Simultaneous Detection of Chlorzoxazone and Paracetamol Using a Greener Reverse-Phase HPTLC-UV Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Chromatographic Analysis
2.3. Calibration Curves and Quality Control (QC) Samples for CZN and PCT
2.4. Sample Preparations for Simultaneous Detection of CZN and PCT in Marketed Tablets and Capsules
2.5. Validation Parameters
2.6. Application of a Greener HPTLC-UV Method in Simultaneous Detection of CZN and PCT in Commercial Tablets and Capsules
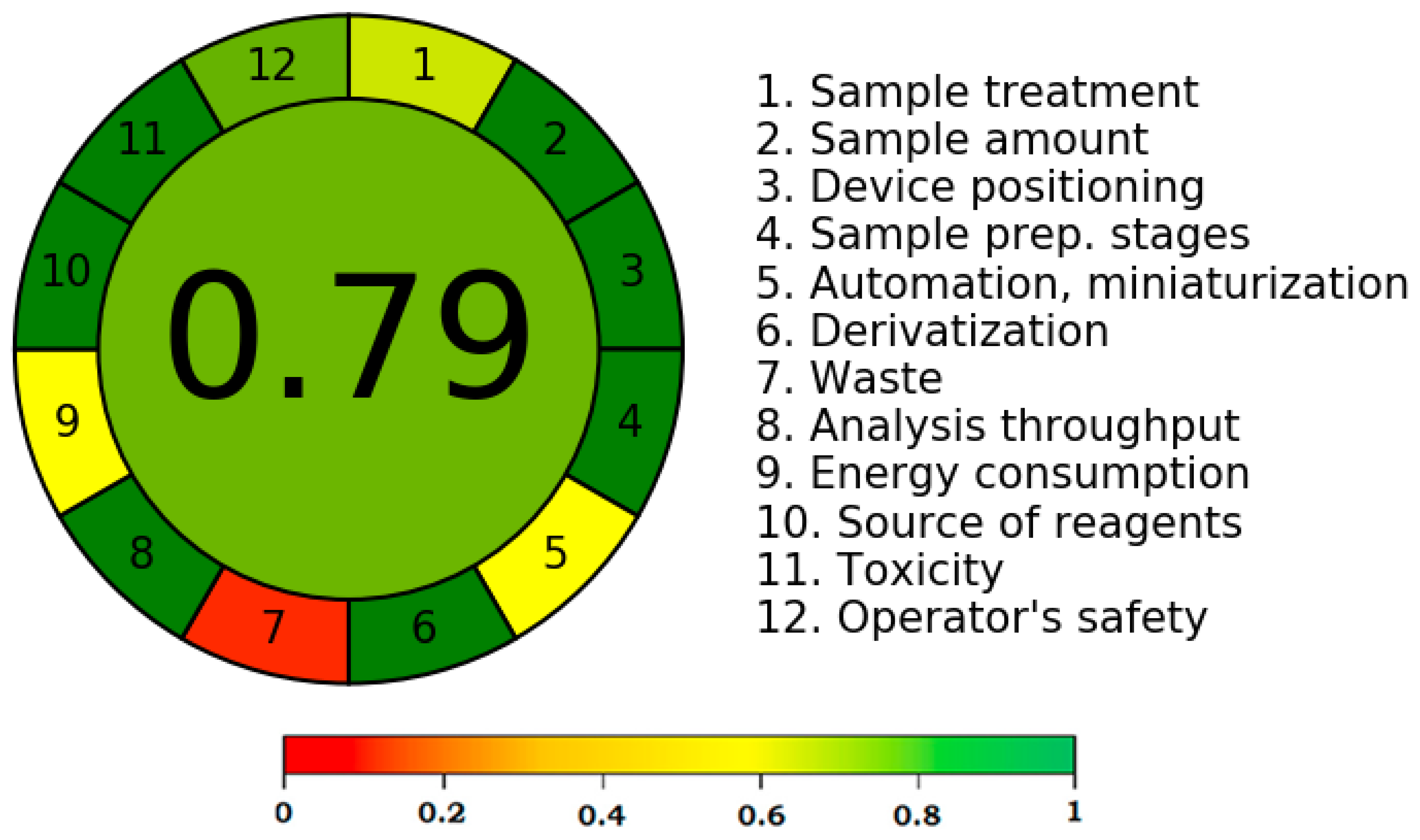
2.7. Greenness Evaluation Using AGREE Methodology
3. Results and Discussion
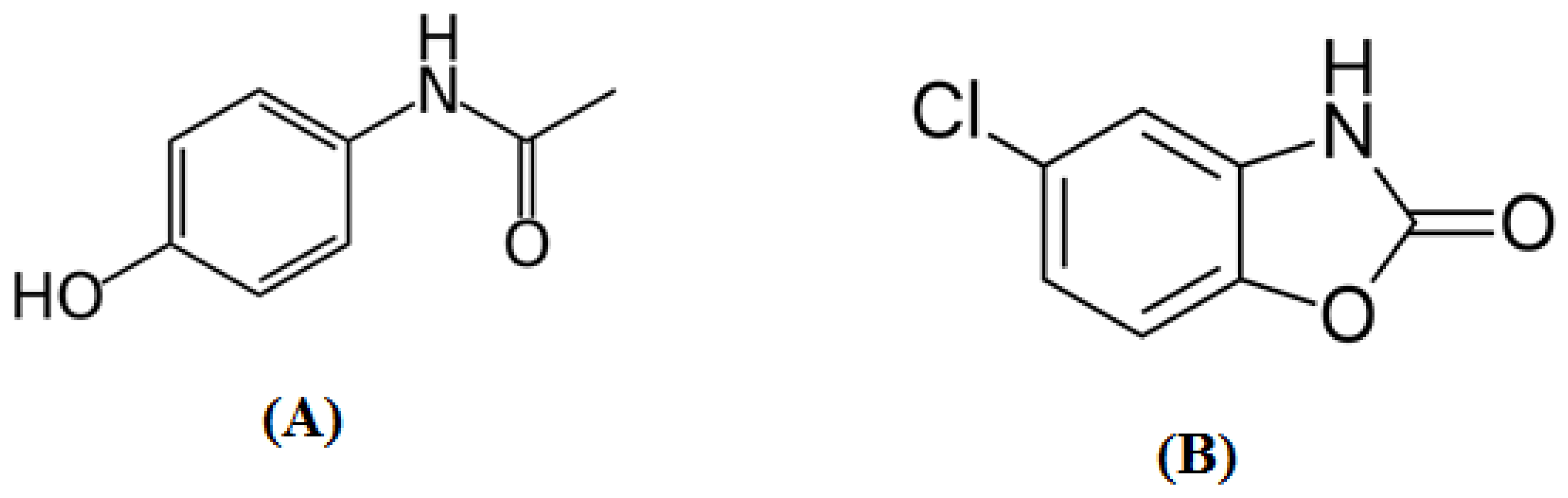
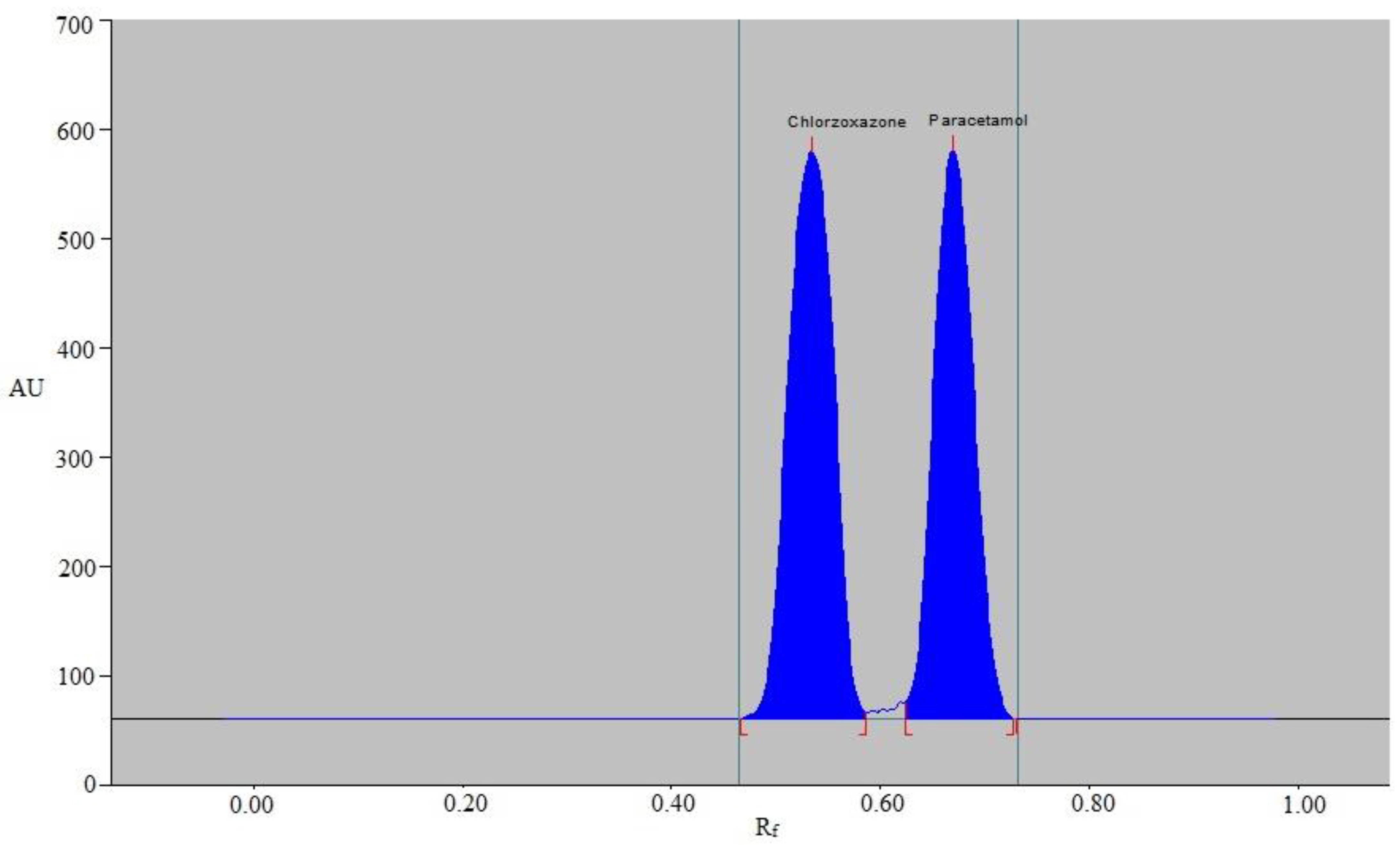
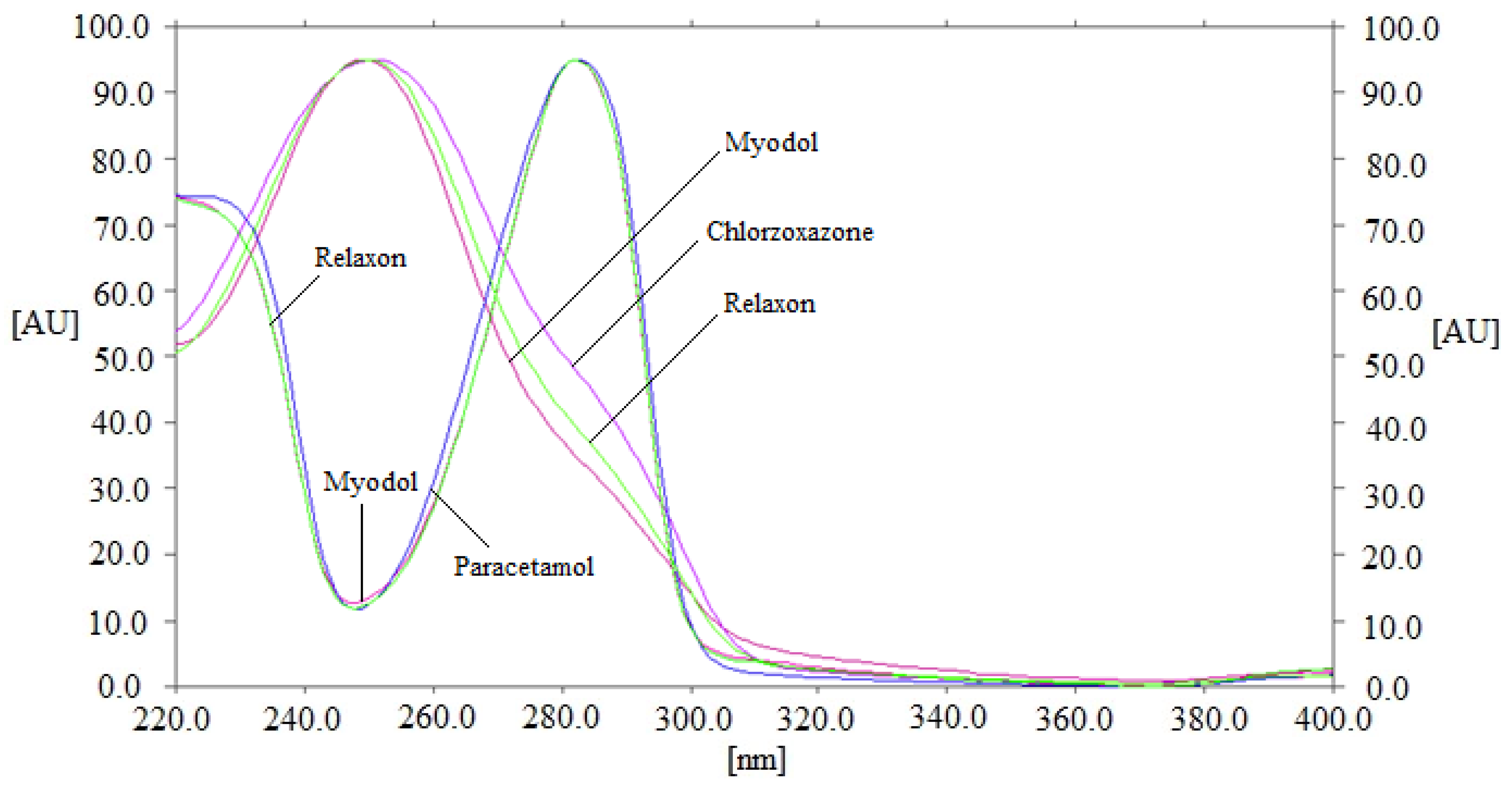
3.1. Method Development
3.2. Validation Parameters
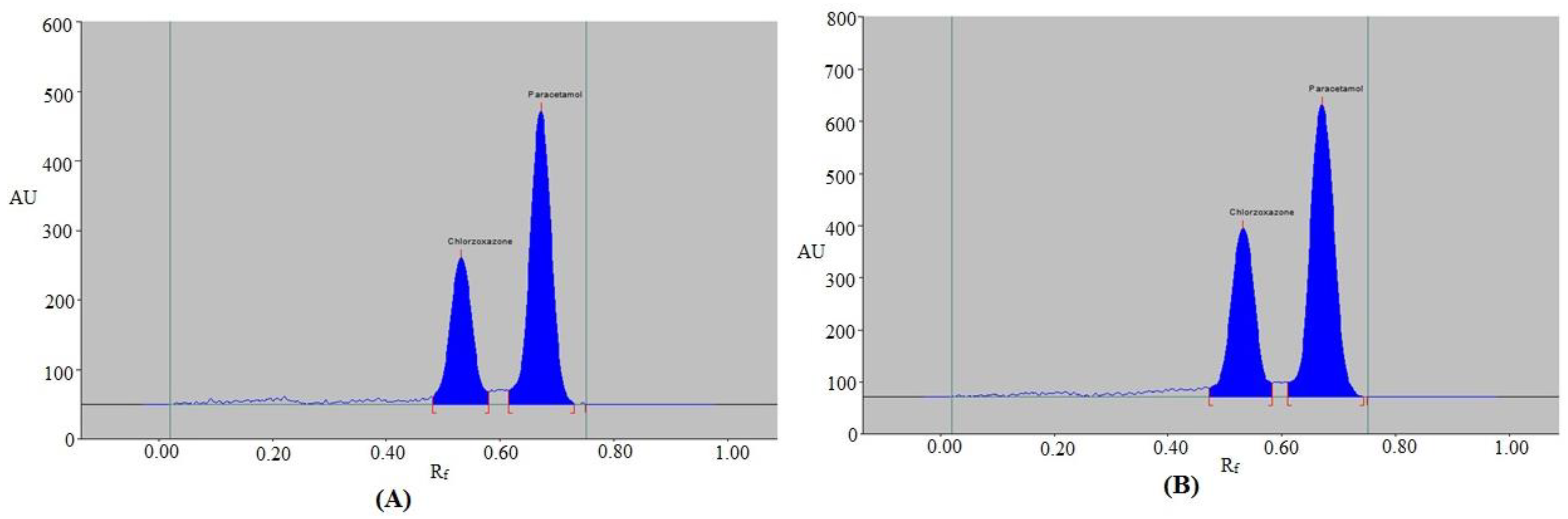
3.3. Application of the HPTLC-UV Method in Simutaneous Detection of CZN and PCT in Marketed Tablets and Capsules
3.4. Greenness Evaluation Using the AGREE Approach
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toubar, S.S.; Hegazy, M.A.; Elshaheda, M.S.; Helmy, M.I. Novel pure component contribution, mean centering of ratio spectra and factor based algorithms for simultaneous resolution and quantification of overlapped spectral signals: An application to recently co-formulated tablets of chlorzoxazone, aceclofenac and paracetamol. Spectrochim. Acta Part A 2016, 163, 89–95. [Google Scholar]
- Jimenez, J.A.; Martinez, F. Thermodynamic study of the solubility of acetaminophen in propylene glycol + water cosolvent mixtures. J. Braz. Chem. Soc. 2006, 17, 125–134. [Google Scholar] [CrossRef]
- Shakeel, F.; Alanazi, F.K.; Alsarra, I.A.; Haq, N. Solubilization behavior of paracetamol in Transcutol-water mixtures at T = (298.15 to 333.15) K. J. Chem. Eng. Data 2012, 58, 3551–3556. [Google Scholar] [CrossRef]
- Petsalo, A.; Turpeinen, M.; Pelkonen, O.; Tolonen, A. Analysis of nine drugs and their cytochrome P450-specific probe metabolites from urine by liquid chromatography tandem mass spectrometry utilizing sub 2 μm particle size column. J. Chromatogr. A 2008, 1215, 107–115. [Google Scholar] [CrossRef]
- Joshi, M.; Tyndale, R.F. Regional and cellular distribution of CYP2E1 in monkey brain and its induction by chronic nicotine. Neuropharmacology 2006, 50, 568–575. [Google Scholar] [CrossRef]
- McCrae, J.C.; Morrison, E.E.; Maclntyre, I.M.; Dear, J.W.; Webb, D.J. Long-term adverse effects of paracetamol-a review. Br. J. Clin. Pharmacol. 2018, 84, 2218–2230. [Google Scholar] [CrossRef]
- Richards, B.L.; Whittle, S.L.; Buchbinder, R. Muscle relaxants for pain management in rheumatoid arthritis. Cochrane Database Syst. Rev. 2012, 1, CD008922. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Jain, C.L.; Sethi, P.D. Simultaneous estimation of chlorzoxazone and acetaminophen in combined dosage forms by an absorbance ratio technique and difference spectrophotometry. J. Pharm. Biomed. Anal. 1989, 7, 693–698. [Google Scholar] [CrossRef]
- Garg, C.; Saraf, W.; Saraf, S. Simultaneous estimation of aceclofenac, paracetamol and chlorzoxazone in tablets. Indian J. Pharm. Sci. 2007, 69, 692–694. [Google Scholar]
- Joshi, R.; Pawar, N.; Sawant, R.; Gaikwad, P. Simultaneous estimation of paracetamol, chlorzoxazone and ibuprofen by validated spectrophotometric methods. Anal. Chem. Lett. 2012, 2, 118–124. [Google Scholar] [CrossRef]
- El-Bagary, R.I.; El-Kady, E.F.; Al-Matari, A.A. Simultaneous spectrophotometric determination of diclofenac sodium, paracetamol, and chlorzoxazone in ternary mixture using chemometric and artificial neural networks techniques. Asian J. Pharm. Clin. Res. 2017, 10, 225–230. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Reid, I.O.A.; Elawni, A. H-point standard additions method for the simultaneous determination of paracetamol and chlorzoxazone in tablets using addition of both analytes and absorbance increments (∆A). Int. J. Adv. Pharm. Anal. 2017, 7, 1–5. [Google Scholar]
- Ravisankar, S.; Vasudevan, M.; Gandhimathi, M.; Suresh, B. Reversed-phase HPLC method for the estimation of acetoaminophen, ibuprofen and chlorzoxazone in formulations. Talanta 1998, 46, 1577–1581. [Google Scholar] [CrossRef]
- Ali, M.S.; Rafiuddin, S.; Ghori, M.; Kahtri, R.A. Simultaneous determination of paracetamol, chlorzoxazone, and related impurities 4-aminophenol, 4’-chloroacetanilide, and p-chlorophenol in pharmaceutical preparations by high-performance liquid chromatography. J. AOAC Int. 2007, 90, 82–93. [Google Scholar] [CrossRef]
- Shaikh, K.A.; Devkhile, A.B. Simultaneous determination of aceclofenac, paracetamol, and chlorzoxazone by RP-HPLC in pharmaceutical dosage form. J. Chromatogr. Sci. 2008, 46, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, V.; Vaidhyalingam; Yuvaraj, G.; Nema, R.K. Simultaneous estimation of paracetamol, chlorzoxazone and aceclofenac in pharmaceutical formulation by HPLC method. Int. J. ChemTech. Res. 2009, 1, 457–460. [Google Scholar]
- Badgujar, M.A.; Pingale, S.G.; Mangaonkar, K.V. Simultaneous determination of paracetamol, chlorzoxazone and diclofenac sodium in tablet dosage form by high performance liquid chromatography. E-J. Chem. 2011, 8, 1206–1211. [Google Scholar] [CrossRef]
- More, S.J.; Tandulwadkar, S.S.; Nikam, A.R.; Rathore, A.S.; Sathianarayanan, L.; Mahadik, K.R. Application of HPLC for the simultaneous determination of paracetamol, chlorzoxazone, and nimesulide in pharmaceutical dosage form. ISRN Chromatogr. 2012, 2012, E252895. [Google Scholar] [CrossRef]
- Vidya, P.; Gawande, V.; Chandewar, A.V.; Dewani, A.P. Development and validation of HPLC-PDA method for simultaneous estimation of famotidine, paracetamol, chlorzoxazone and diclofenac potassium in combined solid dosage from. Der. Pharm. Lett. 2017, 10, 15–27. [Google Scholar]
- El-Yazbi, A.F.; Guirguis, K.M.; Bedair, M.M.; Belal, T.S. Validated specific HPLC-DAD method for simultaneous estimation of paracetamol and chlorzoxazone in the presence of five of their degradation products and toxic impurities. Drug Dev. Ind. Pharm. 2020, 46, 1853–1861. [Google Scholar] [CrossRef]
- Prema Kumari, K.B.; Murugan, V.; Ezhilarasan, V.; Joseph, S.R. Simultaneous determination of paracetamol, aceclofenac and chlorzoxazone in pharmaceutical dosage form by UHPLC method. J. Chromatogr. Sep. Technol. 2017, 8, E1000384. [Google Scholar]
- Bari, V.R.; Dhorda, U.J.; Sundaresan, M. A simultaneous packed column supercritical fluid chromatographic method for ibuprofen, chlorzoxazone and acetoaminophen in bulk and dosage forms. Talanta 1997, 45, 297–302. [Google Scholar] [CrossRef]
- Khan, H.; Ali, M.; Ahuja, A.; Ali, J. Validated UPLC/Q-TOF-MS method for simultaneous determination of aceclofenac, paracetamol and chlorzoxazone in human plasma and its application to pharmacokinetic study. Asian J. Pharm. Anal. 2017, 7, 93. [Google Scholar] [CrossRef]
- Mohamed, D.; Hegazy, M.A.; Elshahed, M.S.; Toubar, S.S.; Helmy, M.I. Liquid chromatography-tandem MS/MS method for simultaneous quantification of paracetamol, chlorzoxazone and aceclofenac in human plasma: An application to a pharmacokinetic study. Biomed. Chromatogr. 2018, 32, E4232. [Google Scholar] [CrossRef]
- Sane, R.T.; Gadgil, M. Simultaneous determination of paracetamol, chlorzoxazone, and nimesulide by HPTLC. J. Planar Chromatogr. 2002, 15, 76–78. [Google Scholar] [CrossRef]
- Yadav, S.S.; Jagtap, A.S.; Rao, J.R. Simultaneous determination of paracetamol, lornoxicam and chlorzoxazone in tablets by high performance thin layer chromatography. Der. Pharm. Lett. 2012, 4, 1798–1802. [Google Scholar]
- Abdelaleem, E.A.; Abdelwahab, N.S. Stability-indicating TLC-densitometry method for simultaneous determination of paracetamol and chlorzoxazone and their toxic impurities. J. Chromatogr. Sci. 2013, 51, 187–191. [Google Scholar] [CrossRef]
- Chhalotiya, U.K.; Patel, D.B.; Shah, D.A.; Mehta, F.A.; Bhatt, K.K. Simultaneous estimation of chlorzoxazone, paracetamol, famotidine and diclofenac potassium in their combined dosage form by thin layer chromatography. J. Pharm. Pharmacol. Sci. 2017, 2, E111. [Google Scholar] [CrossRef]
- Salih, M.E.; Aqel, A.; Abdulkhair, B.Y.; Alothman, Z.A.; Abdulaziz, M.A.; Badjah-Hadj-Ahmed, A.Y. Simultaneous determination of paracetamol and chlorzoxazone in their combined pharmaceutical formulations by reversed-phase capillary liquid chromatography using a polymethacrylate monolithic column. J. Chromatogr. Sci. 2018, 56, 819–827. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Ali, A.; Alqarni, M.H.; Foudah, A.I.; Aljarba, T.M.; Alkholifi, F.K.; Alshehri, S.; Ghoneim, M.M.; Ali, A. Simultaneous determination of caffeine and paracetamol in commercial formulations using normal-phase and reversed-phase HPTLC methods: A contrast of validation parameters. Molecules 2022, 27, 405. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, E105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating green RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, E105960. [Google Scholar] [CrossRef]
- Alam, P.; Salem-Bekhit, M.M.; Al-Joufi, F.A.; Alqarni, M.H.; Shakeel, F. Quantitative analysis of cabozantinib in pharmaceutical dosage forms using green RP-HPTLC and green NP-HPTLC methods: A comparative evaluation. Sustain. Chem. Pharm. 2021, 21, E100413. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). Q2 (R1): Validation of Analytical Procedures–Text and Methodology; International Conference on Harmonization (ICH): Geneva, Switzerland, 2005. [Google Scholar]
| Parameters | CZN | PCT |
|---|---|---|
| Linearity range (ng band−1) | 40–1600 | 30–1600 |
| R2 | 0.9990 | 0.9985 |
| Slope ± SD | 19.29 ± 0.56 | 18.73 ± 0.53 |
| Intercept ± SD | 294.32 ± 5.38 | 900.96 ± 9.87 |
| Standard error of slope | 0.22 | 0.21 |
| Standard error of intercept | 2.19 | 4.03 |
| 95% CI of slope | 18.31–20.28 | 17.80–19.67 |
| 95% CI of intercept | 284.86–303.77 | 883.61–918.30 |
| LOD ± SD (ng band−1) | 13.86 ± 0.21 | 10.21 ± 0.16 |
| LOQ ± SD (ng band−1) | 41.58 ± 0.63 | 30.63 ± 0.48 |
| Parameters | CZN | PCT |
|---|---|---|
| Rf | 0.54 ± 0.02 | 0.67 ± 0.02 |
| As | 1.06 ± 0.03 | 1.04 ± 0.02 |
| N m−1 | 5361 ± 6.12 | 5485 ± 6.28 |
| Conc. (ng band−1) | Conc. Found (ng band−1) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| CZN | |||
| 50 | 50.71 ± 0.54 | 101.42 | 1.06 |
| 400 | 404.64 ± 3.12 | 101.16 | 0.77 |
| 1600 | 1578.95 ± 9.94 | 98.68 | 0.62 |
| PCT | |||
| 50 | 50.48 ± 0.47 | 100.96 | 0.93 |
| 400 | 394.76 ± 1.92 | 98.69 | 0.48 |
| 1600 | 1585.83 ± 7.16 | 99.11 | 0.45 |
| Conc. (ng band−1) | Intra-Day Precision | Inter-Day Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng band−1) ± SD | Standard Error | CV (%) | Conc. (ng band−1) ± SD | Standard Error | CV (%) | |
| CZN | ||||||
| 50 | 49.39 ± 0.48 | 0.19 | 0.97 | 49.52 ± 0.49 | 0.20 | 0.98 |
| 400 | 392.34 ± 3.08 | 1.25 | 0.78 | 389.88 ± 3.14 | 1.28 | 0.80 |
| 1600 | 1581.41 ± 10.22 | 4.17 | 0.64 | 1610.23 ± 12.21 | 4.98 | 0.75 |
| PCT | ||||||
| 50 | 49.61 ± 0.45 | 0.18 | 0.90 | 50.66 ± 0.49 | 0.20 | 0.96 |
| 400 | 406.14 ± 2.14 | 0.87 | 0.52 | 392.87 ± 2.24 | 0.91 | 0.57 |
| 1600 | 1653.54 ± 8.12 | 3.31 | 0.49 | 1581.87 ± 8.55 | 3.49 | 0.54 |
| Conc. (ng band−1) | Mobile Phase Composition (Ethanol/Water) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | (ng band−1) ± SD | % CV | Rf | ||
| CZN | ||||||
| 72:28 | +2.0 | 386.87 ± 3.68 | 0.95 | 0.53 | ||
| 400 | 70:30 | 70:30 | 0.0 | 395.41 ± 3.74 | 0.94 | 0.54 |
| 68:32 | −2.0 | 404.64 ± 3.95 | 0.97 | 0.55 | ||
| PCT | ||||||
| 72:28 | +2.0 | 387.84 ± 3.65 | 0.94 | 0.66 | ||
| 400 | 70:30 | 70:30 | 0.0 | 397.63 ± 3.86 | 0.97 | 0.67 |
| 68:32 | −2.0 | 405.64 ± 4.30 | 1.06 | 0.68 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Aljarba, T.M.; Alshehri, S.; Alam, P. Simultaneous Detection of Chlorzoxazone and Paracetamol Using a Greener Reverse-Phase HPTLC-UV Method. Separations 2022, 9, 300. https://doi.org/10.3390/separations9100300
Foudah AI, Shakeel F, Alqarni MH, Aljarba TM, Alshehri S, Alam P. Simultaneous Detection of Chlorzoxazone and Paracetamol Using a Greener Reverse-Phase HPTLC-UV Method. Separations. 2022; 9(10):300. https://doi.org/10.3390/separations9100300
Chicago/Turabian StyleFoudah, Ahmed I., Faiyaz Shakeel, Mohammed H. Alqarni, Tariq M. Aljarba, Sultan Alshehri, and Prawez Alam. 2022. "Simultaneous Detection of Chlorzoxazone and Paracetamol Using a Greener Reverse-Phase HPTLC-UV Method" Separations 9, no. 10: 300. https://doi.org/10.3390/separations9100300
APA StyleFoudah, A. I., Shakeel, F., Alqarni, M. H., Aljarba, T. M., Alshehri, S., & Alam, P. (2022). Simultaneous Detection of Chlorzoxazone and Paracetamol Using a Greener Reverse-Phase HPTLC-UV Method. Separations, 9(10), 300. https://doi.org/10.3390/separations9100300








