Assessments of Organic Carbon Stabilization Using the Spectroscopic Characteristics of Humic Acids Separated from Soils of the Lena River Delta
Abstract
1. Introduction
- -
- Investigate the elemental composition of HAs separated from soils of the Lena River delta;
- -
- Determine the molecular composition of HAs separated from soils of the Lena River delta;
- -
- Estimate the rate of stabilization of organic matter in the studied soils and buried organic remains.
2. Materials and Methods
2.1. The Study Site
2.2. Sampling Procedure
2.3. Laboratory Analysis
2.4. Protocol for the Extraction of Humic Acids from Soils
- -
- Soil sieving: We manually broke up a portion of the dried soil sample into powder or small portions using a mortar and pestle and sieved the ground sample through 2 mm mesh;
- -
- Extraction of humic substances with alkaline solution: Samples of 50 g for organic soils and 100 g for organo–mineral soils were filled up to 500 mL with NaOH 0.1N solutions and left for 48 h;
- -
- Gravity filtration of the extractant: After 48 h, the soils sedimented to the bottom of the flask, and the supernatant contained NaOH with extracted humic substances. This supernatant was now separated from the soil by gravity filtration;
- -
- Precipitation of the humic substances: For every sample, we calculated the amount of H2SO4 1N solution in the proportion of 50 mL H2SO4 1N per 100 mL of supernatant. After adding the solutions, we let it stand for 24 h;
- -
- Dialysis of humic acids: Over the previous 24 h, the humic substances precipitated to the bottom of the flask. After 24 h, the supernatant liquid (acid-soluble fraction) was siphoned off, and the HA precipitate was squeezed out in a centrifuge at 3000 rpm for 15 min and washed in centrifuge beakers with repeated centrifugation, first with acidified sulfuric acid water and then with pure water until the HA began to disperse. After centrifugation and washing, the HA gel was placed in bags made of dialysis cellophane and placed in large containers with distilled water. During the first 3 days, the water was changed every day, and then it was change after 2 days. Usually, it took 7–10 days to completely remove excess sodium sulfate. The completeness of removal was controlled by the qualitative reaction to sulfates in the water flowing down from the bag;
- -
- Drying of HA preparations: HAs preparations from dialysis bags were transferred into Petri dishes or small crystallizers, after which they were dried in a vacuum oven over containers with dry CaCl2. Further, the HA preparations were transferred into weighing bottles and, if necessary, dried in a desiccator over P2O5. The ash content of the HA preparations obtained by this method did not exceed 5% and significantly depended on the speed and duration of the first centrifugation.
2.4.1. Elemental Composition of HAs
2.4.2. Molecular Composition of HAs
2.4.3. Statistical Survey
3. Results and Discussion
3.1. The Physico–Chemical Characteristics of Study Soils
3.2. Elemental Composition
3.3. Molecular Composition of HAs Separated from the Soils of the Lena River Delta
3.4. The Stabilization of Organic Matter Based on 13C NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, A.; Stolbovoy, V.; Tarnocai, C.; Broll, G.; Spaargaren, O.; Montanarella, L. Soil Atlas of the Northern Circumpolar Region, European Commission; Publications Office of the European Union: Luxembourg, 2010; p. 144. [Google Scholar]
- Davis, T.N. Permafrost: A Guide to Frozen Ground in Transition; University of Alaska Press: Fairbanks, AK, USA, 2001. [Google Scholar]
- Dutta, K.; Schuur, E.A.G.; Neff, J.C.; Zimov, S.A. Potential carbon release from permafrost soils of Northeastern Siberia. Glob. Chang. Biol. 2006, 12, 2336–2351. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial ecosystems and the carbon cycle. Glob. Chang. Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Zubrzycki, S.; Kutzbach, L.; Grosse, G.; Desyatkin, A.; Pfeiffer, E.M. Organic carbon and total nitrogen stocks in soils of the Lena River Delta. Biogeosciences 2013, 10, 3507–3524. [Google Scholar] [CrossRef]
- Zubrzycki, S.; Kutzbach, L.; Pfeiffer, E.M. Permafrost-affected soils and their carbon pools with a focus on the Russian Arctic. Solid Earth 2014, 5, 595–609. [Google Scholar] [CrossRef]
- Semenov, V.M.; Ivannikov, L.A.; Tulina, A.S. Stabilization of organic matter in the soil. Agrochimia 2009, 10, 77–96. [Google Scholar]
- Cauwet, G.; Sidorov, I. The biogeochemistry of Lena River: organic carbon and nutrients distribution. Mar. Chem. 1996, 53, 211–227. [Google Scholar] [CrossRef]
- Ejarque, E.; Abakumov, E. Stability and biodegradability of organic matter from Arctic soils of Western Siberia: insights from 13C-NMR spectroscopy and elemental analysis. Solid Earth 2016, 7, 153–165. [Google Scholar] [CrossRef]
- Knoblauch, C.; Beer, C.; Sosnin, A.; Wagner, D.; Pfeiffer, E.-M. Predicting long-term carbon mineralization and trace gas production from thawing permafrost of Northeast Siberia. Glob. Chang. Biol. 2013, 19, 1160–1172. [Google Scholar] [CrossRef]
- Kutzbach, L.; Wagner, D.; Pfeiffer, E.-M. Effect of microrelief and vegetation on methane emission from wet polygonal tundra, Lena Delta, Northern Siberia. Biogeochemistry 2004, 69, 341–362. [Google Scholar] [CrossRef]
- Lara, R.J.; Rachold, V.; Kattner, G.; Hubberten, H.W.; Guggenberger, G.; Skoog, A.; Thomas, D.N. Dissolved organic matter and nutrients in the Lena River, Siberian Arctic: Characteristics and distribution. Mar. Chem. 1998, 59, 301–309. [Google Scholar] [CrossRef]
- Makeev, O. The Soil Cryology; Russian Academy of Science: Moscow, Russian, 2019; p. 464. [Google Scholar]
- Kholodov, V.A.; Konstantinov, A.I.; Kudryavtsev, A.V.; Perminova, I.V. Structure of humic acids in zonal soils from 13C NMR data. Eurasian Soil Sci. 2011, 44, 976–983. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Kravchenko, E.G.; Konstantinov, A.I.; Perminova, I.V.; Chukov, S.N.; Demin, V.V. Comparison of the Properties of Humic Acids Extracted from Soils by Alkali in the Presence and Absence of Oxygen. Eurasian Soil Sci. 2019, 52, 880–891. [Google Scholar] [CrossRef]
- Piccolo, A.; Nardi, S.; Concheri, G. Macromolecular changes of humic substances induced by interaction with organic acids. Eur. J. Soil Sci. 1996, 47, 319–328. [Google Scholar] [CrossRef]
- Perminova, I.V. Size exclusion chromatography of humic substances: complexities of data interpretation attributable to non-size exclusion effects. Soil Sci. 1999, 164. [Google Scholar] [CrossRef]
- Chukov, S.N.; Abakumov, E.V.; Tomashunas, V.M. Characterization of humic acids from antarctic soils by nuclear magnetic resonance. Eurasian Soil Sci. 2015, 48, 1207–1211. [Google Scholar] [CrossRef]
- Dziadowiec, H.; Gonet, S.; Plichta, W. Properties of humic acids of Arctic tundra soils in Spitsbergen. Pol. Polar Res. 1994, vol. 15, 71. [Google Scholar]
- Lodygin, E.D.; Beznosikov, V.A.; Vasilevich, R.S. Molecular Composition of Humic Substances in Tundra Soils (C-13-NMR Spectroscopic Study). Eurasian Soil Sci. 2014, 47, 400–406. [Google Scholar] [CrossRef]
- Piccolo, A. Chapter 5 - Humus and Soil Conservation. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 225–264. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. Chapter 1 - The Chemical Structure of Humic Substances: Recent Advances. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 1–44. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Cocozza, C.; D’Orazio, V.; Miano, T.M.; Shotyk, W. Characterization of solid and aqueous phases of a peat bog profile using molecular fluorescence spectroscopy, ESR and FT-IR, and comparison with physical properties. Org. Geochem. 2003, 34, 49–60. [Google Scholar] [CrossRef]
- Senesi, N. Application of Electron Spin Resonance (ESR) Spectroscopy in Soil Chemistry. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1990; Volume 14, pp. 77–122. [Google Scholar]
- Thompson, S.O.; Chesters, G. Infra-red spectra and differential thermograms of lignins and soil humic materials saturated with different cations. J. Soil Sci. 1970, 21, 265–272. [Google Scholar] [CrossRef]
- Ovsepyan, L.A.; Kurganova, I.N.; Lopes de Gerenyu, V.O.; Kuzyakov, Y.V.; Rusakov, A.V. Changes in the fractional composition of organic matter in the soils of the forest–steppe zone during their postagrogenic evolution. Eurasian Soil Sci. 2020, 53, 50–61. [Google Scholar] [CrossRef]
- Fedotov, G.N.; Artem’eva, Z.S. Colloidal component of granulodensimetric soil fractions. Eurasian Soil Sci. 2015, 48, 54–62. [Google Scholar] [CrossRef]
- Dai, X.Y.; Ping, C.L.; Michaelson, G.J. Characterizing soil organic matter in Arctic tundra soils by different analytical approaches. Org. Geochem. 2002, 33, 407–419. [Google Scholar] [CrossRef]
- Lupachev, A.; Abakumov, E.; Gubin, S. The Influence of Cryogenic Mass Exchange on the Composition and Stabilization Rate of Soil Organic Matter in Cryosols of the Kolyma Lowland (North Yakutia, Russia). Geosciences 2017, 7, 24. [Google Scholar] [CrossRef]
- Burdelnaya, N.; Bushnev, D.; Mokeev, M.; Dobrodumov, A. Experimental study of kerogen maturation by solid-state 13C NMR spectroscopy. Fuel 2014, 118, 308–315. [Google Scholar] [CrossRef]
- Cao, X.; Lattao, C.; Pignatello, J.J.; Mao, J.; Schmidt-Rohr, K. Sorption Selectivity in Natural Organic Matter Probed with Fully Deuterium-Exchanged and Carbonyl-13C-Labeled Benzophenone and 1H–13C NMR Spectroscopy. Environ. Sci. Technol. 2014, 48, 8645–8652. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.P.; Alam, T.M. Multi-dimensional 1H-13C HETCOR and FSLG-HETCOR NMR study of sphingomyelin bilayers containing cholesterol in the gel and liquid crystalline states. J. Magn. Reson. 2006, 181, 316–326. [Google Scholar] [CrossRef]
- Perminova, I.V.; Garcia-Mina, J.M.; Podgorski, D.C.; Cervantes, F.J.; Efremenko, E.N.; Domingo, J.L. Humic substances and living systems: Impact on environmental and human health. Environ. Res. 2021, 194. [Google Scholar] [CrossRef] [PubMed]
- Bolshiyanov, D.Y.; Makarov, A.S.; Schneider, V.; Stoof, G. Origin and Development of the delta Lena River; AARI: St. Petersburg, Russia, 2013; p. 268. [Google Scholar]
- WRB, F. IUSS Working Group WRB World Reference Base for Soil Resources 2014, Update 2015. p. 195. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 16 June 2021).
- Jahn, R.; Blume, H.P.; Spaargaren, O.; Schad, P. Guidelines for Soil Description; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Bowman, G.; Hutka, J. Particle Size Analysis. In Soil Physical Measurment and Interpritation for Land Evaluation; McKezie, N., Coughlan, K., Cresswell, H., Eds.; CSIRO Publishing: Victoria, Australia, 2002; pp. 224–239. [Google Scholar]
- Swift, R.S. Organic Matter Characterization. Methods Soil Anal. 1996, 1011–1069. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Studies of gas absorption. VI. A graphical representation for the efficiency of physical absorption. Recl. Des Trav. Chim. Des Pays-Bas 1950, 69, 503–508. [Google Scholar] [CrossRef]
- Harwood, H.J. Minimum molecular weight approach for determining empirical formulas. J. Chem. Educ. 1965, 42, 222. [Google Scholar] [CrossRef]
- Orlov, D.S. Soil Chemistry: A Textbook; Moscow State University: Moscow, Russia, 1985. [Google Scholar]
- Izokh, N.; Yazikov, A. Discovery of Early Carboniferous conodonts in Northern Kharaulakh Ranges (lower reaches of the Lena River, northeastern Siberia, Arctic Russia). Revue de Micropaléontologie 2017, 60, 213–232. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Rodina, O.A.; Eskov, A.K. Humification and Humic Acid Composition of Suspended Soil in Oligotrophous Environments in South Vietnam. Appl. Environ. Soil Sci. 2018, 2018, 1026237. [Google Scholar] [CrossRef]
- Orlov, D.S. Soil Humic Acids and General Theory Humification; Moscov State University: Moscow, Russia, 1990; p. 325. [Google Scholar]
- Dobrovolsky, G.V. Soils of floodplains of the center of Russian plain. M. Publ. House Mosc. Univ. 2005, 2025, 293. [Google Scholar]
- Abakumov, E.; Lodygin, E.; Tomashunas, V. 13C NMR and ESR characterization of humic substances isolated from soils of two siberian Arctic Islands. Int. J. Ecol. 2015. [Google Scholar] [CrossRef]
- Abakumov, E.; Maksimova, E.; Tsibart, A. Assessment of postfire soils degradation dynamics: Stability and molecular composition of humic acids with use of spectroscopy methods. Land Degrad. Dev. 2018, 29, 2092–2101. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Schnitzer, M.; Dennis, L.W.; Maciel, G.E. Aromaticity of Humic Substances in Soils. Soil Sci. Soc. Am. J. 1981, 45, 1089–1094. [Google Scholar] [CrossRef]
- Polyakov, V.; Abakumov, E.V. Humic Acids Isolated from Selected Soils from the Russian Arctic and Antarctic: Characterization by Two-Dimensional 1H-13C HETCOR and 13C CP/Mas NMR Spectroscopy. Geosciences 2020, 10. [Google Scholar] [CrossRef]
- Polyakov, V.I.; Chegodaeva, N.A.; Abakumov, E.V. Molecular and elemental composition of humic acids isolated from selected soils of the Russian Arctic. Vestn. Tomsk. Gos. Univ. Biol. 2019, 47, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Beznosikov, V.A.; Lodygin, E.D. High-molecular organic substances in soils. Trans. Komi Sci. Cent. Ural Branch Russ. Acad. Sci. 2010, 1, 24–30. [Google Scholar]
- Schaefer, C.E.G.R.; Simas, F.N.B.; Gilkes, R.J.; Mathison, C.; da Costa, L.M.; Albuquerque, M.A. Micromorphology and microchemistry of selected Cryosols from maritime Antarctica. Geoderma 2008, 144, 104–115. [Google Scholar] [CrossRef]
- Szymański, W.; Skiba, M.; Wojtuń, B.; Drewnik, M. Soil properties, micromorphology, and mineralogy of Cryosols from sorted and unsorted patterned grounds in the Hornsund area, SW Spitsbergen. Geoderma 2015, 253-254, 1–11. [Google Scholar] [CrossRef]
- Pengerud, A.; Dignac, M.-F.; Certini, G.; Strand, L.T.; Forte, C.; Rasse, D.P. Soil organic matter molecular composition and state of decomposition in three locations of the European Arctic. Biogeochemistry 2017, 135, 277–292. [Google Scholar] [CrossRef]
- Vasilevich, R.; Lodygin, E.; Beznosikov, V.; Abakumov, E. Molecular composition of raw peat and humic substances from permafrost peat soils of European Northeast Russia as climate change markers. Sci. Total Environ. 2018, 615, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Vasilevich, R.S.; Beznosikov, V.A.; Lodygin, E.D. Molecular Structure of Humus Substances in Permafrost Peat Mounds in Forest-Tundra. Eurasian Soil Sci. 2019, 52, 283–295. [Google Scholar] [CrossRef]
- Polyakov, V.; Abakumov, E. Stabilization of organic material from soils and soil-like bodies in the Lena River Delta (13C-NMR spectroscopy analysis). Span. J. Soil Sci. 2020, 10, 170–190. [Google Scholar] [CrossRef]
- Fedoros, E.I.; Baldueva, I.A.; Perminova, I.V.; Badun, G.A.; Chernysheva, M.G.; Grozdova, I.D.; Melik-Nubarov, N.S.; Danilova, A.B.; Nekhaeva, T.L.; Kuznetsova, A.I.; et al. Exploring bioactivity potential of polyphenolic water-soluble lignin derivative. Environ. Res. 2020, 191, 110049. [Google Scholar] [CrossRef]
- Lodygin, E.; Beznosikov, V.; Abakumov, E. Humic substances elemental composition of selected taiga and tundra soils from Russian European North-East. Pol. Polar Res. 2017, 38, 125–147. [Google Scholar] [CrossRef][Green Version]
- Lodygin, E.D.; Beznosikov, V.A. The molecular structure and elemental composition of humic substances from Albeluvisols. Chem. Ecol. 2010, 26, 87–95. [Google Scholar] [CrossRef]
- Casey, K.A.; Kaspari, S.D.; Skiles, S.M.; Kreutz, K.; Handley, M.J. The spectral and chemical measurement of pollutants on snow near South Pole, Antarctica. J. Geophys. Res. Atmos. 2017, 122, 6592–6610. [Google Scholar] [CrossRef]
- Szymański, W. Chemistry and spectroscopic properties of surface horizons of Arctic soils under different types of tundra vegetation–A case study from the Fuglebergsletta coastal plain (SW Spitsbergen). Catena 2017, 156, 325–337. [Google Scholar] [CrossRef]
- Stolt, M.H.; Lindbo, D.L. 17 - Soil Organic Matter. In Interpretation of Micromorphological Features of Soils and Regoliths; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 369–396. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Polyakov, V.I.; Orlova, K.S. Podzol development on different aged coastal bars of Lake Ladoga. Vestn. Tomsk. Gos. Univ. Biol. 2019, 48, 6–31. [Google Scholar] [CrossRef] [PubMed]
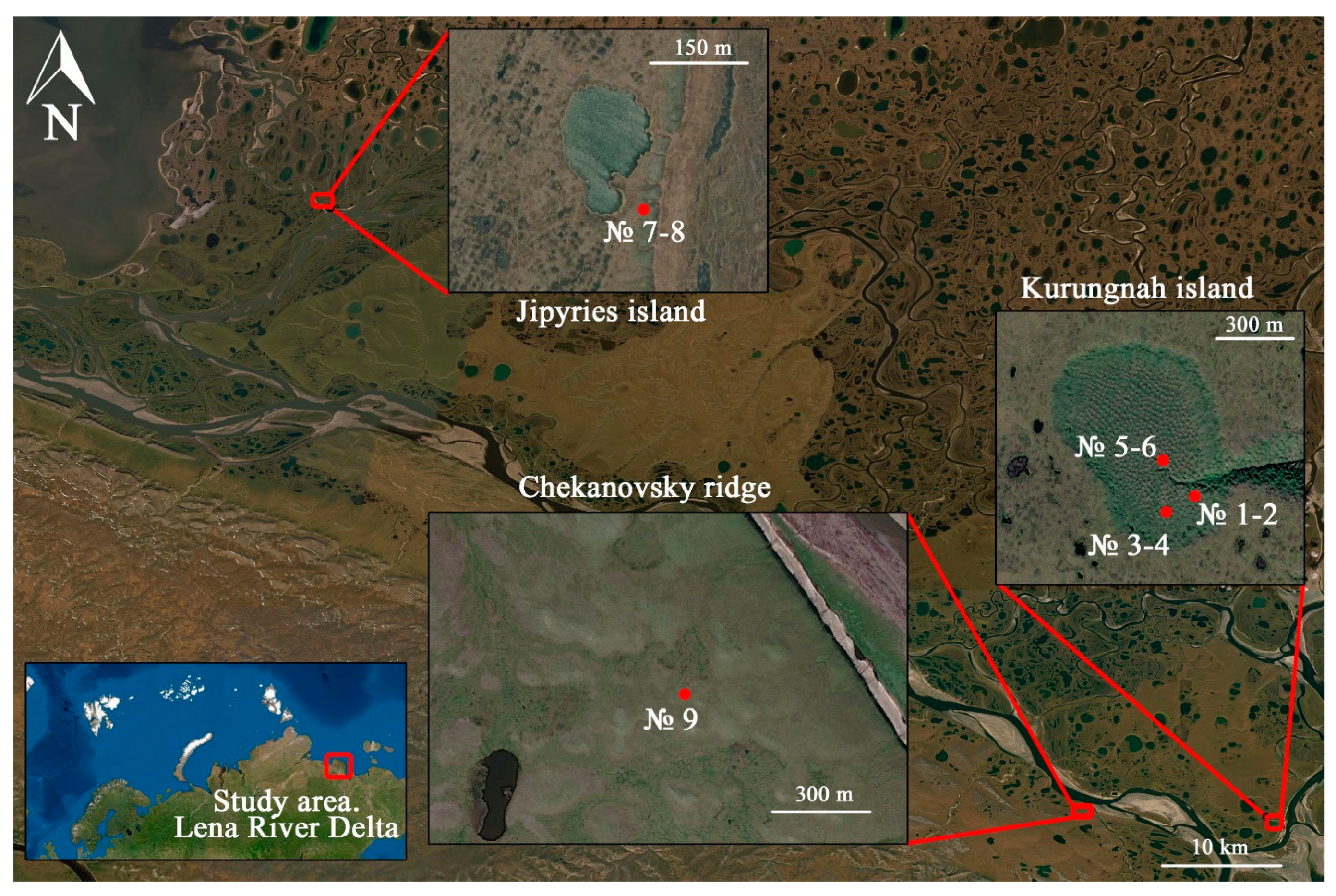
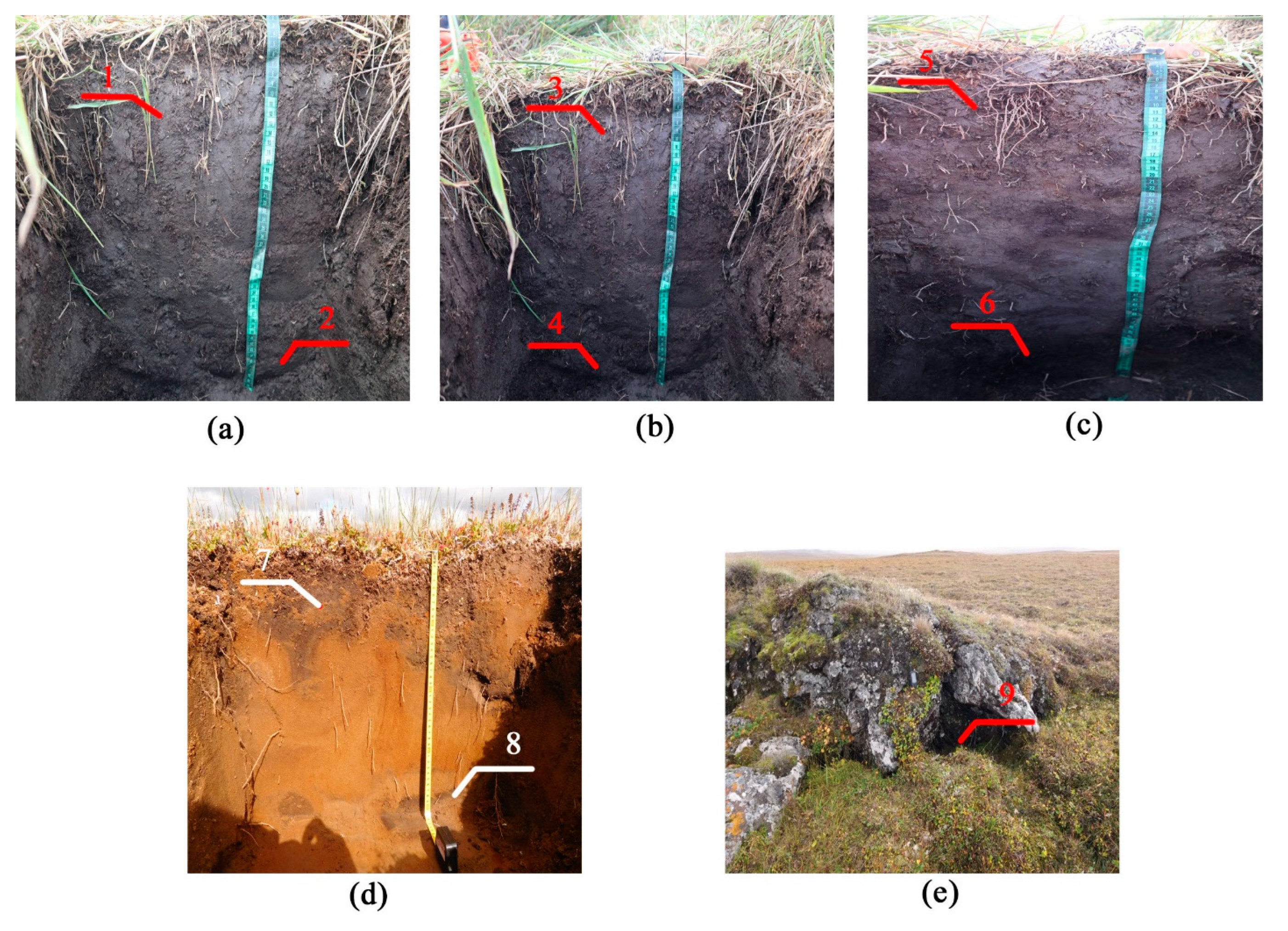
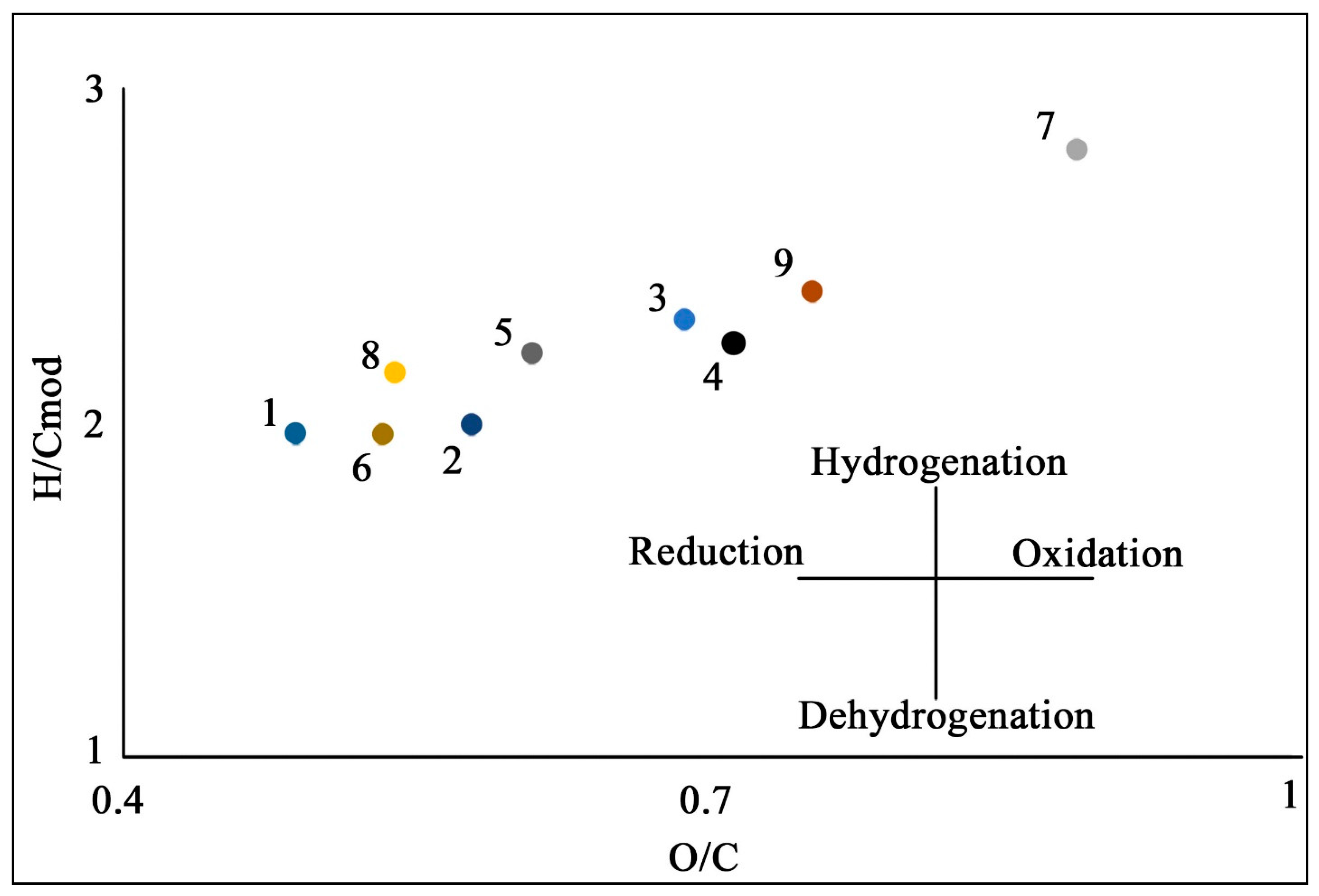
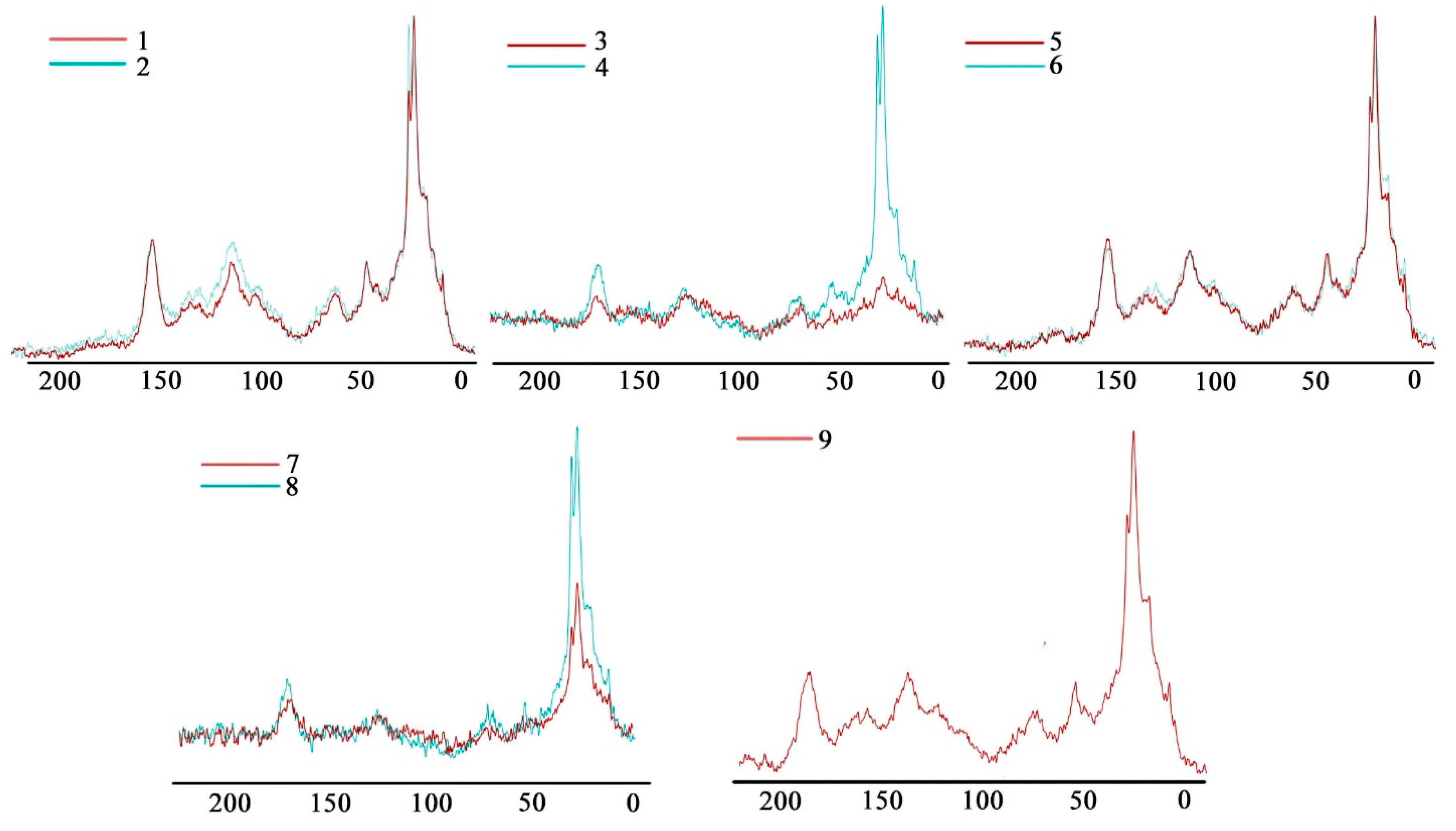
| Site | Soil Profile | Location | Soil ID | Description | Color Index | Vegetation | Soil Name | Sampling Date |
|---|---|---|---|---|---|---|---|---|
| Kurungnah Isl., third terrace | Y-1 | Place of drained lake, pingo with height 1.5 m. N 72°19′19.0″, E 126°15′21.1″ | 1 | Umbric horizon, roots, moderately decomposed organic material. | 10 YR 4/3 | Trisetum, Phragmites. | Umbric Cryosol | 28 August 2019 |
| 2 | Buried, moderately decomposed organic material. Permafrost table from 50 cm. | 10 YR 3/2 | ||||||
| Y-2 | Place of drained lake, pingo with height 2 m. N 72°19′17.7″, E 126°15′20.4″ | 3 | Umbric horizon, roots, moderately decomposed organic material. | 10 YR 4/3 | Trisetum, Phragmites. | Umbric Cryosol | 28 August 2019 | |
| 4 | Buried, moderately decomposed organic material. Permafrost table from 62 cm. | 10 YR 3/2 | ||||||
| Y-3 | Place of drained lake, pingo with height 1.8 m. N 72°19′21.4″, E 126°15′16.3″ | 5 | Umbric horizon, roots, moderately decomposed organic material. | 10 YR 4/3 | Trisetum, Phragmites. | Umbric Cryosol | 5 September 2019 | |
| 6 | Buried, moderately decomposed organic material. Permafrost table from 56 cm. | 10 YR 3/2 | ||||||
| Jipyries Isl., second terrace | S-1 | Border of termocarst lake. N 72°58′55.1″ E 123°48′40.4″ | 7 | Umbric horizon, roots, highly decomposed organic material. | 10 YR 3/2 | Cetraria nivalis, Sphagnum, Carex Aquatilis, Trisetum, Phragmites. | Umbric Cryosol | 5 July 2019 |
| 8 | Buried, highly decomposed organic material. Permafrost table from 41 cm. | 10 YR 3/2 | ||||||
| Chekanovsky Ridge | W-1 | The wind shelter. N 72°19′18.9″, E 125°45′59.1″ | 9 | Highly decomposed organic material in rock shelter. | 10 YR 3/2 | Trisetum, Phragmites, Sphagnum. | Umbric Cryosol | 27 August 2019 |
| Soil ID | pH in Water | C, % | N, % | C/N | Particle Size Distribution | ||
|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | |||||
| Kurungnah Isl., third terrace | |||||||
| 1 | 6.45 | 1.82 | 0.14 | 13 | 3 | 32 | 65 |
| 2 | 5.95 | 2.86 | 0.22 | 13 | 6 | 68 | 24 |
| 3 | 6.63 | 1.52 | 0.11 | 14 | 2 | 41 | 57 |
| 4 | 5.33 | 1.44 | 0.13 | 11 | 2 | 47 | 51 |
| 5 | 6.41 | 2.65 | 0.19 | 14 | 3 | 32 | 65 |
| 6 | 5.44 | 2.24 | 0.13 | 17 | 3 | 15 | 82 |
| Jipyries Isl., second terrace | |||||||
| 7 | 6.32 | 0.55 | 0.06 | 9 | 5 | 13 | 82 |
| 8 | 5.34 | 1.48 | 0.16 | 9 | 6 | 28 | 66 |
| Chekanovsky Ridge | |||||||
| 9 | 5.99 | 4.32 | 0.36 | 12 | 9 | 64 | 27 |
| Standard deviation | 0.51 | 1.08 | 0.09 | 3 | 2 | 19 | 21 |
| Soil ID | Elemental Composition | Atomic Ratio | Extraction Yields, % of SOM | Molecular Weight | |||||
|---|---|---|---|---|---|---|---|---|---|
| N, % | C, % | H, % | O, % | H/C | O/C | H/Cmod | |||
| Kurungnah Isl., third terrace | |||||||||
| 1 | 4 | 52 | 5 | 34 | 1.3 | 0.49 | 1.97 | 51 | 1016 |
| 2 | 3 | 49 | 5 | 38 | 1.2 | 0.58 | 2.00 | 45 | 883 |
| 3 | 3 | 45 | 6 | 41 | 1.4 | 0.69 | 2.31 | 50 | 888 |
| 4 | 2 | 45 | 5 | 43 | 1.3 | 0.72 | 2.25 | 52 | 1257 |
| 5 | 4 | 47 | 6 | 38 | 1.4 | 0.61 | 2.22 | 41 | 719 |
| 6 | 4 | 50 | 5 | 36 | 1.3 | 0.53 | 1.97 | 35 | 1905 |
| Jipyries Isl., second terrace | |||||||||
| 7 | 3 | 40 | 5 | 47 | 1.6 | 0.89 | 2.84 | 49 | 854 |
| 8 | 4 | 50 | 5 | 36 | 1.4 | 0.54 | 2.16 | 20 | 1405 |
| Chekanovsky Ridge | |||||||||
| 9 | 4 | 43 | 5 | 43 | 1.4 | 0.75 | 2.41 | 34 | 1466 |
| Standard deviation | 0.72 | 3.86 | 0.44 | 4.21 | 0.11 | 0.12 | 0.27 | 10 | 383 |
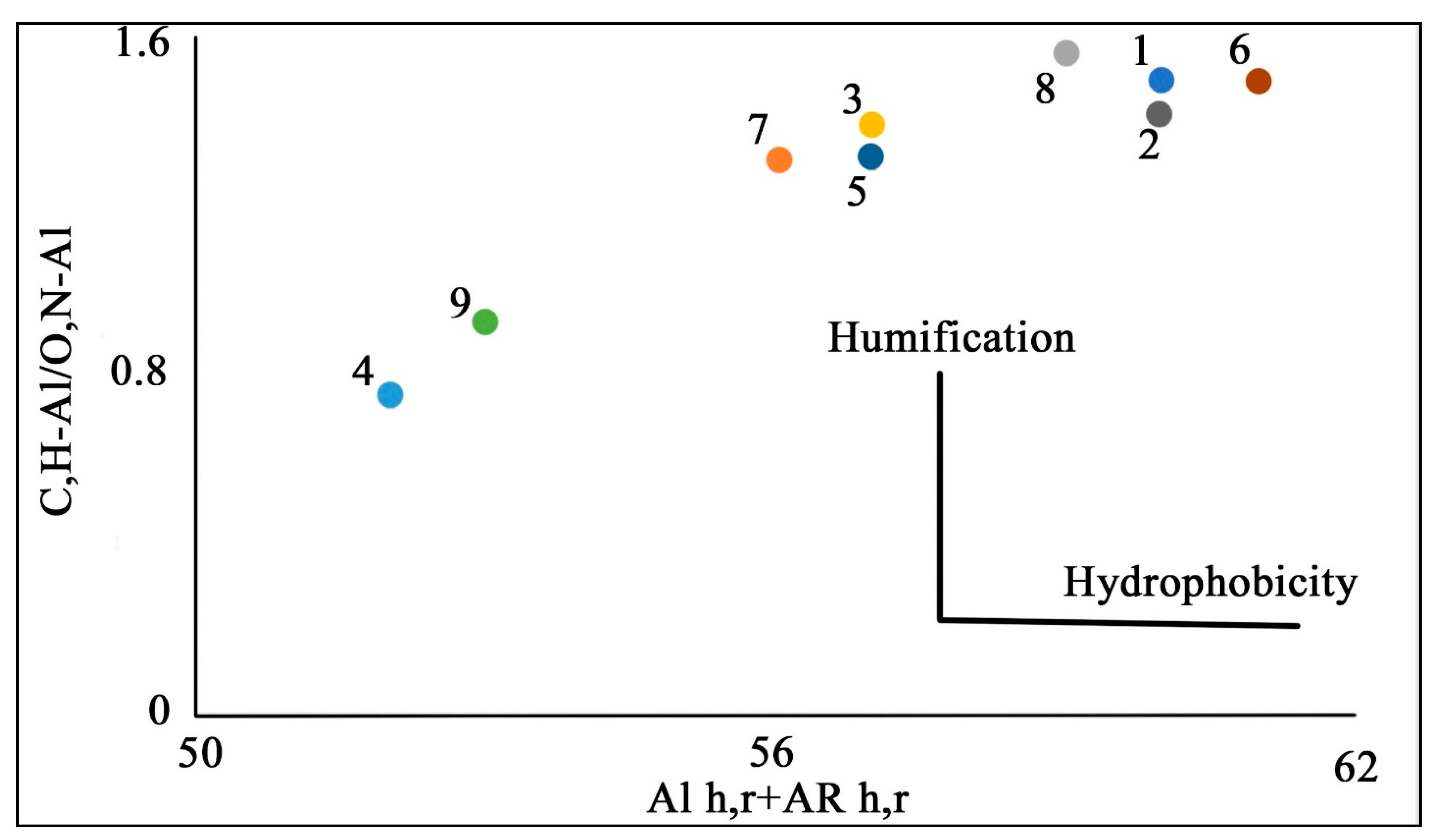
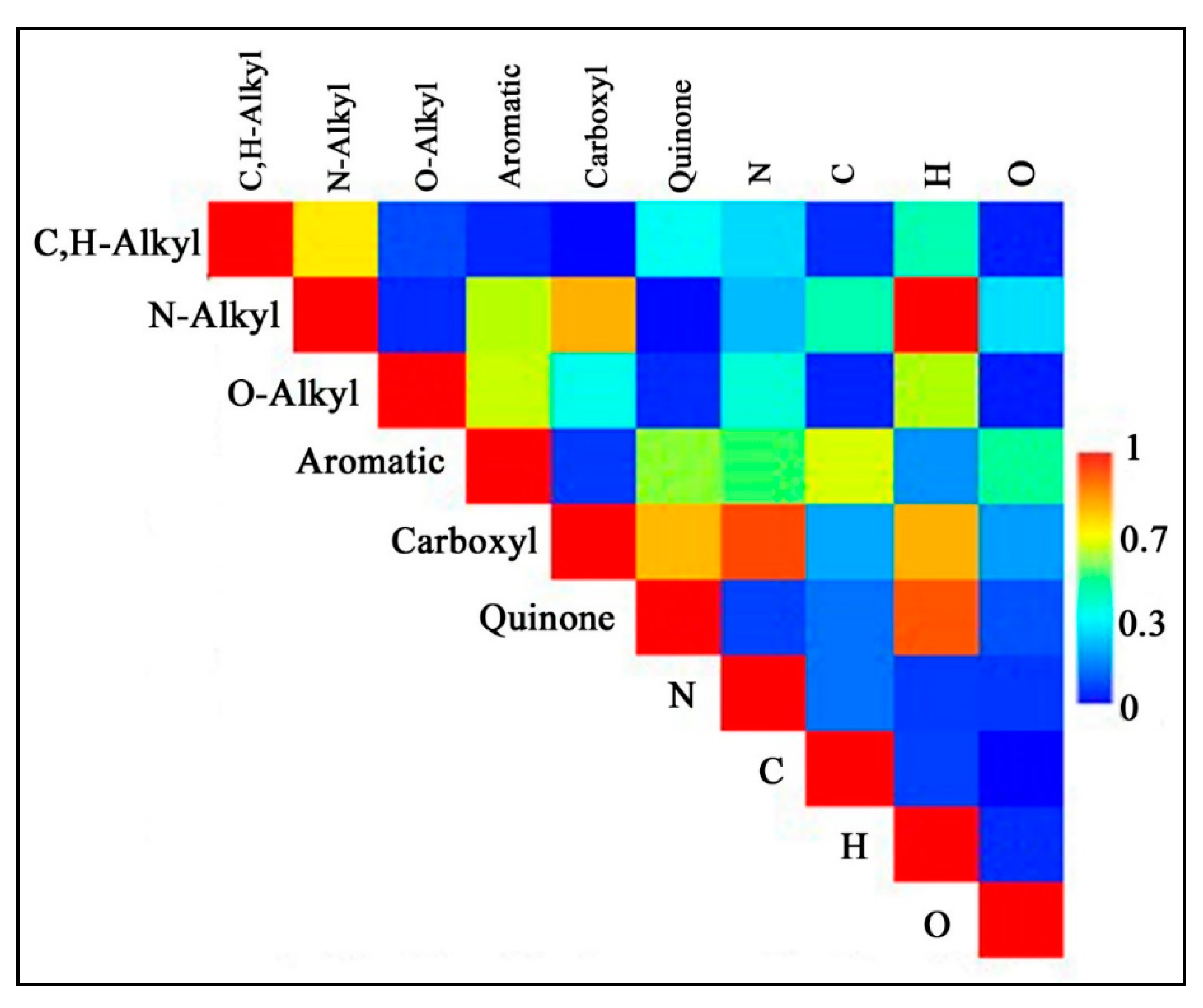
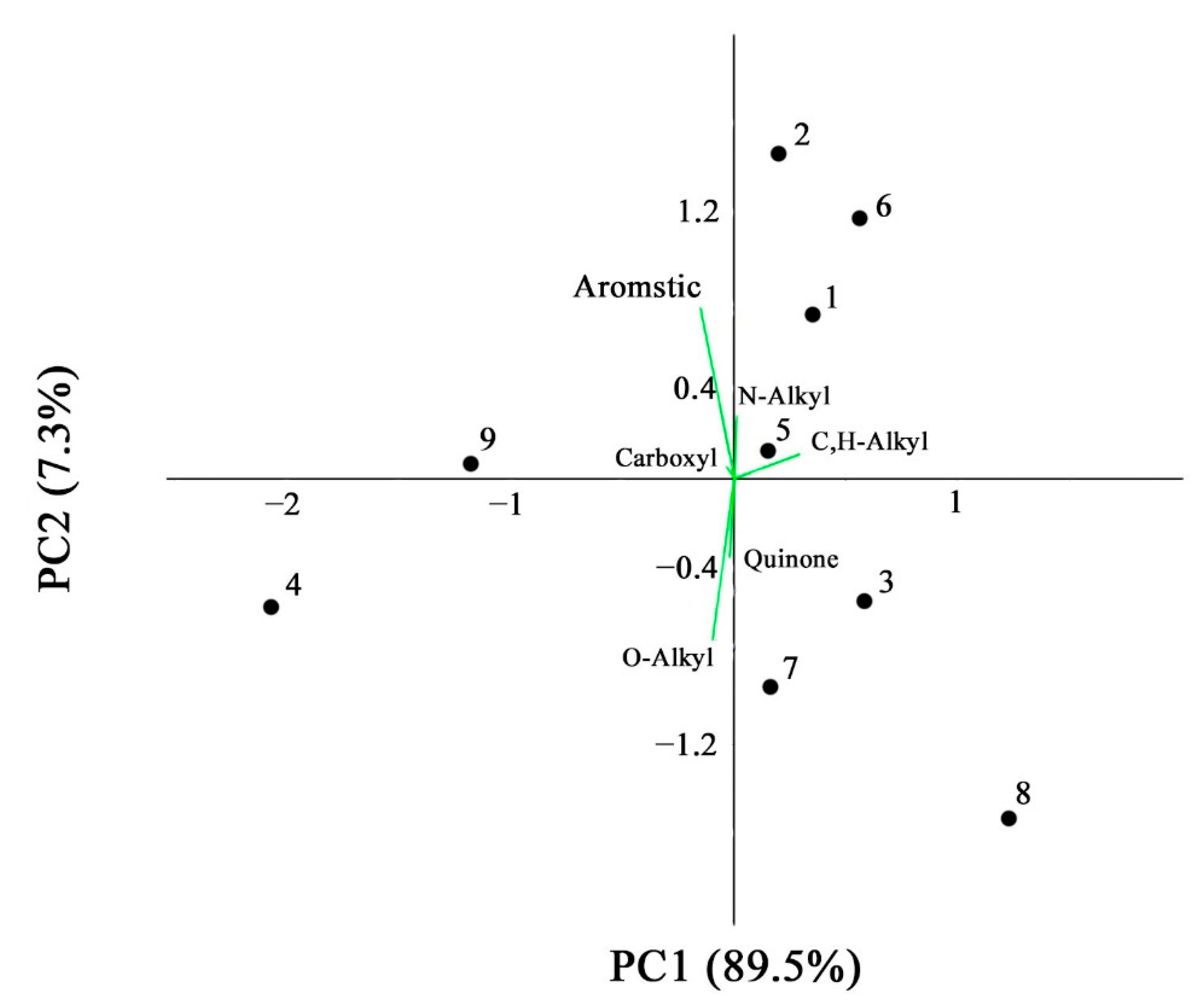
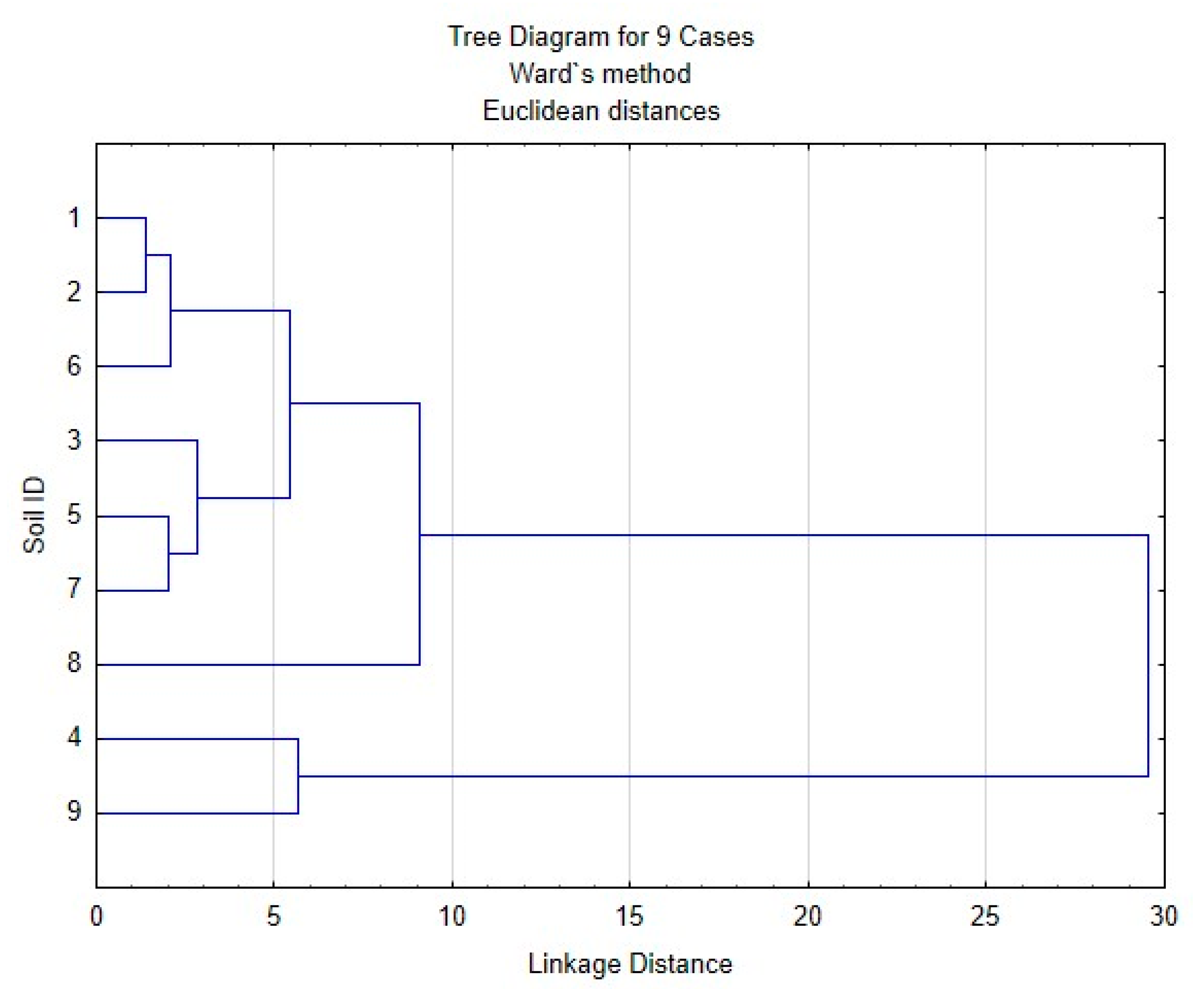
| Soil ID | Chemical Shifts, % | AR | AL | AR/AL | AL h,r + AR h,r * | C,H–AL/O,N–AL ** | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–46 | 46–60 | 60–110 | 110–160 | 160–185 | 185–200 | ||||||
| 1 | 34 | 7 | 17 | 25 | 11 | 6 | 36 | 64 | 0.53 | 59 | 1.50 |
| 2 | 33 | 7 | 17 | 26 | 11 | 6 | 37 | 63 | 0.58 | 59 | 1.42 |
| 3 | 35 | 7 | 18 | 23 | 10 | 7 | 33 | 67 | 0.48 | 57 | 1.40 |
| 4 | 22 | 6 | 23 | 29 | 11 | 9 | 40 | 60 | 0.68 | 51 | 0.76 |
| 5 | 33 | 7 | 18 | 24 | 11 | 7 | 35 | 65 | 0.54 | 57 | 1.32 |
| 6 | 35 | 7 | 17 | 25 | 10 | 6 | 35 | 65 | 0.53 | 60 | 1.50 |
| 7 | 33 | 6 | 19 | 23 | 11 | 8 | 34 | 66 | 0.52 | 56 | 1.32 |
| 8 | 38 | 6 | 19 | 20 | 10 | 7 | 30 | 70 | 0.42 | 58 | 1.56 |
| 9 | 26 | 7 | 21 | 28 | 12 | 6 | 40 | 60 | 0.65 | 54 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakov, V.; Abakumov, E. Assessments of Organic Carbon Stabilization Using the Spectroscopic Characteristics of Humic Acids Separated from Soils of the Lena River Delta. Separations 2021, 8, 87. https://doi.org/10.3390/separations8060087
Polyakov V, Abakumov E. Assessments of Organic Carbon Stabilization Using the Spectroscopic Characteristics of Humic Acids Separated from Soils of the Lena River Delta. Separations. 2021; 8(6):87. https://doi.org/10.3390/separations8060087
Chicago/Turabian StylePolyakov, Vyacheslav, and Evgeny Abakumov. 2021. "Assessments of Organic Carbon Stabilization Using the Spectroscopic Characteristics of Humic Acids Separated from Soils of the Lena River Delta" Separations 8, no. 6: 87. https://doi.org/10.3390/separations8060087
APA StylePolyakov, V., & Abakumov, E. (2021). Assessments of Organic Carbon Stabilization Using the Spectroscopic Characteristics of Humic Acids Separated from Soils of the Lena River Delta. Separations, 8(6), 87. https://doi.org/10.3390/separations8060087






