Abstract
Rapid detection of okadaic acid (OA) in shellfish is crucial for practical application in food safety analysis. In order to establish a rapid, delicate detection scheme, an OA aptamer was utilized to quickly capture OA from the sample solution with polystyrene microspheres as solid phase carriers, and an inner-microchannel dam structure was designed to intercept the aptamer-functionalized microspheres to achieve the separation of OA for detection. Horseradish peroxidase (HRP) is utilized to catalyze the luminescence reaction of luminol-H2O2 solution. Through the direct competition for the aptamer between OA and OA-HRP, the rapid detection of OA can be achieved. The dynamic range of this detection method is 41.3–4.02 ng/mL, and the limit of detection (LOD) and lowest limit of quantitation (LOQ) are 12.4 pg/mL and 41.3 pg/mL, respectively. This miniaturized device enables rapid, ultrasensitive detection of OA, and demonstrates the merits of its field portability and low reagent consumption. The device can be deployed for on-site detection and analysis of marine biotoxins thereof.
1. Introduction
Okadaic acid (OA) is one of the most important marine toxins mainly found in seafood produced by algae of the genera Dinophysis and Prorocentrum [1,2]. Inherently, OA is a low-molecular-weight lipophilic, heat-stable, and highly toxic polyether toxin. When humans consume a sufficient amount of contaminated seafood, a typical gastrointestinal problem known as DSP (diarrheic shellfish poisoning) will occur. OA was first identified by Murata and his co-workers [3] as the main bioactive compound of DSP. Its pathogenic mechanism may relate to protein phosphatases, the enteric nervous system, and serotonin (5-hydroxytryptamine) levels [4,5,6]. Apart from the gastrointestinal tract, other organs, such as the kidneys and placenta, can be affected after poisoning. OA has been proven to be mutagenic, genotoxic, carcinogenic, and neurotoxic as well [7]. Chronic exposure to this polyether toxin will give rise to an increase in levels of tumor promoters and the risk of cancer [8].
A number of analytical methods of detecting okadaic acid (OA) have been reported. Amongst them, a LC-MS scheme was the reference method recommended by the European Commission (EC) [9]. Plenty of novel molecular detection methods have been developed successively to reduce the experimental cost, e.g., enzyme-linked immunosorbent assay (ELISA) [10], real-time polymerase chain reaction (PCR) [11], and in situ hybridization (ISH) [12]. In recent years, with the imperative need for food safety inspection, some rapid, sensitive, and portable test methods have been proposed for prompt and quantitative food analysis [13,14], most of which are frequently utilized immunosensors. However, the relatively long time and high cost of production, poor stability, and the difficulty in modification of antibodies still make the detection unfavorable.
Aptamers are single-stranded oligonucleotides that can be isolated by the systematic evolution of ligands by exponential enrichment (SELEX) protocol [15]. A great number of targets differing in size can be specifically bound by their aptamers such as inorganic metal ions, small organic molecules, proteins, and even whole cells. Compared to antibodies, aptamers as recognition elements can be screened via in vitro selection process, and they are easier to be produced and modified. Moreover, aptamers are more inexpensive and stable, and they have the potential to adapt to a wider variety of targets. Owing to these advantages, aptamers as alternatives or supplements to antibodies can be successfully utilized in the field of biosensor and analytical chemistry. For instance, the aptamer OA34 with a Kd of 77 nM was successfully selected by Eissa et al. [16], and showed good selectivity in the interference of DTX-1 and DTX-2, the analog toxins of which the structures are closely related to OA differing only by two methyl groups. Furthermore, the detection selectivity of OA34 was also confirmed by our teammates and other researchers [17,18,19,20].
To further enhance the analytical capabilities and adaptabilities of OA aptasensors, the development of a customized microfluidic device based on a lab-on-a-chip or micro-total analysis system (μTAS) scheme will offer extra advantages, e.g., faster mass transfer [21], higher sensitivity, less reagent consumption, shorter analysis time and better portability. In most cases, the minute volume fluid is processed and operated in microfluidic device channels ranging from 10 μm to 500 μm, and these channels can be fabricated with photolithographic micromachining by dry etching or wet chemistry processes on appropriate substrates such as glass, quartz, silicon or polymer [22]. In recent years, a number of frontier methods with superior analytical performance based on microfluidic detection devices or systems have been developed successfully [23,24,25].
Integrated sample separation, reaction, and detection using a microfluidic device can provide a better prospect of on-the-spot use of food safety testing schemes. The determination of OA depends on the high-affinity OA34 aptamer. In this work, we used a self-assembled microfluidic glass chip with functionalized polymer microspheres as we reported previously [26] to develop a novel analytical device and investigate the feasibility of highly sensitive rapid assay of hazardous representative small molecules, a direct competitive chemiluminescent method in a microfluidic device for ultra-sensitive determination of okadaic acid was proposed.
2. Materials and Methods
2.1. Material
Purchased or prepared locally are 1-ethyl-3-(3-dimethy-laminopropyl) carbodiimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS), 2-(4-Morpholino) ethanesulfonic acid (MES), bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), carbonate buffer solution, phosphate buffer saline (PBS), Horseradish Peroxidase (HRP), luminol with H2O2 solution (substrate), reaction buffer (536.91 mg MES, 27.5 μL ProClin 300, 55 mL pure water, pH = 5.2), and elution buffer (22.5 mg BSA, 54.5 mg Tris, 22.5 μL ProClin 300, 45 mL pure water, pH = 8.0). Polystyrene microspheres with a diameter of 32 μm were purchased from Suzhou Nanomicro Technology Company. OA standard products were manufactured by Beacon, and OA34 aptamer was synthesized from Sangon Biotech (Shanghai) with the following sequences: 5′-GGTCACCAACAACAGGGAGCGCTACGCGAAGGGTCAATGTGACGTCATGCGGATGTGTGG-3′.
2.2. Coupling of Okadaic Acid with Horseradish Peroxidase
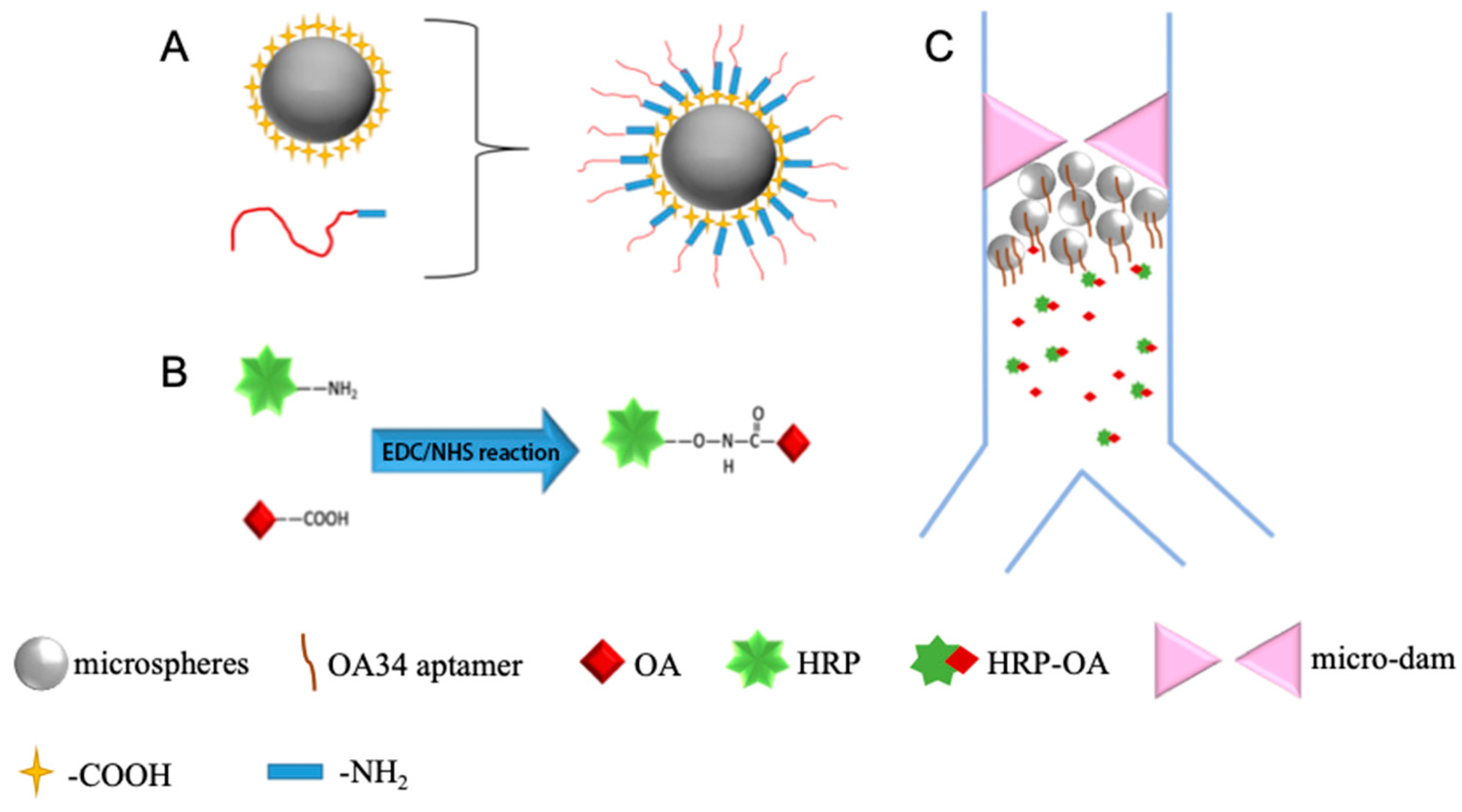
A competitive target molecule or antigen utilized for the detection was produced by the coupling of OA with horseradish peroxidase. Based on the chemical reaction of EDC and NHS, the enzyme-labeled, i.e., OA-HRP is prepared as shown in Figure 1B. First, 0.6 mg EDC, 0.4 mg NHS, and 0.8 mg HRP were dissolved in 0.3 mL carbonate buffer solution (0.1 M, pH = 9.6). Then 2 mg/mL OA solution was added. The resultant mixture reacted at room temperature for two hours to obtain the conjugated OA-HRP, and the dialysis of OA-HRP complexes in 0.01 M PBS for 12 h at room temperature was fulfilled to purify the conjugated OA-HRP as a competitive antigen.
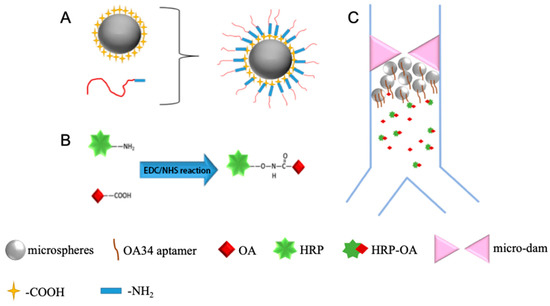
Figure 1.
(A) Diagram of aptamer-functionalized microsphere to isolate OA; (B) diagram of coupling of OA to HRP; (C) diagram of the direct competitive chemiluminescent scheme.
2.3. Immobilization of Aptamers on Polystyrene Microspheres
An OA34 aptamer selected and identified by Eissa et al. [16] was synthesized and modified with an amino group in this work, and the aptamer was immobilized on the microsphere’s surface through the EDC/NHS chemistry as well as shown in Figure 1A.
The confirmation of the successful immobilization of the OA34 aptamer can be characterized by the chemiluminescence analysis of the blank microspheres and the functionalized microspheres, and the comparison of surface morphological roughness of microspheres before and after functionalization, respectively.
The immobilization was experimentally operated as follows: Firstly, 172.4 μL of the carboxylated polystyrene microsphere was prepared by centrifugation at 4000 rpm for 10 min, and then the supernatant was removed. A volume of 400 μL of reaction buffer was added to the remaining solid deposits and the mixture was later centrifuged at 4000 rpm for 10 min and the supernatant was removed subsequently. Another volume of 170 μL of reaction buffer was added to the deposits and a volume of 20 μL of EDC solution was added to the mixture afterward. After blending for 15 min, a volume of 100 μL 0.65 μg/mL aptamer solution was added to the mixture and incubated for 1 h at room temperature. At the end of incubation, the mixture was then subject to centrifugation at 4000 rpm for 10 min and the supernatant was removed. A volume of 400 μL elution buffer was added to the remained deposits for resuspending the mixture of microspheres and aptamers, and then the mixture was centrifuged at 4000 rpm for 10 min. After removing the supernatant, the remaining deposits were resuspended with 400 μL elution buffer and stored at −20 °C for later use.
2.4. The Competitive Chemiluminescent Method Developed in the Microfluidic Device
Aptamer-functionalized microsphere suspension with definite volume was introduced into the microchannel to form a bio-reaction section, OA as a calibrator or OA in the sample and OA-HRP were introduced into the section and competed for the limited number of aptamers after well-mixed, which constitutes the direct aptamer-based competitive assay scheme. The luminol-H2O2 reagent regime was utilized as the substrate system to trigger a chemiluminescent reaction for highly sensitive OA detection with a R1914A photomultiplier tube (PMT) to count the number of emitted photons for precise detection.
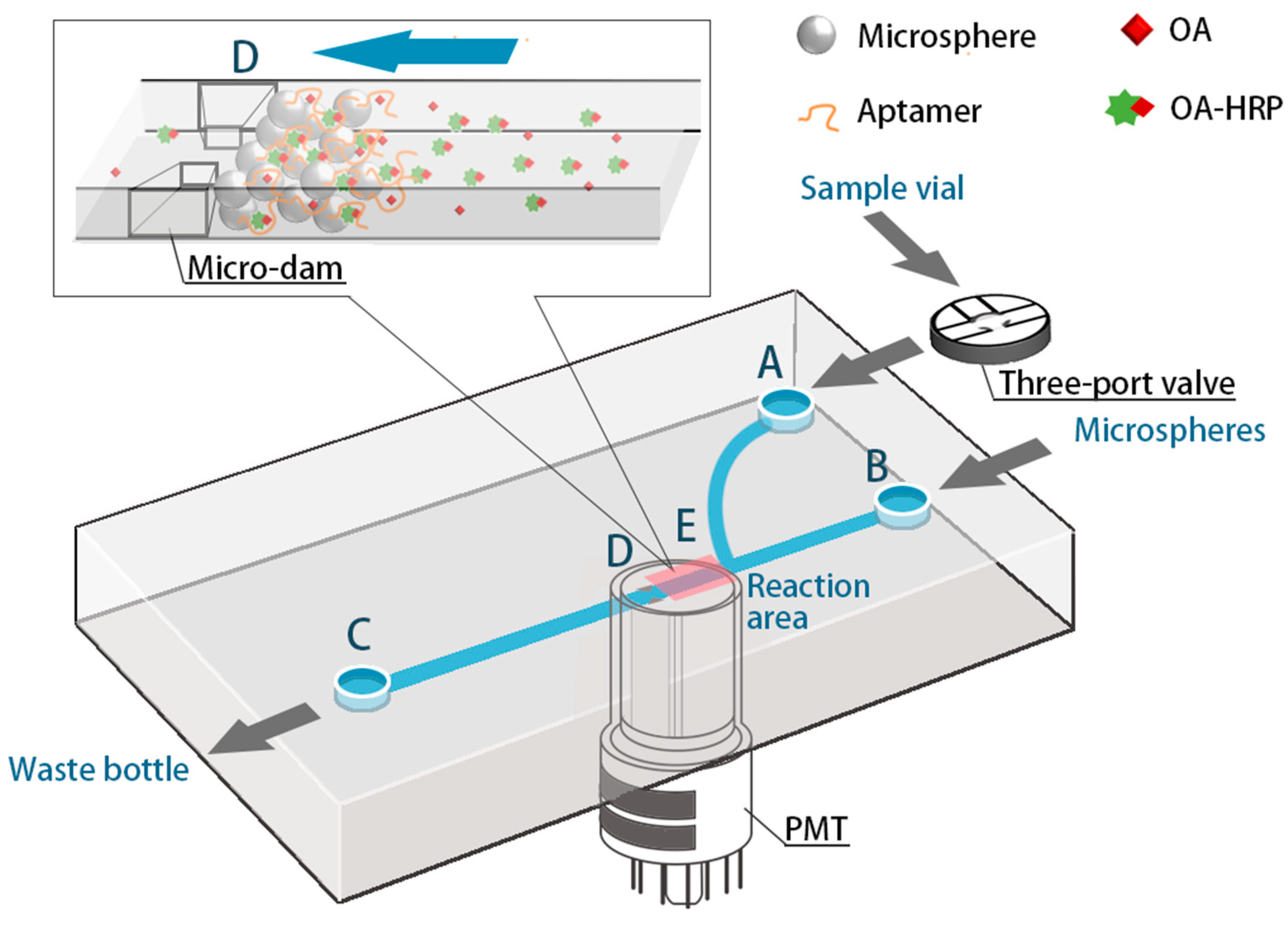
As shown in Figure 2, the detection device is mainly composed of a microfluidic micro-syringe pump, a microfluidic glass chip, a PMT, a three-way valve, and a cassette. the microfluidic chip used in this experiment is a rectangular glass chip with a size of 3 cm × 7 cm, and the channels in the chip have a dendritic Y-type structure. The three-way valve is used to control the introduction of the fluids via the A-end. The microspheres are introduced from the B-end and then closed, and the C-end is the waste liquid port, from which the reaction liquid and the washing liquid during or after the reaction are discharged. The D-end is a micro-dam with a slit width of 20 μm, and the diameter of the microspheres introduced to the bio-reaction section is 32 μm, ensuring the inability of microspheres to be flushed out through the micro-dam slit. In addition, a PMT is aligned to the E position to collect the light signal generated by the reaction, and the PMT is connected to a laptop computer for data acquisition and analysis.
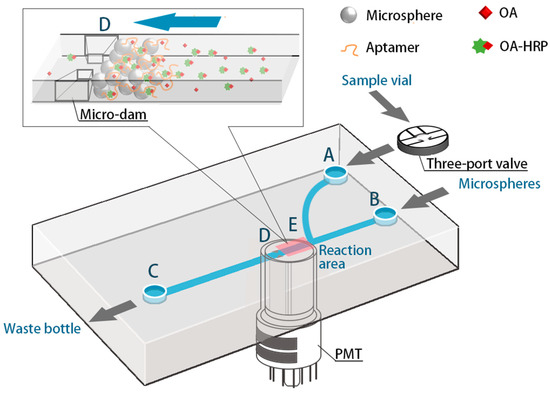
Figure 2.
Diagram of the microfluidic device with a partially enlarged view in the reaction area.
The concentration of OA was, thus, determined as per the following procedures: firstly, micro-channels were washed using pH = 7.4 PBS at least three times. Then, the aptamer-functionalized microspheres were fully introduced into the reaction section in the microchip and excess microspheres were flushed out of the chip. A 15 μL of sample solution and a 15 μL OA-HRP solution were mixed for 20 min beforehand. After that, the 30 μL mixed solution was introduced to the microsphere-loaded channel for 30 s, which makes OA bind with the immobilized aptamer entirely. The non-specific binding was minimized by several post-wash steps with pH = 7.4 PBS. Finally, 30 μL luminol with H2O2 solution, working as the substrate, was injected into the channel at a speed of 0.03 mL/min, and a chemiluminescence reaction was initiated thereafter. The time of the entire pump-in and reaction is 36 s, and the aptamer-functionalized microspheres were finally flushed out after detection. The aptasensor takes only ten minutes to achieve the task.
2.5. Real Sample Preparation for OA Detection
Several kinds of OA-negative fresh shellfish, such as scallops, oysters, and clams were purchased from the local markets in Beijing and Shenzhen, respectively. The edible tissue was separated from the shell and shredded into pieces. Then 0.2 g of prepared tissue was immersed in 9 mL of methanol and the mixture was extracted with an ultrasonic cleaner for 10 min. Further, the mixture was centrifuged at 8000 rpm for 5 min and the supernatant was stored as the extracted sample solution. Moreover, the precipitate was dissolved in 9 mL of methanol and re-extracted once. The two extracts were combined and diluted to 20 mL, and then a volume of 1 mL of the extracts was taken out to a sample well. A volume of 125 μL 2.5 M NaOH was added to each well and incubated for 40 min at 76 °C. After cooling to room temperature, a volume of 125 μL 2.5 M HCL was added to each well, and then the prepared solution was filtered with a filter membrane of 0.22 μm diameter. The solution was blown with nitrogen until it is nearly dry and stored at −20 °C, then re-dissolved with 1 mL of methanol before each measurement.
3. Results and Discussion
3.1. Characterization of the Immobilization of Aptamers
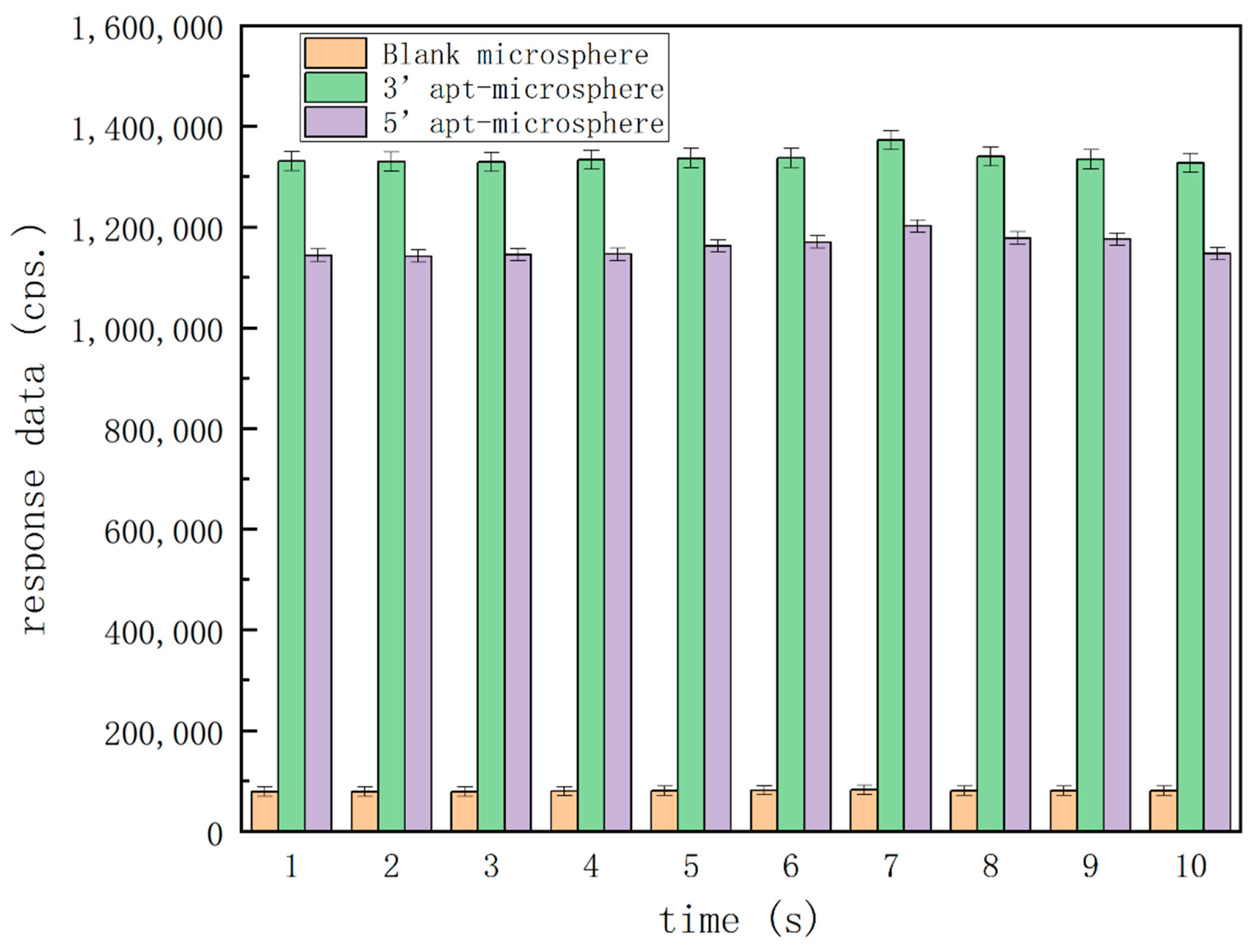
The prerequisite of this method is the successful immobilization of aptamer on the microspheres as solid phase carriers, so as to isolate the target to be tested from the sample solution by combining the with the target in a microfluidic device, and finally realize the quantitative chemiluminescence analysis. To confirm the immobilization, three types of microspheres, i.e., blank microspheres, microspheres functionalized with an aptamer at the 3′-end, and microspheres functionalized with an aptamer at the 5′-end were used for the characterization as per the experimental procedures described in Section 2.4 without the need to add any OA calibrators. The amino groups labeled on OA34 aptamers and the carboxyl groups modified on the surface of microspheres were subjected to EDC/NHS chemistry as usual, and the aptamers could be immobilized with ease on the microspheres by dehydration condensation.
Figure 3 shows the differences in the response data amongst these microspheres. In the blank-type microspheres, the response data were less than 100,000 counts per second (cps). In both types of aptamer-functionalized microspheres, the response data were more than 1 million cps, and microspheres functionalized with aptamer at 3′-end gave relatively higher responses for a more preferable detection sensitivity. Therefore, these characterization results demonstrated that the aptamer immobilization onto the surfaces of microspheres was successful and the detection would be feasible with adding OA as calibrators, the aptamer fixed on the microspheres could successfully isolate the target from the fluid, the microsphere with aptamer (3′-end immobilization) was the most preferential carrier, so we chose this group to perform follow-up experiments.
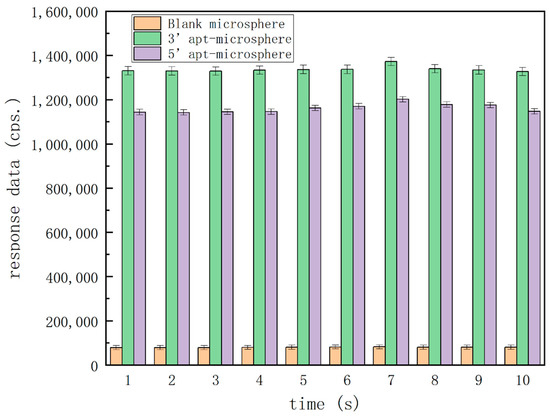
Figure 3.
Confirmatory analysis of the immobilization of aptamers on microspheres.
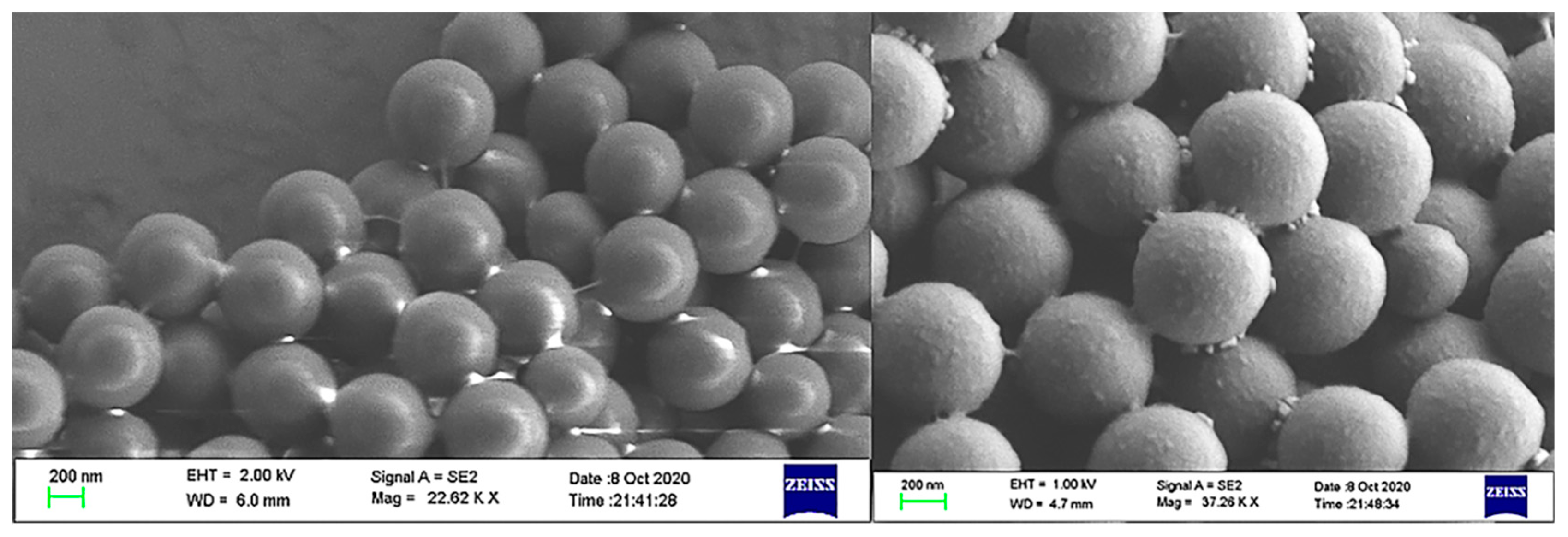
To further validate the characterization, Figure 4 showed the change in surface roughness between the blank microsphere and the aptamer-functionalized microspheres under the scanning electron microscope (SEM). The immobilization of the aptamer increased the surface roughness of the microspheres.
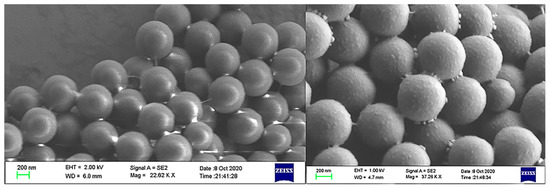
Figure 4.
SEM image of the change in roughness before (the left one) and after(the right one) the microspheres are functionalized.
3.2. Optimizing of Key Experimental Parameters
In order to obtain the optimal condition of the direct competitive chemiluminescent reaction, several important parameters needed to be systematically investigated beforehand. Among these parameters, the working concentration of OA34 aptamer is the most crucial one to ensure a successful detection with high sensitivity.
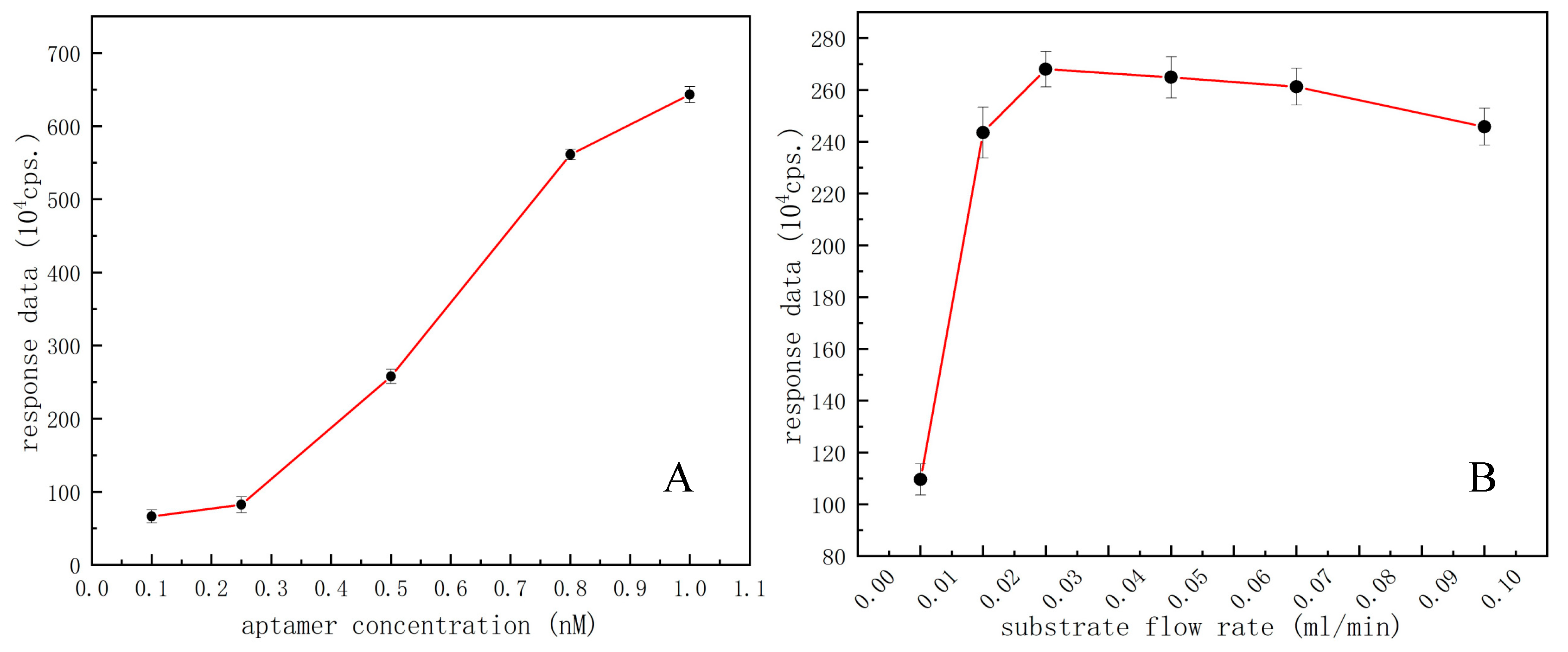
After pre-experiment and consulting related literature reports, the aptamer concentrations ranging from 0.1 to 1 ng/mL were tested by univariable experiments. Different concentrations of aptamer-functionalized microspheres were introduced to the microchip by the syringe pump. The excess microspheres were washed out to keep the same volume/amount of microspheres for microfluidic chemiluminescent reaction each time, and a series of experimental data were obtained with the same operations accordingly. Based on the acquired response traits of PMT signal values versus different concentrations of aptamer for effective functionalization of microspheres, the concentration most sensitive to the response of this experiment is selected as the optimal aptamer concentration for follow-up experiments. As shown in Figure 5A, the aptamer concentration ranging from 0.5 to 0.8 ng/mL showed a maximum slope in this broken line chart, implying the most sensitive concentration range for OA detection, so the midpoint of this range, i.e., 0.65 ng/mL was selected as the optimal aptamer concentration to perform the follow-up experiments.
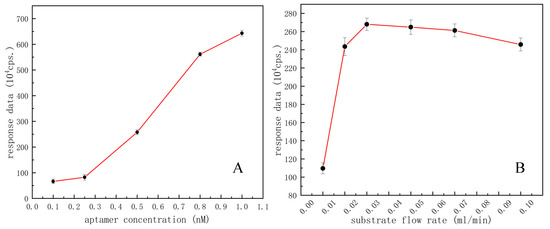
Figure 5.
(A) Optimization of the aptamer concentration with datapoints of black dots; (B) Optimization of the substrate flow rate with datapoints of black dots.
The substrate flow rate pushed by the syringe pump is another key factor affecting the competitive response of the microfluidic chemiluminescent assay. By continuously injecting the substrate solution to make the luminescence reaction continue, it is necessary to select an optimal substrate flow rate for a direct competitive microfluidic chemiluminescent assay. In this part, different flow rates, i.e., 0.01, 0.02, 0.03, 0.05, 0.07, and 0.1 mL/min were set for experiments under the same conditions, respectively. Different luminescence signal data could be obtained without the need to introduce any competitors, i.e., OA molecules, thereby we could obtain the best substrate flow rate from PMT signal readouts. As shown in Figure 5B, when a substrate flow rate is 0.03 mL/min the response value as detected reached its maximum. Therefore, 0.03 mL/min was determined as the best substrate flow rate for a microfluidic competitive chemiluminescent reaction.
3.3. Development of Calibration Curve for Quantitation
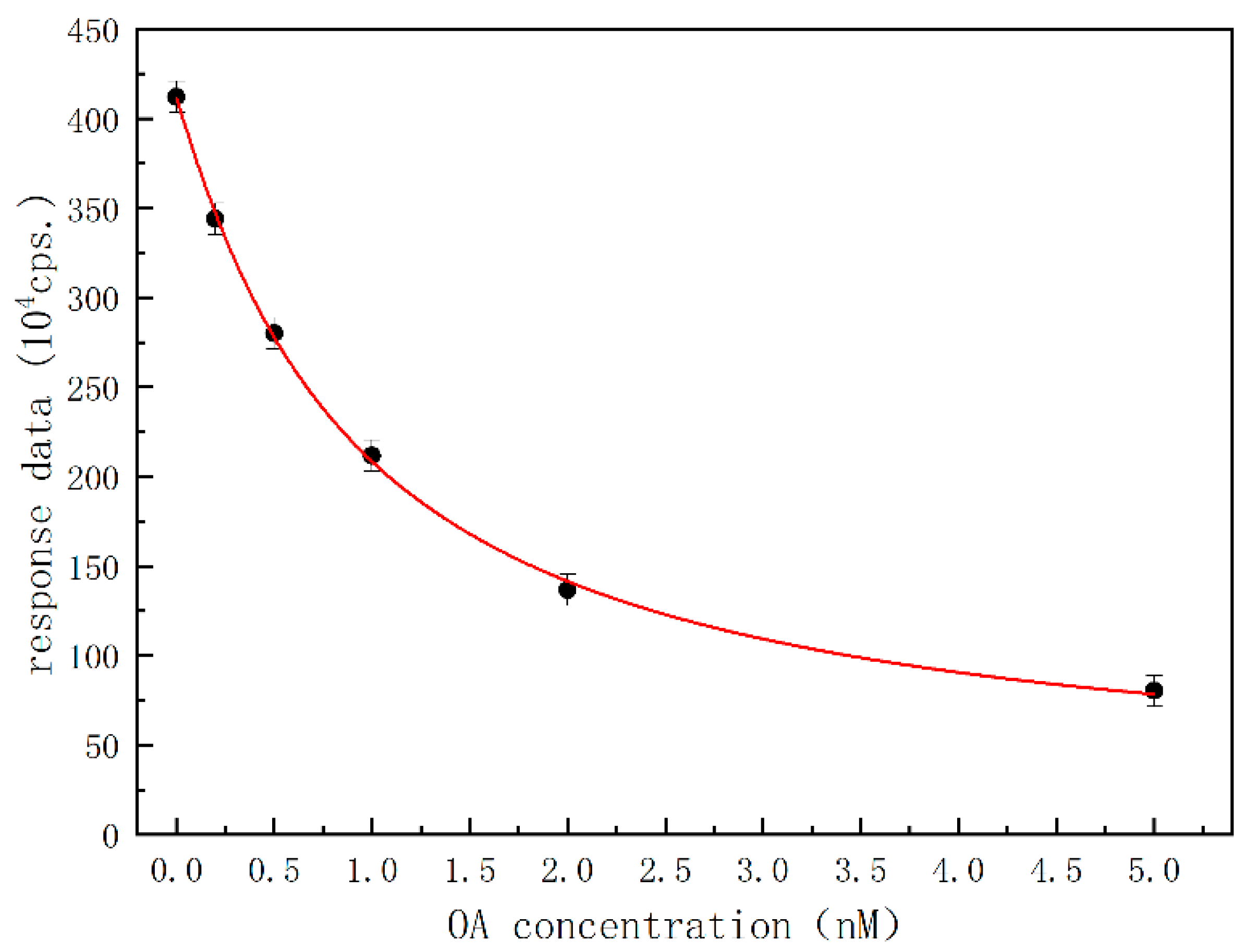
Under optimal conditions, free OA as a calibrator or analyte in the sample and OA-HRP complex formed a competitive relation, so the direct competition method could be realized to determine OA as schematically shown in Figure 1C. Contrary to the varying concentrations/amounts of OA calibrators or OA analyte in the sample, the amount of aptamer immobilized on the solid-phase carrier in the channel was constant, as well as the amount of injected OA-HRP complex. A calibration curve was obtained by experimenting with six different concentrations of OA calibrators, ranging from 0 to 5 ng/mL. Figure 6 shows the response data of microfluidic chemiluminescent reaction varying with the concentration of OA calibrators. As the concentration of OA increased, the response data decreased significantly, demonstrating inhibitive or competitive trends. Each data point of the calibration curve represents an average of three independent trials, and there was a definite relation between response data and the concentration of OA in a range of 41.3–4.02 ng/mL, which can be represented by the non-linear regression fitting equation:
1 + Coa/0.92 = 387.828/(A − 23.293)
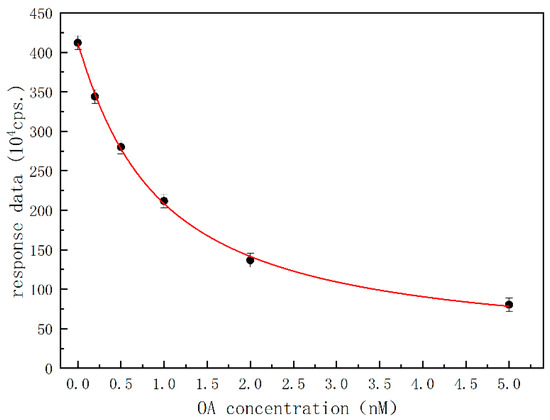
Figure 6.
Calibration curve of OA with datapoints of black dots.
(where A is the response data of microfluidic chemiluminescent reaction (cps), Coa is the calculated concentration of OA (ng/mL), R2 = 0.999). The lowest limit of detection (LOD for S/N = 3) and the lowest limit of quantitation (LOQ for S/N = 10) were computed to be 12.4 pg/mL and 41.3 pg/mL, respectively.
The LOD in this work was found to be lower compared to most previously reported methods as tabulated in Table 1. In addition, this work is advantageous in terms of both detection limit and assay time, and the detection efficiency of this method may be further improved if sample pretreatment and analyte extraction processes can be fully or partially eliminated after rational optimizations in the future.

Table 1.
Comparative table for the detection of OA using different methods.
3.4. Real Samples OA Detection and Method Accuracy Characterization
In order to verify the practicability and method accuracy of the developed microfluidic okadaic acid assay scheme for practical applications, the performance of the method to detect OA spiked fresh shellfish samples using an aptamer-functionalized microfluidic device was evaluated. The fresh mussel samples of different shellfish products were prepared in the steps described in Section 2.5. Different concentrations of OA were spiked into 250-fold diluted mussel samples and analyzed on the microfluidic platform. Results presented in Table 2 showed that the spike recovery for different samples examined was 90.6–107.1% and the coefficient of variation (CV) below 4.4%, indicating the method of an aptamer-based chemiluminescent scheme in a microfluidic device could be used to detect OA in daily life with good accuracy.

Table 2.
Data of accuracy deviation by OA standard and relative standard deviation.
4. Conclusions
Based on the confirmation of the success of aptamer immobilization onto the surface of microspheres to isolate OA with both PMT response and microsphere morphology roughness data, and the optimization of key experimental parameters, a novel method of the directly competitive aptamer-based chemiluminescent scheme was successfully developed and well presented to detect OA in real samples shellfish samples in a lab-assembled microfluidic device. This method provides high sensitivity, high accuracy, and low costs; moreover, the analysis time takes less than 10 min to realize on-site detection. Furthermore, OA detection is accomplished in a dynamic and switchable manner with reagent flowing over the surface of the solid phase carrier, which can hardly be achieved in traditional detection methods, e.g., ELISA with tedious reagent adding and washing steps. In summary, this microfluidic aptasensor combined the chemiluminescence technology with microfluidic chips to realize the integrated operation of separation, reaction, and detection. The aptasensor has the advantages of less reagent consumption, higher sensitivity, more portability, and good dynamic detection ability.
Author Contributions
Conceptualization, L.M. and Y.Y.; formal analysis, Q.Z.; investigation, L.M.; resources, Y.D. and S.W.; data curation, L.M.; writing—original draft preparation, L.M.; writing—review and editing, Q.Z.; visualization, L.M. and Q.Z.; supervision, Y.D.; funding acquisition, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Joint Project of BRC-BC (Biomedical Translational Engineering Research Center of BUCT-CJFH) grant number (RZ2020-02), the National Natural Science Foundation of China (no. 31801620), and the National Key Research and Development Program of China (2016YFF0203703).
Data Availability Statement
All data related to the manuscript are available in the manuscript and in the form graphs, figures, and tables.
Acknowledgments
This work was supported by the Joint Project of BRC-BC (Biomedical Translational Engineering Research Center of BUCT-CJFH) (RZ2020-02), the National Natural Science Foundation of China (no. 31801620), and the National Key Research and Development Program of China (2016YFF0203703).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falconer, I.R. Algal Toxins in Seafood and Drinking Water; Academic Press: Waltham, MA, USA, 1993. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Sugawara, W.; Fukuyo, Y.; Oguri, H.; Igarashi, T.; Fujita, N. Identification of Dinophysis fortii as the Causative Organism of Diarrhetic Shellfish Poisoning. Nippon. Suisan Gakkaishi 1980, 46, 1405–1411. [Google Scholar] [CrossRef]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Nihon-Suisan-Gakkai-Shi 1982, 48, 549–552. [Google Scholar] [CrossRef]
- Traoré, A.; Baudrimont, I.; Ambaliou, S.; Dano, S.D.; Creppy, E.E. DNA breaks and cell cycle arrest induced by okadaic acid in Caco-2 cells, a human colonic epithelial cell line. Arch. Toxicol. 2001, 75, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Fernández, D.A.; Abal, P.; Fraga, M.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Diarrhetic effect of okadaic acid could be related with its neuronal action: Changes in neuropeptide Y. Toxicol. Lett. 2015, 237, 151–160. [Google Scholar] [CrossRef]
- Louzao, M.C.; Costas, C.; Abal, P.; Suzuki, T.; Watanabe, R.; Vilariño, N.; Carrera, C.; Boente-Juncal, A.; Vale, C.; Vieytes, M.R.; et al. Serotonin involvement in okadaic acid-induced diarrhoea in vivo. Arch. Toxicol. 2021, 95, 2797–2813. [Google Scholar] [CrossRef]
- Munday, R. Is protein phosphatase inhibition responsible for the toxic effects of okadaic Acid in animals? Toxins 2013, 5, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Nelis, J.L.D.; Ross, G.M.S.; Jafari, S.; Guercetti, J.; Kopper, K.; Zhao, Y.; Rafferty, K.; Salvador, J.P.; Migliorelli, D.; et al. Critical assessment of recent trends related to screening and confirmatory analytical methods for selected food contaminants and allergens. TrAC Trends Anal. Chem. 2019, 121, 115688. [Google Scholar] [CrossRef]
- Ball, H.J.; Finlay, D.; Reilly, G.A. Sandwich ELISA detection of Mycoplasma bovis in pneumonic calf lungs and nasal swabs. Vet. Rec. 1994, 135, 531–532. [Google Scholar] [CrossRef]
- Rossetti, B.C.; Frey, J.; Pilo, P. Direct detection of Mycoplasma bovis in milk and tissue samples by real-time PCR. Mol. Cell. Probes 2010, 24, 321–323. [Google Scholar] [CrossRef]
- Ekker, M.; Akimenko, M.-A.; Bremiller, R.; Westerfield, M. Regional expression of three homeobox transcripts in the inner ear of zebrafish embryos. Neuron 1992, 9, 27–35. [Google Scholar] [CrossRef]
- Hayat, A.; Barthelmebs, L.; Sassolas, A.; Marty, J.-L. An electrochemical immunosensor based on covalent immobilization of okadaic acid onto screen printed carbon electrode via diazotization-coupling reaction. Talanta 2011, 85, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.L.D.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef]
- Pan, C.; Zhu, X.; Yang, Y.; Wang, H.; Liu, J.; Chen, H.; Dong, Y. Rapid visual detection of okadaic acid based on aptamer. Chin. J. Anal. Lab. 2021, 40, 686–691. [Google Scholar] [CrossRef]
- Yan, X.; Qi, X.; Zhao, Y.; Li, L.; Ma, R.; Wang, L.; Wang, S.; Mao, X. Development of a Label-Free Colorimetric Aptasensor with Rationally Utilized Aptamer for Rapid Detection of Okadaic Acid. J. Ocean. Univ. China 2022, 21, 400–408. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, P.; Chen, Y.; Bai, X.; Du, L.; Chen, W.; Wu, C.; Wang, P. Piezoelectric aptasensor with gold nanoparticle amplification for the label-free detection of okadaic acid. Sens. Actuators B Chem. 2021, 346, 130446. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Zhu, P.; Ge, S.; Zhang, L.; Zhang, Y.; Yu, J. Multiple cooperative amplification paper SERS aptasensor based on AuNPs/3D succulent-like silver for okadaic acid quantization. Sens. Actuators B Chem. 2021, 344, 130174. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Liu, J. Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines 2017, 8, 255. [Google Scholar] [CrossRef]
- Franke, T.A.; Wixforth, A. Microfluidics for miniaturized laboratories on a chip. Chemphyschem 2008, 9, 2140–2156. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.; Chen, G.; Yang, L.; Xu, S.; Xu, W. Aptamer-based surface-enhanced Raman scattering-microfluidic sensor for sensitive and selective polychlorinated biphenyls detection. Anal. Chem. 2015, 87, 9555–9558. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Cai, G.; Liu, N.; Liao, M.; Li, Y.; Zhang, X.; Lin, J. A microfluidic biosensor for online and sensitive detection of Salmonella typhimurium using fluorescence labeling and smartphone video processing. Biosens. Bioelectron. 2019, 140, 111333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; He, P.; Zi, F.; Hu, X.; Wang, Q. Sensitive assay of Escherichia coli in food samples by microchip capillary electrophoresis based on specific aptamer binding strategy. Talanta 2019, 197, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, Y.; Liu, Z.; Fu, Y.; Ohashi, T.; Tanaka, Y.; Mawatari, K.; Kitamori, T. Rapid screening swine foot-and-mouth disease virus using micro-ELISA system. Lab Chip 2011, 11, 2153–2155. [Google Scholar] [CrossRef]
- Chinnappan, R.; AlZabn, R.; Mir, T.A.; Bader, M.; Zourob, M. Fluorometric determination of okadaic acid using a truncated aptamer. Microchim. Acta 2019, 186, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, X.; Zhao, J.; Shi, X.; Sun, A.; Jiao, H.; Xiao, T.; Li, D.; Chen, J. A fluorescence microplate assay based on molecularly imprinted silica coated quantum dot optosensing materials for the separation and detection of okadaic acid in shellfish. Chemosphere 2020, 246, 125622. [Google Scholar] [CrossRef]
- Castanheira, A.; Santos, M.; Rodriguez-Lorenzo, L.; Queirós, R.; Espiña, B. A novel microfluidic system for the sensitive and cost-efficient detection of okadaic acid in mussels. Analyst 2021, 146, 2638–2645. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).