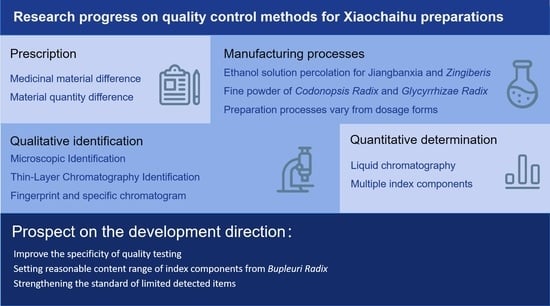
Research Progress on Quality Control Methods for Xiaochaihu Preparations
Abstract
1. Introduction to XCH Formula
2. Formula Differences of Existing XCH Preparations
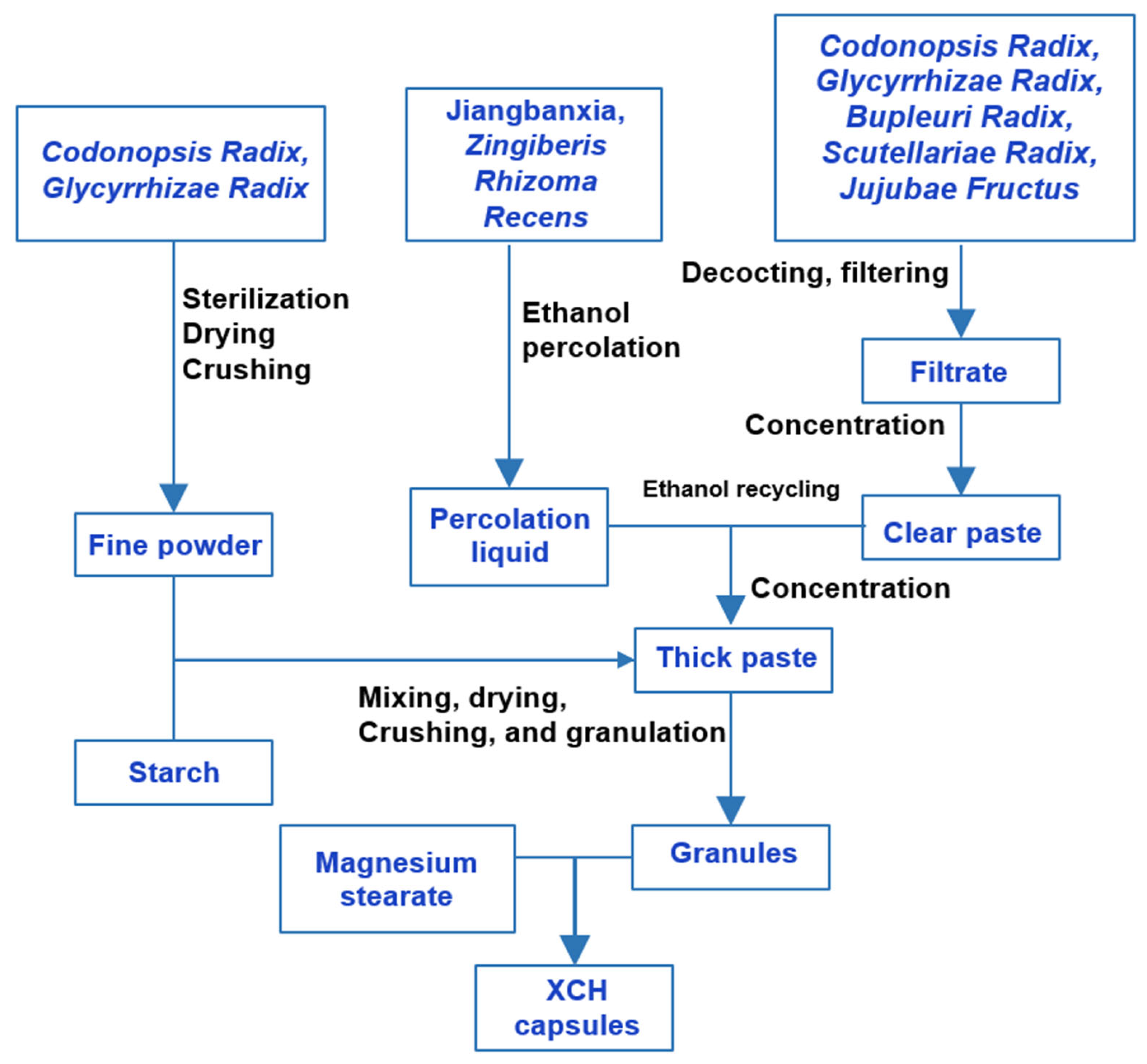
3. Differences in XCH Preparation Methods
4. Differences in Quality Test Indices and Limits of Different XCH Preparations
4.1. Indicators for Qualitative Identification
4.2. Quantitative Determination
4.3. Fingerprint and Specific Chromatogram
| Year | Apparatus | Quantitative Determined Components | Qualitatively Identified Components | Detection Method | Reference | |
|---|---|---|---|---|---|---|
| 1 | 2021 | HPLC-CAD | Saikosaponin A, B1, B2, C, G, H, I | Saikosaponin A, B1, B2, C, G, H, I | Fingerprint chromatogram | [69] |
| 2 | 2021 | UHPLC | Liquiritin, Baicalin, Wogonin, Baicalein, Glycyrrhizin G2, Glycyrrhizic acid, Saikosaponin B2, Saikosaponin B1 | - | Fingerprint chromatogram | [71] |
| 3 | 2018 | UPLC | Liquiritin, Baicalin, Berberine, Wogonoside, Baicalein, Ammonium Glycyrrhizinate | Liquiritin, Baicalin, Berberine, Wogonoside, Baicalein, Ammonium Glycyrrhizinate | Specific chromatogram | [72] |
| 4 | 2017 | HPLC | - | - | Fingerprint chromatogram | [73] |
| 5 | 2017 | HPLC | - | - | Fingerprint chromatogram | [74] |
| 6 | 2016 | HPLC | Lobetyolin, Saikosaponin A | Lobetyolin, Saikosaponin A | Specific chromatogram | [75] |
| 7 | 2016 | HPLC | Baicalin, Ammonium Glycyrrhizinate | Baicalin, Ammonium Glycyrrhizinate | Specific chromatogram | [68] |
| 8 | 2015 | HPLC-ELSD | - | Liquiritin, Ginsenoside Re, Baicalin, Wogonoside, Baicalein, Ginsenoside Rb1 | Fingerprint chromatogram | [76] |
| 9 | 2014 | HPLC | Liquiritin, Baicalin, Wogonoside, Baicalein, Ammonium Glycyrrhizinate, Saikosaponin A, Wogonin | Liquiritin, Baicalin, Wogonoside, Baicalein, Ammonium Glycyrrhizinate, Saikosaponin A, Wogonin | Specific chromatogram | [54] |
| 10 | 2013 | HPLC | - | Liquiritin, Baicalin, Wogonoside, Baicalein, Ammonium Glycyrrhizinate, Saikosaponin A, Wogonin | Fingerprint chromatogram | [77] |
| 11 | 2013 | HPLC | Baicalin, Glycyrrhizic acid | Baicalin, Glycyrrhizic acid | Fingerprint chromatogram | [78] |
| 12 | 2013 | HPLC | - | Liquiritin, Baicalin, Ononin, Wogonoside, Saikosaponin A, Skullcapflavone II, etc. | Fingerprint chromatogram | [79] |
| 13 | 2012 | HPLC-DAD-ESI-MS | Homogentisic acid, Baicalin, Glycyrrhizic acid, Saikosaponin A, 6-Gingerol, Ginsenoside Rg3 | - | Fingerprint chromatogram | [80] |
| 14 | 2012 | HPLC | Baicalin, Wogonoside, Baicalein, Wogonin, Glycyrrhetic acid | Baicalin, Wogonoside, Baicalein, Wogonin, Glycyrrhetic acid | Specific chromatogram | [81] |
| 15 | 2012 | UPLC | - | Glycyrrhizin, Ginsenoside Rg1, Baicalin, Isowogonin, Baicalein, Saikosaponin A | Fingerprint chromatogram | [82] |
| 16 | 2012 | HPLC | Baicalin, Wogonoside, Baicalein, Wogonin | Baicalin, Wogonoside, Baicalein, Wogonin | Specific chromatogram | [83] |
| 17 | 2011 | HPLC-DAD | - | - | Fingerprint chromatogram | [84] |
| 18 | 2009 | HPLC-TOF/MS | - | Liquiritin, Baicalin, Wogonoside, Ginsenoside Rg1, Glycyrrhizic acid | Fingerprint chromatogram | [85] |
| 19 | 2009 | HPLC | - | - | Fingerprint chromatogram | [86] |
5. Prospect on the Development Direction of Quality Control of XCH Preparations
5.1. Improvement in the Specificity of Quality Testing
5.2. Setting Reasonable Content Range of Index Components from Bupleuri Radix
5.3. Strengthening the Standard of Limited Detected Items
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, T.T.; Yao, K.W.; Duan, J.L.; Yu, Z.; Zhang, L.D. Efficacy of Xiaochaihu Decoction on Treating Coronary Heart Disease. Lishizhen Med. Mater. Med. Res. 2019, 30, 2216–2218. [Google Scholar]
- Zhang, W.Z.; Nie, H.; Wang, Z.T.; Mao, D.X. Discussion of ginseng used in Xiaochaihu Decoction. Lishizhen Med. Mater. Med. Res. 2013, 24, 2480–2481. [Google Scholar]
- Zhang, Y.; Zhou, X.L.; Shao, Q.; Fang, Z.E.; Wang, S.S. Anti-inflammatory effect of Xiaochaihutang in rats with collagen-induced arthritis and the mechanism. Immunol. J. 2015, 31, 781–785. [Google Scholar]
- Zhai, Y.X.; Zhao, Y.; Xiang, R.W.; Zhai, F.; Zeng, Y.; Liang, J.K. Study on anti-heatoma mechanism of Xiaochaihu decoction based on weighted network pharmacology. Chin. J. Med. Chem. 2020, 30, 658–668. [Google Scholar]
- Lu, X.W.; Zhu, L.H.; Huang, H.; He, K. Clinical Observations on Modified Xiao Chaihutang in Treatment of Subacute Thyroiditis. Chin. J. Exp. Tradit. Med. Formulae 2018, 22, 153–158. [Google Scholar]
- Zhang, W.Z.; Tian, H.Z.; Zhang, L.J.; Yang, S.J. Clinical Study and Analysis of Modified Xiaochaihu Decoction in Treating Cough Caused by Variant Asthma. World J. Integr. Tradit. Western Med. 2020, 15, 790–793. [Google Scholar]
- Liu, L.; Wang, C.X.; Cui, S.Y.; Zhang, H.X.; Wu, X.X.; Zhang, W. Observation of curative effect of lamivudine combined with compound glycyrrhizin and Xiaochaihu Decoction in the treatment of chronic hepatitis B. Modern J. Integr. Tradit. Chin. Western Med. 2020, 29, 4042–4045. [Google Scholar]
- Luo, X.M. Clinical observation on treating chronic renal failure with the Xiaochaihu decoction. Clin. J. Chin. Med. 2012, 4, 99. [Google Scholar]
- Wang, D.X.; Liu, X.Z.; Lv, C.Y.; Fan, H.; Sun, Y.F.; Yang, S.L. Changes of Platelet- Associated Parameters in Patients with Refractory Immune Thrombocytopenia Treated with Xiaochaihu Decoction and Erzhi Pill. J. Hebei North Univ. Nat. Sci. Ed. 2020, 36, 5–9. [Google Scholar]
- Jiang, M. Effect observation of Minor Decoction of Bupleurum treating acute viral myocarditis. World Latest Med. Inf. 2015, 15, 1–14. [Google Scholar]
- Ma, R.; Liu, H.; Zheng, R.W.; Xu, Z.T.; Jiang, Z.Q.; Zhang, J.H.; Jiang, Y.F.Y.; Bi, C. Pharmacological analysis and confirmation of antipyretic mechanism of Xiaochaihu Granules. J. Pharm. Res. 2021, 8, 497–503. [Google Scholar]
- Li, X.; Li, Y.Y.; Wen, C.X.; Liang, Y.Y.; Zhou, Z.L. Study on the mechanism of Xiaochaihu Decoction in treatment of hepatocellular carcinoma based on network pharmacology and bioinformatics. Anti-Tumor Pharm. 2021, 4, 456–462. [Google Scholar]
- Ma, R.; Liu, H.; Jiang, Y.F.Y.; Zheng, R.; Jiang, Z.Q.; Bi, C.; Zhang, J.H.; He, Y.X.; Du, H.Y. A New Use of Xiaochaihu Granules Combined with Antibiotics. Guangdong Province Patent CN111529671A, 14 August 2020. [Google Scholar]
- Liu, H.; Huang, W.; Cheng, X.Y.; Bi, C.; Zheng, R.W.; Jiang, Z.Q.; Zhang, J.H.; He, Y.X.; Du, H.Y. New Application of Xiaochaihu Granules in the Control of Syncytial Virus and Adenovirus. Patent CN111467466A, 31 July 2020. [Google Scholar]
- Zheng, R.W.; Liu, H.; Situ, W.H.; Jiang, Z.Q.; Bi, C.; Zhang, J.; He, Y.X.; Du, H.Y. New Application of Xiaochaihu Granules in Inhibiting Influenza a Virus. Patent CN111358929A, 3 July 2020. [Google Scholar]
- China National Knowledge Infrastructure. Available online: https://kns.cnki.net/kns8/defaultresult/index (accessed on 9 October 2021).
- Chinese Pharmacopoeia Commission. The Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2020; pp. 602–606.
- Pharmaceuticals and Medical Devices Agency. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch/ (accessed on 22 September 2021).
- Japanese Pharmacopoeia Committee. The Japanese Pharmacopoeia, 17th ed.; The Ministry of Health, Labour and Welfare: Tokyo, Japan, 2016; pp. 1988–1990.
- Kim, H.M.; Kim, Y.Y.; Jang, H.Y.; Moon, S.J.; An, N.H. Action of Sosiho-Tang on systemic and local anaphylaxis by anal administration. Immunopharm. Immunot. 1999, 21, 635–643. [Google Scholar] [CrossRef]
- Zhong, L.Y.; Wu, H. Current researching situation of mucosal irritant components in Acacae family plants. China J. Tradit. Chin. Med. Pharm. 2006, 18, 1561–1563. [Google Scholar]
- Zhang, L.M.; Bao, Z.Y.; Huang, Y.Y.; Huang, W.; Zhang, Y.N.; Sun, R. Experimental Study on the “Dosage-Time-Toxicity” Relationship of Acute Hepatotoxicity induced by Water Extraction from Rhizoma Pinelliae in Mice. Chin. J. Pharmacovigil. 2011, 8, 11–15. [Google Scholar]
- Jin, X.Q.; Huang, C.Q.; Zhang, G. Toxic Components and Processing Mechanism of Rhizoma Pinelliae. Lishizhen Med. Mater. Med. Res. 2019, 30, 1717–1720. [Google Scholar]
- Zhang, K.; Shan, J.J.; Xu, J.Y.; Wang, M.M. Research progress and prospects of pregnancy toxicity of pinellia. China J. Tradit. Chin. Med. Pharm. 2016, 31, 938–941. [Google Scholar]
- Zhu, F.G.; Yu, H.L.; Wu, H.; Shi, R.J.; Tao, W.T.; Qiu, Y.Y. Correlation of Pinellia ternata agglutinin and Pinellia ternata raphides’ toxicity. China J. Chin. Mater. Med. 2012, 37, 1007–1011. [Google Scholar]
- Zhong, L.Y.; Wu, H.; Zhang, K.W.; Wang, Q.R. Study on irritation of calcium oxalate crystal in raw Pinellia ternate. China J. Chin. Mater. Med. 2006, 20, 1706–1710. [Google Scholar]
- Su, T.; Zhang, W.W.; Zhang, Y.M.; Cheng, C.Y.B.; Fu, X.Q.; Li, T.; Guo, H.; Li, Y.X.; Zhu, P.L.; Cao, H.; et al. Standardization of the manufacturing procedure for Pinelliae Rhizoma Praeparatum cum Zingibere et Alumine. J. Ethnopharmacol. 2016, 193, 663–669. [Google Scholar] [CrossRef]
- Shi, R.J.; Wu, H.; Yu, H.L.; Chen, L. The advance of the research that zingiber officinale rosc detoxify the tuber of pinellia. Chin. J. Inf. Tradit. Chin. Med. 2010, 17, 108–110. [Google Scholar]
- Jiang, X.; Yan, F.J.; Jiang, L.; Si, G.M. Different Decocting Methods Influence 9 Kinds of Ingredients of Xiao Chaihutang by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 98–103. [Google Scholar]
- Megumi, S.; Yuko, S.; Fumio, I.; Yoshiro, H.; Takao, N. Extraction Efficiency of Shosaikoto (Xiaochaihu Tang) and Investigation of the Major Constituents in the Residual Crude Drugs. Evid.-Based Compl. Altern. Med. 2012, 2012, 7. [Google Scholar]
- Sakata, K.; Kim, S.J.; Yamada, H. Effects of commercially available mineral waters on decoction of Kampo Medicines. Kampo Med. 2000, 51, 225–232. [Google Scholar] [CrossRef]
- Honma, S.; Ogawa, A.; Kobayashi, D.; Ueda, H.; Fang, L.; Numajiri, S.; Kimura, S.; Teresawa, S.; Morimoto, Y. Effect of hardness on decoction of Chinese medicine. J. Tradit. Med. 2003, 20, 208–215. [Google Scholar]
- Jiang, S.Z.; Fu, S.Q.; Wang, N.S. Overview of chemical constituents of gingerol. Tradit. Chin. Drug Res. Clin. Pharmacol. 2006, 05, 386–389. [Google Scholar]
- Yu, N.; Zeng, H.Y.; Deng, X.; Zeng, M.X.; Feng, B. Study on Extraction, Identification and Antioxidation of Gingerol. Food Sci. 2007, 8, 201–204. [Google Scholar]
- Zhao, W.Z.; Zhang, R.X.; Yu, Z.P.; Wang, X.K.; Li, J.R.; Liu, J.B. Research process in ginger chemical composition and biological activity. Sci. Technol. Food Ind. 2016, 37, 383–389. [Google Scholar]
- Liu, D.; Zhang, C.H.; An, R.H.; Feng, X.Q.; Zhou, F.; Deng, Y.J.; Zhang, J.L.; Liu, H. Advances on extraction and application of ginger bioactive ingredient. Sci. Technol. Food Ind. 2016, 37, 391–400. [Google Scholar]
- Zhang, D.K.; Fu, C.M.; Lin, J.Z.; Ke, X.M.; Zou, W.Q.; Xu, R.C.; Han, L.; Yang, M. Study on theory and application value of “unification of medicines andexcipients” in Chinese materia medica preparations. Chin. Tradit. Herb. Drugs 2017, 48, 1921–1929. [Google Scholar]
- Du, Y.; Xue, J.L.; Zhang, L.P.; Liang, G.X.; Ding, H. A Xiaochaihu Sustained Release Tablet along with Its Preparation Methods. Patent CN105106914B, 12 March 2019. [Google Scholar]
- Yang, M.J. A Nanoscale Xiaochaihu Pharmaceutical Preparation along with Its Preparation Method. Patent CN1362246, 7 August 2002. [Google Scholar]
- Li, C.H. A Fast Classification and Screening Device for Xiaochaihu Granules. Patent CN208960363U, 11 June 2019. [Google Scholar]
- Xu, D.J.; Chi, H.C.; Feng, Z.W.; Li, W.Z. A Preparation Method of Xiaochaihu Granules. Patent CN108853027A, 23 November 2018. [Google Scholar]
- Liu, A.X.; Xu, T.T.; Yan, Y.; Wu, Q.Y.; Zhou, D.D.; Sha, Y.W. Study on determination of seven components in Xiaochaihu Granules by QAMS. Drug Eval. Res. 2020, 43, 2217–2221. [Google Scholar]
- Qu, N.N. Determination of 6-Gingerol Content in Minor Bupleurum Tablets by HPLC Method. Heilongjiang Med. J. 2018, 31, 718–720. [Google Scholar]
- Shao, J.X.; Lin, L.F.; Liu, Y.L.; Wang, C.M.; Li, H.; Yang, Y.J. Determination of five saponins in Ginseng Radix et Rhizoma-Bupleuri Radix herb pair and its preparations by QAMS method. Chin. Tradit. Herb. Drugs 2018, 49, 2873–2877. [Google Scholar]
- Chen, L.L.; Lin, R.X.; Li, R.M. Effects of Different Decoction Methods of Xiaochaihu Decoction on Active Chemical Constituents of Scutellaria Baicalensis Georgi. World Chin. Med. 2018, 13, 743–745+750. [Google Scholar]
- Li, C.D.; Wang, X.; Pan, X.B.; Zhang, W. Detection of Four Kinds of Ingredients in Modified Xiao ChaiHu Capsules by HPLC Wavelength Switching Method Simultaneously. Western J. Tradit. Chin. Med. 2017, 30, 26–28. [Google Scholar]
- Tang, F.S.; Zhang, X.H.; Lan, X.; Meng, C.; Wang, Y.H. Study on the Dissolution of Xiaocaihu Pill from Different Manufacturers. China Pharm. 2016, 27, 4272–4274. [Google Scholar]
- Li, J.; Yang, P.P.; Tang, W.X.; Li, H.J.; Xiang, W.; Jiang, H.; Yang, S.H.; Li, W.; Hou, Y. NIR Calibration Model to Detect Baicalin Content of Xiaochaihu Granules by Using Characteristic Spectrum Selection. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 72–77. [Google Scholar]
- Wang, J.L.; Wang, S.T. Simultaneous determination the saikosaponin a and saikosaponin d in Sho-Saiko Granule with HPLC-MSMS. Anhui Med. Pharm. J. 2015, 19, 453–456. [Google Scholar]
- Yuan, H.X.; Wang, T.T.; Yan, Y. Simultaneous Determination of Five Components in Ethyl Acetate Extract of Xiao Chaihu Tang by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 64–66. [Google Scholar]
- Lan, X.; Tang, F.H.; Chen, Z.N.; Zhang, Y.; Wang, W.; Zhou, X.M. Determination of baicalin in Xiaochaihu decoction by HPLC. J. Zunyi Med. Univ. 2015, 38, 435–438. [Google Scholar]
- Xu, C.C. Determination of active components in Xiaochaihu Decoction by HPLC. Asia-Pac. Tradit. Med. 2015, 11, 39–40. [Google Scholar]
- Jiang, S.Z.; Huang, X.B.; Chen, C.L.; Wu, H.X.; Xiang, Y.Y.; Qiu, Z.J.; Qiu, J.T. Effect of Ginger on Dissolution of Saikosaponin a and Baicalin in Xiaochaihu Decoction Determined by HPLC. Chin. Arch. Tradit. Chin. Med. 2014, 32, 1652–1654. [Google Scholar]
- Zhang, H.; Yan, L.; Lin, L.F.; Dong, X.X.; Shen, M.R.; Qu, C.H.; Ni, J. Determination of seven index components in Xiaochaihu Granules by HPLC. Drugs Clin. 2014, 29, 162–165. [Google Scholar]
- Li, P.T.; Lin, L.R.; Lin, L.H.; Wang, S.L.; Liu, S.Q.; Gao, X.L. Determination of Saikosaponin b2 in Xiaochaihu Granules by HPLC. Tradit. Chin. Drug Res. Clin. Pharm. 2014, 25, 356–359. [Google Scholar]
- Zhang, S.Y. Determination of Saikosaponin, Baicalin and Glycyrrhizic Acid Content in Xiaochaihu Granule by HPLC. J. Shanxi Univ. Chin. Med. 2013, 14, 20–22. [Google Scholar]
- Yi, R.Q.; Song, F.Y. Determination of Saikosaponin a and Saikosaponin d in Xiaochaihu Granules by Capillary Electrophoresis. Chin. J. Pharm. 2012, 43, 47–50. [Google Scholar]
- Zhang, Y.J.; Mai, Y.M.; Mo, Z.J. Investigation of Determination and Evaluation for in vitro Dissolution of Chinese Medicine Preparations by Co-Chromatography and UV. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 17–21. [Google Scholar]
- Liu, L.N.; Qi, Z.H.; Guo, Z.W.; Zhang, Y.; Zhang, H.F.; Zhang, W.J. Study on quality standard of xiaochaihutang dripping pill. Pract. Pharm. Clin. Remedies 2012, 15, 742–744. [Google Scholar]
- Ji, S.G.; Liu, X.F.; Zhu, Z.Y.; Liang, S.S.; Zhao, L.; Zhang, H.; Chai, Y.F. High performance liquid chromatography in determination of baicalin and wogonoside contents in Xiaochaihu decoction. Acad. J. Sec. Mil. Med. Univ. 2010, 31, 1010–1013. [Google Scholar] [CrossRef]
- Liu, Q.C.; Zhao, J.N.; Yan, L.C.; Yi, J.H.; Song, J. Simultaneous determination in Xiaochaihu Tang by HPLC. China J. Chin. Mater. Med. 2010, 35, 708–710. [Google Scholar]
- Zhang, J.Y.; Xie, H.; Pan, S.L. Determination of saikosaponin a in four common bupleurum preparations by HPLC. Chin. Tradit. Pat. Med. 2010, 32, 81–83. [Google Scholar]
- Zhou, B.Y.; Zhu, Z.Y.; Wang, B.; Zhao, L.; Zhang, H.; Xu, Q.; Chai, Y.F. HPLC-DAD combined with TOF/MS technique in determination of saikosaponin a, baicalin and glycyrrhizic acid in Xiaochaihu Decoction. Acad. J. Sec. Mil. Med. Univ. 2007, 5, 527–530. [Google Scholar]
- Shi, Y. Determination of baicalin in Xiaochaihu Decoction pill by HPLC. Chin. Tradit. Pat. Med. 2007, 7, 1115–1117. [Google Scholar]
- Zhu, Z.Y.; Liu, Y.; Zhao, B.Y.; Zhang, H.; Li, X.; Chai, Y.F. Simultaneous determination of two effective components in Xiaochaihu decoction by HPLC/DAD. J. Pharm. Pract. 2006, 6, 331–334. [Google Scholar]
- Chen, P.; Li, C.; Liang, S.P.; Song, G.Q.; Sun, Y.; Shi, Y.H.; Xu, S.L.; Zhang, J.W.; Sheng, S.Q.; Yang, Y.M. Characterization and quantification of eight water-soluble constituents in tubers of Pinellia ternata and in tea granules from the Chinese multiherb remedy Xiaochaihu-tang. J. Chrom. Banalytical Technol. Biomed. Life Sci. 2006, 843, 83–193. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.W.; Li, C.; Shen, H.W.; Nan, F.J. Determination of Saikosaponin Derivatives in Radix bupleuri and in Pharmaceuticals of the Chinese Multiherb Remedy Xiaochaihu-tang Using Liquid Chromatographic Tandem Mass Spectrometry. Anal. Chem. 2004, 76, 4208–4216. [Google Scholar] [CrossRef]
- Wang, D.Q.; Chang, F.M.; Wang, Y.H.; Zhao, P.; Wang, Y.G. Multi-wavelength HPLC analysis of Xiaochaihu Granules and medicinal materials-preparation peak pattern matching analysis. J. Chin. Med. Mater. 2016, 39, 810–812. [Google Scholar]
- Liu, A.X.; Xu, T.T.; Yang, W.N.; Zhou, D.D.; Sha, Y.W. Quantitative Determination of 7 Saikosaponins in Xiaochaihu Granules Using High-Performance Liquid Chromatography with Charged Aerosol Detection. J. Anal. Methods Chem. 2021, 2021, 6616854. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Peng, B.; Li, Z.Y.; Pan, R.L. Study on Transformation Rule of Saikosaponin d Under Different Extraction Conditions by UPLC-QTof-MS. Nat. Prod. Res. Dev. 2014, 26, 716–720. [Google Scholar]
- Zhang, X.; Wu, H.W.; Lin, L.N.; Tang, S.H.; Liu, H.H.; Yang, H.J. The development and application of a quality evaluation method for Xiaochaihu Granules granules which is based on “benchmark sample”. China J. Chin. Mater. Med. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Yang, M.L.; Wu, H.H.; Zhou, A.J.; Chen, S.H.; Liu, Y.W. Study on the Quality Standard for Modified Xiaochaihu Granules. China Pharmacist. 2018, 21, 1299–1303. [Google Scholar]
- Zheng, R.W.; Jiang, Z.Q.; Cheng, G.H.; Hu, W.L.; Zhang, J.H. Study on HPLC Fingerprint for Xiaochaihu Granules. Eval. Anal. Drug-Use Hosp. China. 2017, 17, 1089–1093. [Google Scholar]
- Li, X.D.; Gong, W. Multiwavelength fingerprints of Xiaochaihu Granules preparation and analysis of medicinal materials-preparation peak pattern matching analysis. China Health Ind. 2017, 14, 42–43. [Google Scholar]
- Wang, X.; Li, C.D.; Pan, X.B.; Zhang, W. Quality Standard for Flavored Xiaochaihu Yigan Capsules by HPLC with Wavelength Switch. China Pharmacist. 2016, 19, 1597–1599. [Google Scholar]
- Yuan, H.X.; Liu, Y.Q.; Li, H.F.; Pei, M.R. Fingerprint of n-butanol effective fraction of Xiao Chaihu Tang by HPLC-ELSD. J. Shenyang Pharm. Univ. 2015, 32, 623–632. [Google Scholar]
- Yan, L.; Lin, L.F.; Zhang, H.; Dang, X.F.; Ni, J. Study on fingerprint of Xiaochaihu granules sold in the market. China J. Chin. Mater. Med. 2013, 38, 3498–3501. [Google Scholar]
- Chen, W.X.; Wu, J.L.; Ma, D.; Zhang, P.; Gao, X.; Hu, F.D. Fingerprint study on Xiaochaihu Granules-medicinal materials based on peak pattern matching. Chin. Tradit. Herb. Drugs 2013, 44, 3154–3161. [Google Scholar]
- Jin, Y.; Sun, L.; Qiao, S.Y. Establishment of HPLC fingerprint of Xiaochaihu Decoction. J. Inter. Pharm. Res. 2013, 40, 224–232. [Google Scholar]
- Yang, H.J.; Ma, J.Y.; Weon, J.B.; Lee, B.Y.; Ma, C.J. Qualitative and quantitative simultaneous determination of six marker compounds in soshiho-tang by HPLC-DAD-ESI-MS. Arch. Pharm. Res. 2012, 35, 1785–1791. [Google Scholar] [CrossRef]
- Zhuang, Y.H.; Cai, H.; Liu, X.; Cai, B.C. Simultaneous determination of five main index components and specific chromatograms analysis in Xiaochaihu granules. Acta Pharm. Sin. 2012, 47, 84–87. [Google Scholar]
- Yang, J.; Huang, D.X.; Lu, X.M.; Wang, F.; Li, F.M. Analysis on chemical constituents in Xiaochaihu Decoction and their in vivo metabolites in depressed rats. Chin. Tradit. Herb. Drugs 2012, 43, 1691–1698. [Google Scholar]
- Li, C.H.; Mei, Z.Q.; He, B.; Tian, J. Simultaneous determination of 4 components in Xiaochaihu granules by HPLC. J. Southwest Med. Univ. 2012, 35, 399–401. [Google Scholar]
- Wu, Y.Q.; Gou, Y.Q.; Han, J.; Bi, Y.Y.; Feng, S.L.; Hu, F.D.; Wang, C.M. Evaluation preparation technology of Xiaochaihu granules using fingerprint-peak pattern matching. J. Pharm. Anal. 2011, 1, 119–124. [Google Scholar] [CrossRef][Green Version]
- Liu, X.F.; Lou, Z.Y.; Zhu, Z.Y.; Zhang, H.; Zhao, L.; Chai, Y.F. HPLC-TOF/MS in rapid identification of chemical compositions in Xiaochaihu decoction. Acad. J. Sec. Mil. Med. Univ. 2009, 30, 941–946. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Yin, W.P. Study on the HPLC Fingerprint of Saikosaponins in Radix Bupleuri and Its Water Extract. Lishizhen Med. Mater. Med. Res. 2009, 20, 2205–2206. [Google Scholar]
- Chen, W.; Zhang, C.; Sun, L.; Xue, M. Combination of fingerprint and chemometrics for identification of four processed products of Pinelliae Rhizoma based on nucleosides and nucleobases. Chin. J. Pharm. Anal. 2021, 41, 919–928. [Google Scholar]
- Chinese Academy of Sciences. Flora of China. Available online: http://www.iplant.cn/info/Bupleurum?t=z (accessed on 28 July 2021).
- Zhao, J.F.; Guo, Y.Z.; Meng, X.S. The toxic principles of Bupleurum longiradiatum. Acta Pharm. Sin. 1987, 7, 507–511. [Google Scholar]
- Liu, X.X.; Luo, Z.Y.; Ji, Z.Z.; Li, H.; Zhang, W.Q.; Chen, F. A Detection Method of Adulteration of B. Marginatum var.Stenophyllum in Xiaochaihu Granules Preparation and Its Chinese Herbal Medicine Raw Materials. Patent CN110779993A, 11 February 2020. [Google Scholar]
- Liang, H.L.; Tan, C.C.; Jiang, X.J.; Xiao, X.Y.; Xu, W.B. Study on Rapid Identification on Xiaochaihu Granules from Different Manufacturers by Near Infrared Spectroscopy. Guiding J. Tradit. Chin. Med. Pharm. 2021, 27, 62–64. [Google Scholar]
- Lai, J.; Shi, Z.F.; Song, P.S.; Wang, Z.X.; Ni, L.; Teng, B.X. Identification of Bupleurum marginatum var.stenophyllum Adulterated Bupleurum chinese by PCR Amplification of Based on ITS Sequences. Res. Pract. Chin. Med. 2021, 35, 18–21. [Google Scholar]
- Xie, D.H.; Cai, B.C.; An, Y.Q.; Li, X.; Jia, X.B. The research progerss in saikosaponin and its pharmacology action. J. Nanjing Univ. Tradit. Chin. Med. 2007, 1, 63–65. [Google Scholar]
- Lv, X.H.; Sun, Z.X.; Su, R.Q.; Fan, J.W.; Zhao, Z.Q. The pharmacology research progress in bupleurum and its active constituents. Chin. J. Inf. Tradit. Chin. Med. 2012, 29, 105–107. [Google Scholar]
- Liu, Y.M.; Liu, X.M.; Pan, R.L. The research progress in toxic effect of bupleurum. Chin. Tradit. Pat. Med. 2012, 34, 1148–1151. [Google Scholar]
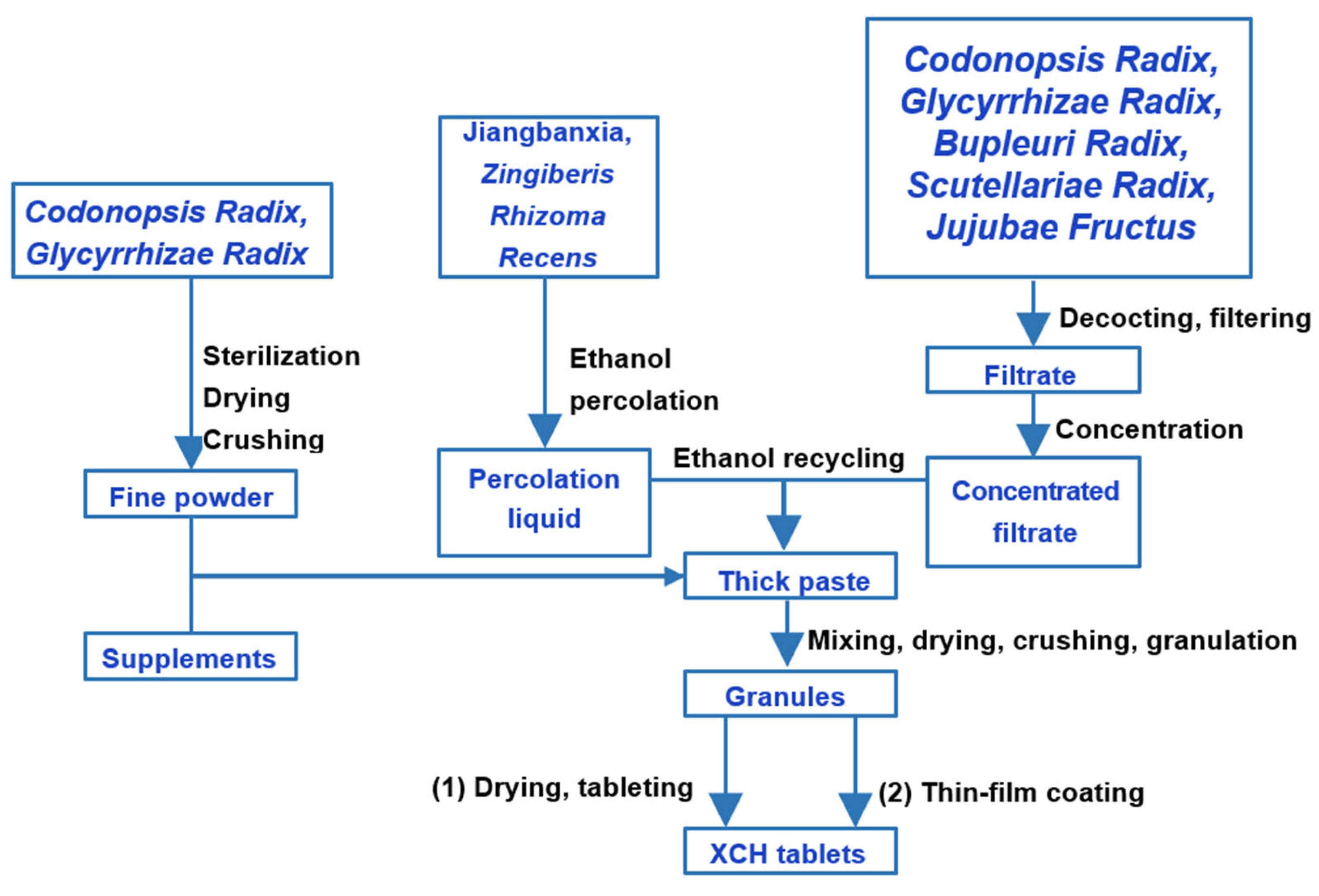
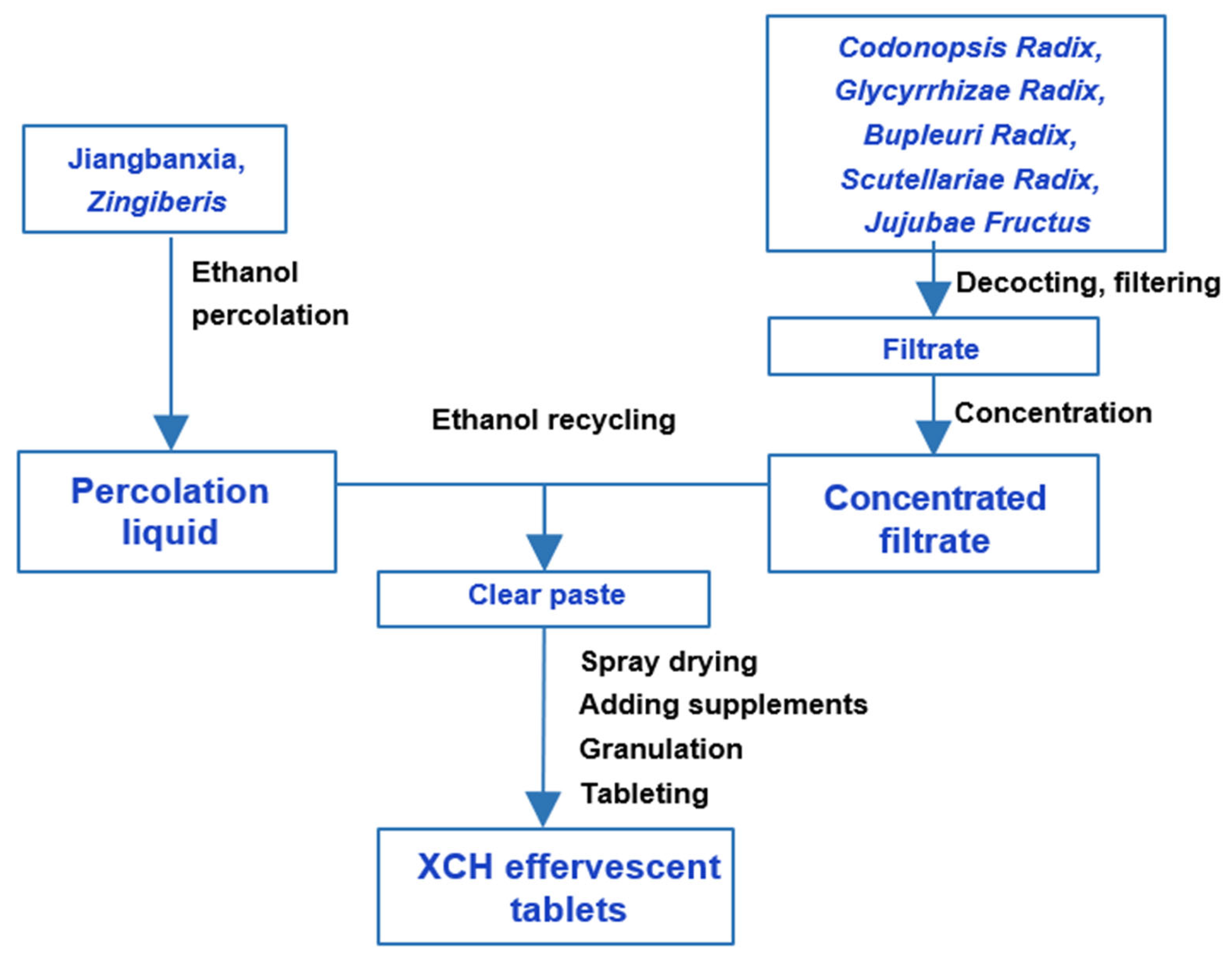
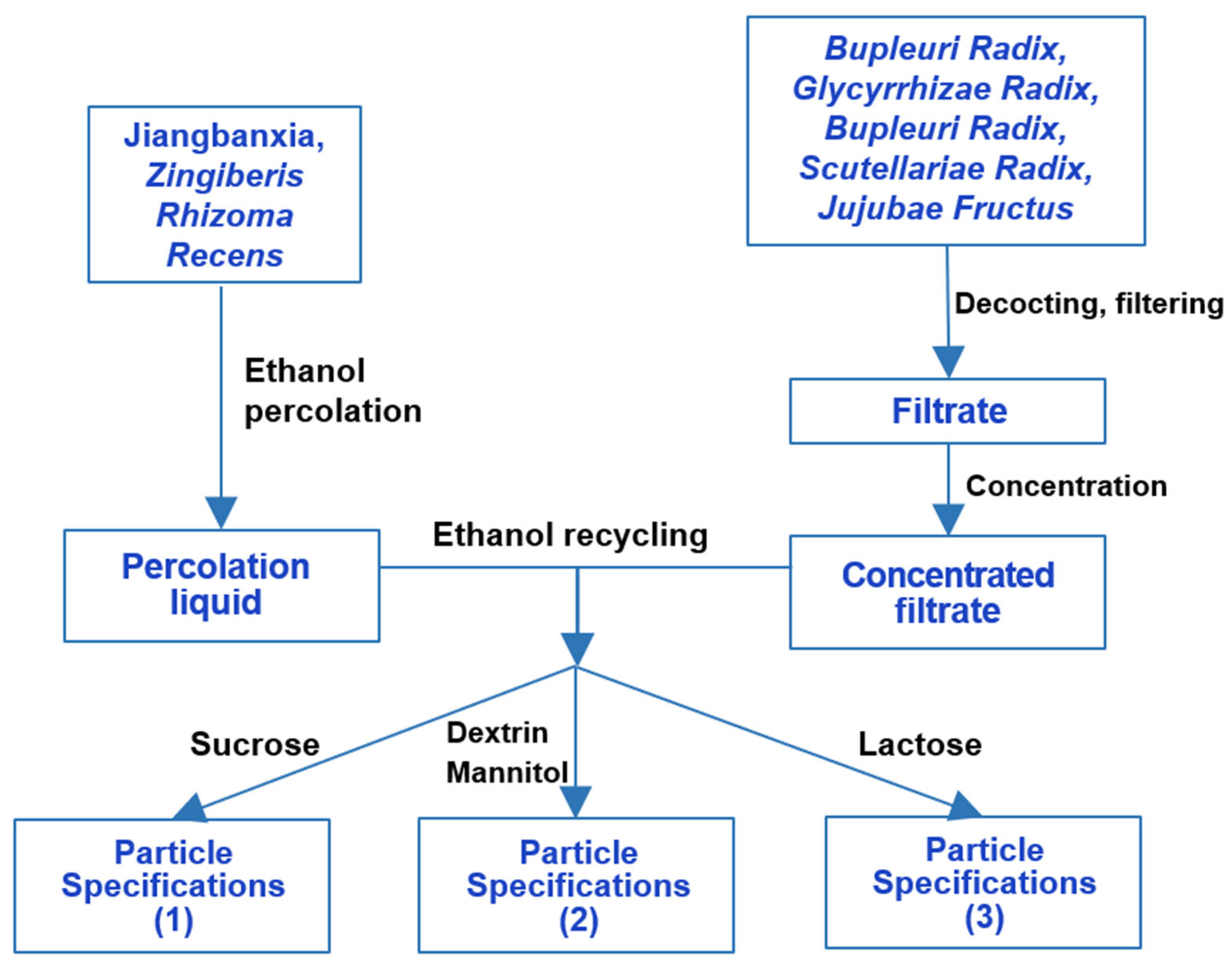
| Raw Materials | XCH Tablets & XCH Capsules | XCH Effervescent Tablets | XCH Granules | Shosaikoto Extract (Japanese) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| — | Amount (g) | Mass Ratio (%) | Amount (g) | Mass Ratio (%) | Amount (g) | Mass Ratio (%) | Amount (g) | Mass Ratio (%) | Amount (g) | Mass Ratio (%) |
| Bupleuri Radix | 445 | 29.6 | 1550 | 31.0 | 150 | 31.0 | 7 | 29.2 | 6 | 26.1 |
| Jiangbanxia | 222 | 14.8 | 575 | 11.5 | 56 | 11.5 | - | - | - | - |
| Pinelliae Rhizoma | - | - | - | - | - | - | 5 | 20.8 | 5 | 21.7 |
| Scutellariae Radix | 167 | 11.1 | 575 | 11.5 | 56 | 11.5 | 3 | 12.5 | 3 | 13.0 |
| Codonopsis Radix | 167 | 11.1 | 575 | 11.5 | 56 | 11.5 | - | - | - | - |
| Ginseng Radix | - | - | - | - | - | - | 3 | 12.5 | 3 | 13.0 |
| Glycyrrhizae Radix | 167 | 11.1 | 575 | 11.5 | 56 | 11.5 | 2 | 8.33 | 2 | 8.70 |
| Zingiberis Rhizoma Recens | 167 | 11.1 | 575 | 11.5 | 56 | 11.5 | 1 | 4.17 | 1 | 4.35 |
| Jujubae Fructus | 167 | 11.1 | 575 | 11.5 | 56 | 11.5 | 3 | 12.5 | 3 | 13.0 |
| Preparation amount | XCH tablets: 1000 tablets, 0.4 g each; XCH capsules: 1000 capsules, 0.4 g each; | XCH effervescent tablets: 1000 tablets, 2.5 g each | XCH granules: 1000 g (combined with sucrose); 400 g (combined with mannitol); 250 g (combined with lactose) | Not specified | ||||||
| Preparations | Identification Method | TLC Reference Substance | TLC Control Crude Drug |
|---|---|---|---|
| XCH tablets | Microscopic Identification, Thin-Layer Chromatography Identification | Baicalin | Glycyrrhizae Radix |
| XCH effervescent tablets | Thin-Layer Chromatography Identification | Baicalin | Glycyrrhizae Radix, Bupleuri Radix, Codonopsis Radix |
| XCH capsules | Microscopic Identification, Thin-Layer Chromatography Identification | - | Glycyrrhizae Radix, Bupleuri Radix, Codonopsis Radix |
| XCH granules | Thin-Layer Chromatography Identification | Baicalin | Glycyrrhizae Radix, Bupleuri Radix |
| Shosaikoto Extract (Japanese) | Thin-Layer Chromatography Identification | Saikosaponin B2, 6-Gingerol, Wogonin, Ginsenoside Rb1, Liquiritin | - |
| Preparations | Detection Component | Prescribed Limit |
|---|---|---|
| XCH tablets | Baicalin | Not lower than 2.0 mg per tablet/capsule |
| XCH capsules | ||
| XCH effervescent tablets | Not lower than 20.0 mg per tablet/package | |
| XCH granules | ||
| Shosaikoto Extract (Japanese) | Saikosaponin B2, baicalin and glycyrrhizic acid | Saikosaponin B2: 2–8 mg |
| Year | Apparatus | Quantified Components | Other Instructions | Reference | |
|---|---|---|---|---|---|
| 1 | 2020 | HPLC | Baicalin, Wogonoside, Baicalein, Wogonin, Ammonium Glycyrrhizinate, Saikosaponin B2, Saikosaponin B1 | QAMS | [42] |
| 2 | 2018 | HPLC | 6-Gingerol | - | [43] |
| 3 | 2018 | HPLC | Ginsenoside Rg1, Ginsenoside Re, Ginsenoside Rb1, Saikosaponin A, Saikosaponin D | QAMS | [44] |
| 4 | 2018 | HPLC | Baicalin, Baicalein, Wogonin | - | [45] |
| 5 | 2017 | HPLC | Saikosaponin A, Saikosaponin D, Saikosaponin B1, Baicalin, Ginsenoside Rb1, Ginsenoside Re, 6-Gingerol, Liquiritin, Ammonium Glycyrrhizinate | - | [29] |
| 6 | 2017 | HPLC | Lobetyolin, Liquiritin, Baicalin, Baicalein | Detection wavelength switched | [46] |
| 7 | 2016 | HPLC | Baicalin | - | [47] |
| 8 | 2016 | UPLC | Baicalin | - | [48] |
| 9 | 2015 | HPLC-MSMS | Saikosaponin A, Saikosaponin D | - | [49] |
| 10 | 2015 | HPLC | Liquiritin, Baicalin, Wogonoside, Baicalein, Wogonin | - | [50] |
| 11 | 2015 | HPLC | Baicalin | - | [51] |
| 12 | 2015 | HPLC | Saikosaponin, Baicalin, Ginsenoside Rg1, Liquiritin, Ephedrine, 6-Gingerol | - | [52] |
| 13 | 2014 | HPLC | Saikosaponin A, Baicalin | - | [53] |
| 14 | 2014 | HPLC | Liquiritin, Baicalin, Wogonoside, Baicalein, Ammonium Glycyrrhizinate, Saikosaponin A, Wogonin | - | [54] |
| 15 | 2014 | HPLC | Saikosaponin B2 | - | [55] |
| 16 | 2013 | HPLC | Saikosaponin, Baicalin, Glycyrrhizic acid | - | [56] |
| 17 | 2012 | Capillary electrophoresis | Saikosaponin A, Saikosaponin D | - | [57] |
| 18 | 2012 | HPLC | Baicalin | - | [58] |
| 19 | 2012 | HPLC | Baicalin | - | [59] |
| 20 | 2010 | HPLC | Baicalin, Wogonoside | - | [60] |
| 21 | 2010 | HPLC | Baicalin, Baicalein, Wogonoside, Wogonin, Glycyrrhizic acid | - | [61] |
| 22 | 2010 | HPLC | Saikosaponin A | - | [62] |
| 23 | 2007 | HPLC-DAD-MS | Saikosaponin A, Baicalin, Glycyrrhizic acid | - | [63] |
| 24 | 2007 | HPLC | Baicalin | - | [64] |
| 25 | 2006 | HPLC/DAD | Baicalin, Glycyrrhizic acid | - | [65] |
| 26 | 2006 | HPLC-MSMS | Cytidine, Tyrosine, Uridine, Adenine, Guanosine, Phenylalanine, Adenosine, Tryptophan | - | [66] |
| 27 | 2004 | HPLC-MSMS | Saikosaponin A, B1, B2, C, D, G, H, I | - | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Wang, H.; Deng, Y.; Xie, K.; Zhao, W.; Gong, X. Research Progress on Quality Control Methods for Xiaochaihu Preparations. Separations 2021, 8, 199. https://doi.org/10.3390/separations8110199
Xu G, Wang H, Deng Y, Xie K, Zhao W, Gong X. Research Progress on Quality Control Methods for Xiaochaihu Preparations. Separations. 2021; 8(11):199. https://doi.org/10.3390/separations8110199
Chicago/Turabian StyleXu, Guangzheng, Hui Wang, Yingqian Deng, Keyi Xie, Weibo Zhao, and Xingchu Gong. 2021. "Research Progress on Quality Control Methods for Xiaochaihu Preparations" Separations 8, no. 11: 199. https://doi.org/10.3390/separations8110199
APA StyleXu, G., Wang, H., Deng, Y., Xie, K., Zhao, W., & Gong, X. (2021). Research Progress on Quality Control Methods for Xiaochaihu Preparations. Separations, 8(11), 199. https://doi.org/10.3390/separations8110199