Quality Distinguish of Red Ginseng from Different Origins by HPLC–ELSD/PDA Combined with HPSEC–MALLS–RID, Focus on the Sugar-Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Instrumentation
2.3. Simultaneous Determination of Disaccharides in Rg Using the Hplc–Elsd Method
2.3.1. Preparation of Samples
2.3.2. Chromatographic Conditions on the Determination of Disaccharides
2.3.3. Methodological Study on the Determination of Disaccharides
2.3.4. Content Determination of Disaccharides
2.4. Determination of Molecular Weight and Compositional Monosaccharides of Rg Polysaccharides
2.4.1. Molecular Weight Analysis of Rg Polysaccharides
Extraction of Polysaccharides
Preparation of the RG Polysaccharide’s Samples
Preparation of the Glucan Solution
Chromatographic Conditions on the Determination of Molecular Weight
2.4.2. Analysis of Compositional Monosaccharides of RG Polysaccharides
Preparation of Hydrolyzed Polysaccharides
Preparation of the Mixed Monosaccharides Solution
Chromatographic Conditions on the Determination of Compositional Monosaccharides
Methodological Study on the Determination of Compositional Monosaccharides of Polysaccharides
2.4.3. Content Determination of Molecular Weights and Compositional Monosaccharides of Rg Polysaccharides
Determination of Molecular Weights of Polysaccharides
Determination of Compositional Monosaccharides of Polysaccharides
3. Results and Discussion
3.1. Conditions Optimization
3.2. Results on the Determination of Sucrose and Maltose
3.3. Quantitative Analysis Results
3.4. Analysis on the Polysaccharides Molecular Weight Determination
3.5. Results of Determinations of the Compositional Monosaccharides of Polysaccharides
3.6. Analysis on the Monosaccharide Composition Determination of Polysaccharides
3.6.1. PCA Results
3.6.2. Results of Systematic Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020.
- Zheng, X.; Zhou, Y.; Chen, W.; Chen, L.; Lu, J.; He, F.; Li, X.; Zhao, L. Ginsenoside 20(S)-Rg3 Prevents PKM2-Targeting miR-324-5p from H19 Sponging to Antagonize the Warburg Effect in Ovarian Cancer Cells. Cell. Physiol. Biochem. 2018, 51, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Han, S.J.; Moon, D.; Kwon, S.; Lee, J.W.; Kim, K.S. Effects of Red Ginseng on the Elastic Properties of Human Skin. J. Ginseng Res. 2020, 44, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiao, C.; Ma, Y.; Chen, J.; Wu, W.; Liu, S. Anti-Ageing Effect of Red Ginseng Revealed by Urinary Metabonomics Using RRLC-Q-TOF-MS. Phytochem. Anal. 2018, 29, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, E.J.; Cheon, J.M.; Nam, K.J.; Oh, T.H.; Kim, K.S. Antioxidant and Hepatoprotective Effects of Fermented Red Ginseng against High Fat Diet-Induced Hyperlipidemia in Rats. Lab. Anim. Res. 2016, 32, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.W.; Yue, C.Y.; Wu, S.; Zhao, X.Q.; Zhang, Y.; Gong, L.H. Study on Antioxidant Ability of Red Ginseng Polysaccharide in Mice. China Food Addit. 2019, 30, 68–71. [Google Scholar]
- Zhou, Q.L.; Xu, W.; Yang, X.W. Chemical constituents of Chinese red ginseng. Chin. J. Chin. Mater. Med. 2016, 41, 233–249. [Google Scholar]
- Zhou, Q.Q.; Ren, W.M.; Wang, Y.H.; Yang, D.; Wang, G.M.; Li, Y.R. Research Progress on Processing Drugs Methods, Chemical Composition and Pharmacological Activity of Red Ginseng. Shanghai J. Tradit. Chin. Med. 2016, 50, 97–100. [Google Scholar]
- Liu, C.L.; Xie, Q.S.; Li, Q.Y.; Gao, T.Y.; Liu, H.X.; Jiang, H. Determination of Total Ginsenosides, Ginsenosides Rg1, Re and Rb1 in Red Ginseng. J. Pharm. Res. 2021, 40, 87–90. [Google Scholar]
- Gao, T.Y.; Jiang, Y.Q.; Li, Q.Y.; Hu, F.D.; Xie, Q.S.; Wang, H. Determination of 12 Ginsenosides in Red Ginseng by High Performance Liquid Chromatography. J. Food Saf. Food Qual. 2021, 12, 175–181. [Google Scholar]
- Da, J.; Wang, Q.R.; Wang, Y.; Yao, S.; Huang, Y.; Wei, W.L.; Liang, J.; Shen, Y.; Franz, G.; Guo, D.A. Quantitative Analysis of Eight Ginsenosides in Red Ginseng Using Ginsenoside Rg1 as Single Reference Standard. World J. Tradit. Chin. Med. 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Lee, D.H.; Cho, H.J.; Kang, H.Y.; Rhee, M.H.; Park, H.J. Total Saponin from Korean Red Ginseng Inhibits Thromboxane A2 Production Associated Microsomal Enzyme Activity in Platelets. J. Ginseng Res. 2012, 36, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abashev, M.; Stekolshchikova, E.; Stavrianidi, A. Quantitative Aspects of the Hydrolysis of Ginseng Saponins: Application in HPLC-MS Analysis of Herbal Products. J. Ginseng Res. 2021, 45, 246–253. [Google Scholar] [CrossRef]
- Jeong, H.C.; Hong, H.D.; Kim, Y.C.; Rhee, Y.K.; Choi, S.Y.; Kim, K.T.; Kim, S.S.; Lee, Y.C.; Cho, C.W. Quantification of Maltol in Korean Ginseng (Panax Ginseng) Products by High-Performance Liquid Chromatography-Diode Array Detector. Pharmacogn. Mag. 2015, 11, 657–664. [Google Scholar] [CrossRef] [Green Version]
- In, G.; Ahn, N.G.; Bae, B.S.; Han, S.T.; Noh, K.B.; Kim, C.S. New Method for Simultaneous Quantification of 12 Ginsenosides in Red Ginseng Powder and Extract: In-House Method Validation. J. Ginseng Res. 2012, 36, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.L.; Zhu, D.N.; Yang, X.W.; Xu, W.; Wang, Y.P. Development and Validation of a UFLC-MS/MS Method for Simultaneous Quantification of Sixty-Six Saponins and Their Six Aglycones: Application to Comparative Analysis of Red Ginseng and White Ginseng. J. Pharm. Biomed. Anal. 2018, 159, 153–165. [Google Scholar] [CrossRef]
- Kim, I.W.; Cha, K.M.; Wee, J.J.; Ye, M.B.; Kim, S.K. A New Validated Analytical Method for the Quality Control of Red Ginseng Products. J. Ginseng Res. 2013, 37, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Ji, S.H.; Choi, B.R.; Choi, D.J.; Lee, Y.G.; Kim, H.G.; Kim, G.S.; Kim, K.; Lee, Y.H.; Baek, N.I.; et al. UPLC-QTOF/MS-Based Metabolomics Applied for the Quality Evaluation of Four Processed Panax ginseng products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Sun, L.; Zhang, Z.; Guo, Y.; Liu, S. Profiling and Multivariate Statistical Analysis of Panax Ginseng Based on Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Liu, L.; Zhao, D.Q.; Zhao, Y.; Bai, X.Y.; Zhang, H.M.; Guan, Y.Y.; Zhao, S.N. Comparative Study of Sugar Content in Panax Ginseng, P. quinquefolium and Red Ginseng. J. China Pharm. 2013, 24, 616–618. [Google Scholar]
- Li, L.; Ma, L.; Guo, Y.; Liu, W.; Wang, Y.; Liu, S. Analysis of Oligosaccharides from Panax Ginseng by Using Solid-Phase Permethylation Method Combined with Ultra-High-Performance Liquid Chromatography-Q-Orbitrap/Mass Spectrometry. J. Ginseng Res. 2020, 44, 775–783. [Google Scholar] [CrossRef]
- Lee, S.J.; In, G.; Han, S.T.; Lee, M.H.; Lee, J.W.; Shin, K.S. Structural Characteristics of a Red Ginseng Acidic Polysaccharide Rhamnogalacturonan I with Immunostimulating Activity from Red Ginseng. J. Ginseng Res. 2020, 44, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Kim, S.W.; Youn, S.H.; Hyun, S.H.; Kyung, J.S.; In, G.; Park, C.K.; Jung, H.R.; Moon, S.J.; Kang, M.J.; et al. Biological Effects of Korean Red Ginseng Polysaccharides in Aged Rat Using Global Proteomic Approach. Molecules 2020, 25, 3019. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.H.; Lee, S.M.; Han, C.K.; In, G.; Park, C.K.; Hyun, S.H. Immune Activity of Polysaccharide Fractions Isolated from Korean Red Ginseng. Molecules 2020, 25, 3569. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Han, B.; Shin, M.S.; Hwang, G.S. Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide. Polymers 2020, 12, 2186. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Nam, Y.; Park, Y.H.; Kim, M.J.; Lee, E.; Jeon, S.G.; Bae, B.S.; Seo, J.; Shim, S.L.; Kim, J.S.; et al. Therapeutic Effects of Non-Saponin Fraction with Rich Polysaccharide from Korean Red Ginseng on Aging and Alzheimer’s Disease. Free Radic. Biol. Med. 2021, 164, 233–248. [Google Scholar] [CrossRef]
- Shin, S.J.; Park, Y.H.; Jeon, S.G.; Kim, S.; Nam, Y.; Oh, S.M.; Lee, Y.Y.; Moon, M. Red Ginseng Inhibits Tau Aggregation and Promotes Tau Dissociation in Vitro. Oxid. Med. Cell. Longev. 2020, 7829842, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.X.; Zhang, H.Z.; Xu, P.Y.; Chen, Z.Q.; Yan, Y.Y. Ethanol Fractional Purification and Antioxidant Activities of Polysaccharides from Polygonum Cuspidatum. Sci. Technol. Food Ind. 2019, 40, 92–95. [Google Scholar]
- Ying, X. Purification and Structural Analysis of Polysaccharides from Red Ginseng; Northeast Normal University: Changchun, China, 2018. [Google Scholar]
- Aa, J. Analysis of Metabolomic Data: Principal Component Analysis. Chin. J. Clin. Pharm. Ther. 2010, 15, 481–489. [Google Scholar]
- Tai, Y.N.; Wu, X.; Fan, L.M.; Wu, Z.N.; Weng, Y.H.; Lin, Q.Q.; Chu, K.D.; Xie, R.H. Simultaneous Determination of Sixteen Components in Alismatis Rhizoma by UPLC-MS/MS. Chin. J. Pharm. Anal. 2018, 38, 1337–1350. [Google Scholar]
- Wu, D.T.; Li, W.Z.; Chen, J.; Zhong, Q.X.; Ju, Y.J.; Zhao, J.; Anton, B.; Li, S.P. An Evaluation System for Characterization of Polysaccharides from the Fruiting Body of Hericium Erinaceus and Identification of ITS Commercial Product. Carbohydr. Polym. 2015, 124, 201–207. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yu, L.S.; Liang, J.; Yang, B.Y.; Kuang, H.X. Chromatography and Mass Spectrometry-Based Approaches for Perception of Polysaccharides in wild and Cultured Fruit Bodies of Auricularia Auricular-Judae. Int. J. Biol. Macromol. 2019, 137, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Wu, D.T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.J.; Li, S.P. Qualitation and Quantification of Specific Polysaccharides From Panax Species Using GC-MS, Saccharide Mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yu, S.Q.; Zhang, F.; Xiao, L.; Nie, J. Determination and PCA Analysis of 10 Chemical Components in Chysanthemi Flos by UPLC-UV. China Pharm. 2018, 21, 1374–1378. [Google Scholar]

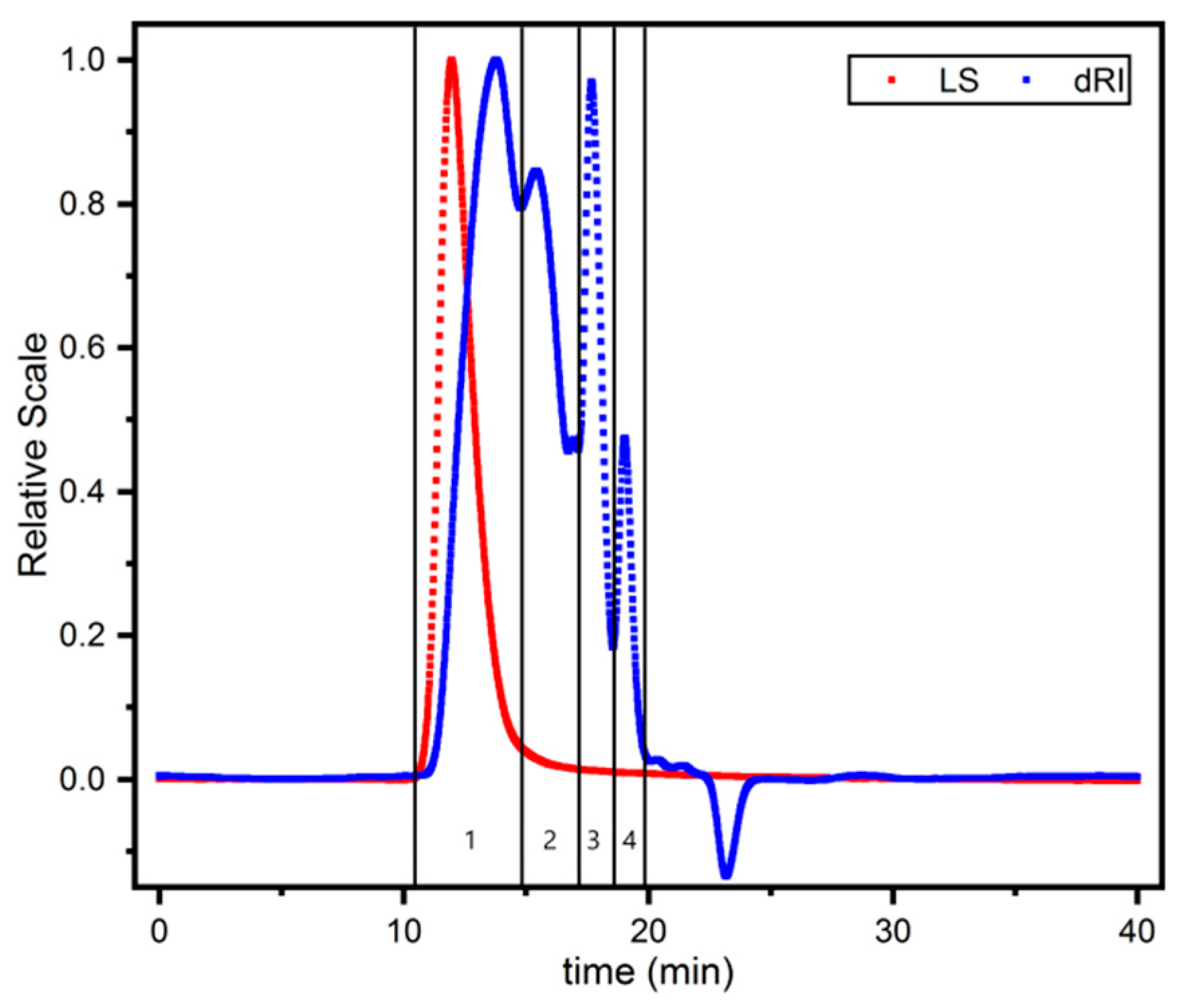
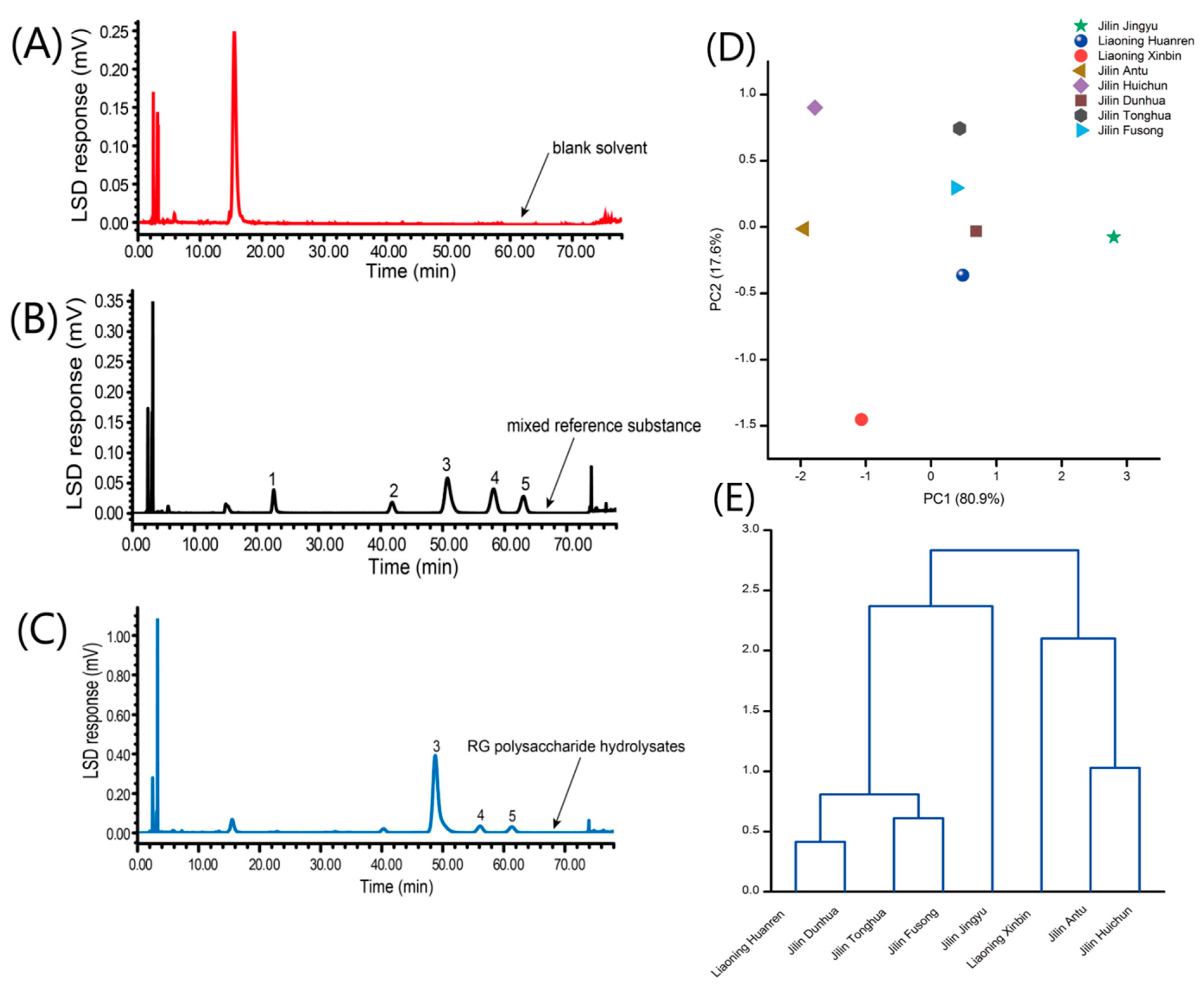
| Localities | Sucrose (mg/g) | Maltose (mg/g) |
|---|---|---|
| Jilin Tonghua | 50.37 | 186.12 |
| Liaoning Xinbin | 57.89 | 92.02 |
| Liaoning Huanren | 56.29 | 162.39 |
| Jilin Jingyu | 70.78 | 145.96 |
| Jilin Huichun | 52.46 | 172.92 |
| Jilin Antu | 70.07 | 179.93 |
| Jilin Fusong | 78.22 | 155.05 |
| Jilin Dunhua | 84.10 | 194.62 |
| Localities | Mw a (kDa) and Error of Peak 1 | Mw/Mn b and Error of Peak 1 | Mw (kDa) and Error of Peak 2 | Mw/Mn and Error of Peak 2 |
|---|---|---|---|---|
| Jilin Tonghua | 2996.6 (±1.2%) | 2.943 (±1.584%) | 235.9 (±2.0%) | 1.027 (±2.900%) |
| Liaoning Xinbin | 3708.5 (±1.3%) | 2.886 (±1.679%) | 322.0 (±1.8%) | 1.019 (±2.546%) |
| Liaoning Huanren | 3502.4 (±1.5%) | 3.158 (±2.097%) | 126.3 (±7.6%) | - c |
| Jilin Jingyu | 785.1 (±1.9%) | 3.577 (±5.558%) | 18.7 (±9.2%) | - |
| Jilin Huichun | 3157.7 (±1.1%) | 3.187 (±1.305%) | 238.0 (±1.2%) | 1.020 (±1.681%) |
| Jilin Antu | 3809.1 (±1.3%) | 2.532 (±1.645%) | 475.2 (±1.7%) | 1.016 (±2.417%) |
| Jilin Fusong | 2351.0 (±1.3%) | 3.299 (±1.779%) | 81.4 (±4.6%) | - |
| Jilin Dunhua | 2463.4 (±1.3%) | 3.147 (±1.978%) | 83.9 (±6.4%) | - |
| Localities | Glucose (mg/mL) | Galactose (mg/mL) | Arabinose (mg/mL) |
|---|---|---|---|
| Jilin Tonghua | 1.0999 | 0.0509 | 0.0410 |
| Liaoning Xinbin | 0.7816 | 0.0519 | 0.0382 |
| Liaoning Huanren | 0.9876 | 0.0548 | 0.0430 |
| Jilin Jingyu | 1.1636 | 0.0664 | 0.0497 |
| Jilin Huichun | 0.9833 | 0.0410 | 0.0314 |
| Jilin Antu | 0.8839 | 0.0454 | 0.0302 |
| Jilin Fusong | 1.0553 | 0.0542 | 0.0396 |
| Jilin Dunhua | 1.0366 | 0.0553 | 0.0426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Peng, S.; Li, F.; Cui, P.; Zhao, C.; Yan, X.; Suo, T.; Wang, C.; He, Y.; Li, Z. Quality Distinguish of Red Ginseng from Different Origins by HPLC–ELSD/PDA Combined with HPSEC–MALLS–RID, Focus on the Sugar-Markers. Separations 2021, 8, 198. https://doi.org/10.3390/separations8110198
Cheng Q, Peng S, Li F, Cui P, Zhao C, Yan X, Suo T, Wang C, He Y, Li Z. Quality Distinguish of Red Ginseng from Different Origins by HPLC–ELSD/PDA Combined with HPSEC–MALLS–RID, Focus on the Sugar-Markers. Separations. 2021; 8(11):198. https://doi.org/10.3390/separations8110198
Chicago/Turabian StyleCheng, Qian, Shuhuan Peng, Fangyi Li, Pengdi Cui, Chunxia Zhao, Xiaohui Yan, Tongchuan Suo, Chunhua Wang, Yongzhi He, and Zheng Li. 2021. "Quality Distinguish of Red Ginseng from Different Origins by HPLC–ELSD/PDA Combined with HPSEC–MALLS–RID, Focus on the Sugar-Markers" Separations 8, no. 11: 198. https://doi.org/10.3390/separations8110198
APA StyleCheng, Q., Peng, S., Li, F., Cui, P., Zhao, C., Yan, X., Suo, T., Wang, C., He, Y., & Li, Z. (2021). Quality Distinguish of Red Ginseng from Different Origins by HPLC–ELSD/PDA Combined with HPSEC–MALLS–RID, Focus on the Sugar-Markers. Separations, 8(11), 198. https://doi.org/10.3390/separations8110198






