Abstract
Ribavirin, a nucleoside analog, is used to treat chronic hepatitis C (HCV) infections. Therapeutic drug monitoring for ribavirin is useful for predicting the effect of treatment. In this study, the selective extraction of ribavirin from serum samples and the HPLC-UV detection method were investigated using a monolithic silica disk-packed spin column with phenylboronate moieties. In this study, 0.6% ammonia and 1% formic acid solutions were used as the conditioning and elution solutions, respectively, and recoveries of >90% were obtained. Ribavirin was separated on an InertSustain AQ-C18 column by isocratic elution. The mobile phase consisted of a mixture of 7 mM Na2SO4 and 60 mM H3PO4 in H2O. Linear regression curves were observed for calibrations over a concentration range of 0.25–25 µg/mL. The lower limit of detection was 0.05 µg/mL, and the lower limit of quantification was 0.1 µg/mL. The intra- and inter-day precisions were below 3.2 and 3.1%, respectively. This method can be applied to quantify ribavirin levels in human serum and may be useful for pharmacokinetic studies.
1. Introduction
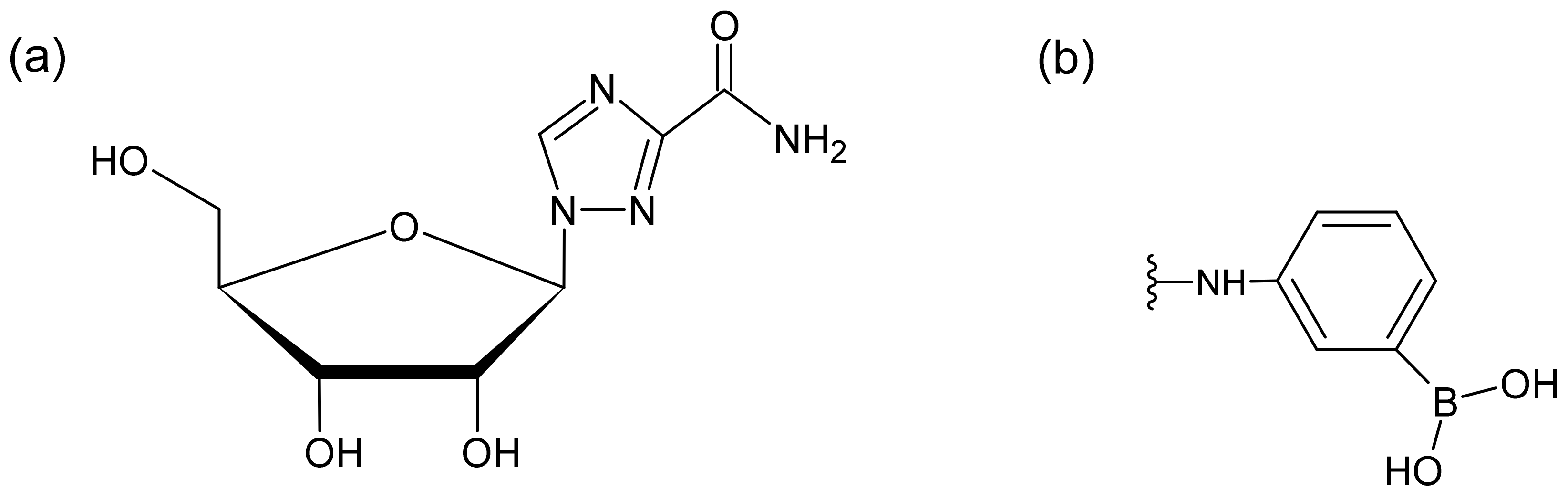
Ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide, Figure 1a), a synthetic triazole guanosine analog that is active against both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) viruses, is used in combination with pegylated interferon to treat chronic hepatitis C infections [1,2]. Different mechanisms of ribavirin action have been proposed, such as inositol monophosphate dehydrogenase inhibition, mutagenesis, and a direct inhibition of RNA-dependent RNA polymerase.
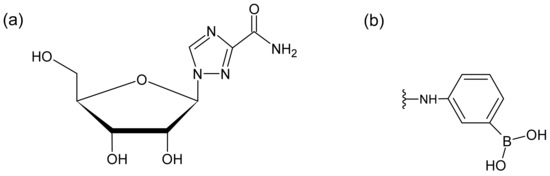
Figure 1.
(a) Chemical structure of ribavirin. (b) Functional group of MonoSpin PBA.
Typically, ribavirin is used for therapeutic drug monitoring (TDM). Ribavirin is rapidly absorbed after oral administration (time to maximum concentration = 1.5 h), followed by rapid distribution, and prolonged elimination phases, which result in a long half-life, large inter-individual variability of the dose-concentration relationship, and narrow therapeutic zone [3,4]. Therefore, monitoring the ribavirin blood levels in patients with chronic hepatitis C infections using interferon alpha-2b and ribavirin combination therapy may be useful for predicting the effect of treatment and examining the relationship between changes in the treatment method and side effects.
There are several analytical methods available for ribavirin detection in biological samples, including immunoassays, gas chromatography–mass spectrometry (GC-MS), and high-performance liquid chromatography (HPLC) combined with ultraviolet (UV) detection or tandem mass spectrometry (MS/MS) [5,6,7,8,9]. The measurement of blood samples in TDM requires careful consideration of a pretreatment method, which selectively extracts ribavirin from endogenous biomolecules in the blood. Svensson et al. used phenylboronic acid-containing solid-phase extraction combined with an HPLC analysis [9]. As is typical in conventional solid-phase extractions, tedious procedures, such as the application of a vacuum, were necessary. To achieve a simpler pretreatment method, the use of monolithic silica disk-packed spin columns with phenylboronate moieties, MonoSpin phenyl boric acid (PBA) (Figure 1b), were investigated in this study.
2. Materials and Methods
2.1. Reagents
Ribavirin was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). All solvents were of HPLC grade and obtained from Kishida Chemical Co., Ltd. (Osaka, Japan). All chemicals were of analytical grade and used as received. Water was purified using a Milli-Q Integral 3 instrument (Merck Millipore, Darmstadt, Germany).
2.2. Apparatus and Chromatographic Conditions
Chromatographic analysis was performed using an LC800 system equipped with a UV-VIS detector (GL Sciences, Tokyo, Japan). Detection was performed at 220 nm. The column used was InertSustain AQ-C18 (3 μm, 150 mm × 2.1 mm I.D., GL Sciences). The column temperature was maintained at 25 °C. Isocratic elution was performed with the mobile phase of a mixture of 7 mM Na2SO4 and 60 mM H3PO4 in H2O. The flow rate of the mobile phase was 0.3 mL/min.
2.3. Preparation of Standards
A stock solution (10 mg/mL) of ribavirin was prepared in water and stored at 4 °C. Working solution mixtures were prepared by mixing appropriate volumes of the stock solution with water. As spiked samples, the ribavirin solution was mixed with an equal volume of the serum sample.
2.4. Sample Preparation and Extraction
Solid-phase extraction was performed to selectively extract ribavirin from the serum samples (Nissui Pharmaceutical, Tokyo, Japan). For sample loading, washing, and elution of the analytes, the Monospin PBA spin column was installed in a microtube (2 mL). Firstly, 0.4 mL of the 0.6% ammonia solution was poured into the column, which was centrifuged at 2300× g for 2 min. Next, the sample solution (0.4 mL) was directly placed in the spin column, and the column was centrifuged at 2300× g for 2 min. Subsequently, the column was rinsed with a 0.6% ammonia solution using centrifugation. Lastly, the column was installed in a new microtube, and the adsorbed analytes were eluted using a 1% formic acid water solution (0.2 mL).
3. Results
3.1. Optimization of Elution Conditions for Ribavirin Using a Spin Column
The spin column used in this study had phenylboronate moieties bonded to the monolithic silica [10,11,12,13,14]. The structure of monolithic silica comprised a support body with a relatively large surface area per unit volume compared with that of silica particles. Purification steps, such as sample loading, washing, and elution of target compounds, were all achieved by centrifugation of the spin column. Furthermore, this extraction method could be used to prepare many samples simultaneously, without evaporation. Therefore, a pretreatment method using a monolithic silica disk-packed spin column is suitable for simple ribavirin analysis.
The principle of separation using this spin column is based on the fact that ribavirin, which has vicinal hydroxy groups, selectively binds to phenylboronate moieties at a high pH. Conversely, at a lower pH, ribavirin is released from the phenylboronate moieties and may be extracted. To obtain a high recovery of ribavirin from serum samples, the elution conditions, including conditioning and elution solutions, were evaluated.
Firstly, the conditioning solution content was investigated. A 0.6% ammonia solution, 5% ammonia solution, and 20 mM phosphate buffer (pH 8.0) were tested as conditioning solutions, and the ribavirin recoveries were >90, >90, and 15%, respectively. The phosphate buffer had very low recoveries, and approximately half of the ribavirin was detected in the flow-through solution, which indicated that a pH of 8 was not suitable to facilitate the binding of ribavirin to phenylboronate moieties.
Subsequently, the elution solution content was investigated using 0.6 and 5% ammonia solutions as the conditioning solutions. Formic acid solutions (1 and 2%) were evaluated as elution solutions to release the bound ribavirin from phenylboronate. When the 0.6% ammonia solution was applied as the conditioning solution, the ribavirin recovery of >90% was obtained for both formic acid solutions. However, when the 5% ammonia solution was applied as a conditioning solution, the ribavirin recoveries were >90% and 70–80% for the 2% and 1% formic acid solutions, respectively. Based on the results, 1% formic acid was insufficient to neutralize 5% ammonia. Consequently, the 0.6% ammonia and 1% formic acid solutions were selected as the conditioning and elution solutions, respectively.
3.2. Optimization of Separation Conditions for Ribavirin on an Octadecylsilyl (ODS) Column
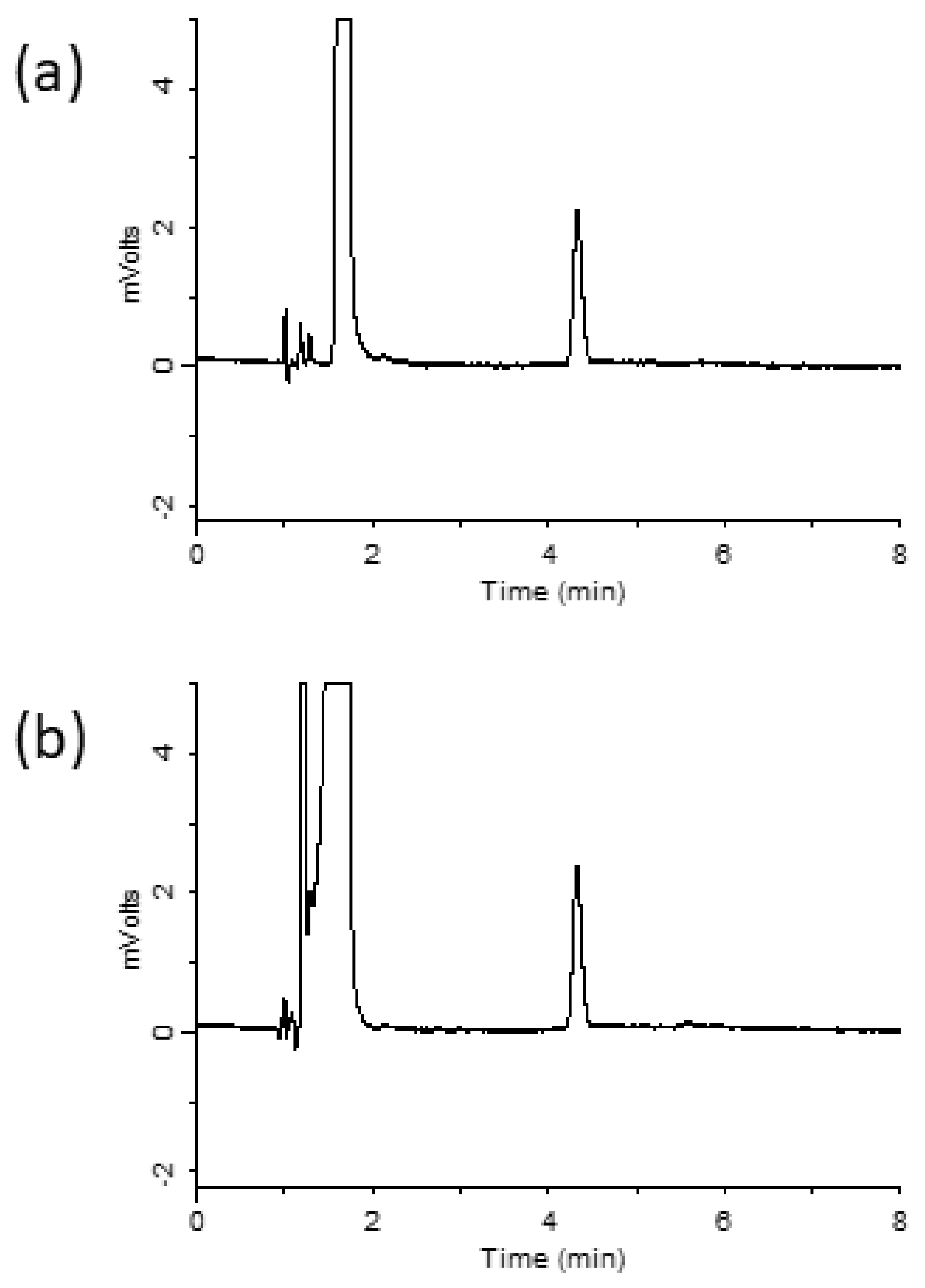
Ribavirin is a hydrophilic compound that is not easily retained on octadecylsilyl (ODS) columns. Hydrophilic interaction chromatography (HILIC) was used to retain and separate ribavirin [15]. However, HILIC columns have a few disadvantages, such as low stability, limited sample composition, and extended equilibration time [16]. Hence, in this study, the InertSustain AQ-C18 column was designed to strongly retain highly polar compounds, compared with ordinary ODS columns, by introducing ODS groups in a manner that optimizes bond spacing. Using the prepared mobile phase previously described in the Materials and Methods section, ribavirin was retained for 4.3 min. A chromatogram obtained under the optimized conditions is shown in Figure 2a. Ribavirin-spiked serum samples were analyzed after extraction using the MonoSpin PBA column, and the resulting chromatogram is shown in Figure 2b. A ribavirin peak was observed without any interference from the serum sample, which indicated that ribavirin can be extracted from serum samples using a MonoSpin PBA column.
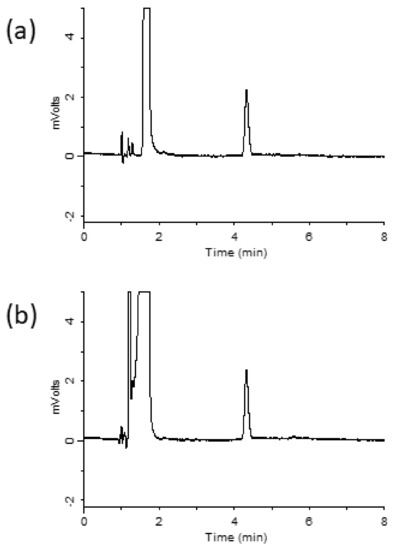
Figure 2.
Chromatograms of (a) ribavirin standard, (b) serum sample after purification with MonoSpin PBA.
3.3. Method Validation
Calibration curves were obtained by plotting the peak area of ribavirin against the standard concentrations of ribavirin (µg/mL). The concentration range was linear from 0.25 to 25 µg/mL with a good linearity (r2 = 0.9998). The linear regression equation was y = 16682x–2514. The limit of quantification was 0.1 µg/mL. A target concentration of at least 2–2.5 µg/mL is recommended to maximize the realization of a sustained virological response [17]. Hence, the proposed method is suitable for ribavirin quantification and has adequate sensitivity for the determination of ribavirin concentrations in serum samples. Inter- and intra-day precisions were evaluated by an analysis of ribavirin with spiked concentrations of 0.25, 2.5, 5, and 25 µg/mL in serum samples. As shown in Table 1, the intra- and interday precisions were below 3.2 and 3.1 %, respectively. The accuracies were found to be 98–101% (intraday) and 97–108% (interday).

Table 1.
Intra- and inter-day precision and accuracy in spiked serum samples (n = 6).
4. Conclusions
The selective extraction of ribavirin from serum samples was achieved using a monolithic silica disk-packed spin column with phenylboronate moieties. Under optimized extraction conditions, the recovery of ribavirin from serum samples was >90%. Pretreatment using a MonoSpin column followed by LC separation on an ODS column combined with UV detection provided accurate ribavirin quantification, without matrix effects from the serum sample. This method is easy to apply in routine therapeutic drug monitoring, and it has sufficient sensitivity for pharmacokinetic studies.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McHutchison, J.G.; Gordon, S.C.; Schiff, E.R.; Shiffman, M.L.; Lee, W.M.; Rustgi, V.K.; Goodman, Z.D.; Ling, M.H.; Cort, S.; Albrecht, J.K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 1998, 339, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Loustaud-Ratti, V.; Debette-Gratien, M.; Jacques, J.; Alain, S.; Marquet, P.; Sautereau, D.; Rousseau, A.; Carrier, P. Ribavirin: Past, present and future. World J. Hepatol. 2016, 18, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Glue, P. The clinical pharmacology of ribavirin. Semin. Liver Dis. 1999, 19, 17–24. [Google Scholar] [PubMed]
- Chan, A.H.W.; Partovi, N.; Ensom, M.H.H. The utility of therapeutic drug monitoring for ribavirin in patients with chronic hepatitis C—A critical review. Ann. Pharmacother. 2009, 43, 2044–2063. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Ribavirin: Analytical determinations since the origin until today. J. Pharm. Biomed. Anal. 2007, 45, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.H.A.; Gilbert, B.E. Quantification of ribavirin in biological fluids and tissues by high performance liquid chromatography. J. Chromatogr. 1987, 414, 202–210. [Google Scholar] [CrossRef]
- Zironi, E.; Gazzotti, T.; Lugoboni, B.; Barbarossa, A.; Scagliarini, A.; Pagliuca, G. Development of a rapid LC–MS/MS method for ribavirin determination in rat brain. J. Pharm. Biomed. Anal. 2011, 54, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Magdy, N.; Hussein, L.A.; El-Kosasy, A.M. Validated RP-HPLC Method for Simultaneous Determination of Ribavirin, Sofosbuvir and Daclatasvir in Human Plasma: A Treatment Protocol Administered to HCV Patients in Egypt. J. Chromatogr. Sci. 2019, 57, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.-O.; Bruchfeld, A.; Schvarcz, R.; Stahle, L. Determination of Ribavirin in Serum Using Highly Selective Solid-Phase Extraction and High-Performance Liquid Chromatography. Ther. Drug Monit. 2000, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Aoyama, C.; Ota, S.; Tamura, T.; Funatsu, T. Extraction of catecholamines from urine using a monolithic silica disk-packed spin column and high-performance liquid chromatography-electrochemical detection. Anal. Methods 2011, 3, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Hirayama, M.; Ohno, K.; Tsuda, T. A simple analytical method involving the use of a monolithic silica disk-packed spin column and HPLC-ECD for determination of l-DOPA in plasma of patients with Parkinson’s disease. Anal. Methods 2013, 5, 5161–5164. [Google Scholar] [CrossRef]
- Suzuki, R.; Funatsu, T.; Tsunoda, M. Identification of methylated tubulin through analysis of methylated lysine. Anal. Bioanal. Chem. 2018, 410, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Zhuang, H. Investigation of the interaction between serotonin-related compounds and phenylboronate moieties in monolithic silica disk-packed spin columns. Biomed. Chromatogr. 2019, 33, e4650. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Funatsu, T.; Tsunoda, M. Improved Extraction Method for Catecholamines Using Monolithic Silica Disk-Packed Spin Column. Chromatography 2020, 41, 137–140. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, C.; Wang, W.; Qian, M.; Xu, J.; Yang, H. Chromatography column comparison and rapid pretreatment for the simultaneous analysis of amantadine, rimantadine, acyclovir, ribavirin, and moroxydine in chicken muscle by ultra high performance liquid chromatography and tandem mass spectrometry. J. Sep. Sci. 2016, 39, 3998–4010. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M.; Kanamori, T.; Funatsu, T.; Tsunoda, M. Recent advances in hydrophilic interaction chromatography for quantitative analysis of endogenous and pharmaceutical compounds in plasma samples. Bioanalysis 2014, 6, 2421–2439. [Google Scholar] [CrossRef] [PubMed]
- Morello, J.; Rodríguez-Novoa, S.; Jiménez-Nácher, I.; Soriano, V. Usefulness of monitoring ribavirin plasma concentrations to improve treatment response in patients with chronic hepatitis C. J. Antimicrob. Chemother. 2008, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).