Membrane-Based Electrochemical Detection of Uranium: A Review
Abstract
1. Introduction
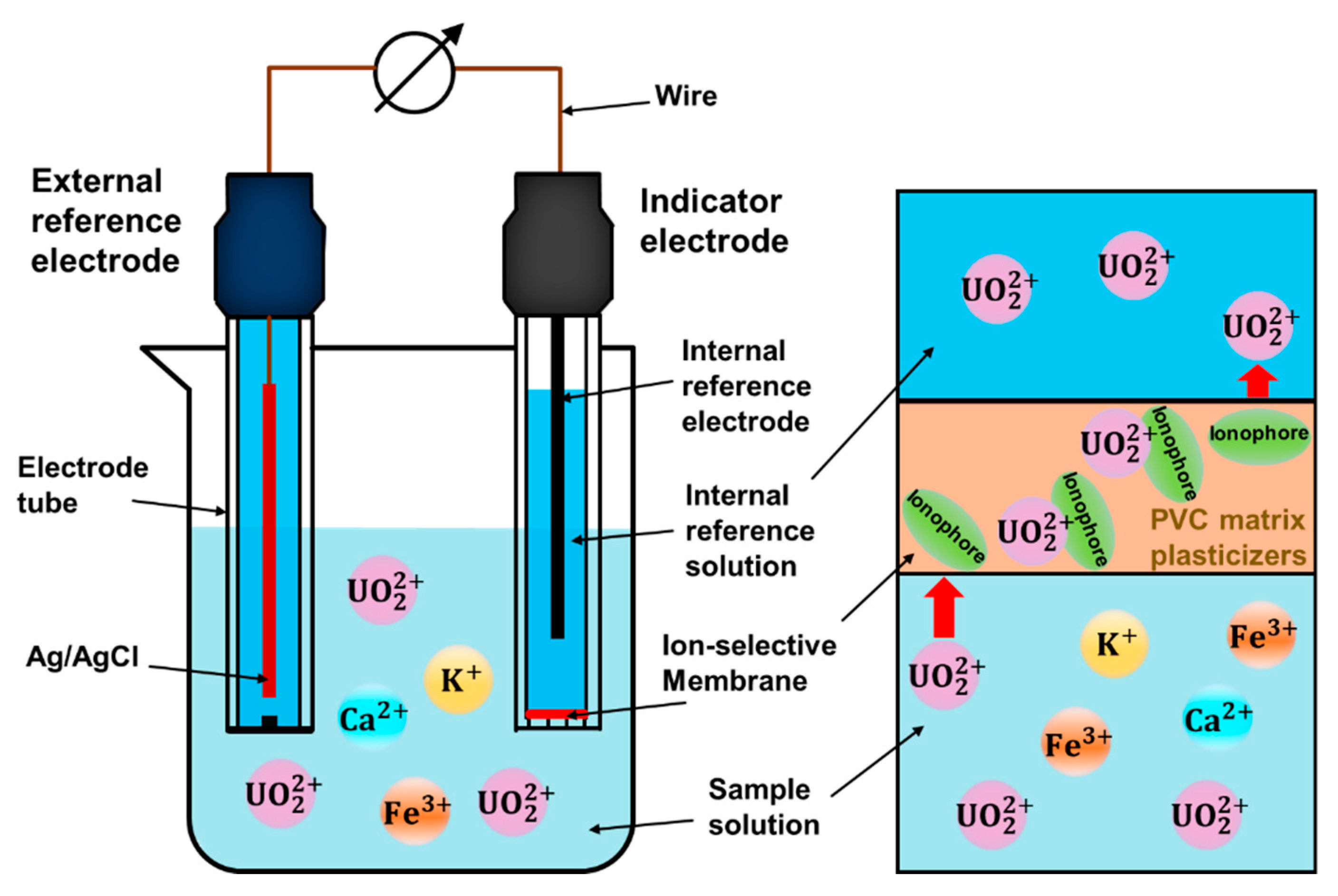
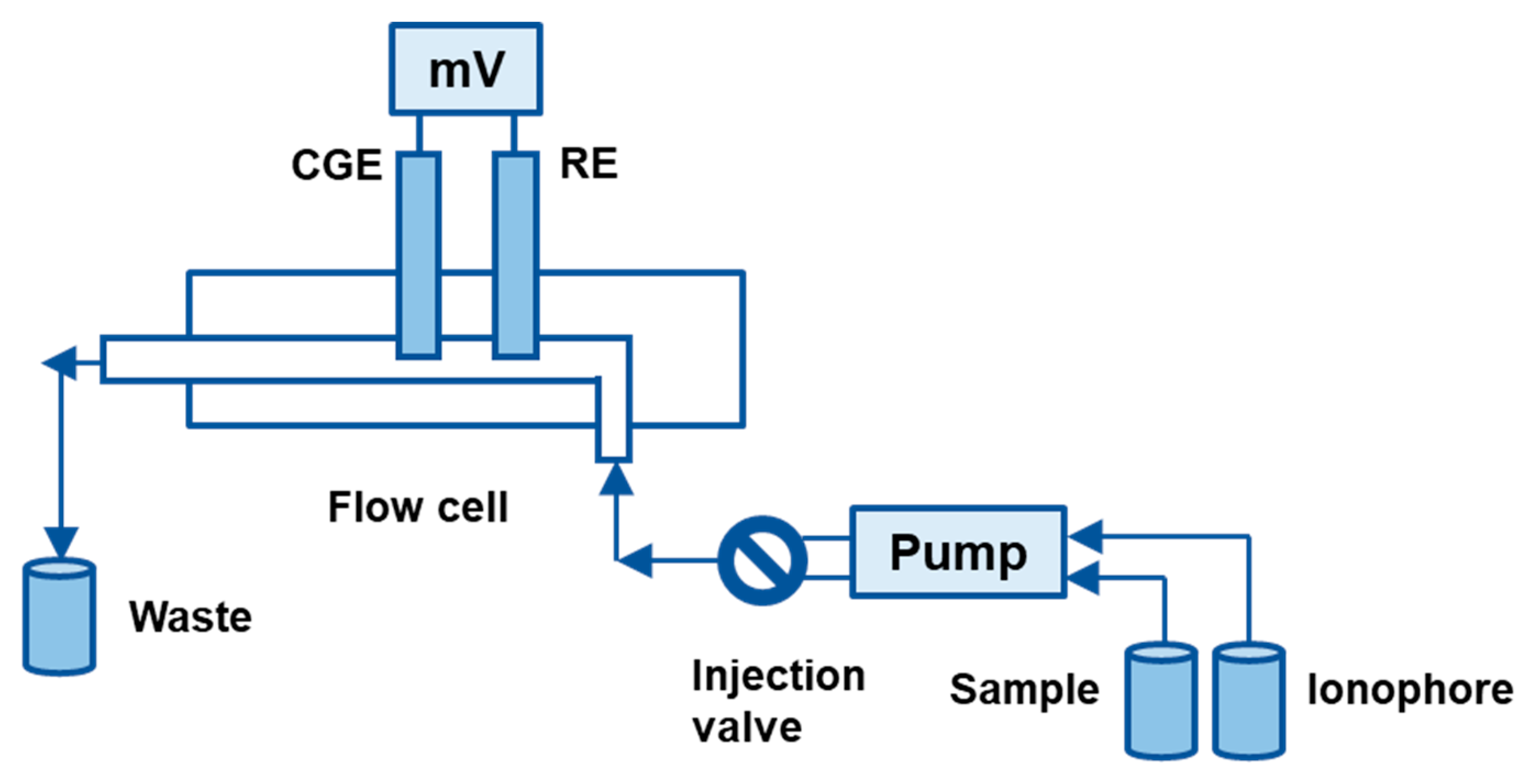
2. Ion-Selective Membranes for Uranium Detection
3. Polymeric Matrix
4. Plasticizer
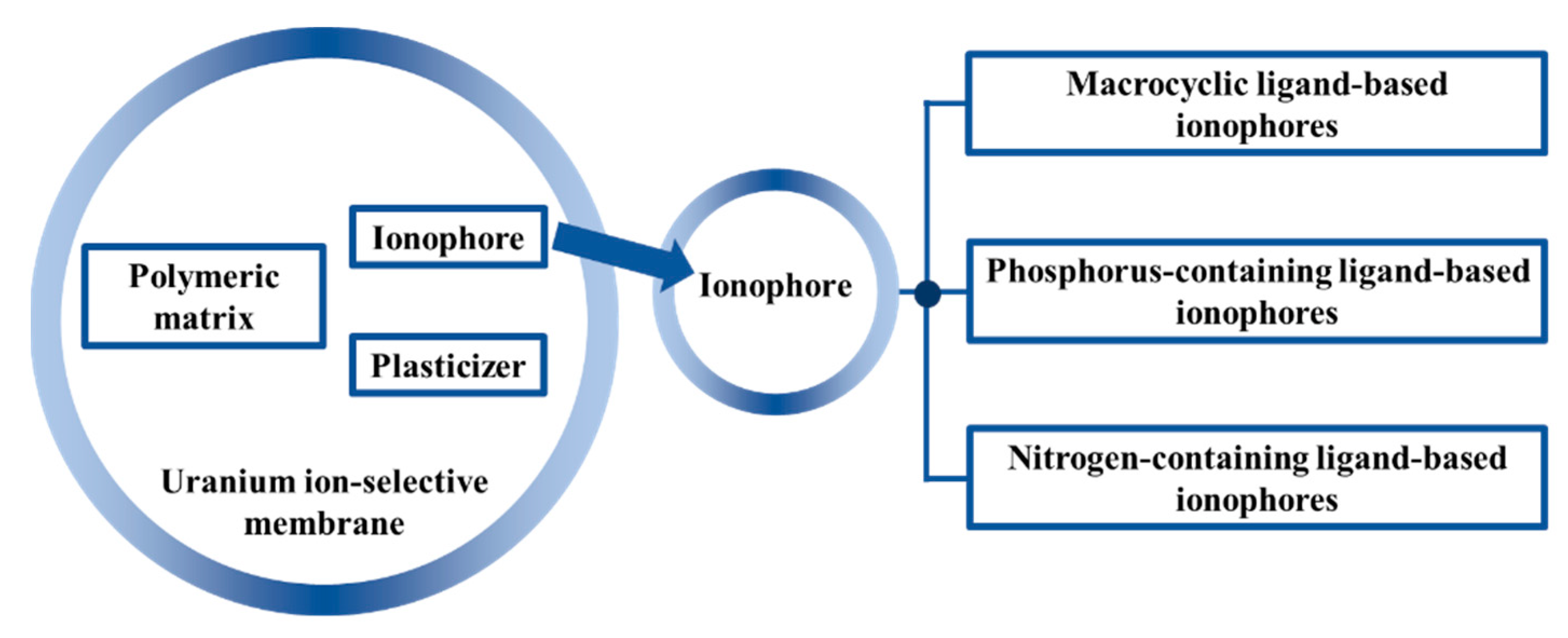
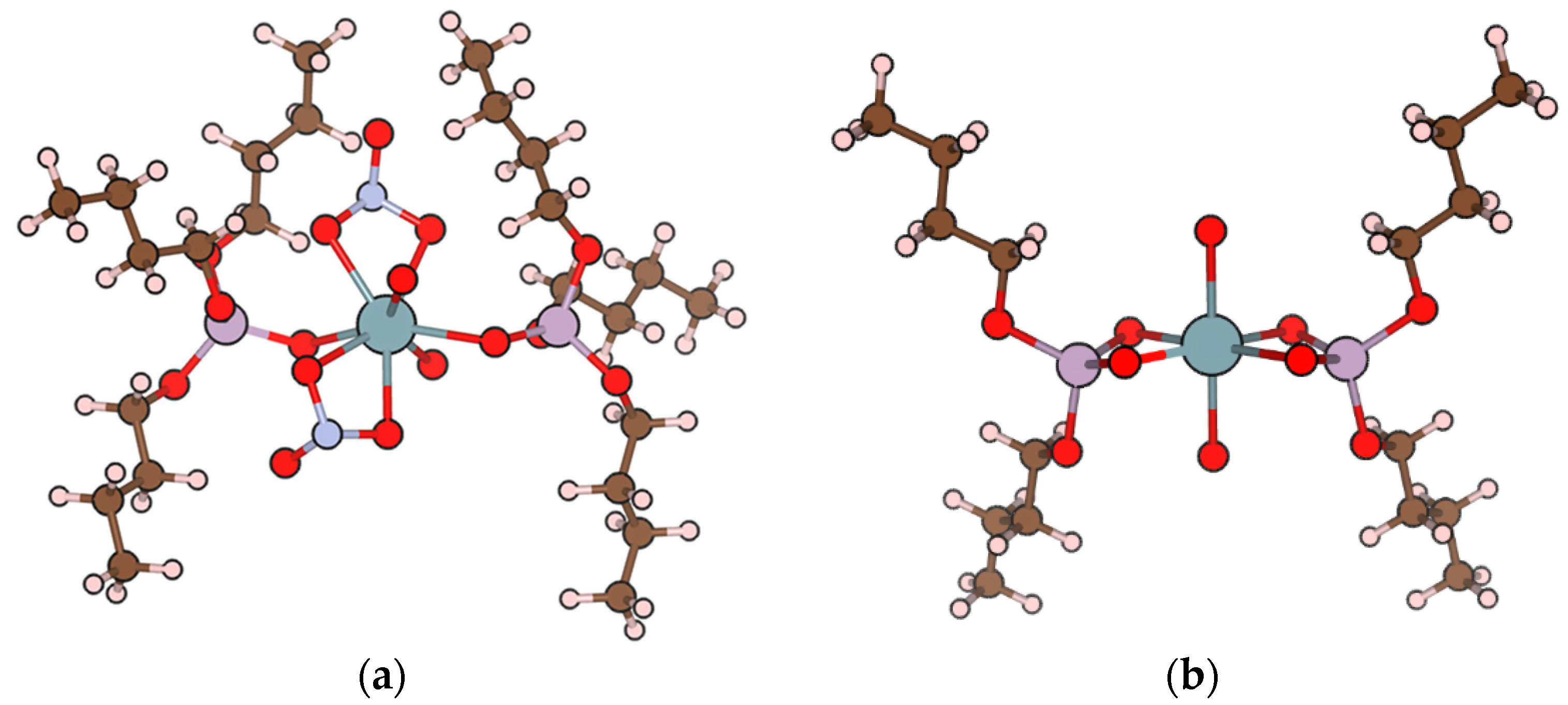
5. Ionophores
5.1. Macrocyclic Ligand-Based Ionophores
5.2. Phosphorus-Containing Ligand-Based Ionophores
5.3. Nitrogen-Containing Ligand-Based Ionophores
6. Response Times and Lifetime
6.1. Response Times
6.1.1. Membrane Thickness
6.1.2. Concentrations
6.1.3. Types of Uranium Solution and Competing Ions
6.2. Lifetime
7. Limitation and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.; Weber, K.A. Natural Uranium Contamination in Major US Aquifers Linked to Nitrate. Environ. Sci. Technol. Lett. 2015, 2, 215–220. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). National Primary Drinking Water Regulations; Radionuclides; Final Rule, December 7, 2000, 40 CFR Parts 9, 141 and 142; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Wu, X.; Huang, Q.; Mao, Y.; Wang, X.; Wang, Y.; Hu, Q.; Wang, H.; Wang, X. Sensors for determination of uranium: A review. TrAC Trends Anal. Chem. 2019, 118, 89–111. [Google Scholar] [CrossRef]
- Dietrich, W.; Manning, D. Uranium-Sensitive Electrode Membrane. U.S. Patent No. 3864233, 4 February 1975. [Google Scholar]
- Miller, D. Ion-Selective Electrode Determination of Fluoride Ion. Chem Lab Man. 2011, 321, 23–27. [Google Scholar]
- Gupta, V.K.; Mangla, R.; Khurana, U.; Kumar, P. Determination of uranyl ions using poly(vinyl chloride) based 4-tert-butylcalix[6]arene membrane sensor. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 1999, 11, 573–576. [Google Scholar] [CrossRef]
- Shamsipur, M.; Mizani, F.; Mousavi, M.F.; Alizadeh, N.; Alizadeh, K.; Eshghi, H.; Karami, H. A novel flow injection potentiometric graphite coated ion-selective electrode for the low level determination of uranyl ion. Anal. Chim. Acta 2007, 589, 22–32. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, Y.; Zhang, W.; Zhao, S.; Wang, Y. PVC-based hybrid membranes containing metal-organic frameworks for Li+/Mg2+ separation. J. Membr. Sci. 2020, 596, 117724. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, X.; He, Q.; Deng, T.; Zhang, C.; Zhang, W. Stable ionic liquid-based polymer inclusion membranes for lithium and magnesium separation. Sep. Purif. Technol. 2022, 288, 120626. [Google Scholar] [CrossRef]
- Lindner, E.; Cosofret, V.; Ufer, S.; Buck, R.; Kao, W.; Neuman, M.; Anderson, J. Ion-selective membranes with low plasticizer content: Electroanalytical characterization and biocompatibility studies. J. Biomed. Mater. Res. 1994, 28, 591–601. [Google Scholar] [CrossRef]
- Verma, P.; Kumari, N.; Pathak, P.; Sadhu, B.; Sundararajan, M.; Aswal, V.; Mohapatra, P. Investigations on preferential Pu(IV) extraction over U(VI) by N, N-dihexyloctanamide versus tri-n-butyl phosphate: Evidence through small angle neutron scattering and DFT studies. J. Phys. Chem. A 2014, 118, 3996–4004. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Chen, J. Novel mesoporous silicas bearing phosphine oxide ligands with different alkyl chains for the binding of uranium in strong HNO3 media. J. Mater. Chem. A 2013, 1, 12706–12709. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, X.; Mu, Y.; Wang, Y.; Chen, J. Constructing adjacent phosphine oxide ligands confined in mesoporous Zr-MOFs for uranium capture from acidic medium. J. Mater. Chem. A 2021, 9, 16685–16691. [Google Scholar] [CrossRef]
- Powell, B.A.; Navratil, J.D.; Thompson, M.C. Compounds of hexavalent uranium and dibutylphosphate in nitric acid systems. Solvent Extr. Ion Exch. 2003, 21, 347–368. [Google Scholar] [CrossRef]
- Rufus, A.; Dhanesh, M.; Velmurugan, S. Dissolution of synthetic uranium dibutyl phosphate (U-DBP) in sodium EDTA and sodium carbonate based formulations. Prog. Nucl. Energy 2017, 100, 373–379. [Google Scholar] [CrossRef]
- Rounaghi, G. Selective uranyl cation detection by polymeric ion selective electrode based on benzo-15-crown-5. Mater. Sci. Eng. C 2011, 31, 1637–1642. [Google Scholar]
- Hassan, S.S.; Ali, M.M.; Attawiya, A.M. PVC membrane based potentiometric sensors for uranium determination. Talanta 2001, 54, 1153–1161. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Ghaedi, M.; Montazerozohori, M.; Khanjari, N.; Najibzadeh, M. Construction of a New Uranyl-Selective Electrode Based on a New Ionophore: Comparison of the Effect Additive on Electrode Responses. J. Chin. Chem. Soc. 2009, 56, 812–821. [Google Scholar] [CrossRef]
- Senkyr, J.; Ammann, D.; Meier, P.; Morf, W.; Pretsch, E.; Simon, W. Uranyl ion selective electrode based on a new synthetic neutral carrier. Anal. Chem. 1979, 51, 786–790. [Google Scholar] [CrossRef]
- Florido, A.; Casas, I.; García-Raurich, J.; Arad-Yellin, R.; Warshawsky, A. Uranyl-selective electrode based on a new bifunctional derivative combining the synergistic properties of phosphine oxide and ester of phosphoric acid. Anal. Chem. 2000, 72, 1604–1610. [Google Scholar] [CrossRef]
- Duncan, D.M.; Cockayne, J. Application of calixarene ionophores in PVC based ISEs for uranium detection. Sens. Actuators B Chem. 2001, 73, 228–235. [Google Scholar] [CrossRef]
- Johnson, S.; Moody, G.; Thomas, J.; Kohnke, F.; Stoddart, J. Poly(vinyl chloride) matrix membrane uranyl ion-selective electrodes based on cyclic and acyclic neutral carrier sensors. Analyst 1989, 114, 1025–1028. [Google Scholar] [CrossRef]
- Hassan, S.S.; Attawiya, A.M. A novel uranyl membrane sensor with potentiometric anionic response. Talanta 2006, 70, 883–889. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Memari, Z.; Norouzi, P.; Shaabani, B.; Emamalizadeh, M.; Hanifehpour, Y.; Faridbod, F. Uranyl Microsensor: An Asymmetric Potentiometric Membrane Sensor Based on a New Calix[4]arene. Anal. Lett. 2010, 43, 2220–2233. [Google Scholar] [CrossRef]
- Biswas, S.; Pathak, P.; Roy, S. Carrier facilitated transport of uranium across supported liquid membrane using dinonyl phenyl phosphoric acid and its mixture with neutral donors. Desalination 2012, 290, 74–82. [Google Scholar] [CrossRef]
- Zhang, C.; Mu, Y.; Zhao, S.; Zhang, W.; Wang, Y. Lithium extraction from synthetic brine with high Mg2+/Li+ ratio using the polymer inclusion membrane. Desalination 2020, 496, 114710. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Extraction and adsorption of U(VI) from aqueous solution using affinity ligand-based technologies: An overview. Rev. Environ. Sci. Bio Technol. 2019, 18, 437–452. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rawashdeh, N.A.; Al-Mheidat, I.R.; Nassory, N.S. Preparation and evaluation of new uranyl imprinted polymer electrode sensor for uranyl ion based on uranyl–carboxybezotriazole complex in pvc matrix membrane. Sens. Actuators B Chem. 2016, 227, 336–345. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KA, Bpot values (Technical Report). Pure Appl. Chem. 1995, 67, 507–518. [Google Scholar] [CrossRef]
- Agrahari, S.; Kumar, S.; Srivastava, A. Ion selective electrode for uranium based on composite multiwalled carbon nanotube-benzo-15-crown-5 in PVC matrix coated on graphite rod. J. Anal. Chem. 2014, 69, 36–44. [Google Scholar] [CrossRef]
- Ghanbari, M.; Rounaghi, G.H.; Ashraf, N. An uranyl solid state PVC membrane potentiometric sensor based on 4,13-didecyl-1,7,10,16-tetraoxa-4,13-diazacyclooctadecane and its application for environmental samples. Int. J. Environ. Anal. Chem. 2017, 97, 189–200. [Google Scholar] [CrossRef]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Massah, A.R. Uranyl-selective PVC membrane electrodes based on some recently synthesized benzo-substituted macrocyclic diamides. Talanta 2002, 58, 237–246. [Google Scholar] [CrossRef]
- Shamsipur, M.; Saeidi, M.; Yari, A.; Yaganeh-Faal, A.; Mashhadizadeh, M.H.; Azimi, G.; Naeimi, H.; Sharghi, H. ion-selective membrane electrode based on a naphthol-derivative Schiff’s base 2,2′-[1,2-ethandiyl bis(nitriloethylidene)]bis(1-naphthalene). Bull. Korean Chem. Soc. 2004, 25, 629–633. [Google Scholar]
- Kiegiel, K.; Steczek, L.; Zakrzewska-Trznadel, G. Application of calixarenes as macrocyclic ligands for Uranium(VI): A review. J. Chem. 2013, 2013, 762819. [Google Scholar] [CrossRef]
- Shinkai, S.; Araki, K.; Manabe, O. Chemical Communications. Does the calixarene cavity recognise the size of guest molecules? On the ‘hole-size selectivity’in water-soluble calixarenes. J. Chem. Soc. Chem. Commun. 1988, 3, 187–189. [Google Scholar] [CrossRef]
- Hu, S.-X.; Li, W.-L.; Dong, L.; Gibson, J.K.; Li, J. Crown ether complexes of actinyls: A computational assessment of AnO2(15-crown-5)2+(An= U, Np, Pu, Am, Cm). Dalton Trans. 2017, 46, 12354–12363. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, J.; Nayak, S.; Maiti, B.J. Transport of uranyl ion across a bulk liquid membrane using calixarene and synergistic agents as carriers. J. Membr. Sci. 2002, 196, 203–210. [Google Scholar] [CrossRef]
- Sessler, J.L.; Melfi, P.J.; Pantos, G.D. Uranium complexes of multidentate N-donor ligands. Coord. Chem. Rev. 2006, 250, 816–843. [Google Scholar] [CrossRef]
- Casellato, U.; Tamburini, S.; Tomasin, P.; Vigato, P. Uranyl(VI) complexes with [1+1] asymmetric compartmental ligands containing a Schiff base and a crown ether-like chamber. Inorg. Chim. Acta 2002, 341, 118–126. [Google Scholar] [CrossRef]
- Metilda, P.; Prasad, K.; Kala, R.; Gladis, J.; Rao, T.P.; Naidu, G. Ion imprinted polymer based sensor for monitoring toxic uranium in environmental samples. Anal. Chim. Acta 2007, 582, 147–153. [Google Scholar] [CrossRef]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To extract, or not to extract uranium from phosphate rock, that is the question. Environ. Sci. Technol. 2017, 51, 753–754. [Google Scholar] [CrossRef]
- Singh, D.; Mondal, S.; Chakravartty, J. Recovery of uranium from phosphoric acid: A review. Solvent Extr. Ion Exch. 2016, 34, 201–225. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Chen, J. New insights into the uranium adsorption behavior of mesoporous SBA-15 silicas decorated with alkylphosphine oxide ligands. RSC Adv. 2016, 6, 1210–1217. [Google Scholar] [CrossRef]
- Pinaeva, U.; Dietz, T.; Al Sheikhly, M.; Balanzat, E.; Castellino, M.; Wade, T.; Clochard, M. Bis[2-(methacryloyloxy)ethyl] phosphate radiografted into track-etched PVDF for uranium(VI) determination by means of cathodic stripping voltammetry. React. Funct. Polym. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Merdivan, M.; Buchmeiser, M.R.; Bonn, G. Phosphonate-based resins for the selective enrichment of uranium(VI). Anal. Chim. Acta 1999, 402, 91–97. [Google Scholar] [CrossRef]
- Kukkonen, E.; Virtanen, E.J.; Moilanen, J.O. α-Aminophosphonates,-Phosphinates, and-Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [Google Scholar] [CrossRef] [PubMed]
- Badr, I.H.; Zidan, W.; Akl, Z. Cyanex based uranyl sensitive polymeric membrane electrodes. Talanta 2014, 118, 147–155. [Google Scholar] [CrossRef]
- Badr, I.H.; Zidan, W.; Akl, Z. A novel neutral carrier for uranyl ion based on a commercially available aminophosphate derivative: Evaluation in membrane electrodes and nuclear safeguards applications. Electroanalysis 2012, 24, 2309–2316. [Google Scholar] [CrossRef]
- Zhang, W.; Bu, A.; Ji, Q.; Min, L.; Zhao, S.; Wang, Y.; Chen, J. pKa-Directed Incorporation of Phosphonates into MOF-808 via Ligand Exchange: Stability and Adsorption Properties for Uranium. ACS Appl. Mater. Interfaces 2019, 11, 33931–33940. [Google Scholar] [CrossRef]
- Nassory, N. Uranium-sensitive electrodes based on the uranium—Di(octylphenyl) phosphate complex as sensor and alkyl phosphate as mediator in a PVC matrix membrane. Talanta 1989, 36, 672–674. [Google Scholar] [CrossRef]
- Luo, C.S.; Chang, F.C.; Yeh, Y.C. Uranyl selective electrode based on organophosphorus compounds. Anal. Chem. 1982, 54, 2333–2336. [Google Scholar] [CrossRef]
- Saleh, M. A uranyl selective electrode based on neutral bidentate organo-phosphorus compounds. Indian J. Chem. 1992, 31, 12–16. [Google Scholar]
- Moody, G.; Slater, J.M.; Thomas, J. Poly(vinyl chloride) matrix membrane uranyl ion-selective electrodes based on organophosphorus sensors. Analyst 1988, 113, 699–703. [Google Scholar] [CrossRef]
- Manning, D.; Stokely, J.; Magouyrk, D. Uranyl organophosphorus compounds in a poly(vinyl chloride) (PVC) matrix as ion sensors for uranium. Anal. Chem. 1974, 46, 1116–1119. [Google Scholar] [CrossRef]
- Goldberg, I.; Meyerstein, D. Influence of ion exchanger and diluent structure on uranyl ion selective electrode response. Anal. Chem. 1980, 52, 2105–2108. [Google Scholar] [CrossRef]
- Petrukhin, O.M.; Avdeeva, E.N.; Zhukov, A.F.; Polosuchina, I.B.; Krylova, S.A.; Rogatinskaya, S.L.; Bodrin, G.V.; Nesterova, N.P.; Polikarpov, Y.M.; Kabachnik, M.I. Bidentate organophosphorus compounds as ionophores for calcium-and uranyl-selective electrodes. Analyst 1991, 116, 715–719. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, K.W.; Yang, M.H.; Kim, T.H.; Mahajan, R.K.; Kim, J.S. Selective uranyl ion detection by polymeric ion-selective electrodes based on salphenH2 derivatives. Talanta 2007, 74, 223–228. [Google Scholar] [CrossRef]
- Bertrand, P.A.; Choppin, G.R.; Rao, L.F.; Bunzli, J.C.G. Membrane electrode for the determination of actinyl(VI) cations. Anal. Chem. 1983, 55, 364–367. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, V.; Khurana, U.; Singh, L.P. A new membrane sensor for ions based on 2-hydroxyacetophenoneoxime-thiourea-trioxane resin. Electroanalysis 1997, 9, 857–860. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, K.W.; Yang, M.H.; Kim, J.E.; Lee, S.S.; Kim, J.S. Salphen H2 as a Neutral Carrier for the Uranyl Ion-Selective PVC Membrane Sensor. Bull. Korean Chem. Soc. 2006, 27, 899–902. [Google Scholar]
- Ali, T.A.; Mohamed, G.G.; Aglan, R.F.; Mourad, M.A. A novel screen-printed and carbon paste electrodes for potentiometric determination of uranyl(II) ion in spiked water samples. Russ. J. Electrochem. 2018, 54, 201–215. [Google Scholar] [CrossRef]
- Saleh, M.B.; Hassan, S.S.; Gaber, A.A.A.; Kream, N.A.A. A novel uranyl ion-selective PVC membrane sensor based on 5,6,7,8-tetrahydro-8-thioxopyrido[4′,3′,4,5] thieno [2,3-d] pyrimidine-4(3H) one. Sens. Actuators B Chem. 2003, 94, 140–144. [Google Scholar] [CrossRef]
- Akl, Z.F. Rapid electrochemical sensor for uranium (VI) assessment in aqueous media. RSC Adv. 2022, 12, 20147–20155. [Google Scholar] [CrossRef] [PubMed]
- Maccà, C. Response time of ion-selective electrodes: Current usage versus IUPAC recommendations. Anal. Chim. Acta 2004, 512, 183–190. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, H.; Mittal, S.K. Electrochemical sensor based on ion-selective membrane of silica/polyaniline nano-composites for selective determination of uranyl ions. Talanta Open 2022, 6, 100158. [Google Scholar] [CrossRef]
- Moreno, T.V.; Malacarne, L.C.; Baesso, M.L.; Qu, W.; Dy, E.; Xie, Z.; Fahlman, J.; Shen, J.; Astrath, N.G. Potentiometric sensors with chalcogenide glasses as sensitive membranes: A short review. J. Non-Cryst. Solids 2018, 495, 8–18. [Google Scholar] [CrossRef]
- Gindler, J.E. The Radio-Chemistry of Uranium; Nuclear Science Series, NAS-NS 3050; National Academy of Sciences, National Research Council: Washington, DC, USA, 1962; pp. 136–156.
- Rechnitz, G.A.; Lin, Z.-F. Potentiometric measurements with calcium-selective liquid-liquid membrane electrodes. Anal. Chem. 1968, 40, 696–699. [Google Scholar] [CrossRef]
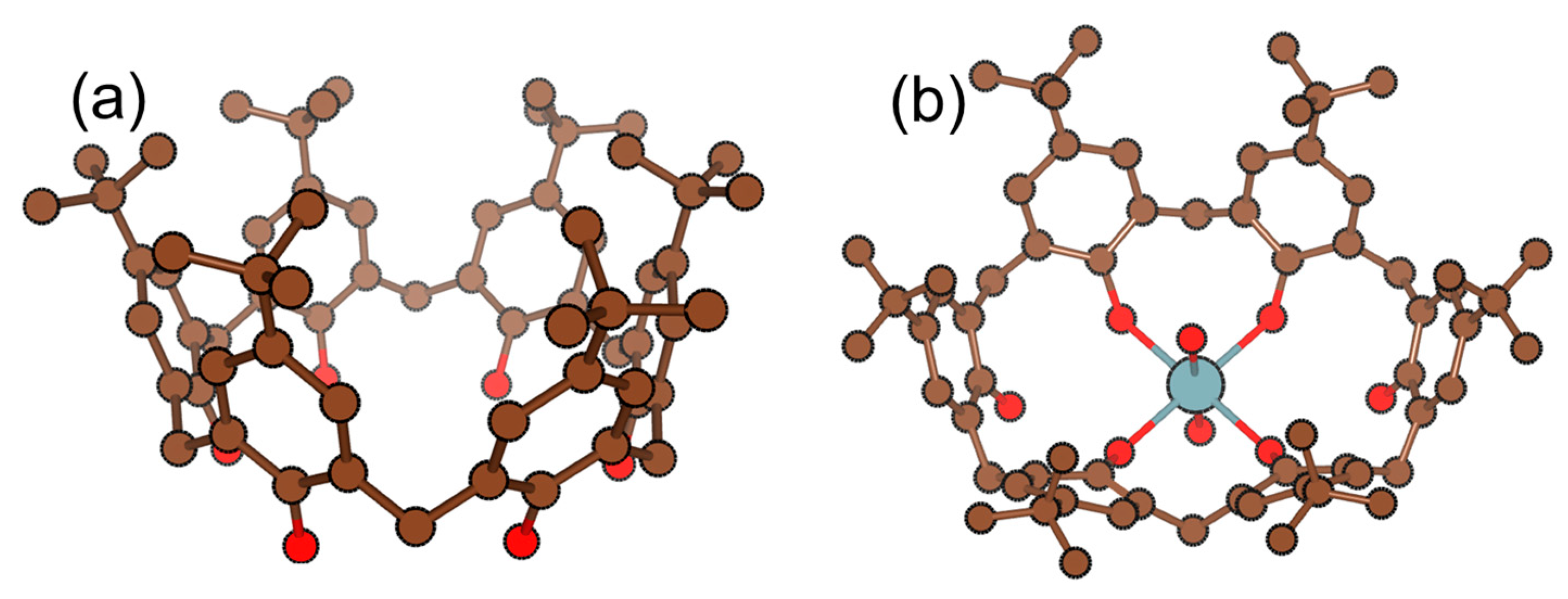
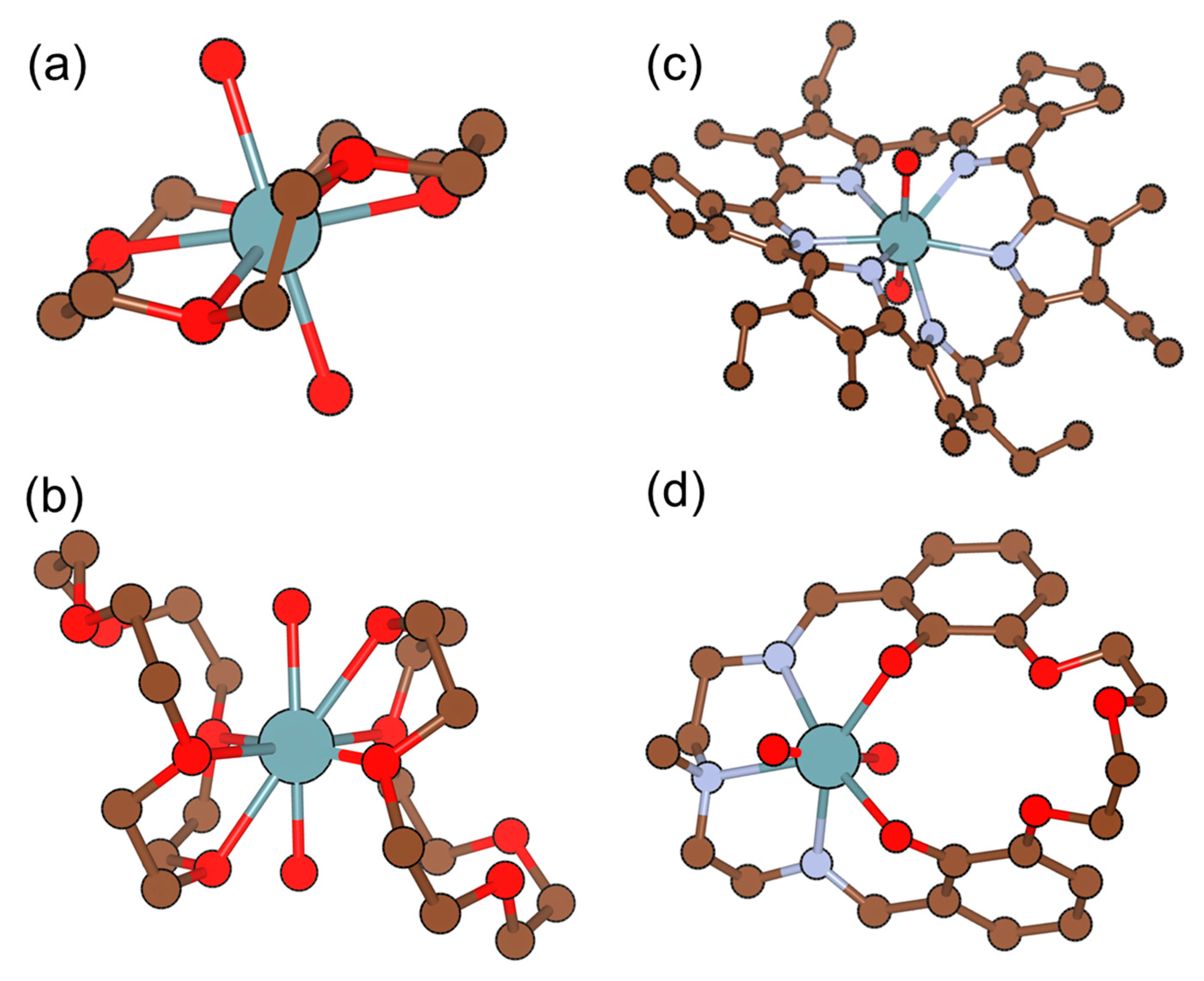
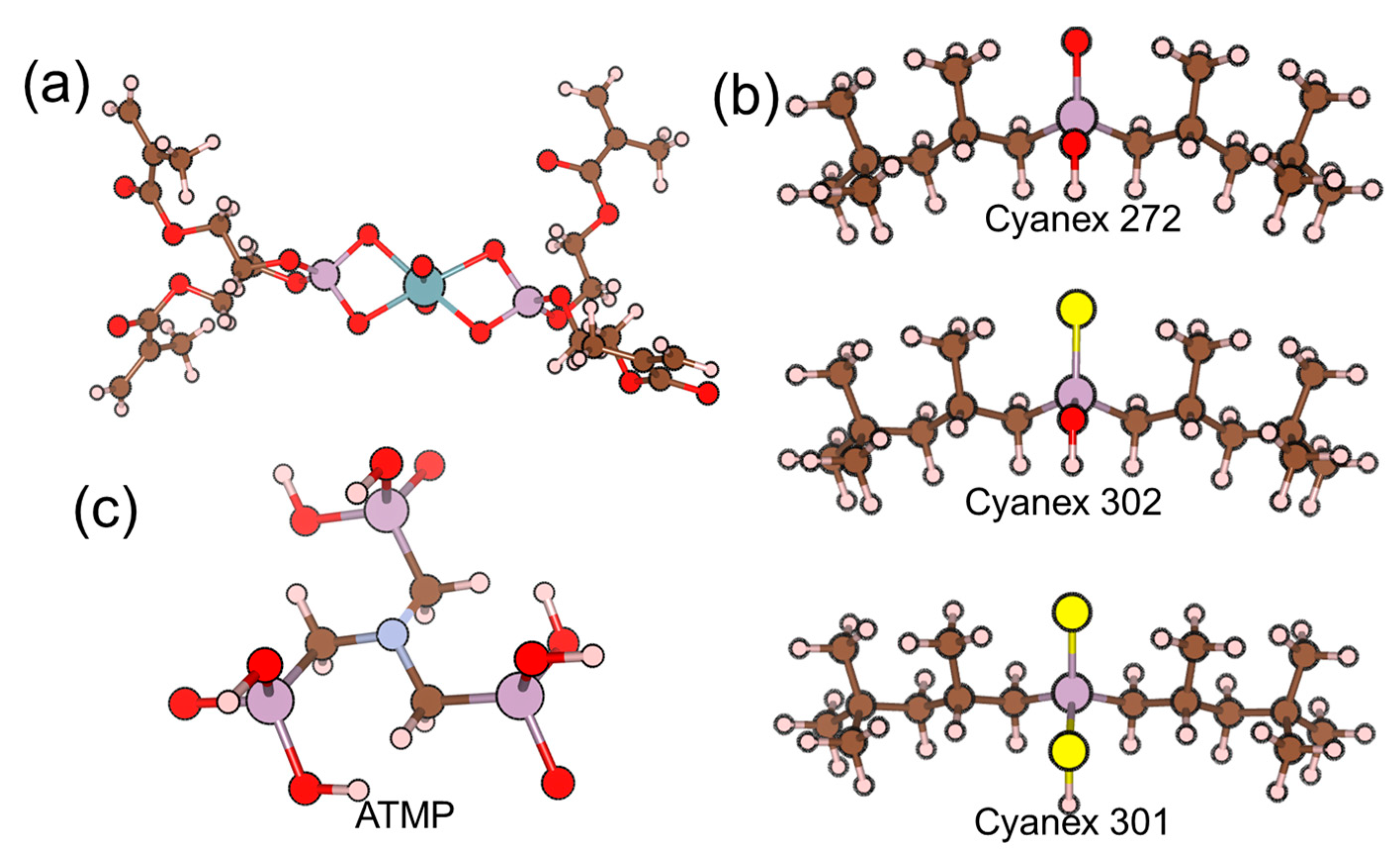

| Polymeric Matrix | Plasticizer | Components (wt. %) | Ref. | |
|---|---|---|---|---|
| Polymeric Matrix | Plasticizer | |||
| PVC | Dioctylphtalate (DOP) | 56 | 20 | [16] |
| PVC | Tributyl phosphate (TBP) | 52 | 35 | [6] |
| PVC | Tris(2-ethylhexyl)phosphate (TEHP) | 48.5 | 48.5 | [17] |
| PVC | Benzyl acetate (BA) | 33 | 57 | [7] |
| PVC | Dimethylsebacate (DMS) | 32.8 | 65.6 | [18] |
| PVC | 1-chloronaphthalene | 32.7 | 66.7 | [19] |
| PVC | Bis(2-ethylhexyl) sebacate (DOS) | 32 | 66 | [20] |
| PVC | 2-nitrophenyl octylether (NPOE) | 32 | 64 | [21] |
| PVC | Dioctylphenyl phosphonate (DOPP) | 31 | 67 | [22] |
| PVC | Dibutylsebacate (DBS) | 24.6 | 71.8 | [23] |
| PVC | Dibutyl phthalate (DBP) | 20 | 73 | [24] |
| Ionophore | pH Stability | Sensitivity [mV Per Decade] | Linear Range [mol L−1] | Selectivity Coefficients (>10−2) | Ref. |
|---|---|---|---|---|---|
| 5,11,17,23-tetra-tert-butyl-25,27-bis(hydroxy)-26-(ethoxycarbonylmethoxy)-28-(diethylcarbamoyl-methoxy) calix [4]arene | 5.5–8.5 | 36.4 | - | Ca2+, Na+, | [21] |
| 4-tert-butylcalix [6]arene | 2.2–3.2 | 29.1 | 1 × 10−1 to 3.9 × 10−5 | Na+, Ag+, NH4+, Li+, K+ | [6] |
| 5,11,17,23-tetra-tertio butyl(25,27),-bis)2-)n-]2-hydroxy-5-dinitridphenilonitrilidine) amino etoxy-(26,28)-di hydroxy calix [4]arene (HAECA) | 2.2–3.6 | 28.5 ± 0.3 | 1 × 10−4 to 1 × 10−10 | No interference | [24] |
| Benzo-15-crown-5 (B15C5) | 2.5–4.5 | 29.9 ± 0.4 | 1 × 10−1 to 1 × 10−7 | No interference | [30] |
| Benzo-15-crown-5 (B15C5) | 4–7 | 29.5 ± 2 | 1 × 10−1 to 1 × 10−4 | No interference | [16] |
| 4,13-didecyl-1,7,10,16-tetraoxa-4,13-diazacyclooctadecane (Kryptofix 22DD) | 1.5–4 | 29.6 | 1 × 10−1 to 1 × 10−4 | K+, NH4+ | [31] |
| 1,18-diaza-3,4;15,16-dibenzo-5,8,11,14,21,24-hexaoxacyclohexaeicosane-2,17-dione | 3–3.5 | 29.8 ± 0.4 | 8.2 × 10−3 to 3 × 10−6 | No interference | [32] |
| 1,11-bis(2-benzyloxy-5-formylphenoxy)-3,6,9-trioxaundecane | ~3 | 22.7 | - | Ba2+ | [22] |
| 5,7-dichloroquinoline-8-ol-4-vinyl pyridine (biomimetic) | 6.0–8.0 | 15.0–29.0 | 1 × 10−2 to 2 × 10−8 | No interference | [40] |
| 6,7,9,10,12,13,15,16,23,24,25,26-dodecahydrodibenzo[n,v][1,4,7,10,13,17,20]pentaoxadiazacyclotricosine-22,27-dione | 2.9–3.7 | 30.1 | 1 × 10−1 to 1 × 10−6 | No interference | [7] |
| Phosphorus Containing Ligand-Based Ionophores for Ionophore | pH Stability | Sensitivity [mV Per Decade] | Linear Range [mol L−1] | Selectivity Coefficients (>10−2) | Ref. |
|---|---|---|---|---|---|
| Tris(2-ethylhexyl)phosphate (TEHP) | 2.8–3.6 | 25.0 ± 0.2 | 1 × 10−1 to 2 × 10−5 | Fe3+, Ca2+, V4+, F− | [17] |
| O-(1,2-dihydro-2-oxo-1-pyridyl)-N,N,N′,N′-bis(tetra-methylene)uranium hexafluorophosphate (TPTU) | 2.5–3.5 | 27.5 ± 0.2 | 1 × 10−1 to 5 × 10−5 | Fe3+, Th4+, F− | [17] |
| Di-[4-(n-octyl)phenyl]phosphoric acid | - | 30 | 1 × 10−1 to 1 × 10−5 | Sr2+ | [50] |
| Di-[4-(l,1,3,3-tetramethylbutyl)phenyl]phosphoric acid | - | 30 | 1 × 10−1 to 1 × 10−5 | No interference | [50] |
| Bis(2,4,4-trimethylpentyl) phosphinic acid | 2.1–3.7 | 29.4 | 1 × 10−1 to 5.3×10−4 | Th4+ | [47] |
| Bis(2,4,4-trimethylpentyl) monothiophosphinic acid | 2.1–3.7 | 28.0 | 1 × 10−1 to 5.5×10−5 | Fe3+, Th4+ | [47] |
| Bis(2,4,4-trimethylpentyl) dithiophosphinic acid | 2.2–3.7 | 29.3 | 1 × 10−1 to 5×10−6 | Th4+ | [47] |
| Tri-n-octylphosphine oxide (TOPO) | 3.0 | 59 | 1 × 10−1 to 1×10−4 | Ni2+ | [51] |
| Tetraphenyl-o-xylyldiphosphine dioxide | 2.75–3.25 | 26–29 | 1 × 10−1 to 1 × 10−4 | No interference | [52] |
| Bis[di [4-(l,l,3,3-tetramethylbutyl)phenyl] phosphate] | 3 | 29–31 | 1 × 10−2 to 1 × 10−4 | No interference | [53] |
| Di(2-ethylhexyl)phosphoric acid | ~3 | 25 ± 2 | 1 × 10−1 to 1 × 10−4 | Fe3+ | [54] |
| Tris(chloroethyl)phosphite | - | 29 | 1 × 10−1 to 1 × 10−6 | Ce4+, Cr3+ | [55] |
| O-methyldihexyl phosphine oxide O′-hexyl-2-ethyl-phosphoric acid | - | 70–83 | - | Mn2+, Fe3+, Zn2+, Co2+, Ni2+, F−, Cu2+, H2PO42− | [20] |
| [O-methyldihexyl phosphine oxide O′-hexyl-2-ethyl-phosphoric acid (HL)]’s uranyl complex (UO2L2) | 2.5–4 | 25–31 | 1.47 to 2.10 × 10−5 | Fe3+, Mn2+, F−, H2PO42− | [20] |
| Tetraphenyl-o-xylylenediphosphine dioxide (o-PXDO) | - | 28 ± 1.3 | 1 × 10−2 to 1 × 10−4 | - | [56] |
| Tetratolyl-o-xylylenediphosphine dioxide (o-TXDO) | 2.70 ± 0.05 | 30 ± 2.2 | 1 × 10−3 to 1 × 10−5 | - | [56] |
| Bis [2-(methacryloyloxy)ethyl] phosphate (B2MP) | 3 | - | - | Zn2+, Ni2+, Cu2+, Co2+ | [44] |
| Amino(trimethyl) phosphate (ATMP) | 2–3.5 | 29.4 | 1 × 10−1 to 5.4 × 10−5 | No interference | [48] |
| Ionophore | pH Stability | Sensitivity [mV Per Decade] | Linear Range [mol L−1] | Selectivity Coefficients (>10−2) | Ref. |
|---|---|---|---|---|---|
| Triethylenetetramine | 2.5–3.8 | −26.5 | 10−1 to 10−5 | Fe3+, Th4+ | [23] |
| 2,2′-[1,2-ethanediyl bis (nitriloethylidene)]bis(1-naphthalene) | 3–4 | 28.5 | 10−1 to 10−7 | Mg2+, Ca2+, Ag+, Fe3+, Zn2+, Na+ | [33] |
| Bis(2-hydroxyacetophenone)ethylenediimine | 3.0–4.5 | 29.3 | 10−2 to 10−6 | K+, Ag+, Fe3+, Cu2+, Zn2+ | [18] |
| N,N′-bis[(11-ethoxycarbonyl)undecyl]-N,N′,4,5-tetramethyl-3,6-dioxaoctane diamide | - | 21.6 | - | - | [22] |
| 6,6-Dimethyl-4,8-dioxaundecanedioic nitrile | ~3 | - | 10−2 to 10−4 | H+ | [19] |
| N,N′-Diheptyl-N,N′,6,6-tetramethyl-4,8-dioxaundecanediamide | 2–4.3 | 45–55 | 10−2 to 10−5 | H+ | [58] |
| 2-hydroxyacetophenoneoximethiourea-trioxane resin | 2.5–3.5 | 39.0 | 10−1 to 10−5 | Na+, K+, Tl+, Li+, Ag+, NH4+, Ca2+, Ba2+, Sr2+, Mn2+, Pb2+, Zn2+, Ni2+, Cu2+, Mg2+, Hg2+, Al3+, La3+, Fe3+ | [59] |
| N,N′-4,5-(ethylenedioxy)benzenebis(salicylideneimine) (SalphenH2) | 1.5–4.0 | 28.0–30.9 | 10−2 to 10−6 | Cu2+, Pb2+ | [60] |
| N,N′-(propylenedioxy)benzenebis(salicylideneimine) | 1.0–5.0 | 27.0 | 10−2 to 10−6 | No interference | [57] |
| N,N′-4,5-(propylenedioxy)benzenebis(3,5-di-tert-butylsalicylideneimine) | 1.0–5.0 | 28.8 | 10−2 to 10−6 | No interference | [57] |
| Poly-(1-4)-2-amino-2-deoxy-β-D-glucan | 3–8 | 28.2–29.7 | 10−1 to 10−5 | Ce3+, Cu2+, Fe2+, NH4+, Na+, K+ | [61] |
| 5,6,7,8-Tetrahydro-8-thioxopyrido [4′,3′,4,5]thieno [2,3-d]pyrimidine-4(3H)one | 2.0–3.5 | 30 | 10−1 to 10−5 | No interference | [62] |
| Ionophores | Membrane Composition [wt. %] | Thickness of Membrane | Uranyl Solution | Response Time [s] | Lifetime | Ref. |
|---|---|---|---|---|---|---|
| 4-(1-((1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) imino) ethyl)-1-dodecylpyridin-1-ium bromide | PVC (32.50) : ionophore (2.00) : o-NPOE (65.00) : KTpClPB (0.50) | 5 mm | Uranyl nitrate solution | 9 | 8 weeks | [63] |
| 4-tert-Butylcalix[6]arene | PVC (52.00) : ionophore (7.00) : TBP (35.00) : NaTPB (6.00) | - | Uranyl nitrate solution | 10 | 5 months | [6] |
| 5,11,17,23-tetra-tertio butyl(25,27),-bis)2-)n-]2-hydroxy-5-dinitridphenilonitrilidine) amino etoxy-(26,28)-di hydroxy calix [4]arene (HAECA) | PVC (20.00) : ionophore (5.00) : dibutylphthalate (73.00) : NaTPB (2.00) | - | Uranyl nitrate solution | 20–25 | 6 weeks | [24] |
| Benzo 15-crown-5 | PVC (56.00) : ionophore (4.00) : dioctylphtalate (20.00) : carbon powder (20.00) | - | Uranyl nitrate solution | 15 | 6 weeks | [16] |
| 5,7-dichloroquinoline-8-ol-4-vinyl pyridine (biomimetic) | - | 0.6 mm | Uranyl nitrate solution | 120 | 3 months | [40] |
| Benzo 15-crown-5 | PVC (33.00) : ionophore (2.50) : MWNT (2.00): o-NPOE (60.50) : NaTPB (2.00) | - | Uranyl nitrate solution | 4 | 6 months | [30] |
| 4,13-didecyl-1,7,10,16-tetraoxa-4,13-diazacyclooctadecane (Kryptofix 22DD) | PVC (30.00) : ionophore (3.60) : dibutylphetalate (60.00) : graphite powder (6.40) | - | Uranyl nitrate solution | 10 | 12 weeks | [31] |
| 6,7,9,10,12,13,15,16,23,24,25,26-dodecahydrodibenzo[n,v][1,4,7,10,13,17,20]pentaoxadiazacyclotricosine-22,27-dione | PVC (33.00) : ionophore (7.00) : NPOE (57.00) : STPB (3.00) | 0.3 mm | Uranyl nitrate solution | 5 | 13 weeks | [7] |
| Bis(2,4,4-trimethylpentyl) phosphinic acid | - | 0.2 mm | Uranyl nitrate solution | 26 | 10 weeks | [47] |
| Bis(2,4,4-trimethylpentyl) monothiophosphinic acid | - | 0.2 mm | Uranyl nitrate solution | 35 | 10 weeks | [47] |
| Bis(2,4,4-trimethylpentyl) dithiophosphinic acid | - | 0.2 mm | Uranyl nitrate solution | 20 | 10 weeks | [47] |
| Tri-n-octylphosphine oxide (TOPO) | Ionophore (18.18) : TBP (72.73) : NaTPB (9.09) | 0.3 mm | Uranyl chloride and sulfate solution | - | 1 months | [51] |
| Tetraphenyl-o-xylyldiphosphine dioxide | PVC (33.00) : ionophore (1.00) : o-NPOE (65.50) : KTpClPB (0.50) | 0.2 mm | Uranyl chloride and nitrate solution | 120 | 4 months | [52] |
| Bis[di [4-(l,l,3,3-tetramethylbutyl)phenyl] phosphate] | - | - | Uranyl nitrate solution | 20 | 10 months | [53] |
| Di(2-ethylhexyl)phosphoric acid | - | - | Uranyl chloride solution | a few minutes | 4 to 8 weeks | [54] |
| Tris(chloroethyl)phosphite | PVC (77.95) : ionophore (2.01) : bis(2-ethylhexyl) (2-ethylhexyl)phosphonate (20.04) | 0.3–1.2 mm | Uranyl chloride solution | 10 | 3 months | [55] |
| [O-methyldihexyl phosphine oxide O′-hexyl-2-ethyl-phosphoric acid (HL)]’s uranyl complex (UO2L2) | PVC (32.00) : ionophore (2.00) : bis(2-ethylhexyl) sebacate (66.00) | 0.3 mm | Uranyl nitrate solution | 10–30 | 20 days | [20] |
| Amino(trimethyl) phosphate (ATMP) | - | 0.2 mm | Uranyl nitrate solution | 10–20 | 7 weeks | [48] |
| Tris(2-ethylhexyl)phosphate (TEHP) | PVC (48.50) : ionophore (48.5) : NaTPB (3.00) | 0.3 mm | Uranyl nitrate solution | 50 | 4 weeks | [17] |
| O-(1,2-dihydro-2-oxo-1-pyridyl)-N,N,N′,N′-bis(tetra-methylene)uranium hexafluorophosphate (TPTU) | - | 0.3 mm | Uranyl nitrate solution | 30 | 6 weeks | [17] |
| Triethylenetetramine | PVC (24.60) : ionophore (3.60) : o-NPOE (71.80) | 0.3 mm | Uranyl sulphate solution | 30 | 12 weeks | [23] |
| 2,2′-[1,2-ethanediyl bis (nitriloethylidene)]bis(1-naphthalene) | PVC (30.50) : ionophore (4.00) : DOP (63.50) : NaTPB (2.00) | 0.3 mm | Uranyl nitrate solution | 20 | 2 months | [33] |
| Bis(2-hydroxyacetophenone)ethylenediimine | PVC (32.80) : ionophore (1.00) : DOP (65.60) : MTOACl (0.60) | - | Uranyl nitrate solution | 5 | 2 months | [18] |
| 6,6-Dimethyl-4,8-dioxaundecanedioic nitrile | PVC (32.70) : ionophore (0.60) : 1-chloronaphthalene (66.70) | - | Uranyl nitrate solution | 20 | - | [19] |
| 2-hydroxyacetophenoneoximethiourea-trioxane resin | PVC (40.00) : ionophore (30.00) : DBP (30.00) | 0.5 mm | Uranyl nitrate solution | 30 | 4 months | [59] |
| N,N′-4,5-(ethylenedioxy)benzenebis(salicylideneimine) (SalphenH2) | PVC (32.66) : ionophore (0.99) : NPOE (65.91) : KTpClPB (0.44) | 0.2 mm | Uranyl nitrate solution | 60 | - | [60] |
| N,N′-(propylenedioxy)benzenebis(salicylideneimine) | PVC (33.02) : ionophore (1.00) : TEHP (65.98) | 0.2 mm | Uranyl nitrate solution | 20 | 10 months | [57] |
| N,N′-4,5-(propylenedioxy)benzenebis(3,5-di-tert-butylsalicylideneimine) | PVC (33.13) : ionophore (1.00) : TEHP (65.87) | 0.2 mm | Uranyl nitrate solution | 20 | 10 months | [57] |
| Poly-(1-4)-2-amino-2-deoxy-β-D-glucan | PVC (50.86) : ionophore (0.31) : DBP (18.31) : carbon powder (30.52) | - | Uranyl nitrate solution | 3 | 115 days | [61] |
| 5,6,7,8-Tetrahydro-8-thioxopyrido [4′,3′,4,5]thieno [2,3-d]pyrimidine-4(3H)one | PVC (32.52) : ionophore (1.63) : o-NPOE (65.04) : KTClPB (0.81) | 0.2 mm | Uranyl nitrate solution | 40 | 2 months | [62] |
| Uranyl-carboxybenzotriazol | - | 0.5 mm | Uranyl nitrate solution | 60 | 2 months | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; He, Q.; Zhang, W. Membrane-Based Electrochemical Detection of Uranium: A Review. Separations 2022, 9, 404. https://doi.org/10.3390/separations9120404
Zhang J, He Q, Zhang W. Membrane-Based Electrochemical Detection of Uranium: A Review. Separations. 2022; 9(12):404. https://doi.org/10.3390/separations9120404
Chicago/Turabian StyleZhang, Jingyue, Qing He, and Wen Zhang. 2022. "Membrane-Based Electrochemical Detection of Uranium: A Review" Separations 9, no. 12: 404. https://doi.org/10.3390/separations9120404
APA StyleZhang, J., He, Q., & Zhang, W. (2022). Membrane-Based Electrochemical Detection of Uranium: A Review. Separations, 9(12), 404. https://doi.org/10.3390/separations9120404






