Abstract
The title ligand (L”x), methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate was synthesized following the Morita-Baylis-Hillman reaction scheme. Spectroscopic techniques such as: UV- Visible, FT-IR, ESI-MS, and 1H NMR helped in characterization of the L”x. Complexes of Cr3+, Co3+, Ni2+, Mn2+, Cu2+ with L”x were prepared and characterized by UV- Visible, FT-IR and powder-XRD. FTIR spectrum of the L”x generated through DFT B3LYP method and 6-311++ G (d,p) basis set was found in good agreement with experimental spectrum. Additionally, the semi-empirical PM6 method optimization helped propose the most suitable geometries of the complexes with Cr3+, Co3+ possessing octahedral, Ni2+ square planner, Mn2+ and Cu2+ tetrahedral geometries. Powder-XRD patterns of the complexes have revealed cubic crystal class for Cr3+ and Co3+, whereas hexagonal, orthorhombic, and monoclinic for Ni2+, Mn2+, and Cu2+ complexes were observed, respectively. In addition, the nano-particle size was found in the range of 8.2560–4.5316 nm for complexes. Antibacterial activity against S. aureus, E. coli, B. pumilis and S. typhi confirmed a substantially high potential, as endorsed by their Molecular docking studies, of Ni2+ and Cu2+ complexes against each bacterial strain. Moreover, all compounds exhibited positive antioxidant activities, but have no antifungal potential except L”x. The current study demonstrates the usefulness of these novel transition metal complexes as possible potent antibacterial and antioxidant agents.
1. Introduction
In synthetic organic chemistry, the carbon-carbon bond formation is one of the essential tools to prepare novel compounds and hence is a challenging and fascinating field [1,2] for synthetic chemists. The primary requirement in such a synthesis is the production of a C-C bond at a particular site when more than one competitive position is available. Such selections tend to improve the procedure for manufacturing an organic network through inspiration, novelty, and logical contribution [3,4,5]. In this regard, the Morita-Baylis-Hillman (MBH) reaction has proven to be one of the best approaches due to its ease of execution and methodology [6]. MBH adducts are prepared via coupling between alkenes or alkynes at α-position and aldehydes in the presence of a suitable catalyst, most probably tertiary amines or phosphine. The reaction has been reported to be extremely useful in synthesizing a novel class of bioactive compounds with the potential to discover new cheaper, and more efficient drugs [7].
Moreover, this reaction congruently provides an approved atom economical approach for synthesizing medicinally useful multifunctional molecules. The aromatic aldehydes offer a unique class of compounds when introduced with multifunctional groups rather than the aliphatic ones [8,9]. The prepared adduct-type molecules have been utilized extensively as starting materials in preparing organic and inorganic molecules for various medicinal applications [10,11]. Numerous protocols have been adopted to achieve a pure high yield in a minimum period of time [12].
Moreover, MBH adduct derivatives bearing numerous functional groups such as acetate, ester, alcoholic, nitrates, and halogen in a single compound [7,13] have demonstrated prominent pharmacological activities [14,15,16].
As a part of our ongoing research on MBH adducts, we report herein the synthesis and characterization of a new multifunctional MBH adduct ligand Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate (L”x) and its corresponding metal complexes with Cr3+, Co3+, Ni2+, Mn2+, and Cu2+ ions. Nature has endowed metals with unique characteristics such as variable coordination modes, redox activity, catalysis, and reactivity towards organic substrates to make them suitable as essential cellular components to perform several functions in biochemical processes for living organisms. [17]. Additionally, the transition metal complexes based on the leading group of transition series have attracted greater attention due to the change in coordination state after coordination, regular geometry, formation of color compounds, binding variety, and hybridization [18]. Though all of these properties are not accessible in organic drug compounds because the carbon atom does not exceed the coordination number of four, owing to the presence of transition metal, the complex compounds retain these properties in their molecular structures [19]. Hence, it is considered that the coordination of organic-based biomolecules with transition metals should improve their biological properties [20].
Among natural sciences, synthetic compounds’ critical implications for treating diseases in the living system are one of the essential properties [21,22] in the field of bioinorganic chemistry. In the various steps of drug discovery and designing, the computational science of chemicals and their methodologies belong to a set of valuable scientific tools often practiced [23]. Furthermore, to better understand bioactive compounds’ behavior, quantum calculations and molecular mechanics are widely used in the modern pharmaceutical industry for chemical research [24]. Comparison of the molecular modeling data with the experimental characterization procedures helps expand the frontiers of medicinal and structural chemistry [25].
The objective of the present study is to discuss the ease of synthesis of a new MBH multifunctional adduct through a simple one-pot economical procedure, its characterization, and evaluation of the possibility of its participation in developing new MBH adduct-based drugs as a ligand or in the form of metal complexes.
2. Experimental
2.1. Materials and Methods
The current study used various A.R. grades solvents, such as n-hexane, methanol, and ethyl acetate. Additionally, Methyl Acrylate, NaCl, MgSO4 anhydrous, NaOH (Dae-Jung Kosdaq Reagents and Chemicals), DABCO (1,4-diazabicyclo [2.2.2]octane), NiCl2·H2O, CuCl2·2H2O, MnCl2·2H2O, CoCl3·6H2O, CrCl3·6H2O, and 4-Cyanobenzaldehyde, (Sigma Aldrich, St. Louis, MO, USA) were also used. A U.V. lamp (monitoring TLC card), digital hotplate with constant temperature and stirring system, Gallen Kamp melting point instrument (SPW, Ambala Cantt, Haryana, India), LAMBDA 1050+ U.V./Vis/NIR spectrophotometer, FTIR spectrophotometer Shimadzu Company (Kanda Nishiki-cho, Chiyoda-ku, Tokyo 101-8448, Japan) prestige-21 (4000–500 cm−1), AVANCE NEO 1H-NMR spectrophotometer (500 MHz in CDCl3), LECO CHNS 932 model microanalytical elemental analyzer instrument (Model: AV500, BRUKER, Bath, UK) (±0.3%), JEOL 600H-1 Mass spectrophotometer (ionization mode EI+) by Direct Probe Inlet and XRD Panalytical company (Lelyweg 1, Almelo, Netherland) X-pro serial No. DYH313 (Goniometer Radius mm = 240.00 and Dist. Focus-Diverg. Slit mm = 91.00) with Cu- anode material, Kα [Å] = 1.54060 and generator settings 30 mA, 40 kV) were the main instruments used during this research. Thermal stability of the complexes was studied by using a TA Instruments Trios V4.5.1.42498 SDT 650 Simultaneous Thermal Analyzer, (TA Instruments, New Castle, DE, USA). The analysis was carried out under an N2 atmosphere with the flow time of 1 mg min−1 at a heating rate of 20 °C min−1.
2.2. Synthesis of Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate MBH Adduct
Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate (L”x) was synthesized by mixing five equivalent methyl acrylate and one equivalent 4-cyano benzaldehyde in the presence of 0.65 equivalents of DABCO (catalyst) in a round-bottomed flask at room temperature and constant stirring [26]. The reaction mixture was checked after half-hour intervals using TLC plates under a UV lamp. The completion of the reaction took almost one day, as revealed by TLC results. A gummy crude material was obtained 24 h after an excess of the methyl acrylate was evaporated [11]. The glutinous crude was diluted by adding 15 mL each of ethyl acetate and brine solution. The complete organic and inorganic layers were separated in a separating funnel, and the mixture was collected thrice after running through the solvent. Through evaporation of EtOAc under reduced pressure, the organic phase was concentrated.
Furthermore, crude was subjected to column chromatography for further purification. The mobile phase system was constituted in a 10:90 to 20:70 (v/v) ratio of ethyl acetate and n-hexane. The prominent spot was collected as a pure product with an 85% yield, further confirmed through spectral analysis [2,7,27]. Scheme 1 here shows the whole setup for the preparation of MBH Adduct.
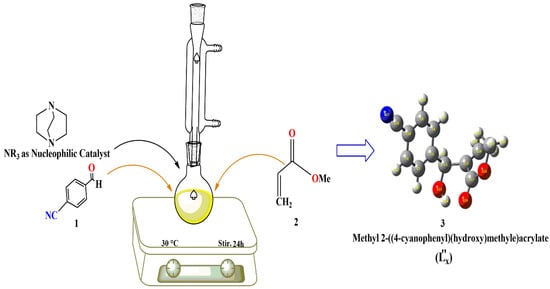
Scheme 1.
Synthesis of Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate MBH adduct (L”x).
Analytical Data for L”x (Compound 3)
M.P. 120.5–123.4 °C; 217.07 (g/mol) yield 87%. Color: Milky white. λ (nm): 269. ε (mol−1cm−1L): 762.63. FT-IR (KBr, ῡ=cm−1): 3437 alcoholic (-OH), 2954 (=C-H), 3000 (sp3 C-H stretch), 2229 (-C≡N), 1718 (ester C=O), 1629 (aromatic C=C), 1600, 1053 (-OH bend). 1H NMR (500MHz, CDCl3-D-1): δ 3.203 (s, 1H, OH), 3.693 (s, 3H, OCH3), 5.488 (s, 1H, CH2), 5.804 (s, 1H, CH2), 6.307 (s, 1H, CH), 7.240 (m, 4H, H-2, H-3, H-5, H-6). Mass spectrum [m/z (%)]: 216 (52) M+, 185 (48), 184 (15), 158 (17), 157 (80), 156 (11), 142 (14), 140 (19), 130 (100), 128 (10), 102 (17), 104 (23), 77 (12), 55 (14). Anal. Calculated for C12H11NO3 (%): 217.07 (100), Found (%):218.08 (52.2). Elemental Anal. Calc. (%): C, 66.35; H, 5.10; N, 6.45, Found: C, 66.36; H, 4.93; N, 6.48
2.3. Synthesis of Metal Complexes of L”x
About 20 mL of 0.042M solution of L”x in hot ethanol and 0.0201M transition metal salt solutions of CoCl3·6H2O, NiCl2·6H2O, MnCl2·2H2O, CrCl3·6H2O and CuCl2·2H2O in distilled water were prepared separately. The metal ions were added dropwise to the L”x solution with continuous stirring. The pH was maintained at ten by adding a few drops of NaOH solution, and the mixture was refluxed at 60–70 °C for about 5–6 h until a solid mass was formed. The progress of the reaction was continuously checked via TLC under a UV lamp [28]. After the completion of the response, as indicated by a single spot under the UV lamp, the cooled product was collected employing vacuum filtration. Though the single crystal formation of these complexes remained unsuccessful despite many attempts of different procedures for crystallization, we collected the product in an amorphous state for subsequent analyses. The product was washed with water and ethanol thrice and dried in a desiccator [29,30]. Each metal complex was characterized through UV- Visible, FTIR, and percentage analysis to determine the molecular and structural formula. Computational studies geometrically optimized the Synthesized compounds. Further, the crystal class of these novel crystal compounds of metal complexes was obtained through XRD powder diffraction. Scheme 2 outlines the synthesis of these metal complexes of L”x.
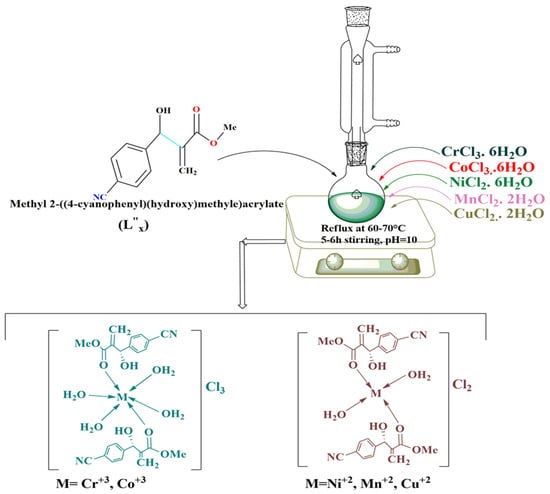
Scheme 2.
Synthesis of the L”x Tansition Metal Complexes.
2.4. Analytical Data of Transition Metal Complexes
2.4.1. Cr3+L’’x Complex (Compound 4)
M.P: >300 °C. 558.50 (g/mol): Color: Sky blue. λmax (nm): 371.5. ε (mol−1cm−1L): 238.09. FT-IR (KBr, ῡ=cm−1): 3450–3380 alcoholic (-OH), 2850 (=C-H), 2900–2800 (sp3 C-H stretch), 2374 (-C≡N), 1608 (aromatic C=C), 850 (M-OH2), 607 (M-O). Mass spectrum [m/z (%)], for C24H30CrN2O103+M+: Anal. Calculated for (%):186.04 (100.0), Found (%): 185.47 (83.3). Elemental Anal. Calc. (%): C, 51.61; H, 5.41; N, 5.02, Found: C, 51.58; H, 4.89; N, 5.10.
2.4.2. Co3+L”x Complex (Compound 5)
M.P: >300 °C. 565.43 (g/mol): Color: Teal blue. λmax (nm): 361. ε (mol−1cm−1L): 224.23. FT-IR (KBr, ῡ=cm−1): 3500–3350 alcoholic (-OH), 2900 (=C-H), 2850 (sp3 C-H stretch), 2210 (-C≡N), 1716 small (ester C=O) 1635 (aromatic C=C), 835 (M-OH2), 653 (M-O). Mass spectrum [m/z (%)], for C24H30CoN2O103+M+ (%): 188.37 (100), Found (%): 188.39 (26.6). Elemental Anal. Calc. (%): C, 50.98; H, 5.35; N, 4.95, Found: C, 50.80; H, 5.54; N, 4.78.
2.4.3. Ni2+L”x Complex (Compound 6)
M.P: >300 °C. 529.16 (g/mol): Color: Cadet blue. λmax (nm): 360. ε (mol−1cm−1L): 309.81. FT-IR (KBr, ῡ=cm−1): 3550–3450 alcoholic (-OH), 2950 (=C-H), 2850 (sp3 C-H stretch), 1640 (ester C=O), 1625 (aromatic C=C), 605 (M-O). Mass spectrum [m/z (%)] for C24H26N2NiO82+ M+ (%): 264.05 (100.0), Found (%): 265.10 (38.7). Elemental Anal. Calc. (%): C, 54.47; H, 4.95; N, 5.29, Found: 54.50; H, 4.87; N, 4.94.
2.4.4. Mn2+L”x Complex (Compound 7)
M.P: >300 °C. 525.41 (g/mol): Color: Wine red. λmax (nm): 372. ε (mol−1cm−1L): 168.57. FT-IR (KBr, ῡ=cm−1): 3450–3350 alcoholic (-OH), 2954 (=C-H), 2875 (sp3 C-H stretch), 2229 min (-C≡N), 1722 (ester C=O), 1625 (aromatic C=C), 613 (M-O). Mass spectrum [m/z (%)], for C24H26MnN2O82+ M+ (%): 262.55 (100.0), Found (%): 263.10 (26.6). Elemental Anal. Calc. (%): C, 54.86; H, 4.99; N, 5.33, Found: C, 54.45; H, 4.41; N, 5.03.
2.4.5. Cu2+L”x Complex (Compound 8)
M.P: >300 °C. 533.06 (g/mol): Color: Hunter green. λmax (nm): 371. ε (mol−1cm−1L): 120.11. FT-IR (KBr, ῡ=cm−1): 3500–3470 alcoholic (-OH), 2900 (=C-H), 2875 (sp3 C-H stretch), 1673 (aromatic C=C), 603 (M-O). Mass spectrum [m/z (%)], for [C24H26CuN2O8]2+ M+, Calc. (%): 266.55 (100.0), Found (%): 265.87 (49.3). Elemental Anal. Calc. (%): C, 53.98; H, 4.91; N, 5.25, Found: C, 54.02; H, 4.95; N, 5.31.
2.5. Antibacterial Assay for Compounds 3–8
The agar disc diffusion method [31,32] was used to determine the antibacterial activities of the compounds 3–8 against four bacterial strains Staphylococcus aureus, Escherichia coli (Gram-negative), and Bacillus pumilis, Salmonella typhi (Gram-positive). A sterile membrane syringe filter of pore size 0.45 µm (Millipore) was used for extraction. To 100 mL antibiotic agar No.11 (45 °C), 1mL of culture suspension (25 T%, 530 nm) of each strain was added and intermingled. 25mL of each agar medium was poured into separate Petri dishes (20 × 100 mm) and solidified. Four holes were made after solidifying by expending a sterile borer of 8mm diameter with (6 mm inner diameter). Each extract (100 µL) was poured into the marked holes and incubated at 37 °C for 24 h. These experiments were performed under a severe aseptic environment in triplicate. The inhibition (mm) of the incubation zone produced via individual extract demonstrated antibacterial activity. Finally, the % inhibition was computed. The standard antibiotic Gentamycin (0.3%) was used as a standard [33].
2.6. Determination of DPPH Radical Scavenging Activity of Compounds 3–8
The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging method has been extensively used to determine free extreme scavenging action [34]. The same was applied in our work with some modifications. 0.3 mM solution of DPPH was prepared in methanol. 10μL of varying concentrations (25–100 mg) of each compound 3–8 was mixed with 1.0 mL of DPPH solution. At 37 °C, the reaction mixture was incubated for about half an hour. The absorbance was measured at 515 nm. In contrast to methanol-treated control percent, radical scavenging activity was determined when ascorbic acid was used as a standard for sample analysis. The antioxidant activity was determined [9,35].
2.7. Antifungal Assay for Compounds 3–8
The antifungal activity of the compounds 3–8 was measured by well-known quantitative agar well diffusion method [36,37]. About 1 mL of the compound suspension was mixed with 100 mL of 4.0% Sabouraud Dextrose Agar (SDA) and transferred into plate. Wells of 8 mm were prepared and 100µL of test compounds were poured to positive control into respective wells after the solid mass have been prepared. The test plates were left for 5 days at room temperature and then incubated for further 5 days at same temperature. Zones of inhibitions were measured with reference to the plant extract of 100 µL to be assayed and Ketoconazole 1.0 mg mL−1 was used for instance positive control [36,38].
2.8. Computational Studies
All the optimization and frequency calculations were performed with the Gaussian 16W [39] program package on an ACPI x64 Core i7 laptop with a 3.0 GHz processor and 16 GB Ram. The molecular structure of the ligand (L”x) was optimized by using the DFT B3LYP method and 6-311++ G (d,p) basis set [40,41,42]. All metal complexes’ molecular structures were optimized using the semi-empirical PM6 method [43], which was justified by the absence of imaginary frequency. The assignment of the calculated wavenumbers was aided by the animation option of Gauss View 6.0 graphical interface for the Gaussian program, which gives a visual presentation of the shape of the vibrational modes. Furthermore, for molecular docking, structures of ligand and complexes were taken from their optimized geometries calculated by Gaussian. The crystal structures of Escherichia coli, Staphylococcus aureus, and Salmonella typhi proteins were fetched from the Protein data bank (PDB) using IDs; 1AJ6 [44], 4UR0 [45], and 1WVG [46], respectively. Polar hydrogen atoms and Kollman united atom charges were added using AutoDock Tools [47]. Docking calculations were performed with AutoDock Vina using the Lamarckian Genetic Algorithm [48]. A grid box size of 31 × 37 × 35 Å points for E.coli, 35 × 30 × 38 Å points for Staphylococcus aureus, and 40 × 35 × 37 Å points for Salmonella typhi with a grid spacing of 0.375 Å was generated using AutoGrid. The coordinates of active site for E.coli are x = 60.29, y = −8.53, z = 36.67, for Staphylococcus aureus are x = 2.51, y = −54.72, z = −13.36, and for Salmonella typhi are x = 63.95, y = 21.80, z = 49.35, respectively.
3. Results and Discussion
3.1. Physical Characteristics and Elemental Analysis
The MBH adduct ligand (L”x) and its corresponding metal complexes were analyzed by physical as well as comparative elemental analysis (Table 1). It has already been stated that pure L”x is a viscous liquid. In contrast, its corresponding metal complexes are amorphous solids (compounds 4–8). The prepared complexes (compounds 4–8) are all colored compounds, stable at room temperature, insoluble in water, and most organic solvents, such as acetone, DMF, methanol, and ethanol, but soluble to a lesser extent in DMSO. Several oxygen atom-containing organic ligands prefer an essential medium for complexation when more than one chelation site is available in a compound for coordination [49,50]. Under such conditions, hydrated hydroxide can be formed, which can further participate in complex formation if the incoming ligand is substantial [51]. The physical analysis, e.g., color, melting point, and molecular formula, are preliminary examination-based tests to observe the formation of new species. For example, manganese forms a stable complex in the essential medium of dark red to brown shades. Color change of transition metals after the formation of the complexes is meaningful property as it can be a consequence of either charge transfer or changes in the d-d transitions of the metal ions [52]. The yield was found to be in the range of 60–85%.

Table 1.
Physical Properties and analytical data of compounds 3–8.
The elemental analysis (%) results confirmed that the counter ions might not contain carbon, hydrogen, and nitrogen atoms; if they are present, then there will be higher percentages of these elements rather than observed [49]. The results have also confirmed the formation of complexes of L”x with desired metal ions (Cr3+, Co3+, Ni2+, Cu2+, Mn2+) in a molar ratio of 2:1 (L”x: M). The molecular formulas of complexes were confirmed by computational data obtained through the semi-empirical PM6 method. These transition metal complexes with organic adduct-based ligands have also been studied and reported in the literature [23,53,54,55]. The physical data, along with the estimated elemental analysis, have been summarized in Table 1.
3.2. Electronic Spectroscopy
The UV-Vis spectra of compounds 3–8 have provided knowledge about the changes that occurred upon complex formation, as reported in Table 2. The bands at 251 and 265 nm in the UV spectrum of compound 3 in DMSO are assigned to the π→π* transitions. The spectrum has also presented a half curve at 269 nm and a strong band at 272 nm due to the n→π* evolution of the unshared pairs of electrons found in the cyno-group of the nitrogen at the para position of the aromatic ring (Table 2). The n→π* transition is also a characteristic of the aldehyde groups in a compound [56,57]. It has been observed that the π→π* and n→π* transitions were shifted after the metal ions were coordinated to L”x. This change supports that the oxygen atoms of the esoteric carbonyl group in L”x contribute to the coordination towards the central transition metal ions. By observing changes in the electronic spectrum of L”x upon complex formation with the metal ions, it can be concluded that compounds 4–8 show a characteristic band in the region of 360–372 nm that is absent in compound 3. These bands have shallow values of molar absorptivity coefficients and can be assigned to d-d transitions in the metal complexes.

Table 2.
Investigated UV-Vis data for the Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate MBH adduct and and its formed complexes.
3.3. Theoratical and Experimental FT-IR of Ligand L”x (Compound 3)
The FTIR spectra of MBH Adduct (L”x) and its metal complexes were recorded between 4000–500 cm−1 using a KBr disk, and the data is presented in Table 3. The FTIR spectral data of compound 3 has shown the characteristics -OH broad peak at 3437 cm−1, a prominent stretch of =C-H at 2954 cm−1 and sp3 C-H stretch at 3000 cm−1 [58], and the band observed at 1718 cm−1 is due to ester carbonyl vibration. Furthermore, the ῡ of C=C aromatic, -CH3, C-O, and C-CN were assigned as 1629 cm−1, 1440 cm−1, 1247 cm−1, and 2229 cm−1, respectively (Figure 1a). Moreover, in theoretical IR analysis, it was observed that -OH peak is somewhat different in shape, may be due to some content of moisture present in the sample during the examination, from the observed spectrum however it retains its position. Furthermore, the rest of the fragments above (Figure 1b) show a good agreement of frequencies in both the experimental and theoretical spectrum. A slight change might be observed because of the theoretical calculations of the range corresponding to the isolated functional groups in the gaseous state. At the same time, the experimental results belong to the molecules in a solid state [59,60]. The close resemblance of DFT-based IR and calculated ῡ results have made us suggest the molecular structure of Methyl 2-((4-cyanophenyl)(hydroxy)methyl)acrylate MBH Adduct (L”x) as presented in Figure 2a.

Table 3.
Characteristic FTIR bands of the MBH Adduct (L”x) and its metal complexes (cm−1) ῡ.
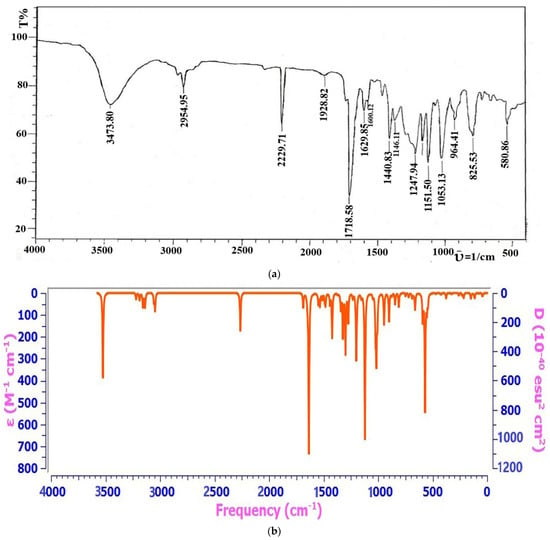
Figure 1.
(a) Experimental FT-IR Spectrum of Compound 3. (b) Theoretical FT-IR Spectrum of Compound 3.
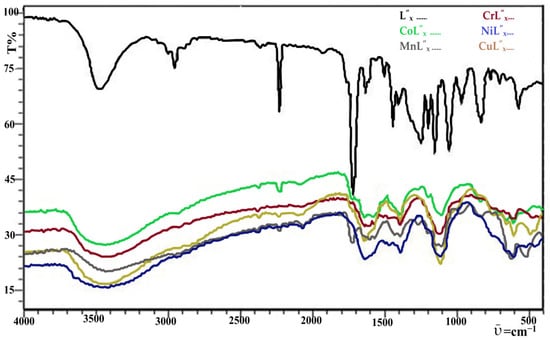
Figure 2.
Experimental FT-IR Spectrum of Transition Metals Complexes with MBH Adduct (L”x).
3.4. Experimental FT-IR Spectroscopyof Complexes 4–8
A detailed explanation of experimental FT-IR spectroscopy results has been summarized in Table 3. In experimental FT-IR, a characteristic band of the –OH functional group was observed in the region of 3350–3550 cm−1 in the IR spectra of the compounds 4–8, proposing its nonparticipation in coordinate bond formation towards all metal ions in these compounds. Furthermore, the broadness of this peak was increased after coordination, clearly seen in Figure 2. This may either be due to coordinated water molecules in the complexes or the moisture in the sample [23,61,62]. Common and robust evidence regarding the coordination of Ligand (L” x) towards the central metal ion is the disappearance of the C=O peak, which originated at 1680–1720 cm−1 (Figure 2) in both theoretical and experimental spectrum (Figure 1a,b) of the compound 3. The results in Table 3 revealed the disappearance of the characteristic peak of the C=O group from compounds 4–8 except compound 6 (Ni2+L” x), where the mountain has merely shifted to 1640 cm−1. The disappearance of the peak endorses the formation of a coordination bond through the oxygen of the C=O group with the central metal ion, which eventually diminishes the strength of the carbonyl group in complexes after bonding. The literature also supports such a way of coordination [63,64]. In compound 6, we can observe that the peak intensity is also very low rather than the more intense appearing in the Ligand [61]. This significant change is good support for justifying that the coordination of Ligand towards the Ni2+ metal ions is from this definite functional group (Figure 2).
Table 3 also shows the shift of Ligand’s ester (C-O) group from 1247 cm−1 to 1390 cm−1 in compound 4 may be attributable to the environmental effect after complex formation. Moreover, the additional peaks have also been observed at 607 and 550 cm−1 and assigned to the Cr-O and Cr-OH2, respectively [61]. Further, it is inferred that the C=C group doesn’t participate in complexation. Because there were no significant changes in the IR frequency of the C=C double bond seen upon complex formation, based on these observations, the molecular and structural formulae of the compound 4 were deduced as [Cr(L”x)2(H2O)4]·3Cl−. Both compounds 4 and 5 have shown peak shifting with low intensity of the Ligand’s cyano (-C≡N) group to a region of 2374–2210 cm−1. The little shift and peak intensity difference might be due to the environmental effect [65] and ligand coordination [66]. The involvement of the carbonyl group (1718 cm−1) (L”x) in coordinate bond formation has been proved and already reported in the literature [67], thus confirming the coordination of this Ligand’s carbonyl site towards the Co+3 metal ions. Table 3 shows an exceptional signal attributable to Co-O appearing at 653 cm−1 and another noticeable difference in Co-OH2 frequency from 535 cm−1. Based on comprehensive information, the molecular formula [Co(L”x)2(H2O)4]Cl−3 was established for compound 5.
The detailed inspection of the data based on the FT-IR analysis (Table 3) has led us to elucidate the structural formula for compounds 6–8 and presented peaks for all the conspicuous functional groups, which revealed the complex formation along with some informative shifts. Such as an extremely weak band, almost negligible at 1640 cm−1, corresponds to the C=O group, which was comparatively at a lower frequency than its precursor C=O absorption (1718 cm−1) in compound 6. In addition, the –OH and =CH groups exhibited peaks at 3450–3350 cm−1 (broad) and 2950 cm−1, respectively. Furthermore, we have also observed peaks for the C=C bond at 1625 cm−1 with no noticeable shift, simultaneously signifying that C=C does not participate in coordination bond formation. Further, in compound 7, we have also observed a broad peak at 3450–3350 cm−1 of the same (-OH) functional group. The difference from precursor ligand –OH is may due to the presence of moisture in the sample, which has provided interference during the recording of the spectrum. Similarly, the remaining functional groups show a slight change in the calculated values, as is evident from their sharp pointed peaks at exact frequencies. FTIR results for compound 8 have revealed the presence of the –OH functional group by a height almost at a similar position (3500–3470 cm−1) as that of the ligand forerunner. Around about similar results were gained of the complex for the presence of sp2 and sp3 C-H bonds, i.e., their peaks were observed at 2900, 2875 cm−1, respectively. The ester carbonyl absorption has disappeared as compared to that of the ligand carbonyl (1718 cm−1), suggesting its participation in complex formation. No remarkable change was observed in the frequency due to aliphatic and aromatic C=C double bonds not contributing to complex formation. These assumptions were further supported by the appearance of IR peaks at 605 cm−1 and 520 cm−1 in Compound 6 were assigned to Ni-O and Ni-OH2 groups, respectively while in Compound 7 revealed the ῡ at 603 cm−1, and 527 cm−1 due to the presence of Cu-O and Cu-OH2 bond (Table 3, Figure 2). Upon physical, analytical and spectral results basis the prepared complexes (compounds 6–8) were assigned the structure presented above in Scheme 2 formulated as [M(L”x)2(H2O)2]Cl−2, where, M= Ni2+, Mn2+, Cu2+.
3.5. Mass Spectroscopy
The molecular weight of the Compound 3 was determined through mass spectroscopy based. The results have shown m/z 130, 70.2%, with a total of 349 ions. The finest ions with the lowest % are 77 and appear because of the phenyl group (C6H6+) at the lowest m/e (12%) comparatively. A fragment detected with a mass of 104 matches with C6H6CN+ at 23%, while the ionic chip (130) next to this ion is present in the highest m/z, i.e., 100%. This molecular ion fragment is the precise result of the molecular weight of C7H8OCN+. Further, it was observed in the mass spectrum that the C9H11OCN+ with the molar mass 157 appears at 80% m/e. Additionally, the molecular ion fragments C11H13O2CN+ (185) and C11H13O3CN+ (216) were also observed at 56% and 51%, respectively. Hence, these results also confirm the purity of compound 3 obtained through this technique [15,68,69].
Experimentally, mass spectroscopy of compound 4–8 could not be possible due to the insolubility of these metal complexes in any suitable solvent other than pure DMSO [70,71]. However, the estimated Mass spectrum of the Cr3+ complex showed a molecular ion peak M+ at m/z 185.47 that is equivalent to its molecular mass, while that of Co3+ at m/z 188.39 is attributable to [M+]. Ni2+, Mn2+, and Cu2+ complexes are suggested to have two water molecules and thereby display [M+] fragments at m/z 265.10, 265.87, and 263.10, respectively. Furthermore, [M+] peaks are in good agreement with the structures proposed (Scheme 2) by estimated elemental analysis, spectral data, and molar stoichiometry [58,72,73].
3.6. 1H NMR Spectroscopy
1H NMR spectrum of ligand L”x (compound 3) was observed in CDCl3 solvent at 500MHz by keeping TMS as internal reference. The data obtained in the form of spectrum showed five signals as singlet at 3.203, 3.693, 5.488, 5.804, and 6.307 ppm, because of the -OH, -OCH3, =CH2, =CH2, and –CH protons, respectively. However, only one signal of multiplet, which is certainly due to the aromatic protons at 7.240 ppm was also observed. These aromatic protons were located at the same chemical shift, because of their identical chemical environment [58].
3.7. Molecular Modeling
DFT B3LYP method and 6-311++ G (d,p) basis set were used for geometrical optimization and frequency calculation of the ligand (L”x). Consequently, the FT-IR spectrum of the synthesized ligand was computed theoretically and found in good agreement with the experimental FT-IR range. The ligand (L”x) was subsequently used in the synthesis of five novel transition metal complexes (compounds 4–8) (Scheme 2). These metal complexes were further analyzed by physical, analytical, and spectral analyses to confirm their proposed structures. The synthesized metal complexes’ best possible geometry has been submitted using the semi-empirical PM6 method. Geometrical optimization of metal complexes demonstrates their proposed designs best fit the experimental findings. For example, the Cr3+ complex (compound 4) is optimized in tetrahedral geometry with high spin and quartet multiplicity. The Co3+ complex (compound 5) is found in distorted octahedral geometry with low spin and singlet multiplicity. Mn2+ and Cu2+ complexes (compounds 6 and 7) attain identical spin (down) as well as geometries (tetrahedral). The assortment adjusted in theoretical calculations is sextet for Mn2+ (d5), while doublet is found for Cu2+ (d9). However, Ni2+ (d8) complex (compound 8) is observed in a low spin with singlet multiplicity and square planner geometry. This structural analysis for geometry optimization of metal complexes is well presented in the literature [74,75,76]. Computational studies on the synthesized ligand and its metal complexes provided reasonable geometrical shapes, which correlate well with the experimental results. The results of these optimized structures are presented in Figure 3.
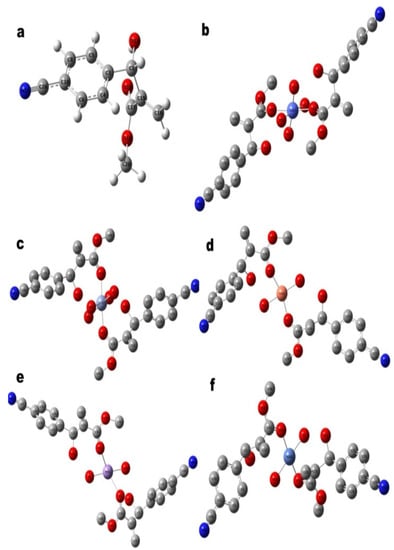
Figure 3.
Theoretically Optimized Geometries of (a) MBHA (L”x), and its Corresponding Complexes, (b) CoL”x, (c) CrL”x, (d) CuL”x, (e) MnL”x and (f) NiL”x. The results have confirmed that each metal is coordinated by two ligand molecules. Cr+3, Co+3 are octahedral and Ni+2 is square planner, whereas, Mn+2 and Cu+2 are tetrahedral as per optimized geometrical structures. Hydrogen atoms are removed from metal complexes for geometry clarity.
3.8. X-ray Diffraction
Powder X-ray diffraction was performed to confirm the crystal structure of transition metal complexes with L”x. Single crystal XRD of these synthesized compounds was not possible because the crystals of the complexes could not be grown despite many attempts to crystallize a single crystal perfect for the analysis. However, powder XRD has also proved to be a handy tool for determining crystal class whenever we have the product in the crystalline form [67,77,78]. The patterns of these diffractograms for compounds 4–8 are given in Figure 4. The ways provide an idea of the coordination of oxygen atoms from the ligand to the metal ions. Furthermore, these patterns also indicate the crystalline nature of all the complexes [58]. It can be observed that mainly the remaining diffractograms of all these compounds differ due to their different crystalline structures [79]. This difference might be due to the coordinated water molecules in the coordination sphere [80] that give an appropriate and unique crystal class [78]. Their subsequent XRD results, such as; calculated density, specified volume, radius intensity ratio, and lattice parameters (a, b, c, α, β, γ) with crystal class,, have been given in Table 4.
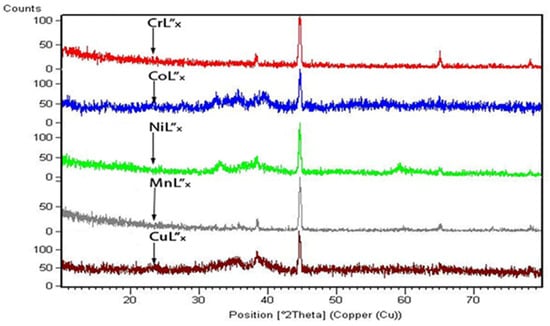
Figure 4.
X-ray Diffraction Patterns of Compounds 4–8.

Table 4.
Crystallographic data and observed refinement parameters for compounds 4–8.
Powder X-ray diffraction was performed to confirm the crystal structure of transition metal complexes with L”x. Single crystal XRD of these synthesized compounds was not possible because the crystals of the complexes could not be grown despite many attempts to crystallize a single crystal perfect for the analysis. However, powder XRD has also proved to be a handy tool for determining crystal class whenever we have the product in the crystalline form [67,77,78]. The patterns of these diffractograms for compounds 4–8 are given in Figure 4. The ways provide an idea of the coordination of oxygen atoms from the ligand to the metal ions. Furthermore, these patterns also indicate the crystalline nature of all the complexes [58]. It can be observed that mainly the remaining diffractograms of all these compounds differ due to their different crystalline structures [79]. This difference might be due to the coordinated water molecules in the coordination sphere [80] that give an appropriate and unique crystal class [78]. Their subsequent XRD results, such as; calculated density, specified volume, radius intensity ratio, and lattice parameters (a, b, c, α, β, γ) with crystal class, have been given in Table 4. It is essential that the complex Mn2+L”x (compound 7) was found to have orthorhombic crystalline nature, having space group Pnma (62) with a Z value of 4. The indexing related [67,81] to their structure elucidation, peak intensities, and diffraction angles (2θ) is given in Table 5. Compound 8 belongs to the monoclinic crystal class, which provides it with a unique identity among these compounds. The other important properties, including the space group, unit cell number, and radius intensity ratio, depending upon the crystal class [80,81]. These findings, together with the results from computational modeling, have strengthened our conclusions on the energy and mass of our metal complexes.

Table 5.
Crystal lattice Data and Summary of Data Collection and Refinement for Compounds 4–8.
Moreover, the peaks of the highest intensities of all complexes, along with the values of miller indices, d-spacing, and position (2θ), are given in Table 5. The grain size of amorphous compounds is also significant to confirm their stability. XRD- powder diffraction is a method used for this purpose. Scherer’s equation can calculate the size of the grain. This equation is formulated as:
In Formula (1), λ shows X-ray wavelength, θ shows Bragg’s diffraction angle, m is a constant value (0.94), and β shows full width of half maximum intensity (FWHMI). In this research the average grain size (S) in nm of a complex was calculated from the highest peak intensity (Table 5).
3.9. Thermal Analysis
Thermal stability of Co and Ni complexes (compounds 5 and 6) was studied by TGA. It is an essential technique that has been used to determine the compound’s stability [82]. TGA measurements were carried out from room temperature to 1000 °C and obtained curves for the metal complexes are illustrated in Figure 5A,B). Figure 5A presents three decomposition steps for compound 5. The first miniature weight loss with respect to temprature 31.30 °C probably shows the removal of any moisture present in the sample. After 17 min at the temperature of about 355 °C, 31.46% weight loss was observed (calc. 32.5%), which might be due to the water molecules, OCH3, -CH2 as well as -OH fragments from the ligand (L”x). The residual complex was stable upto the temperature 614.20 °C and then decomposed with weight loss 37.11% (calc. 37.1%). This degradation was a little larger than the previous one, because of the removal of cyanide from the coordinated ligand molecules (Figure 5A). Upon further heating, most of the part of the ligand in the form of gases was evaporated and the metal oxide was left as final residue 56.98% (calc. 57.12%) (Figure 5A). Almost similar changes have been observed in Ni L”x (compound 6) TGA curve (Figure 5B). Howover, the stability of intermediate residue is less than compound 5 and the complex gradually decomposes completely at comparatively low temperature. The loss of water molecules and the organic moities results in 26.5% (calc. 27.7%) residue at about 200 °C. The second weight loss 33.6% (32.30%) was observed at 320 °C, again due to the first weightt loss along with the cyanide group from both of the ligands. In last step, the final residue left over 55.7% (calc. 56.4%), because of the Ni2+ oxides. The stabilities of other complexes of these transition metals are known and support our findings too [83,84].
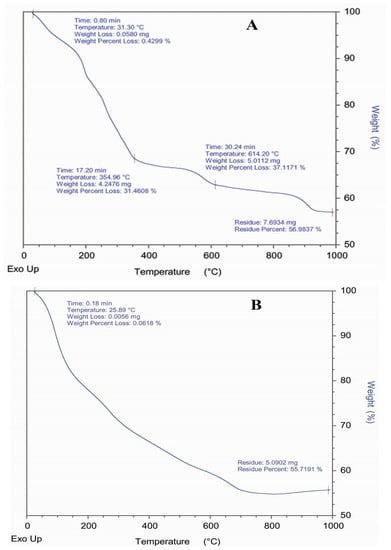
Figure 5.
(A) Thermo Gravimetric Analysis (TGA) Curve of Compound 5. (B) Thermo Gravimetric Analysis (TGA) Curve of Compound 6.
3.10. Antibacterial Assay
The antibacterial assay of compounds 3–8 was performed using the agar disc diffusion method [85] against four bacterial strains (S. aureus, E. coli, B. pupils, and S. typhi) employing Gentamycin as standard (Table 6). The following relation has been used to calculate the activity index of the tested compounds.

Table 6.
Antibacterial Activities of Compound 3–8.
L”x (compound 3), MnL”x (compound 7), and CuL”x (compound 8) were found incompetent against the S. aureus strain. At the same time, it’s CoL”x and NiL”x (compounds 5 and 6) metal complexes presented significant potential, i.e., 57.14% and 71.4%, respectively. This change has been attributed to the coordination of ligands with the metal ions, which enhances the capability to fight against the bacterial pathogenic strain [58,86]. Moreover, compounds 7 & 8 were inactive against all the music selected for this study. All other compounds show at least some activity against these strains with variable potential in each case. It is interesting to notice that compound 5 has a higher inhibition potency toward E. coli as compared to L”x (compound 3), and NiL”x complex (compound 6) has also exhibited fairly well (Figure 6).
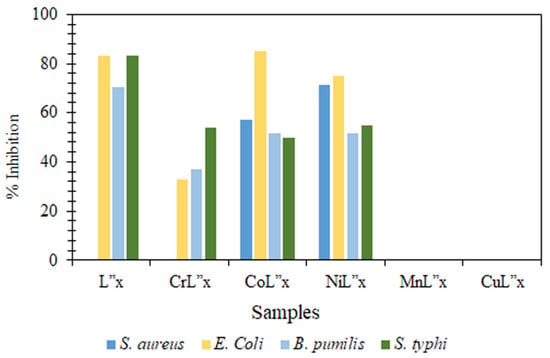
Figure 6.
Comparative Histograms Showing Inhibition Zone in Antibacterial Assays of Compounds 3–8.
3.11. Antioxidant Activity
The synthesized compounds were tested to determine their efficiency as an antioxidant comparable to ascorbic acid as standard by using DPPH (α,α-diphenyl-β-picrylhydrazyl) free radical scavenging method [87]. The potential compounds were mixed with DPPH solution, and absorbance was noted after a definite period. However, with the progression and superiority in instrumental methods, the process has been modified, but the fundamental aspects remain similar [88,89]. The absorbance at 515 nm was measured by spectrophotometer, and radical scavenging activity (%) was determined compared to the methanol-treated control according to the following relation. Where ‘E’ is the absorbance of the control (extreme). The results of the study have been presented graphically in Figure 7.
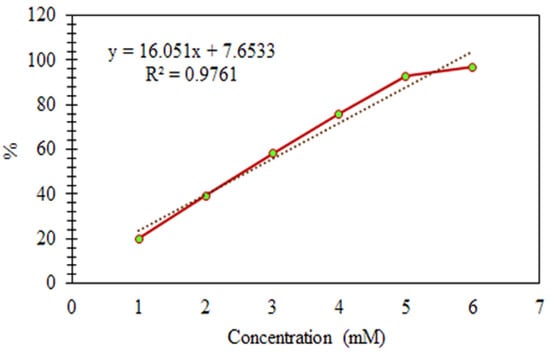
Figure 7.
Antioxidant Activities of the Standard (Ascorbic Acid).
The results of compounds 3–8 have been manipulated in Figure 8. Compound 3 contains an alcoholic, an ester functional group, and an aromatic ring in its structure. These types of compounds are known to be exceptionally active in exhibiting antioxidant potential [9,90]. Moreover, the results also signify that compound 3 has the highest antioxidant activity compared to its novel transition metal complexes synthesized here.
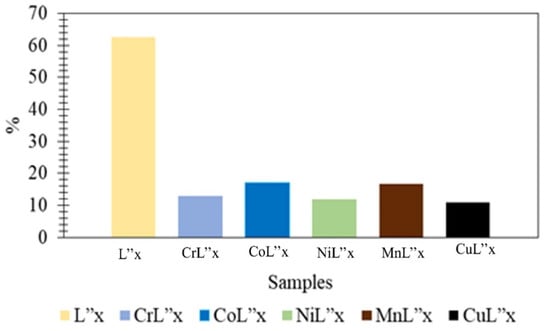
Figure 8.
Graphical Representation of Antioxidant Activities of Compounds 3–8.
3.12. Antifungal Activity
The fungal strains A. niger, A. flavus, A. fumigatus, and C. albicans were selected to determine the antifungal potential of compounds 3–8. The quantitative agar well diffusion method was employed [7] for this purpose, and the results have been reported in Figure 9. Out of these compounds, only compound 3 presented antifungal activity. In contrast, the other compounds were found inactive. The highest activity was achieved against A. fumigatus (88.6%). For C. albicans, the movement found was 60%, whereas it was 61.90% for A. niger. The remaining fungal strains have presented comparatively low percentages.
where,
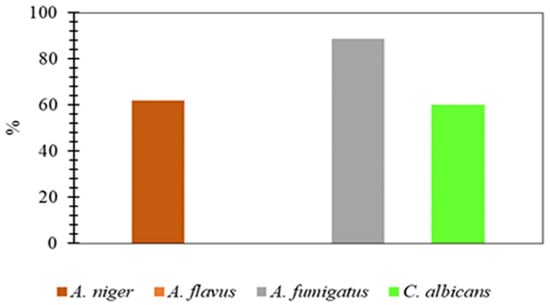
Figure 9.
Antifungal Activity of Compound 3 Against Some Fungi.
- ΔD = Dc − Dt
- Dc = average diameter of fungal colony in negative control
- Dt = average diameter of fungal colony in experimental plates
3.13. Docking Studies
To check the binding ability of the synthesized ligand and its complexes towards certain antibacterial enzymes, molecular docking studies were performed using the ligand-bound crystal structures of E. coli, S. aureus, and S. typhi from PDB. Results from docking studies agree with the obtained antibacterial activities of ligand and metal complexes. Figure 10A–C panels are related to the synthesized MBH adduct ligand (L”x), CoL”x, and NiL”x binding with the ATP active site of E. coli DNA gyrase B. The ATPase binding site residues of E. coli DNA gyrase B from PDB-ID: 1AJ6 are N46, D73, R76, V120, R136, and T165 and are found to be occupied with the well-known antibacterial compound novobiocin. Ligand (L”x), CoL”x, and NiL”x are found well settled inside the ATPase binding site of E. coli DNA gyrase B. Figure 10D is related to the NiL”x binding with S. aureus. The ATPase binding site residues of S. aureus DNA-gyrase from PDB-ID: 4UR0 are S55, A64, N65, V79, D89, T164, and T173 and they were found engaged with novobiocin. NiL”x is found bound inside the ATPase binding site of S. aureus DNA gyrase B. Figure 10E panel is related to the MBH adduct ligand (L”x) binding with S. typhi. The crystal structure presents the CDP-D-glucose 4,6-dehydratase from S. typhi complexed with CDP-D-xylose. The substrate binding site residues of S. typhi dehydratase from PDB-ID: 1WVG are S134, D135, K136, Y159, N197, and R208. The ligand (L”x) was found active and occupied the xylose binding site of S. typhi dehydratase.
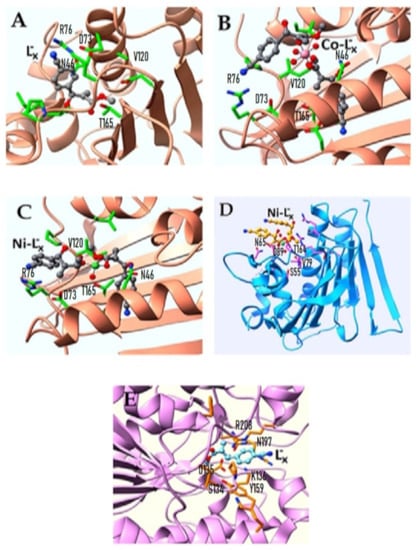
Figure 10.
The Binding Conformations of Ligand and Corresponding Metal Complexes Active against (A–C) E. coli, (D) S. aureaus and (E) S. typhi.
4. Conclusions
A well-recognized MBH (Morita-Baylis-Hillman) reaction method has been utilized in the current study to prepare an aromatic adduct, methyl 2-((4-cyanophenyl)(hydroxy)methyl) acrylate (compound 3) presented in Scheme 1. The prepared ligand was characterized by physical, analytical, and spectral analysis, and its molecular and structural formulas were determined. Moreover, the ligand’s geometrical optimization was achieved using DFT B3LYP method and 6-311++ G (d,p) basis set. FT-IR spectrum of the synthesized ligand (L”x) was resolved computationally and found in good agreement with the observed FT-IR spectrum. The five novel transition metal complexes (Cr3+, Co3+, Ni2+, Mn2+, Cu2+) were synthesized successfully (Scheme 2) by utilizing this bioactive adduct subsequently (compounds 4–8). These metal complexes were further analyzed by physical, analytical, and spectral analyses to confirm their proposed structures. Semi-empirical PM6 optimization and molecular docking studies of metal complexes provided their reasonable geometric shapes and binding conformations well correlated with our experimental findings. The results have suggested that compounds 4 and 5 have four water molecules in respective complexes (Scheme 2). Whereas compounds 6–8 have two water molecules in their structures. XRD-Powder diffraction analysis confirmed that all the synthesized metal complexes are crystalline. Additionally, chromium and cobalt metal complexes (compounds 4 and 5) with the same oxidation state (3+) were found in cubic crystalline nature. Nickel, Manganese, and Copper (oxidation state = 2+) were in the hexagonal, orthorhombic, and monoclinic crystal class, respectively (compounds 6–8). Further, the antibacterial activity has been performed to check the comparative potency of all synthesized compounds (compounds 3–8) in contrast to S. aureus, E. coli, B. pumilis, and S. typhi bacterial strains. It was concluded that Cobalt and Nickel complexes (compounds 5 and 6) exhibited the highest antibacterial potential compared to MBH adduct (L”x) against S. aureus and E. coli. Additionally, against the remaining two strains (B. pumilis and S. typhi) they also showed positive results. However, both ligand (L”x) and its Chromium-based metal complex (compounds 3 and 4) are inactive against S. aureus, while, active against remaining three bacterial strains (E. coli, B. pumilis and S. typhi). Likewise, our studies demonstrated that both Manganese and Copper-based complexes (compounds 7 and 8) were non-active against the four selected bacterial strains. In addition, the antioxidant activity (DPPH radical scavenging assay) of compounds 3–8 verified the positive inhibition with perspective to the ascorbic acid (standard). Furthermore, it has been observed that the MBH adduct L”x (compound 3) is an excellent antifungal drug; besides it, our newly synthesized metal complexes are non-active against these fungal strains (A. niger, A. flavus, A. fumigatus, and C. albicans). This research can be continued by synthesizing new MBH adduct ligands and their novel metal complexes to recognize the most suitable drugs as medication in treating infectious disorders.
Author Contributions
Conceptualization, S.I. (Shazia Ishfaq), S.N. and A.S.A.; methodology, S.I. (Shazia Ishfaq) and M.S.J.; software, S.I. (Sadaf Iqbal); validation, A.Q., N.F. and F.A.A.; formal analysis, S.I. (Sadaf Iqbal) and A.S.A.; investigation, S.I. (Shazia Ishfaq) and A.S.A.; resources, A.S.A.; data curation, E.M.T.E.D.; writing—original draft preparation, S.I. (Shazia Ishfaq); writing—review and editing, S.N. and A.S.A.; visualization, F.A.A. and M.S.J.; supervision, S.N.; project administration, P.B. and F.A.A.; funding acquisition, E.M.T.E.D. and M.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project Number (RSP-2021/259) King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to University of Karachi for providing research laboratory and other necessary facilities to accomplish this project. We are extremely grateful to School of Pharmacy University of Karachi, PCSIR laboratories complex-Karachi Pakistan and NED University of Engineering and Technology for their cooperation in TGA, bioactivity studies and XRD analysis. Authors are thankful to the Supercomputing Center of Lanzhou University. This work was funded by the Researchers Supporting Project Number (RSP-2021/259) King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Basavaiah, D.; Rao, K.V.; Reddy, R.J. The Baylis–Hillman Reaction: A Novel Source of Attraction, Opportunities, and Challenges in Synthetic Chemistry. Chem. Soc. Rev. 2007, 36, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Junior, C.G.L.; De Assis, P.A.C.; Silva, F.P.L.; Sousa, S.C.O.; De Andrade, N.G.; Barbosa, T.P.; Nerís, P.L.N.; Segundo, L.V.G.; Anjos, Í.C.; Carvalho, G.A.U.; et al. Efficient Synthesis of 16 Aromatic Morita-Baylis-Hillman Adducts: Biological Evaluation on Leishmania Amazonensis and Leishmania Chagasi. Bioorg. Chem. 2010, 38, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.A.; Dos Santos, A.A. A Facile, Versatile, and Mild Morita-Baylis-Hillman-Type Reaction for the Modular One-Pot Synthesis of Highly Functionalized MBH Adducts. Eur. J. Org. Chem. 2012, 3431–3436. [Google Scholar] [CrossRef]
- Du, J.; Ma, Y.; Meng, F.; Zhang, R.; Wang, R.; Shi, H.; Wang, Q.; Fan, Y.; Huang, H.; Cui, J.; et al. Lewis Base-Catalyzed [4 + 3] Annulation of Ortho -Quinone Methides and MBH Carbonates: Synthesis of Functionalized Benzo[b]Oxepines Bearing Oxindole ScaffOlds. Org. Lett. 2019, 21, 465–468. [Google Scholar] [CrossRef]
- Ketata, E.; Elleuch, H.; Neifar, A.; Mihoubi, W.; Ayadi, W.; Marrakchi, N.; Rezgui, F.; Gargouri, A. Anti-Melanogenesis Potential of a New Series of Morita-Baylis-Hillman Adducts in B16F10 Melanoma Cell Line. Bioorg. Chem. 2019, 84, 17–23. [Google Scholar] [CrossRef]
- Mohan, R.; Rastogi, N.; Namboothiri, I.N.N.; Mobin, S.M.; Panda, D. Synthesis and Evaluation of α-Hydroxymethylated Conjugated Nitroalkenes for Their Anticancer Activity: Inhibition of Cell Proliferation by Targeting Microtubules. Bioorg. Med. Chem. 2006, 14, 8073–8085. [Google Scholar] [CrossRef] [PubMed]
- Lima-Junior, C.G.; Vasconcellos, M.L.A.A. Morita-Baylis-Hillman Adducts: Biological Activities and Potentialities to the Discovery of New Cheaper Drugs. Bioorg. Med. Chem. 2012, 20, 3954–3971. [Google Scholar] [CrossRef]
- Dadwal, M.; Mohan, R.; Panda, D.; Mobin, S.M.; Namboothiri, I.N.N. The Morita-Baylis-Hillman Adducts of β-Aryl Nitroethylenes with Other Activated Alkenes: Synthesis and Anticancer Activity Studies. Chem. Commun. 2006, 3, 338–340. [Google Scholar] [CrossRef]
- Elleuch, H.; Mihoubi, W.; Mihoubi, M.; Ketata, E.; Gargouri, A.; Rezgui, F. Potential Antioxidant Activity of Morita-Baylis-Hillman Adducts. Bioorg. Chem. 2018, 78, 24–28. [Google Scholar] [CrossRef]
- de Souza, R.O.M.A.; Pereira, V.L.P.; Muzitano, M.F.; Falcão, C.A.B.; Rossi-Bergmann, B.; Filho, E.B.A.; Vasconcellos, M.L.A.A. High Selective Leishmanicidal Activity of 3-Hydroxy-2-Methylene-3-(4-Bromophenyl) Propanenitrile and Analogous Compounds. Eur. J. Med. Chem. 2007, 42, 99–102. [Google Scholar] [CrossRef]
- Amarante, G.W.; Cavallaro, M.; Coelho, F. Hyphenating the Curtius Rearrangement with Morita-Baylis-Hillman Adducts: Synthesis of Biologically Active Acyloins and Vicinal Aminoalcohols. J. Braz. Chem. Soc. 2011, 22, 1568–1584. [Google Scholar] [CrossRef]
- Santos, M.S.; Fernandes, D.C.; Rodrigues, M.T.; Regiani, T.; Andricopulo, A.D.; Ruiz, A.L.T.G.; Vendramini-Costa, D.B.; De Carvalho, J.E.; Eberlin, M.N.; Coelho, F. Diastereoselective Synthesis of Biologically Active Cyclopenta[b]Indoles. J. Org. Chem. 2016, 81, 6626–6639. [Google Scholar] [CrossRef]
- Pirovani, R.V.; Ferreira, B.R.V.; Coelho, F. Highly Functionalized Spirocyclohexadienones from Morita-Baylis-Hillman Adducts. Synlett 2009, 14, 2333–2337. [Google Scholar] [CrossRef]
- Calvo, B.C.; Buter, J.; Minnaard, A.J. Applications to the synthesis of natural products. In Copper-Catalyzed Asymmetric Synthesis; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 9783527332, pp. 373–448. [Google Scholar] [CrossRef]
- Rabello, A.; Rubinger, M.; Souza, R.; Guilardi, S.; de Lima, G.; Tavares, E.; Zanon, É.; Silva, G.; Zambolim, L.; Ellena, J. Molecular Structure Studies on Allyl Sulfonamides: Synthesis, Theoretical Treatment and Evaluation of Biological Activity. J. Braz. Chem. Soc. 2021, 32, 2033–2046. [Google Scholar] [CrossRef]
- Amarante, G.W.; Cavallaro, M.; Coelho, F. Highly Diastereoselective Total Synthesis of the Anti-Tumoral Agent (±)-Spisulosine (ES285) from a Morita-Baylis-Hillman Adduct. Tetrahedron Lett. 2010, 51, 2597–2599. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel Metals and Metal Complexes as Platforms for Cancer Therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Hanif, M.; Hartinger, C.G. Anticancer Metallodrugs: Where Is the next Cisplatin? Future Med. Chem. 2018, 10, 615–617. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A Review on Biological Activities and Chemical Synthesis of Hydrazide Derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis and Antimicrobial Activity of Copper(II) and Silver(I) Complexes of Hydroxynitrocoumarins: X-Ray Crystal Structures of [Cu(Hnc)2(H2O)2] · 2H2O and [Ag(Hnc)] (HncH = 4-Hydroxy-3-Nitro-2H-Chromen-2-One). Polyhedron 2005, 24, 949–957. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, S.; Wu, W.; Yang, K.; Zhang, Y.F.; Tumukunde, E.; Wang, S.; Wang, Y. Functional Analysis of Peptidyl-Prolyl Cis-Trans Isomerase from Aspergillus Flavus. Int. J. Mol. Sci. 2019, 20, 2206. [Google Scholar] [CrossRef]
- Wu, W.Z.; Ahmad, S.; Wang, S.; Zhang, Y.F.; Yang, H.; Wang, S.H.; Wang, Y. Expression and Antibody Preparation of Small Ubiquitin-like Modifier (SUMO) from Aspergillus Flavus. IOP Conf. Ser. Earth Environ. Sci. 2019, 346. [Google Scholar] [CrossRef]
- Abdi, Y.; Bensouilah, N.; Siziani, D.; Hamdi, M.; Silva, A.M.S.; Boutemeur-Kheddis, B. New Complexes of Manganese (II) and Copper (II) Derived from the Two New Furopyran-3, 4-Dione Ligands: Synthesis, Spectral Characterization, ESR, DFT Studies and Evaluation of Antimicrobial Activity. J. Mol. Struct. 2020, 1202, 127307. [Google Scholar] [CrossRef]
- Zhang, C.; Han, X.; Korshin, G.V.; Kuznetsov, A.M.; Yan, M. Interpretation of the Differential UV–Visible Absorbance Spectra of Metal-NOM Complexes Based on the Quantum Chemical Simulations for the Model Compound Esculetin. Chemosphere 2021, 276, 130043. [Google Scholar] [CrossRef]
- Brito, V.B.M.; Santos, G.F.; Silva, T.D.S.; Souza, J.L.C.; Militão, G.C.G.; Martins, F.T.; Silva, F.P.L.; Oliveira, B.G.; Araújo, E.C.C.; Vasconcellos, M.L.A.A.; et al. Synthesis, Anti-Proliferative Activity, Theoretical and 1H NMR Experimental Studies of Morita–Baylis–Hillman Adducts from Isatin Derivatives. Mol. Divers. 2020, 24, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Sanchez, V.; Simirgiotis, M.J.; Santos, L.S. The Morita-Baylis-Hillman Reaction: Insights into Asymmetry and Reaction Mechanisms by Electrospray Ionization Mass Spectrometry. Molecules 2009, 14, 3989–4021. [Google Scholar] [CrossRef]
- da Câmara Rocha, J.; da Franca Rodrigues, K.A.; do Nascimento Néris, P.L.; da Silva, L.V.; Almeida, F.S.; Lima, V.S.; Peixoto, R.F.; da Câmara Rocha, J.; de Azevedo, F.d.L.A.A.; Veras, R.C.; et al. Biological Activity of Morita-Baylis-Hillman Adduct Homodimers in L. Infantum and L. Amazonensis: Anti-Leishmania Activity and Cytotoxicity. Parasitol. Res. 2019, 118, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Hills, I.D.; Fu, G.C. The First General Method for the Synthesis of Transition-Metal π Complexes of an Electronically Diverse Family of 1,2-Azaborolyls. Organometallics 2002, 21, 4323–4325. [Google Scholar] [CrossRef]
- Aiyelabola, T.; Akinkunmi, E.; Ojo, I.; Obuotor, E.; Adebajo, C.; Isabirye, D. Syntheses, Characterization, Resolution, and Biological Studies of Coordination Compounds of Aspartic Acid and Glycine. Bioinorg. Chem. Appl. 2017, 2956145. [Google Scholar] [CrossRef]
- Jeslin Kanaga Inba, P.; Annaraj, B.; Thalamuthu, S.; Neelakantan, M.A. Cu(II), Ni(II), and Zn(II) Complexes of Salan-Type Ligand Containing Ester Groups: Synthesis, Characterization, Electrochemical Properties, and in Vitro Biological Activities. Bioinorg. Chem. Appl. 2013, 2013. [Google Scholar] [CrossRef]
- Kirbag, S.; Erecevit, P.; Zengin, F.; Guvenc, A.N. Antimicrobial Activities of Some Euphorbia Species. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 305–309. [Google Scholar] [CrossRef]
- Harmon, K.G.; Clugston, J.R.; Dec, K.; Hainline, B.; Herring, S.; Kane, S.F.; Kontos, A.P.; Leddy, J.J.; McCrea, M.; Poddar, S.K.; et al. American Medical Society for Sports Medicine Position Statement on Concussion in Sport. Br. J. Sports Med. 2019, 53, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ikram, M.; Subhan, F.; Sinnokrot, M.; Khan, W. Antibacterial Activities of Transition Metal Complexes of Mesocyclic Amidine 1,4-Diazacycloheptane (DACH). Open Chem. 2019, 17, 936–942. [Google Scholar] [CrossRef]
- Alfredo Rodriguez, V.; Alfredo, V. Formulation of Nanoparticle Systems for Drug Delivery Application in Biomedical Sciences. Master’s Thesis, The University of Texas at El Paso, El Paso, TX, USA, 2018. [Google Scholar]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On Practical Problems in Estimation of Antioxidant Activity of Compounds by DPPH Method (Problems in Estimation of Antioxidant Activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Surapuram, V.; Setzer, W.N.; McFeeters, R.L.; McFeeters, H. Antifungal Activity of Plant Extracts against Aspergillus Niger and Rhizopus Stolonifer. Nat. Prod. Commun. 2014, 9, 1603–1605. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria Turcica. J. Nanomater. 2020, 9535432. [Google Scholar] [CrossRef]
- Pusnik, M.; Imeri, M.; Deppierraz, G.; Bruinink, A.; Zinn, M. The Agar Diffusion Scratch Assay—A Novel Method to Assess the Bioactive and Cytotoxic Potential of New Materials and Compounds. Sci. Rep. 2016, 6, 20854. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16 2016. Available online: https://gaussian.com/ (accessed on 18 August 2022).
- Ahmed, H.; Hashim, A.; Abduljalil, H. Analysis of Structural, Electrical and Electronic Properties of (Polymer Nanocomposites/Silicon Carbide) for Antibacterial Application. Egypt. J. Chem. 2019, 62, 767–776. [Google Scholar] [CrossRef]
- Becke, A.D. The Effect of the Exchange-Only Gradient Gradient Correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.I.; Alam, M.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Siddiqui, M.R.H. A New Ladder-Type Dichloro(2,2-Dimethyl-1,3-Diaminopropane) Copper Complex: Synthesis, Structural Studies and Selective Sensing Behavior towards a Ketone Molecule. Polyhedron 2019, 170, 287–293. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Holdgate, G.A.; Tunnicliffe, A.; Ward, W.H.J.; Weston, S.A.; Rosenbrock, G.; Barth, P.T.; Taylor, I.W.F.; Pauptit, R.A.; Timms, D. The Entropic Penalty of Ordered Water Accounts for Weaker Binding of the Antibiotic Novobiocin to a Resistant Mutant of DNA Gyrase: A Thermodynamic and Crystallographic Study. Biochemistry 1997, 36, 9663–9673. [Google Scholar] [CrossRef] [PubMed]
- Bommer, M.; Kunze, C.; Fesseler, J.; Schubert, T.; Diekert, G.; Dobbek, H. Structural Basis for Organohalide Respiration. Science 2014, 346, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.M.; Holden, H.M. Structure of CDP-D-Glucose 4, 6-Dehydratase from Salmonella Typhi Complexed with CDP-D-Xylose. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 61, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Taft, C.A.; Da Silva, V.B. Current Topics in Computer-aided Drug Design. J. Pharm. Sci. 2008, 97, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- El-Halim, H.F.A.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational Behaviour of Lomefloxacin Drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO 2(VI) Ions: Synthesis, Structural Characterization and Biological Activity Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Batra, N. Synthesis, Characterization and Antimicrobial Activities of Mixed Ligand Transition Metal Complexes with Isatin Monohydrazone Schiff Base Ligands and Heterocyclic Nitrogen Base. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Desnoyer, A.N.; Nicolay, A.; Rios, P.; Ziegler, M.S.; Tilley, T.D. Bimetallics in a Nutshell: Complexes Supported by Chelating Naphthyridine-Based Ligands. Acc. Chem. Res. 2020, 53, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; Khedr, A.M.; Elsharkawy, M. Characterization and Thermal Studies of Nano-Synthesized Mn(II), Co(II), Ni(II) and Cu(II) Complexes with Adipohydrazone Ligand as New Promising Antimicrobial and Antitumor Agents. Appl. Organomet. Chem. 2017, 31, 1–14. [Google Scholar] [CrossRef]
- Köse, D.A. Synthesis and Characterization of Bis(Nicotinamide) m-Hydroxybenzoate Complexes of Co(II), Ni(II), Cu(II), and Zn(II). Russ. J. Inorg. Chem. 2007, 52, 1384–1390. [Google Scholar] [CrossRef]
- Kang, Q.P.; Li, X.Y.; Wang, L.; Zhang, Y.; Dong, W.K. Containing-PMBP N2O2-Donors Transition Metal(II) Complexes: Synthesis, Crystal Structure, Hirshfeld Surface Analyses and Fluorescence Properties. Appl. Organomet. Chem. 2019, 33, e5013. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New Trends for Metal Complexes with Anticancer Activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Spectroscopy of Organic Compounds. Dept. Chem. 2006, 66, 1–36. [Google Scholar]
- Shaghaghi, Z. Spectroscopic Properties of Some New Azo–Azomethine Ligands in the Presence of Cu2+, Pb2+, Hg2+, Co2+, Ni2+, Cd2+ and Zn2+ and Their Antioxidant Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khan, A.; Hussain, I.; Khan, M.A.; Gul, S.; Iqbal, M.; Inayat-Ur-Rahman; Khuda, F. Spectral, XRD, SEM and Biological Properties of New Mononuclear Schiff Base Transition Metal Complexes. Inorg. Chem. Commun. 2013, 35, 104–109. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Kalaichelvan, S.; Meganathan, C.; Joshua, B.D.; Cornard, J. FT-IR, FT-Raman Spectra and Ab Initio HF and DFT Calculations of 4-N,N′-Dimethylamino Pyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, M.; Cinar, M.; Kurt, M.; Sundaraganesan, N. Experimental and Theoretical FTIR and FT-Raman Spectroscopic Analysis of 1-Pyrenecarboxylic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Aiyelabola, T.; Akinkunmi, E.; Obuotor, E.; Olawuni, I.; Isabirye, D.; Jordaan, J. Synthesis Characterization and Biological Activities of Coordination Compounds of 4-Hydroxy-3-Nitro-2H-Chromen-2-One and Its Aminoethanoic Acid and Pyrrolidine-2-Carboxylic Acid Mixed Ligand Complexes. Bioinorg. Chem. Appl. 2017, 2017, 6426747. [Google Scholar] [CrossRef] [PubMed]
- Anacona, J.R.; Santaella, J.; Al-shemary, R.K.R.; Amenta, J.; Otero, A.; Ramos, C.; Celis, F. Ceftriaxone-Based Schiff Base Transition Metal(II) Complexes. Synthesis, Characterization, Bacterial Toxicity, and DFT Calculations. Enhanced Antibacterial Activity of a Novel Zn(II) Complex against S. aureus and E. coli. J. Inorg. Biochem. 2021, 223, 111519. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic Activity of Cerium Complexes with Coumarin Derivatives. Molecular Modeling of the Ligands. Arch. der Pharm. Int. J. Pharm. Med. Chem. 2000, 333, 93–98. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K.; Abou El-Enein, S.A.; El-Sheikh-Amer, M.M. Design of New Metal Complexes of 2-(3-Amino-4,6-Dimethyl-1H-Pyrazolo[3,4-b]Pyridin-1-Yl)Aceto-Hydrazide: Synthesis, Characterization, Modelling and Antioxidant Activity. J. Mol. Struct. 2017, 1127, 386–396. [Google Scholar] [CrossRef]
- Singh, S.K.; Eng, J.; Atanasov, M.; Neese, F. Covalency and Chemical Bonding in Transition Metal Complexes: An Ab Initio Based Ligand Field Perspective. Coord. Chem. Rev. 2017, 344, 2–25. [Google Scholar] [CrossRef]
- Xu, F.; Huang, W.; You, X.Z. Novel Cyano-Bridged Mixed-Valent Copper Complexes Formed by Completely in Situ Synthetic Method via the Cleavage of C-C Bond in Acetonitrile. Dalt. Trans. 2010, 39, 10652–10658. [Google Scholar] [CrossRef] [PubMed]
- Martí-Rujas, J. Structural Elucidation of Microcrystalline MOFs from Powder X-Ray Diffraction. Dalt. Trans. 2020, 49, 13897–13916. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.C.; Rubinger, M.M.M.; Filho, E.V.; Oliveira, M.R.L.; Piló-Veloso, D.; Ellena, J.; Guilardi, S.; Souza, R.A.C.; Zambolim, L. Tetraphenylphosphonium Allyldithiocarbimates Derived from Morita-Baylis-Hillman Adducts: Synthesis, Characterization, Crystal Structure and Antifungal Activity. J. Mol. Struct. 2016, 1106, 130–140. [Google Scholar] [CrossRef]
- Amarante, G.W.; Milagre, H.M.S.; Vaz, B.G.; Ferreira, B.R.V.; Eberlin, M.N.; Coelho, F. Dualistic Nature of the Mechanism of the Morita-Baylis-Hillman Reaction Probed by Electrospray Ionization Mass Spectrometry. J. Org. Chem. 2009, 74, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Szabó, P.T.; Kele, Z. Electrospray Mass Spectrometry of Hydrophobic Compounds Using Dimethyl Sulfoxide and Dimethylformamide as Solvents. Rapid Commun. Mass Spectrom. 2001, 15, 2415–2419. [Google Scholar] [CrossRef]
- Borges, V.; Henion, J. Determination of Pharmaceutical Compounds in Aqueous Dimethyl Sulfoxide by Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 415–423. [Google Scholar] [CrossRef]
- Saad, F.A. Synthesis, Spectral, Electrochemical and X-Ray Single Crystal Studies on Ni(II) and Co(II) Complexes Derived from 1-Benzoyl-3-(4-Methylpyridin-2-Yl) Thiourea. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Şahin, Ö.; Özdemir, Ü.Ö.; Seferoğlu, N.; Genc, Z.K.; Kaya, K.; Aydıner, B.; Tekin, S.; Seferoğlu, Z. New Platinum (II) and Palladium (II) Complexes of Coumarin-Thiazole Schiff Base with a Fluorescent Chemosensor Properties: Synthesis, Spectroscopic Characterization, X-Ray Structure Determination, in Vitro Anticancer Activity on Various Human Carcinoma Ce. J. Photochem. Photobiol. B Biol. 2018, 178, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Atalay, V.E.; Ölüç, I.B.; Karahan, M. Modeling of BSA–Metal Ion-Acrylic Acid Complex by Theoretical Methods: Semi-Empirical PM6 and Docking Study. Acta Phys. Pol. A 2018, 134, 1200–1203. [Google Scholar] [CrossRef]
- Pourjavid, M.R.; Sehat, A.A.; Arabieh, M.; Yousefi, S.R.; Hosseini, M.H.; Rezaee, M. Column Solid Phase Extraction and Flame Atomic Absorption Spectrometric Determination of Manganese(II) and Iron(III) Ions in Water, Food and Biological Samples Using 3-(1-Methyl-1H-Pyrrol-2-Yl)-1H-Pyrazole-5-Carboxylic Acid on Synthesized Graphene Oxide. Mater. Sci. Eng. C 2014, 35, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.S.A.; Ismail, E.H. A Combined Experimental and Modelling Investigations on Mixed Bioligand Complexes of Divalent Cobalt and Copper in β-Cyclodextrin. Appl. Organomet. Chem. 2020, 34, e5280. [Google Scholar] [CrossRef]
- Mahapatra, B.B.; Mishra, R.R.; Sarangi, A.K. Synthesis, Characterisation, XRD, Molecular Modelling and Potential Antibacterial Studies of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) Complexes with Bidentate Azodye Ligand. J. Saudi Chem. Soc. 2016, 20, 635–643. [Google Scholar] [CrossRef]
- Bouhdada, M.; Amane, M.E.L.; El Hamzaoui, N. Synthesis, Spectroscopic Studies, X-Ray Powder Diffraction Data and Antibacterial Activity of Mixed Transition Metal Complexes with Sulfonate Azo Dye, Sulfamate and Caffeine Ligands. Inorg. Chem. Commun. 2019, 101, 32–39. [Google Scholar] [CrossRef]
- Shukla, K.; Mishra, A.; Sharma, P. Synthesis, Characterization, XRD and EXAFS Studies of Fe(III) Complexes. AIP Conf. Proc. 2019, 2100, 020163. [Google Scholar] [CrossRef]
- Prescimone, A.; Morien, C.; Allan, D.; Schlueter, J.A.; Tozer, S.W.; Manson, J.L.; Parsons, S.; Brechin, E.K.; Hill, S. Pressure-Driven Orbital Reorientations and Coordination-Sphere Reconstructions in [CuF2(H2O)2(Pyz)]. Angew. Chem. 2012, 124, 7608–7612. [Google Scholar] [CrossRef]
- Sinthuja, S.A.; Shaji, Y.C.; Rose, G.L. Synthesis, Characterization and Evaluation of Biological Properties of Transition Metal Chelates with Schiff Base Ligands Derived from Glutaraldehyde with L-Leucine. IJSRST 2018, 4, 587–593. [Google Scholar]
- Sharfalddin, A.A.; Emwas, A.H.; Jaremko, M.; Hussien, M.A. Transition Metal Complexes of 6-Mercaptopurine: Characterization, Theoretical Calculation, DNA-Binding, Molecular Docking, and Anticancer Activity. Appl. Organomet. Chem. 2021, 35, e6041. [Google Scholar] [CrossRef]
- Neha, M.; Biplab, M. TGA Analysis of Transition Metal Complexes Derived from Phenothiazine Ligands Introduction. Int. J. Sci. Eng. Res. 2018, 6, 47–57. [Google Scholar]
- Mohammed Shafeeulla, R.; Krishnamurthy, G.; Bhojynaik, H.S.; Shivarudrappa, H.P.; Shiralgi, Y. Spectral Thermal Cytotoxic and Molecular Docking Studies of N′-2-Hydroxybenzoyl; Pyridine-4-Carbohydrazide Its Complexes. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 332–344. [Google Scholar] [CrossRef]
- Kavitha, B.; Sravanthi, M.; Saritha Reddy, P. DNA Interaction, Docking, Molecular Modelling and Biological Studies of o-Vanillin Derived Schiff Base Metal Complexes. J. Mol. Struct. 2019, 1185, 153–167. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdelhamid, A.A.; Marzouk, A.A.; Shehata, M.R.; Bakheet, M.A.; Almaghrabi, O.A.; Nafady, A. Synthesis and Characterization of New Cr(III), Fe(III) and Cu(II) Complexes Incorporating Multi-Substituted Aryl Imidazole Ligand: Structural, DFT, DNA Binding, and Biological Implications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117700. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef]
- Ortiz, R.; Antilén, M.; Speisky, H.; Aliaga, M.E.; López-Alarcón, C.; Baugh, S. Application of a Microplate-Based Orac-Pyrogallol Red Assay for the Estimation of Antioxidant Capacity: First Action 2012.03. J. AOAC Int. 2012, 95, 1558–1561. [Google Scholar] [CrossRef]
- Tsai, C.E.; Lin, L.H. DPPH Scavenging Capacity of Extracts from Camellia Seed Dregs Using Polyol Compounds as Solvents. Heliyon 2019, 5, e02315. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).