Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms
Abstract
1. Introduction
2. Sources of Tomentosin
3. Traditional Uses of Medicinal Plants Containing Tomentosin against Cancer and Inflammation
4. Toxicity of Medicinal Plants Containing Tomentosin
5. Anticancer Activity
5.1. Cellular Level
5.2. Molecular Level
6. Anti-Inflammatory Effect
6.1. Cellular Level
6.2. Molecular Level
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| Bcl-2 | B-cell lymphoma 2 |
| CAT | Catalase |
| CDK | Cyclin-Dependent Kinase |
| COX-2 | Cyclooxygenase-2 |
| DNA | Deoxyribonucleic acid |
| EC50 | Half-maximal effective concentration |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK | Extracellular signal-Regulated Kinase |
| GCC | Gastric Cancer Cell |
| GSH | Glutathione |
| HC | Hepatocellular Carcinoma |
| HPLC-DAD | High-performance Liquid Chromatographic method with Diode-Array Detection |
| IC50 | Half-maximal inhibitory concentration |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| iNOS | Inducible Nitric Oxide Synthase |
| LC-MS (ESI) | Liquid chromatography-mass spectrometer (electrospray ionisation) |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Mitochondrial Membrane Potential |
| MPO | Myeloperoxidase |
| MPTP | 1-methyl-4-Phenyl-1,2,3,6-Tetrahydro-Pyridine |
| mTOR | Mammalian Target of Rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 |
| NO | Nitric Oxide |
| OGD-R | Oxygen-glucose deprivation/reperfusion |
| PARP | Poly(ADP-ribose) polymérase |
| PBMC | Peripheral Blood Mononuclear Cells |
| PD | Parkinson’s Disease |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol-3-kinase |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TLR4 | Toll-like Receptor 4 |
| TNF-α | Tumour Necrosis Factor-α |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive Profile of Pumpkin: An Overview on Terpenoids and Their Health-Promoting Properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021, in press. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Benjouad, A.; Ameziane El Hassani, R.; Amzazi, S.; Dakka, N.; Bakri, Y. Pharmacological Properties and Mechanism Insights of Moroccan Anticancer Medicinal Plants: What are the next Steps? Ind. Crops Prod. 2020, 147, 112198. [Google Scholar] [CrossRef]
- El Omari, N.; Bakha, M.; Imtara, H.; Guaouguaoua, F.-E.; Balahbib, A.; Zengin, G.; Bouyahya, A. Anticancer Mechanisms of Phytochemical Compounds: Focusing on Epigenetic Targets. Environ. Sci. Pollut. Res. 2021, 28, 47869–47903. [Google Scholar] [CrossRef] [PubMed]
- Mamoci, E.; Cavoski, I.; Simeone, V.; Mondelli, D.; Al-Bitar, L.; Caboni, P. Chemical Composition and in Vitro Activity of Plant Extracts from Ferula Communis and Dittrichia Viscosa against Postharvest Fungi. Mol. Basel Switz. 2011, 16, 2609–2625. [Google Scholar] [CrossRef]
- Borrelli, F.; Borbone, N.; Capasso, R.; Montesano, D.; De Marino, S.; Aviello, G.; Aprea, G.; Masone, S.; Izzo, A.A. Potent Relaxant Effect of a Celastrus Paniculatus Extract in the Rat and Human Ileum. J. Ethnopharmacol. 2009, 122, 434–438. [Google Scholar] [CrossRef]
- Messaoudi, M.; Chahmi, N.; Mzibri, M.E.; Gmouh, S.; Amzazi, S.; Benbacer, L.; Hassouni, M.E. Cytotoxic Effect and Chemical Composition of Inula Viscosa from Three Different Regions of Morocco. Eur. J. Med. Plants 2016, 16, 1–9. [Google Scholar] [CrossRef]
- El Yaagoubi, O.M.; Lahmadi, A.; Bouyahya, A.; Filali, H.; Samaki, H.; El Antri, S.; Aboudkhil, S. Antitumor Effect of Inula Viscosa Extracts on DMBA-Induced Skin Carcinoma are Mediated by Proteasome Inhibition. BioMed Res. Int. 2021, 2021, e6687589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X. Studies on sesquiterpene lactones from Carpesium faberi. Zhongguo Zhong Yao Za Zhi 2016, 41, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Lee, S.-K.; Kim, C.-S.; Kim, K.-S.; Moon, D.-C. Phytochemical Constituents Of Carpesium macrocephalum FR- et SAV-. Arch. Pharm. Res. 2004, 27, 1029–1033. [Google Scholar] [CrossRef]
- Yang, A.M.; Yu, H.T.; Yang, L.; Zeng, Y.; Men, Y.; Shi, X.L.; Gong, H.F. Sesquiterpenoids from Cremanthodium potaninii. Chem. Nat. Compd. 2015, 51, 1191–1192. [Google Scholar] [CrossRef]
- Abou-Douh, A.M. New Eudesmane Derivatives and Other Sesquiterpenes from the Epigeal Parts of Dittrichia Graveolens. Chem. Pharm. Bull. 2008, 56, 1535–1545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamoci, E.; Cavoski, I.; Andres, M.F.; Díaz, C.E.; Gonzalez-Coloma, A. Chemical Characterization of the Aphid Antifeedant Extracts from Dittrichia viscosa and Ferula communis. Biochem. Syst. Ecol. 2012, 43, 101–107. [Google Scholar] [CrossRef]
- Cohen, Y.; Wang, W.; Ben-Daniel, B.-H.; Ben-Daniel, Y. Extracts of Inula Viscosa Control Downy Mildew of Grapes Caused by Plasmopara viticola. Phytopathology 2006, 96, 417–424. [Google Scholar] [CrossRef]
- Fontana, G.; Rocca, S.L.; Passannanti, S.; Paternostro, M.P. Sesquiterpene Compounds from Inula viscosa. Nat. Prod. Res. 2007, 21, 824–827. [Google Scholar] [CrossRef]
- De Laurentis, N.; Losacco, V.; Milillo, M.; Lai, O. Chemical Investigations of Volatile Constituents of Inula viscosa (L.) Aiton (Asteraceae) from Different Areas of Apulia, Southern Italy. Delpinoa 2002, 44, 115–119. [Google Scholar]
- Lu, Y.; Li, Y.; Jin, M.; Yang, J.H.; Li, X.; Chao, G.H.; Park, H.-H.; Park, Y.N.; Son, J.K.; Lee, E.; et al. Inula Japonica Extract Inhibits Mast Cell-Mediated Allergic Reaction and Mast Cell Activation. J. Ethnopharmacol. 2012, 143, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Piao, D.; Kim, T.; Zhang, H.Y.; Choi, H.G.; Lee, C.S.; Choi, H.J.; Chang, H.W.; Woo, M.-H.; Son, J.-K. DNA Topoisomerase Inhibitory Activity of Constituents from the Flowers of Inula Japonica. Chem. Pharm. Bull. 2016, 64, 276–281. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, Q.; Ren, J.; Qin, J.; Zhang, S.; Shen, Y.; Zhu, J.; Zhang, F.; Chang, R.; Zhu, Y.; et al. Sesquiterpene Lactones from Inula falconeri, a Plant Endemic to the Himalayas, as Potential Anti-Inflammatory Agents. Eur. J. Med. Chem. 2011, 46, 5408–5415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-R.; Ye, J.; Ren, J.; Zeng, Q.; Zhang, F.; Qin, J.-J.; Shen, Y.-H.; Zhang, W.-D.; Jin, H.-Z. Terpenoids from Inula sericophylla Franch. and Their Chemotaxonomic Significance. Biochem. Syst. Ecol. 2012, 42, 75–78. [Google Scholar] [CrossRef]
- Nie, L.-Y.; Qin, J.-J.; Huang, Y.; Yan, L.; Liu, Y.-B.; Pan, Y.-X.; Jin, H.-Z.; Zhang, W.-D. Sesquiterpenoids from Inula lineariifolia Inhibit Nitric Oxide Production. J. Nat. Prod. 2010, 73, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-J.; Zhu, J.-X.; Zeng, Q.; Cheng, X.-R.; Zhang, S.-D.; Jin, H.-Z.; Zhang, W.-D. Sesquiterpene Lactones from Inula hupehensis Inhibit Nitric Oxide Production in RAW264.7 Macrophages. Planta Med. 2012, 78, 1002–1009. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Nakamura, S.; Tawfik, W.A.; Abdel-Azim, N.S.; Abdel-Lateff, A.; Matsuda, H.; Paré, P.W. Rare Hydroperoxyl Guaianolide Sesquiterpenes from Pulicaria Undulata. Phytochem. Lett. 2015, 12, 177–181. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfour, H.Z.; Khayat, M.T. Undulaterpene A: A New Triterpene Fatty Acid Ester from Pulicaria Undulata. Pharmacogn. Mag. 2019, 15, 671. [Google Scholar] [CrossRef]
- Meragelman, K.M.; Espinar, L.A.; Sosa, V.E.; Uriburu, M.L.; de la Fuente, J.R. Terpenoid Constituents of Viguiera tucumanensis. Phytochemistry 1996, 41, 499–502. [Google Scholar] [CrossRef]
- Aranjani, J.M.; Manuel, A.; Mallikarjuna Rao, C.; Udupa, N.; Rao, J.V.; Joy, A.M.; Gandhi, P.; Radhakrishnan, E.K. Preliminary Evaluation of in Vitro Cytotoxicity and in Vivo Antitumor Activity of Xanthium Strumarium in Transplantable Tumors in Mice. Am. J. Chin. Med. 2013, 41, 145–162. [Google Scholar] [CrossRef]
- Bui, V.-B.; Liu, S.-T.; Zhu, J.-J.; Xiong, J.; Zhao, Y.; Yang, G.-X.; Xia, G.; Hu, J.-F. Sesquiterpene Lactones from the Aerial Parts of Xanthium sibiricum and Their Cytotoxic Effects on Human Cancer Cell Lines. Phytochem. Lett. 2012, 5, 685–689. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Rubio-Rosas, E.; Ruiz-González, N.; Martínez-Orea, Y.; Cruz-Duran, R.; Ramirez-Garcia, S.A.; Ramón-Canúl, L.G. Lipophilic Constituents and Some Biological Activities of Hexanic Extracts from Zaluzania montagnifolia, (SCH. BIP.) SCH. BIP. (Asteraceae). Agrociencia 2013, 47, 335–346. [Google Scholar]
- Belmimoun, A.; Meddah, B.; Side Larbi, K.; Sonnet, P. Phytochemical study of Zygophyllum album extract. Int. J. Eng. Technol. Manag. Res. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative Activity of Plant Extracts Used Against Cancer in Traditional Medicine. Sci. Pharm. 2010, 78, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A Systematic Review on Ethnomedicines of Anti-Cancer Plants. Phytother. Res. PTR 2017, 31, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A. Traditional Uses of Iraqi Medicinal Plants. IOSR J. Pharm. 2018, 8, 32–96. [Google Scholar]
- Lin, R.; Yu, D.J.; Wu, Z.Y. Inula L Flora of China; Beijing Science Press: Beijing, China, 1989; pp. 263–281. [Google Scholar]
- Saidi, H.; Mofidi, M. Toxic effect of Xanthium strumarium as an herbal medicine preparation. EXCLI J. 2009, 3, 115–117. [Google Scholar]
- Guemmaz, T.; Zerargui, F.; Boumerfeg, S.; Arrar, L.; Aouachria, S.; Khennouf, S.; Charef, N.; Baghiani, A. Anti-Hemolytic, Anti-Lipid Peroxidation, Antioxidant Properties and Acute Toxicity of Xanthium strumarium Leaves Extracts. Annu. Res. Rev. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Aranjani, J.M.; Rao, C.M.; Manuel, A.; Rao, J.V.; Udupa, N.; Hebbar, K. Acute and Subacute Toxicity of Chloroform and Hexane extracts of Root of Xanthium strumarium. Comp. Clin. Pathol. 2012, 21, 1223–1230. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.J.; Yang, L.; Zhang, J.-X.; Hou, A.-J.; Man, W.-J.; Wang, S.; Yang, B.-Y.; Chan, K.; Wang, Q.-H. The Fruits of Xanthium sibiricum Patr: A Review on Phytochemistry, Pharmacological Activities, and Toxicity. World J. Tradit. Chin. Med. 2020, 6, 408–422. [Google Scholar]
- Sung, C.Y.; Cheng, L.Y.; Hsieh, M.C.; Li, C.H.; Kuo, K.W. The toxic principle of Tsang-er-tzu (The seed of Xanthium strumarium L.) and its pharmacological actions. Acta Pharm. Sin. 1962, 11, 678–684. [Google Scholar]
- Ouahchia, C.; Cherif, H.S.; Hamaidi-Chergui, F.; Marzen, L.; Deradji, S.; Hemma, R.; Nouar, N.; Saidi, F. Acute and subacute toxicity of Inula viscosa L. (Dittrichia viscosa L.) methanolic extracts. AgroBiologia 2017, 7, 562–573. [Google Scholar]
- Tchaker, F.Z.; Merah, O.; Djazouli, Z. Toxicity Evaluation of Dittrichia viscosa L’s Aqueous Extracts in Combination with Bio-Adjuvant Silene Fuscata on Chaitophorus Leucomelas Koch. (Hom., Aphididae) and on Biocenotic Resumption of Functional Groups. Jordan J. Agric. Sci. 2016, 12, 797–814. [Google Scholar]
- Schwartz, G.K. Development of Cell Cycle Active Drugs for the Treatment of Gastrointestinal Cancers: A New Approach to Cancer Therapy. J. Clin. Oncol. 2005, 23, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M Arrest and Apoptosis by Sesquiterpene Lactones in Human Melanoma Cell Lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Weng, L.; Gao, X.; Zhao, Y.; Pang, F.; Liu, J.-H.; Zhang, H.-F.; Hu, J.-F. Antiproliferative and Apoptotic Sesquiterpene Lactones from Carpesium faberi. Bioorg. Med. Chem. Lett. 2011, 21, 366–372. [Google Scholar] [CrossRef]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Attaleb, M.; Madoulet, C.; Wenner, T.; Amzazi, S. Tomentosin Induces Telomere Shortening and Caspase-Dependant Apoptosis in Cervical Cancer Cells. J. Cell. Biochem. 2017, 118, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.-H. Tomentosin Displays Anti-Carcinogenic Effect in Human Osteosarcoma MG-63 Cells via the Induction of Intracellular Reactive Oxygen Species. Int. J. Mol. Sci. 2019, 20, 1508. [Google Scholar] [CrossRef] [PubMed]
- See-Hyoung Park. Activation of Apoptosis Signaling by Tomentosin in Hepatocellular Carcinoma Cell Line HepG2 and Huh-7 Cells. International Conference of the Korean Society for Molecular and Cellular Biology; Korean Society for Molecular and Cellular Biology, 2019. Available online: https://scholarworks.bwise.kr/hongik/handle/2020.sw.hongik/1103 (accessed on 31 October 2021).
- Yang, H.; Zhao, H.; Dong, X.; Yang, Z.; Chang, W. Tomentosin Induces Apoptotic Pathway by Blocking Inflammatory Mediators via Modulation of Cell Proteins in AGS Gastric Cancer Cell Line. J. Biochem. Mol. Toxicol. 2020, 34, e22501. [Google Scholar] [CrossRef]
- Yang, L.; Xie, J.; Almoallim, H.S.; Alharbi, S.A.; Chen, Y. Tomentosin Inhibits Cell Proliferation and Induces Apoptosis in MOLT-4 Leukemia Cancer Cells through the Inhibition of MTOR/PI3K/Akt Signaling Pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22719. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, C.M.; Ha, S.H.; Lee, J.; Jang, K.Y.; Park, S.H. Induction of Cell Cycle Arrest and Apoptosis by Tomentosin in Hepatocellular Carcinoma HepG2 and Huh7 Cells. Hum. Exp. Toxicol. 2021, 40, 231–244. [Google Scholar] [CrossRef]
- Shieh, S.-Y.; Ikeda, M.; Taya, Y.; Prives, C. DNA Damage-Induced Phosphorylation of P53 Alleviates Inhibition by MDM2. Cell 1997, 91, 325–334. [Google Scholar] [CrossRef]
- Dothager, R.S.; Putt, K.S.; Allen, B.J.; Leslie, B.J.; Nesterenko, V.; Hergenrother, P.J. Synthesis and Identification of Small Molecules That Potently Induce Apoptosis in Melanoma Cells through G1 Cell Cycle Arrest. J. Am. Chem. Soc. 2005, 127, 8686–8696. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.; McNiff, J.M.; Li, F.; Altieri, D.C. Expression and Targeting of the Apoptosis Inhibitor, Survivin, in Human Melanoma. J. Investig. Dermatol. 1999, 113, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Adida, C.; Altieri, D.C. A Novel Anti-Apoptosis Gene, Survivin, Expressed in Cancer and Lymphoma. Nat. Med. 1997, 3, 917–921. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Reed, J.C. IAP Family Proteins—Suppressors of Apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Pannati, M.; Diadone, M.G. Survivin as a Target for New Anticancer Interventions. J. Cell. Mol. Med. 2005, 9, 360–372. [Google Scholar] [CrossRef]
- Grandin, N.; Charbonneau, M. Protection against Chromosome Degradation at the Telomeres. Biochimie 2008, 90, 41–59. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of Survival, Proliferation, Invasion, Angiogenesis, and Metastasis of Tumor Cells through Modulation of Inflammatory Pathways by Nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Prasad, S.; Ravindran, J.; Aggarwal, B.B. NF-κB and Cancer: How Intimate Is This Relationship. Mol. Cell. Biochem. 2010, 336, 25–37. [Google Scholar] [CrossRef]
- Biswas, D.K.; Cruz, A.P.; Gansberger, E.; Pardee, A.B. Epidermal Growth Factor-Induced Nuclear Factor κB Activation: A Major Pathway of Cell-Cycle Progression in Estrogen-Receptor Negative Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 8542–8547. [Google Scholar] [CrossRef]
- Brotelle, T.; Bay, J.-O. La Voie de Signalisation PI3K-AKT-MTOR: Description, Développement Thérapeutique, Résistances, Marqueurs Prédictifs/Pronostiques et Applications Thérapeutiques En Cancérologie. Bull. Cancer 2016, 103, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and P38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.-C. ERK and Cell Death: Mechanisms of ERK-Induced Cell Death–Apoptosis, Autophagy and Senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Abrham, G.; Dovrat, S.; Bessler, H.; Grossman, S.; Uri, N.; Bergman, M. Inhibition of Inflammatory Cytokine Secretion by Plant-Derived Compounds Inuviscolide and Tomentosin: The Role of NFκB and STAT1. Open Pharmacol. J. 2010, 4, 36–44. [Google Scholar] [CrossRef]
- Park, H.-H.; Kim, S.-G.; Kim, M.J.; Lee, J.; Choi, B.-K.; Jin, M.-H.; Lee, E. Suppressive Effect of Tomentosin on the Production of Inflammatory Mediators in RAW264. 7 Cells. Biol. Pharm. Bull. 2014, 37, 1177–1183. [Google Scholar] [CrossRef]
- He, J.; Wu, H.; Zhou, Y.; Zheng, C. Tomentosin Inhibit Cerebral Ischemia/Reperfusion Induced Inflammatory Response via TLR4/NLRP3 Signalling Pathway–in Vivo and in Vitro Studies. Biomed. Pharmacother. 2020, 131, 110697. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Y.; Sun, J.; Fan, C.; Wan, J. Tomentosin Inhibits Lipopolysaccharide-Induced Acute Lung Injury and Inflammatory Response by Suppression of the NF-κB Pathway in a Mouse Model of Sepsis. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 291–298. [Google Scholar] [CrossRef]
- Fan, Y.; Maghimaa, M.; Chinnathambi, A.; Alharbi, S.A.; Veeraraghavan, V.P.; Mohan, S.K.; Hussain, S.; Rengarajan, T. Tomentosin Reduces Behavior Deficits and Neuroinflammatory Response in MPTP-Induced Parkinson’s Disease in Mice. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 75–84. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Sharon, P.; Ligumsky, M.; Rachmilewitz, D.; Zor, U. Role of Prostaglandins in Ulcerative Colitis: Enhanced Production during Active Disease and Inhibition by Sulfasalazine. Gastroenterology 1978, 75, 638–640. [Google Scholar] [CrossRef]
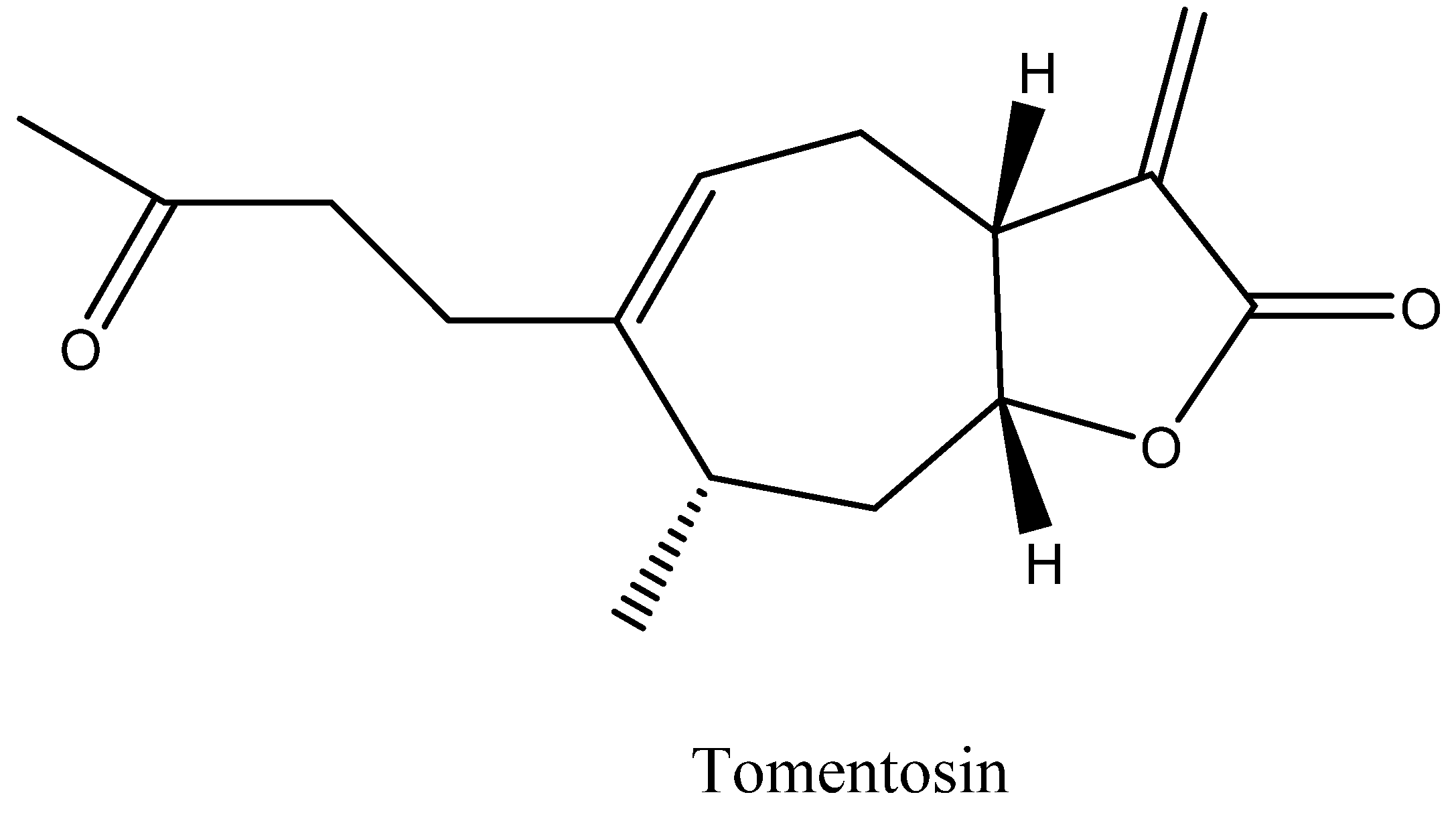
| Sources | Countries | Parts Used | Tomentosin Contents | References |
|---|---|---|---|---|
| Carpesium macrocephalum | Korea | Whole plant | nd | [13] |
| Cremanthodium potaninii | China | Whole plant | nd | [14] |
| Dittrichia graveolens | Egypt | Epigeal parts | nd | [15] |
| Dittrichia viscosa | Italy | Leaves | 235.41 mg/g extract | [16] |
| Dittrichia viscosa | Italy | Leaves | 205.80 mg/g extract | [8] |
| Inula viscosa | Israel | Leaves | 10.6% of the total paste weight | [17] |
| Inula viscosa | Italy | Aerial parts | nd | [18] |
| Inula viscosa | Italy | Leaves | 2% of dry matter | [19] |
| Inula viscosa | Morocco | nd | 22–64% of the dry paste | [10] |
| Inula japonica | Korea | Flowers | nd | [20] |
| Inula japonica | Korea | Flowers | nd | [21] |
| Inula falconeri | China | Aerial parts | nd | [22] |
| Inula sericophylla | China | Whole plant | nd | [23] |
| Inula lineariifolia | China | Aerial parts | nd | [24] |
| Inula hupehensis | China | Aerial parts | nd | [25] |
| Pulicaria undulata | Egypt | Aerial parts | nd | [26] |
| Pulicaria undulata | Saudi Arabia | Aerial parts | nd | [27] |
| Viguiera tucumanensis | Argentina | Aerial parts | nd | [28] |
| Xanthium strumarium | India | Whole plant | nd | [29] |
| Xanthium sibiricum | China | Aerial parts | nd | [30] |
| Zaluzania montagnifolia | Mexico | Leaves | 0.87% | [31] |
| Zygophyllum album | Algeria | Aerial parts | nd | [32] |
| Cells Tested | Methods Used | Key Results | References |
|---|---|---|---|
| MCF-7 human breast cancer cells | MTT assay | Anti-proliferative activity in vitro IC50 values between 3.0 ± 0.1 and 31.9 ± 1.6 μg/mL | [46] |
| AGS gastric cancer cell line | Enzyme-linked immunosorbent assay Western blot analysis | Inhibited the cell proliferation Diminished NF-κB, TNF-α, IL-1β, and IL-6 expression levels Up-regulated the Bcl-2 and Bax expression | [50] |
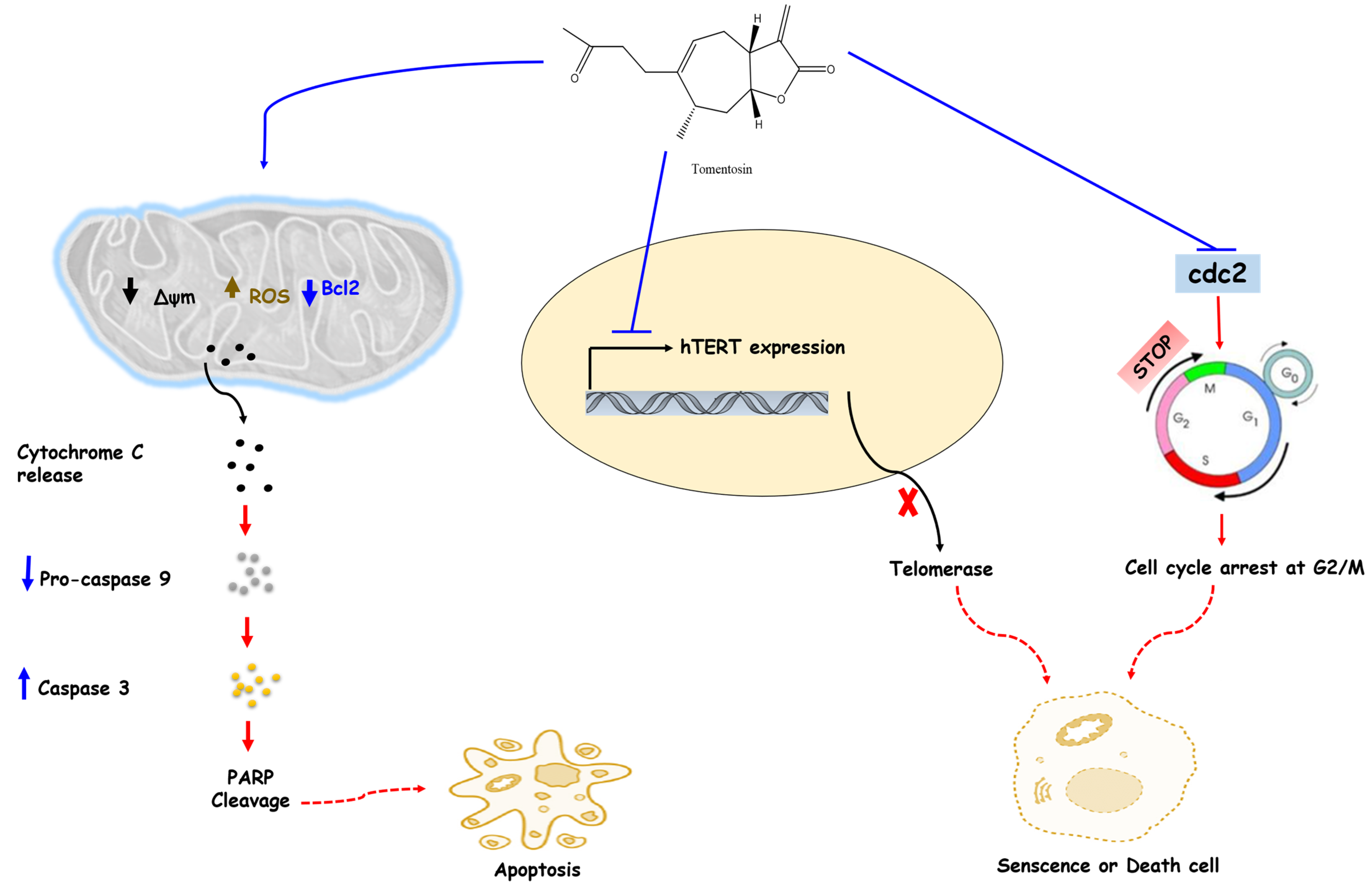
| Human melanoma cell lines | MTT assay Flow cytometry Western blot analysis | Inhibited the proliferation of three human melanoma cells (SK-28, 624 mel, and 1363 mel) Induced the cell cycle arrest at G2/M Induced the apoptosis Reduced protein expression of both Cdc2 (Cdk1, p34) and cyclin b1 Increased the expression level of p53 Decreased survivin expression in SK-28 cells | [45] |
| Human cervical cancer HeLa and SiHa cell lines | MTT assay Western blot analysis Celle cycle analysis | Inhibited the growth of SiHa (IC50 = 7.10 ± 0.78 μM) and HeLa (IC50 = 5.87 ± 0.36 μM) cell lines in a dose- and time-dependent manner Induced apoptosis and cell cycle arrest at G2/M Increased ROS production Decreased Bcl-2 expression Cleavage of the 113kD PARP protein into 89kD fragments | [47] |
| Human osteosarcoma MG-63 cells | Cell migration/viability/proliferation, apoptosis, and ROS analysis assays Cell cycle analysis | Decreased cell viability and migration ability Induced apoptosis, cell cycle arrest, DNA damage, and ROS production Decreased cell viability and induced apoptosis, cell cycle arrest, and DNA damage | [48] |
| Human leukemia (MOLT-4) cell line | qRT-PCR Cytotoxic assay | Inhibited cell proliferation Increased intracellular ROS production Inhibited the mTOR/PI3K/Akt signaling pathway Inhibited the inflammatory transcription factors (NF-κB, TNF-α, IL-1β, and IL-6) Induced the apoptosis in MOLT-4 cells | [51] |
| Hepatocellular carcinoma cell lines (HepG2 and Huh-7 cells) | Western blot analysis | Activation of apoptosis signaling | [49] |
| Human hepatocellular carcinoma cell lines (HepG2 and Huh7 cells) | Cell counting Colony formation assay TUNEL assay Western blot analysis | Decreased the viability and suppressed the proliferation rate of HepG2 and Huh7 cells in a dose- and time-dependent manner Increased population of cells at the SubG1 and G2/M stage Decreased population of cells at the G0/1 stage Increased apoptotic cell population and DNA fragmentation Decreased the expression levels of cell cycle-related proteins (CDK2, CDK4, CDK6, cyclin B1, cyclin D1, cyclin D2, cyclin D3, and cyclin E) and apoptosis-related proteins (PAPR-1, Bcl-2, caspase-3, caspase-7, and caspase-9) in Huh7 and HepG2 cells | [52] |
| Cells Tested/Animal Models | Methods Used | Key Results | References |
|---|---|---|---|
| Peripheral blood mononuclear cells (PBMCs) | In vitro effect on the pro-inflammatory cytokines secretion from human PBMCs Western blot analysis | Decreased production of IL-2, IL-1β, and IFNγ Slightly increased secretion of TNF-α Reduced the protein level of p65/RelA of NF-κB and STAT1 by proteosomal degradation | [67] |
| RAW264. 7 cells | Effect on the production of inflammatory mediators as well as on the activation of NF-κB and MAP kinase | Decreased the NO production by suppressing the protein expression of iNOS Decreased the PGE2 production by suppressing the protein expression of COX-2 Reduced the release of pro-inflammatory cytokines Suppressed the phosphorylation of MAP kinases | [68] |
| Healthy male Sprague Dawley rats (in vivo) SH-SY5Y, human neuroblastoma cells (in vitro) | Inhibited neuro-inflammation in in vivo and in vitro models qPCR analysis MTT assay | Decreased neurological deficient Reduced inflammatory cytokine levels Inhibited the NLRP3 inflammasome activation in rats Inhibited the pro-inflammatory cytokines in OGD-R induced SH-SY5Y cells | [69] |
| Male C57/BL6 mice | MPTP-stimulated neuroinflammation in mice ELISA test Western blot analysis | Decreased pro-inflammatory cytokine levels Inhibited the TLR4/NF-κB signaling pathway Prevented inflammation-mediated neuronal cell damage Reduced glial cell damage Normalized ganglion layers | [71] |
| BALB/c mice (mouse model of sepsis) | Effect against LPS-induced acute lung injury via the suppression of TLR4/NF-κB pathway Western blot analysis | Suppressed the status of pro-inflammatory markers Reduced the activation of iNOS, MPO, COX-2, and PGE2 in the lung Down-regulated the TLR4/NF-κB signaling pathway | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Omari, N.; El Menyiy, N.; Zengin, G.; Goh, B.H.; Gallo, M.; Montesano, D.; Naviglio, D.; Bouyahya, A. Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations 2021, 8, 207. https://doi.org/10.3390/separations8110207
El Omari N, El Menyiy N, Zengin G, Goh BH, Gallo M, Montesano D, Naviglio D, Bouyahya A. Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations. 2021; 8(11):207. https://doi.org/10.3390/separations8110207
Chicago/Turabian StyleEl Omari, Nasreddine, Naoual El Menyiy, Gokhan Zengin, Bey Hing Goh, Monica Gallo, Domenico Montesano, Daniele Naviglio, and Abdelhakim Bouyahya. 2021. "Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms" Separations 8, no. 11: 207. https://doi.org/10.3390/separations8110207
APA StyleEl Omari, N., El Menyiy, N., Zengin, G., Goh, B. H., Gallo, M., Montesano, D., Naviglio, D., & Bouyahya, A. (2021). Anticancer and Anti-Inflammatory Effects of Tomentosin: Cellular and Molecular Mechanisms. Separations, 8(11), 207. https://doi.org/10.3390/separations8110207











