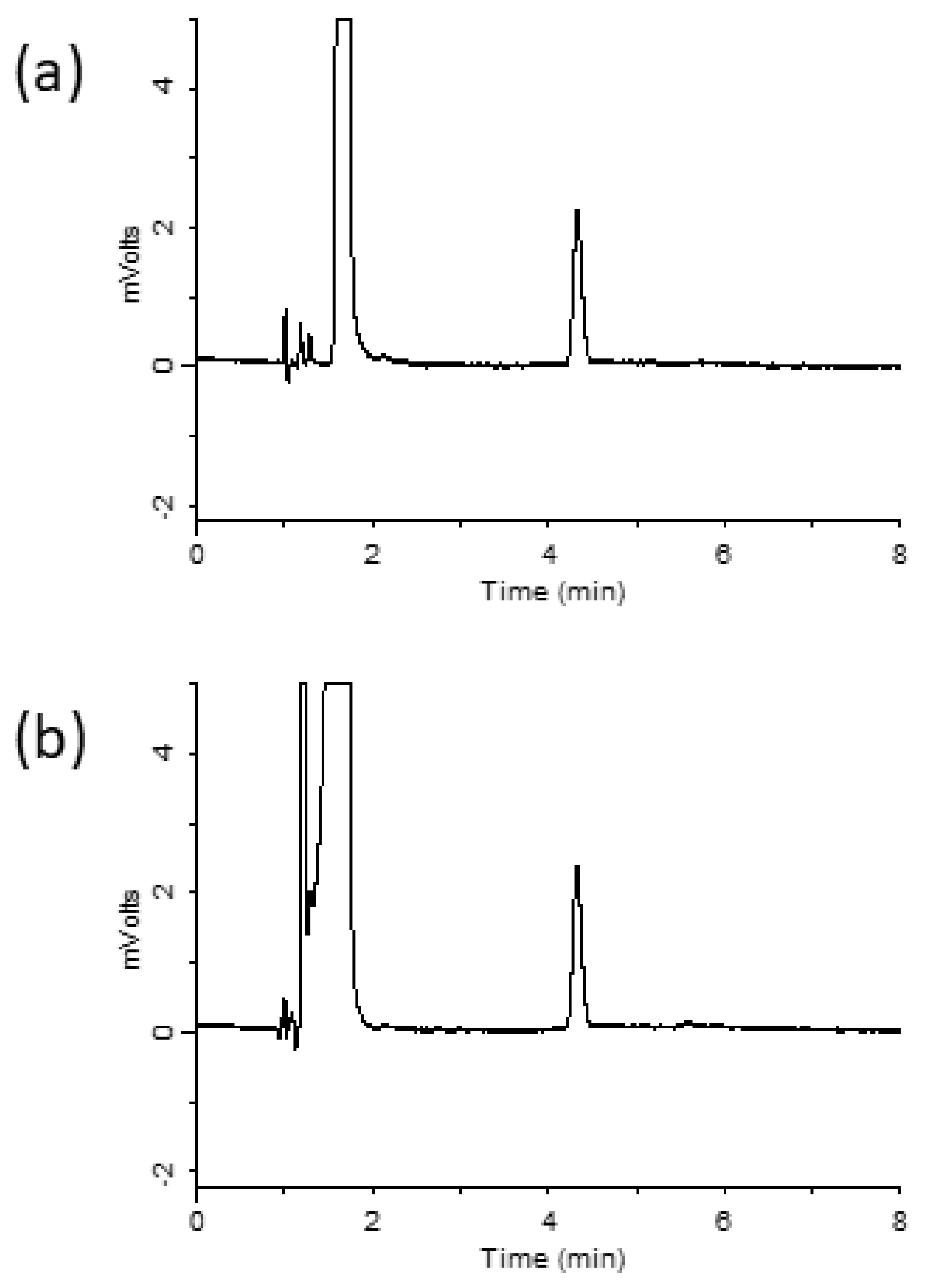
3.1. Optimization of Elution Conditions for Ribavirin Using a Spin Column
3.2. Optimization of Separation Conditions for Ribavirin on an Octadecylsilyl (ODS) Column
3.3. Method Validation
4. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McHutchison, J.G.; Gordon, S.C.; Schiff, E.R.; Shiffman, M.L.; Lee, W.M.; Rustgi, V.K.; Goodman, Z.D.; Ling, M.H.; Cort, S.; Albrecht, J.K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 1998, 339, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Loustaud-Ratti, V.; Debette-Gratien, M.; Jacques, J.; Alain, S.; Marquet, P.; Sautereau, D.; Rousseau, A.; Carrier, P. Ribavirin: Past, present and future. World J. Hepatol. 2016, 18, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Glue, P. The clinical pharmacology of ribavirin. Semin. Liver Dis. 1999, 19, 17–24. [Google Scholar] [PubMed]
- Chan, A.H.W.; Partovi, N.; Ensom, M.H.H. The utility of therapeutic drug monitoring for ribavirin in patients with chronic hepatitis C—A critical review. Ann. Pharmacother. 2009, 43, 2044–2063. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Ribavirin: Analytical determinations since the origin until today. J. Pharm. Biomed. Anal. 2007, 45, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.H.A.; Gilbert, B.E. Quantification of ribavirin in biological fluids and tissues by high performance liquid chromatography. J. Chromatogr. 1987, 414, 202–210. [Google Scholar] [CrossRef]
- Zironi, E.; Gazzotti, T.; Lugoboni, B.; Barbarossa, A.; Scagliarini, A.; Pagliuca, G. Development of a rapid LC–MS/MS method for ribavirin determination in rat brain. J. Pharm. Biomed. Anal. 2011, 54, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Magdy, N.; Hussein, L.A.; El-Kosasy, A.M. Validated RP-HPLC Method for Simultaneous Determination of Ribavirin, Sofosbuvir and Daclatasvir in Human Plasma: A Treatment Protocol Administered to HCV Patients in Egypt. J. Chromatogr. Sci. 2019, 57, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.-O.; Bruchfeld, A.; Schvarcz, R.; Stahle, L. Determination of Ribavirin in Serum Using Highly Selective Solid-Phase Extraction and High-Performance Liquid Chromatography. Ther. Drug Monit. 2000, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Aoyama, C.; Ota, S.; Tamura, T.; Funatsu, T. Extraction of catecholamines from urine using a monolithic silica disk-packed spin column and high-performance liquid chromatography-electrochemical detection. Anal. Methods 2011, 3, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Hirayama, M.; Ohno, K.; Tsuda, T. A simple analytical method involving the use of a monolithic silica disk-packed spin column and HPLC-ECD for determination of l-DOPA in plasma of patients with Parkinson’s disease. Anal. Methods 2013, 5, 5161–5164. [Google Scholar] [CrossRef]
- Suzuki, R.; Funatsu, T.; Tsunoda, M. Identification of methylated tubulin through analysis of methylated lysine. Anal. Bioanal. Chem. 2018, 410, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Zhuang, H. Investigation of the interaction between serotonin-related compounds and phenylboronate moieties in monolithic silica disk-packed spin columns. Biomed. Chromatogr. 2019, 33, e4650. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Funatsu, T.; Tsunoda, M. Improved Extraction Method for Catecholamines Using Monolithic Silica Disk-Packed Spin Column. Chromatography 2020, 41, 137–140. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, C.; Wang, W.; Qian, M.; Xu, J.; Yang, H. Chromatography column comparison and rapid pretreatment for the simultaneous analysis of amantadine, rimantadine, acyclovir, ribavirin, and moroxydine in chicken muscle by ultra high performance liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2016, 39, 3998–4010. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M.; Kanamori, T.; Funatsu, T.; Tsunoda, M. Recent advances in hydrophilic interaction chromatography for quantitative analysis of endogenous and pharmaceutical compounds in plasma samples. Bioanalysis 2014, 6, 2421–2439. [Google Scholar] [CrossRef] [PubMed]
- Morello, J.; Rodríguez-Novoa, S.; Jiménez-Nácher, I.; Soriano, V. Usefulness of monitoring ribavirin plasma concentrations to improve treatment response in patients with chronic hepatitis C. J. Antimicrob. Chemother. 2008, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Intraday | Interday | |||||
|---|---|---|---|---|---|---|
| Added (µM) | Measured (Mean ± SD, µM) | RSD (%) | Accuracy (%) | Measured (Mean ± SD, µM) | RSD (%) | Accuracy (%) |
| 0.25 | 0.25 ± 0.01 | 3.2 | 101 | 0.24 ± 0.01 | 3.1 | 97 |
| 2.5 | 2.44 ± 0.04 | 1.7 | 98 | 2.54 ± 0.04 | 1.6 | 101 |
| 5 | 4.89 ± 0.12 | 2.5 | 98 | 5.31 ± 0.13 | 2.5 | 106 |
| 25 | 24.7 ± 0.3 | 1.2 | 99 | 26.9 ± 0.5 | 1.9 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yui, Y.; Ota, S.; Aoyama, C.; Song, Y.; Tsunoda, M. Investigation of Selective Ribavirin Extraction from Serum Samples Using a Monolithic Silica Disk-Packed Spin Column. Separations 2022, 9, 113. https://doi.org/10.3390/separations9050113
Yui Y, Ota S, Aoyama C, Song Y, Tsunoda M. Investigation of Selective Ribavirin Extraction from Serum Samples Using a Monolithic Silica Disk-Packed Spin Column. Separations. 2022; 9(5):113. https://doi.org/10.3390/separations9050113
Chicago/Turabian StyleYui, Yuko, Shigenori Ota, Chiaki Aoyama, Yanting Song, and Makoto Tsunoda. 2022. "Investigation of Selective Ribavirin Extraction from Serum Samples Using a Monolithic Silica Disk-Packed Spin Column" Separations 9, no. 5: 113. https://doi.org/10.3390/separations9050113
APA StyleYui, Y., Ota, S., Aoyama, C., Song, Y., & Tsunoda, M. (2022). Investigation of Selective Ribavirin Extraction from Serum Samples Using a Monolithic Silica Disk-Packed Spin Column. Separations, 9(5), 113. https://doi.org/10.3390/separations9050113






