Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Amidoxime-Chitosan/Cellulose Hydrogels
2.3. Adsorption of Metal Ions on Hydrogels
3. Results and Discussion
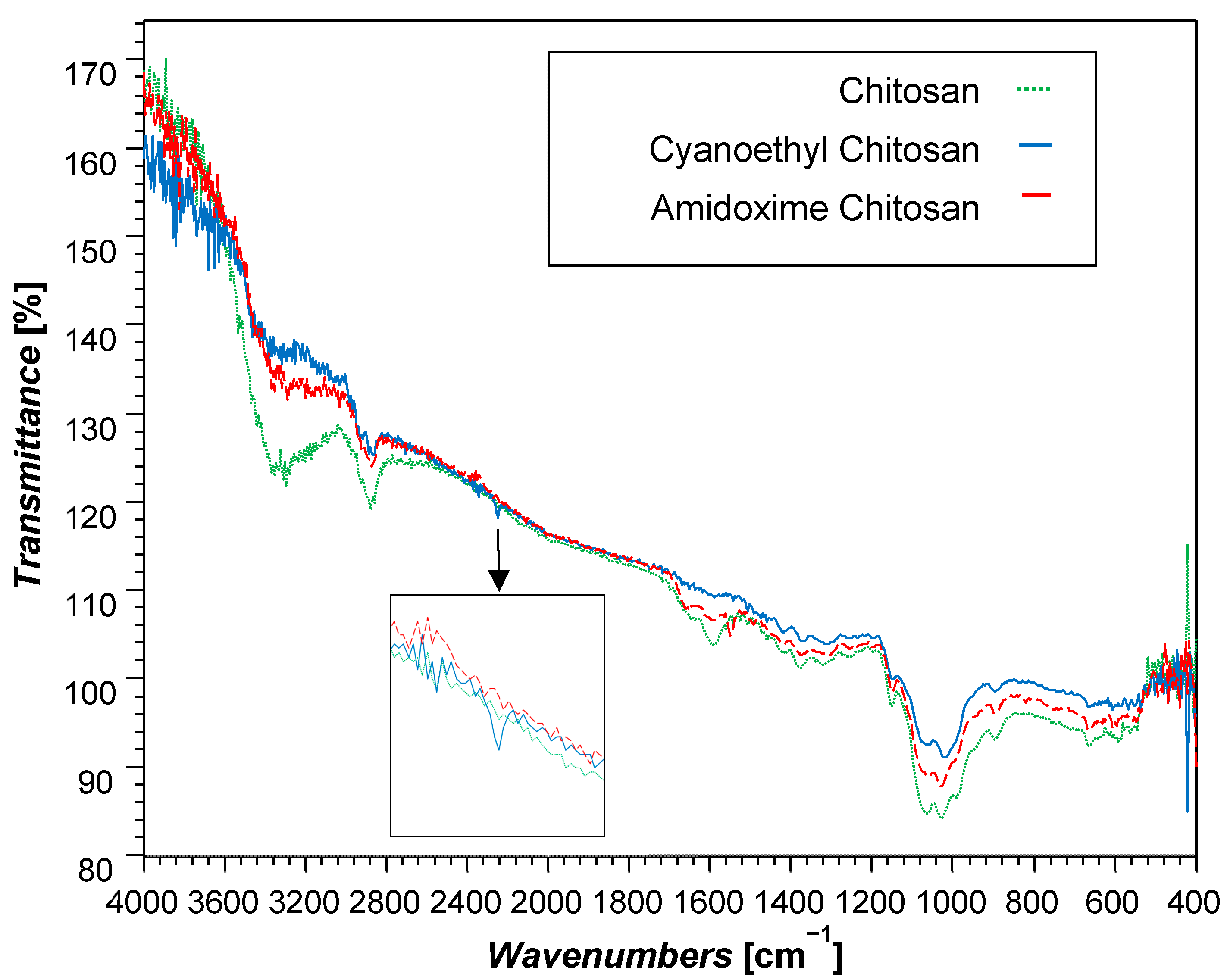
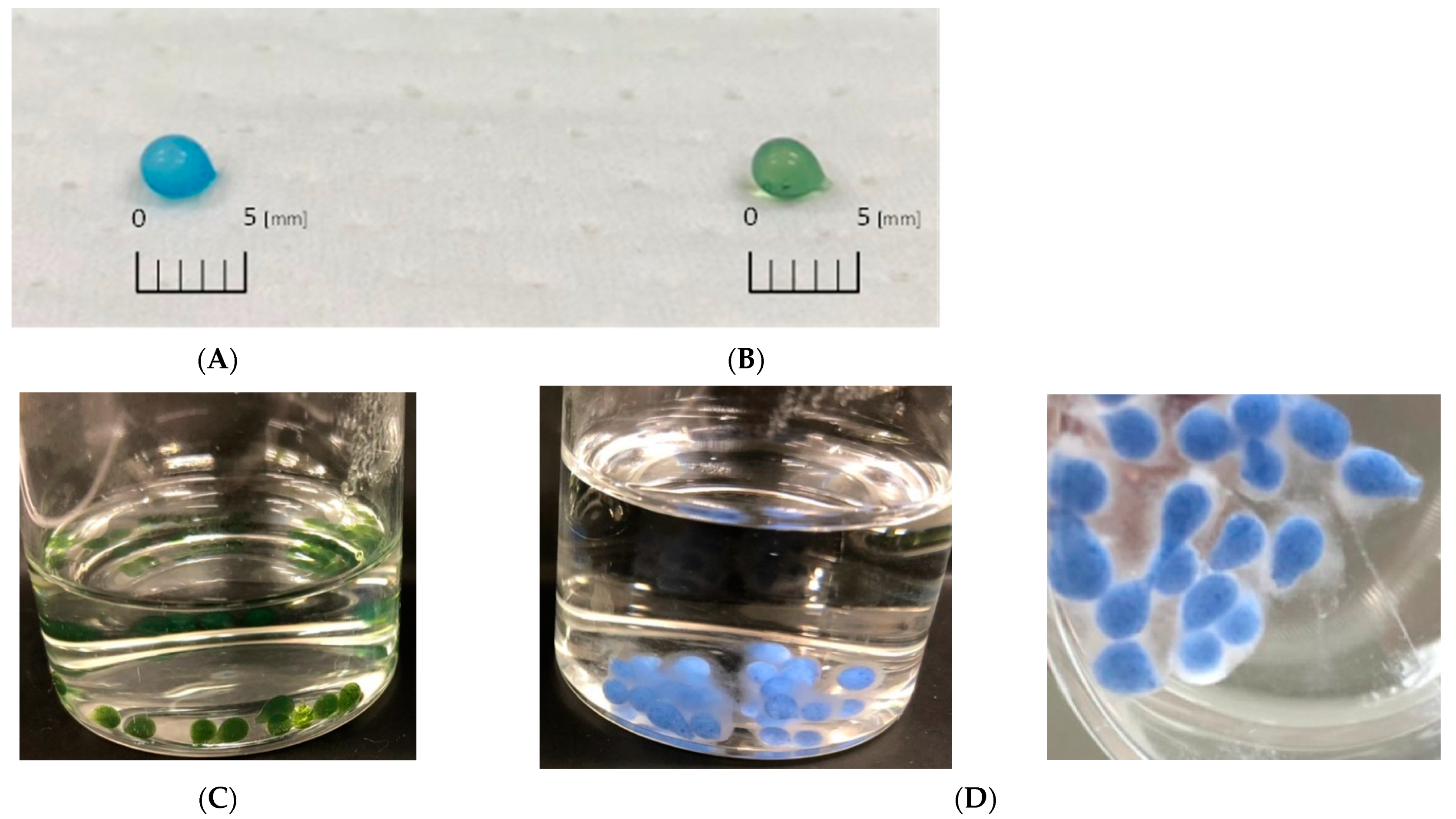
3.1. Characteristics of Amidoxime-Chitosan/Cellulose Hydrogels
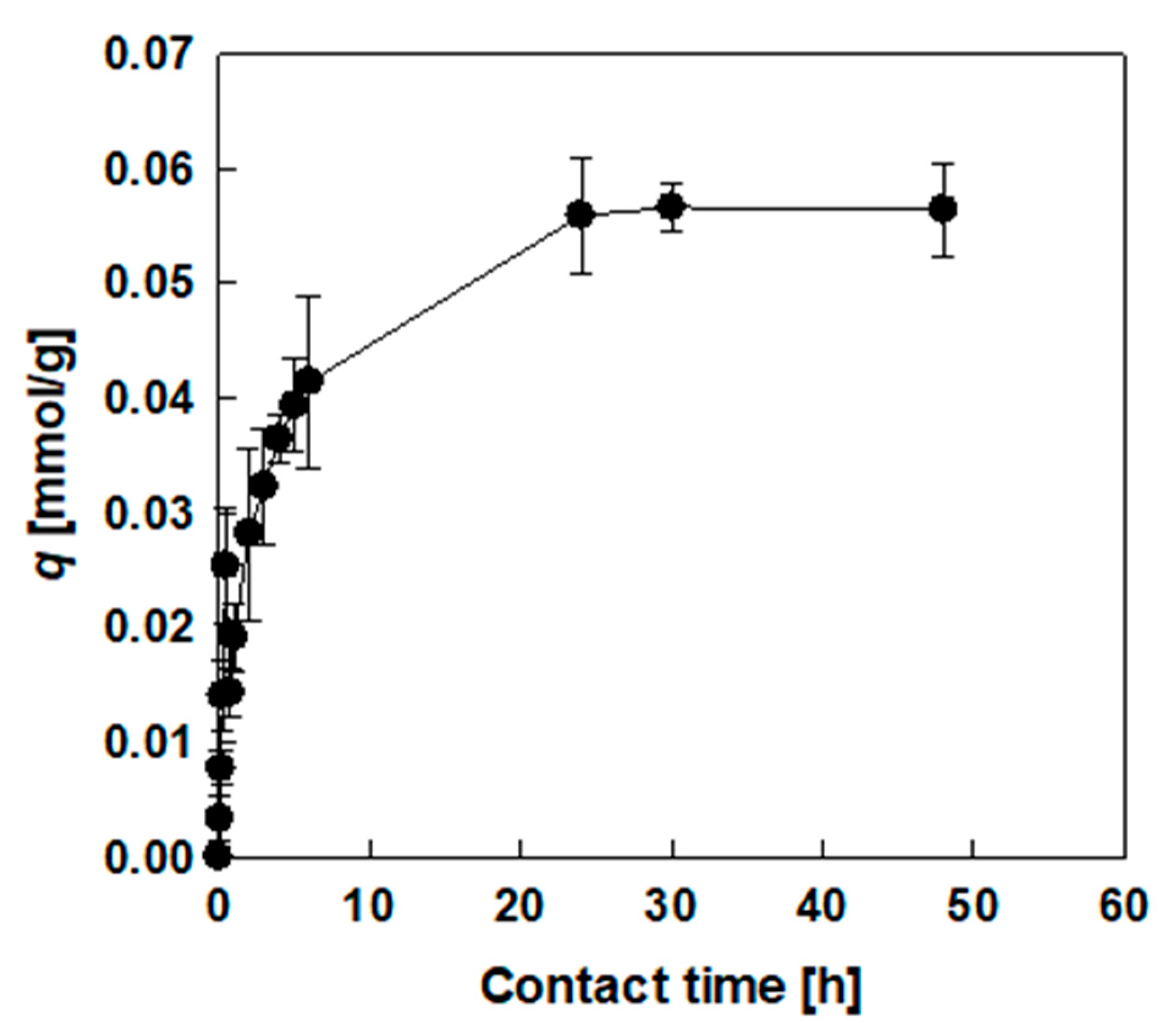
3.2. Effect of Contact Time on Adsorption
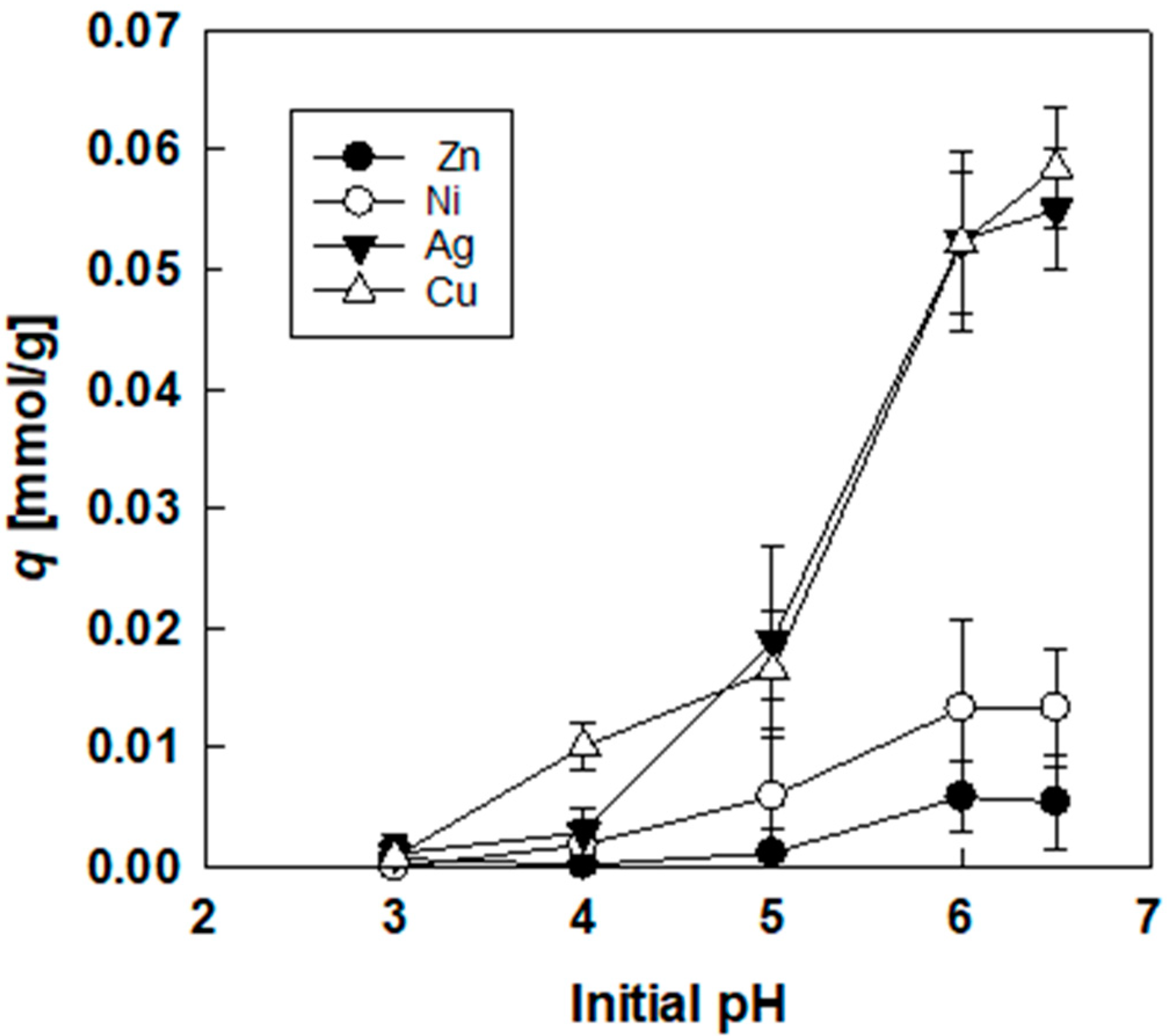
3.3. Effect of Initial pH on Adsorption
3.4. Adsorption Isotherm
3.5. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.; Madan, S.; Madan, R. Removal of heavy metals from wastewater by adsorption. In Heavy Metals—Their Environmental Impacts and Mitigation; Nazal, M., Zhao, H., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of heavy metals by polysaccharide: A review. Polm.-Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.H.N.; Sulong, A.B. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Hiroki, A.; Tran, H.T.; Nagasawa, N.; Yagi, T.; Tamada, M. Metal adsorption of carboxymethyl cellulose/carboxymethyl chitosan blend hydrogels prepared by Gamma irradiation. Radiat. Phys. Chem. 2009, 78, 1076–1080. [Google Scholar] [CrossRef]
- Baroudi, A.; García-Payo, C.; Khayet, M. Structural, mechanical, and transport properties of electron beam-irradiated chitosan membranes at different doses. Polymers 2018, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Peng, B.; Ji, Y.; Chen, J.; Li, D. Chitosan (Chitin)/cellulose composite biosorbents prepared using ionic liquid for heavy metal ions adsorption. AIChE J. 2009, 55, 2062–2069. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ishikawa, S.; Kamigaki, T. Durability of chitosan/cellulose hydrogel beads regenerated from an ionic liquid in acidic and basic media and their metal adsorptive characteristics. Prog. Chem. Appl. Chitin Deriv. 2019, 24, 145–150. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, K.; Wang, J. Fibrous chitosan/cellulose composite as an efficient adsorbent for Co(II) removal. J. Clean. Prod. 2021, 285, 124911. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Lonic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime functionalization of algal/polyethyleneimine beads for the sorption of Sr (II) from aqueous solutions. Molecules 2019, 24, 3893. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Liu, C.; Kong, B.; Sun, J.; Gong, Y.; Liu, K.; Xie, J.; Pei, A.; Cui, Y. Amidoxime-functionalized macroporous carbon self-refreshed electrode materials for rapid and high-capacity removal of heavy metal from water. ACS Cent. Sci. 2019, 5, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.L.; Fui, C.J.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Abdullah, M.H.; Sarkar, S.M.; O’Reilly, E.J. Poly(amidoxime) ligand derived from waste palm fiber for the removal of heavy metals from electroplating wastewater. Environ. Sci. Pollut. Res. 2020, 27, 34541–34556. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga (III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Long, H.; Zhao, Z.; Chai, Y.; Li, X.; Hua, Z.; Xiao, Y.; Yang, Y. Binding mechanism of the amidoxime functional group on chelating resins toward gallium (III) in Bayer liquor. Ind. Eng. Chem. Res. 2015, 54, 8025–8030. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Leggett, C.J.; Parker, B.F.; Zhang, Z.; Arnold, J.; Dai, S.; Abney, C.W.; Bryantsev, V.S.; Rao, L. Origin of the unusually strong and selective binding of vanadium by polyamidoximes in seawater. Nat. Commun. 2017, 8, 1560. [Google Scholar] [CrossRef]
- Ao, J.; Zhang, H.; Xu, C.; Hu, X.; Yao, F.; Ma, L.; Zhang, L.; Ye, B.; Li, Q.; Xu, L.; et al. A novel ion-imprinted amidoxime-functionalized UHMWPE fiber based on radiation-induced crosslinking for selective adsorption of uranium. RSC Adv. 2019, 9, 28588. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yang, X.; Hu, R.; Wu, J. Amidoxime functionalized mesoporous SBA-15 with various mesostructures for highly efficient concentration of U(VI). NANO 2019, 14, 1950027. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wang, H.; Liu, R.; Qiu, M. Highly efficient U(VI) capture by amidoxime/carbon nitride composites: Evidence of EXAFS and modelling. Chemosphere 2021, 274, 129743. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, W.; Yin, Q.; Wang, Y.; Wang, H. A conjugated fluorescent polymer sensor with amidoxime and polyfluorene entities for effective detection of uranyl ion in real samples. Spectrochim. Acta Part A 2021, 244, 118864. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Matsumoto, M.; Okamoto, K. Enhanced adsorption of copper (II) ion on novel amidoxime chitosan resin. J. Chem. Eng. Jpn. 1999, 32, 217–222. [Google Scholar] [CrossRef]
- Higasa, S.; Sogo, M.; Hata, K. Cyanoethylation of cellulose and infrared adsorption spectrum of cyanoethyl cellulose. Tech. Bull. Fac. Agric. Kagawa Univ. 1971, 23, 97–103. [Google Scholar]
- Guo, X.; Huang, L.; Li, C.; Hu, J.; Wu, G.; Huai, P. Sequestering uranium from UO2(CO3)34− in seawater with amine ligands: Density functional theory calculation. Phys. Chem. Chem. Phys. 2015, 17, 14662–14673. [Google Scholar] [CrossRef] [PubMed]
- Ayawel, N.; Ebelegl, A.N.; Wankasl, D. Modeling and interpretation of adsorption isotherms. J. Chem. 2017, 3039817. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Ayadin, S. Modelling of adsorption kinetic processes—Error, theory and application. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]



| Metal | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax [mmol/g] | KL [dm3/mmol] | KL [dm3/mmol] [5] | R2 | 1/n | KF | R2 | KT | BT | R2 | |
| Cu | 0.152 | 3.48 | 0.0121 | 0.879 | 0.185 | 0.0952 | 0.962 | 36.5 | 0.0199 | 0.886 |
| Ag | 0.156 | 0.554 | 0.970 | 0.258 | 0.0677 | 0.959 | 43.3 | 0.0209 | 0.964 | |
| Ni | 0.092 | 0.097 | 0.162 | 0.932 | 0.513 | 0.0127 | 0.885 | 39.6 | 0.0127 | 0.801 |
| Zn | 0.046 | 0.102 | 0.171 | 0.850 | 0.505 | 0.007 | 0.829 | 157 | 0.00392 | 0.723 |
| Kinetic Model | Parameters | |||
|---|---|---|---|---|
| Pseudo-first-order | k1 (h−1) 0.206 | qe (exp) 0.0565 | qe (calc) 0.0458 | R2 0.901 |
| Pseudo-second-order | k1 (g/(mmol h)) 8.97 | qe (exp) 0.0565 | qe (calc) 0.0552 | R2 0.998 |
| Intraparticle | kIP (mmol/(g h0.5)) 0.0166 | CIP (mmol/g) 0.0034 | R2 0.885 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsumi, T.; Tahara, Y.; Matsumoto, M. Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels. Separations 2021, 8, 202. https://doi.org/10.3390/separations8110202
Tatsumi T, Tahara Y, Matsumoto M. Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels. Separations. 2021; 8(11):202. https://doi.org/10.3390/separations8110202
Chicago/Turabian StyleTatsumi, Takaaki, Yoshiro Tahara, and Michiaki Matsumoto. 2021. "Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels" Separations 8, no. 11: 202. https://doi.org/10.3390/separations8110202
APA StyleTatsumi, T., Tahara, Y., & Matsumoto, M. (2021). Adsorption of Metallic Ions on Amidoxime-Chitosan/Cellulose Hydrogels. Separations, 8(11), 202. https://doi.org/10.3390/separations8110202







