Development of an UPLC-MS/MS Method for Quantitative Analysis of Clotrimazole in Human Plasma Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Administration
2.2. Preparation of Stock Solution, Calibration Standard and Quality Control Samples
2.3. Clotrimazole Liquid/Liquid Extraction from Plasma Samples
2.4. Chromatographic and Mass-Spectrometric Conditions
3. Results
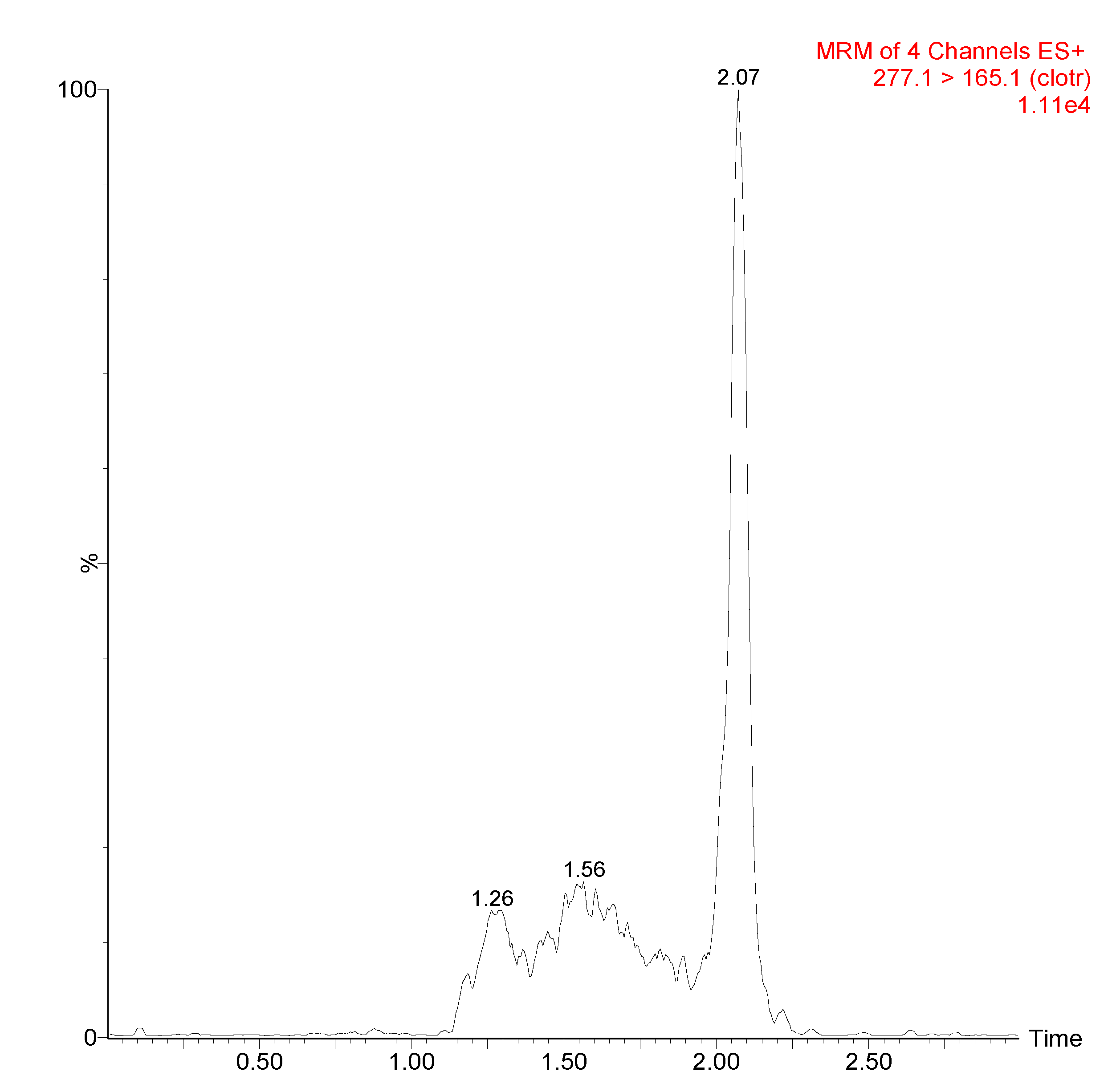
3.1. UPLC/MS-MS Chromatograms
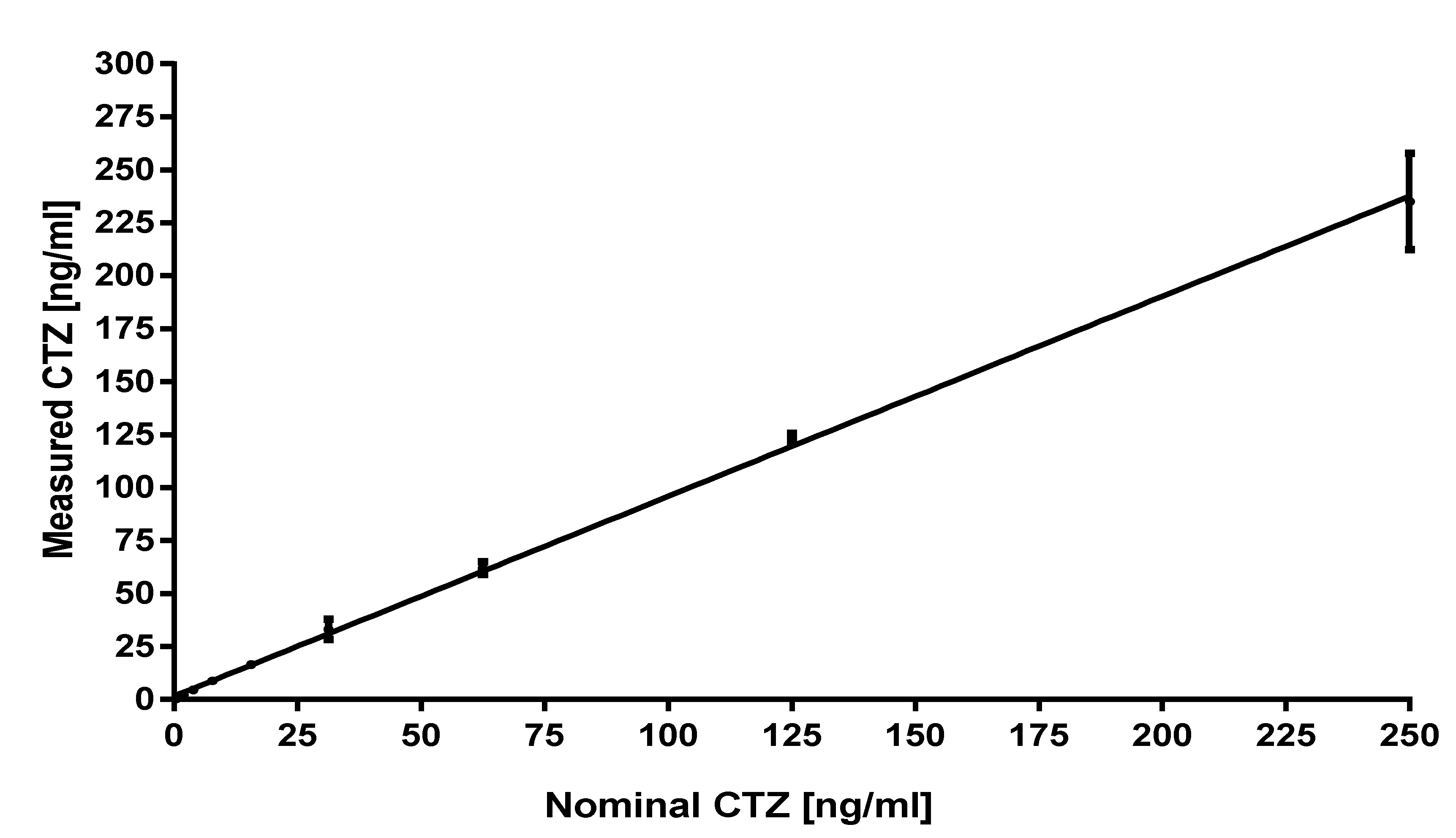
3.2. Validation
3.3. In Vivo Testing
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Ethical Statement
References
- Sawyer, P.R.; Brogden, R.N.; Pinder, R.M.; Speight, T.M. Avery, G.S. Clotrimazole: A review of its antifungal activity and therapeutic efficacy. Drugs 1975, 9, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.medicines.org.uk/emc/product/2598/smpc (accessed on 22 September 2020).
- Ritter, W.; Patzschke, K.; Krause, U.; Stettendorf, S. Pharmacokinetic fundamentals of vaginal treatment with clotrimazole. Chemotherapy 1982, 28 (Suppl. 1), 37–42. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-clinical-requirements-locally-applied-locally-acting-products-containing-known_en.pdf (accessed on 22 September 2020).
- Campestre, C.; Locatelli, M.; Guglielmi, P.; De Luca, E.; Bellagamba, G.; Menta, S.; Zengin, G.; Celia, C.; Di Marzio, L.; Carradori, S. Analysis of imidazoles and triazoles in biological samples after MicroExtraction by packed sorbent. J. Enzym. Inhib. Med. Chem. 2017, 32, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A fabric phase sorptive extraction-High performance liquid chromatography-Photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Mousa, B.A.; El-Kousy, N.M.; El-Bagary, R.I.; Mohamed, N.G. Stability indicating methods for the determination of some anti-fungal agents using densitometric and RP-HPLC methods. Chem. Pharm. Bull. 2008, 56, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgoła-Grześkowiak, A.; Grześkowiak, T. Application of dispersive liquid-liquid microextraction followed by HPLC-MS/MS for the trace determination of clotrimazole in environmental water samples. J. Sep. Sci. 2013, 36, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 22 September 2020).
- Kaza, M.; Karaźniewicz-Łada, M.; Kosicka, K.; Siemiątkowska, A.; Rudzki, P.J. Bioanalytical method validation: New FDA guidance vs. EMA guideline. Better or worse? J. Pharm. Biomed. Anal. 2019, 165, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Sakamoto, M.; Law, T.; Platt, O.; Mikati, M.; Armsby, C.C.; Brugnara, C. HPLC measurement, blood distribution, and pharmacokinetics of oral clotrimazole, potentially useful antisickling agent. Clin. Chem. 1995, 41, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Tawakkol, S.M.; Fayez, Y.M.; Fahmy, N.M.; Lotfy, H.M.; Shehata, M.A. Monitoring of Clotrimazole Degradation Pathway in Presence of its Co-formulated Drug. J. Chromatogr. Sci. 2019, 57, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 22 September 2020).
- Yan, F.; Sun, M.; Hang, T.; Sun, J.; Zhou, X.; Deng, X.; Ge, L.; Qian, H.; Ya, D.; Huang, W. A rapid and sensitive UPLC-MS/MS method for determination of HZ08 in rat plasma and tissues: Application to a pharmacokinetic study of liposome injections. J. Pharm. Biomed. Anal. 2015, 102, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.Y.; Song, M.; Hang, T.J.; Huang, W.L.; Du, Y. Pharmacokinetics of HZ08 in rats by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 856, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.N.; Ashraf, A.; Iqbal, M.; Nazir, A. Analytical method development and validation of hydrocortisone and clotrimazole in topical dosage form using RP-HPLC. Future J. Pharm. Sci. 2020, 6, 49. [Google Scholar] [CrossRef]
- MuthuKumar, S.; Kathram, S.; Navanethan, J.; Selvakumar, D.; Banji, D. Method development and validation of rp-hplc method for simultaneous determination of clindamycinphosphate and clotrimazole in soft gelatinvaginal suppositories. Int. J. Pharm. Ther. 2013, 4, 270–275. [Google Scholar]
- Ferreira, P.G.; Lima, C.G.S.; Noronha, L.L.; De Moraes, M.C.; Da Silva, F.; Vicosa, A.; Futuro, D.O.; Ferreira, V. Development of a Method for the Quantification of Clotrimazole and Itraconazole and Study of Their Stability in a New Microemulsion for the Treatment of Sporotrichosis. Molecules 2019, 24, 2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Hospital Formulary Service (AHFS) Drug Information. 2007. Available online: https://www.ahfsdruginformation.com/ (accessed on 22 September 2020).
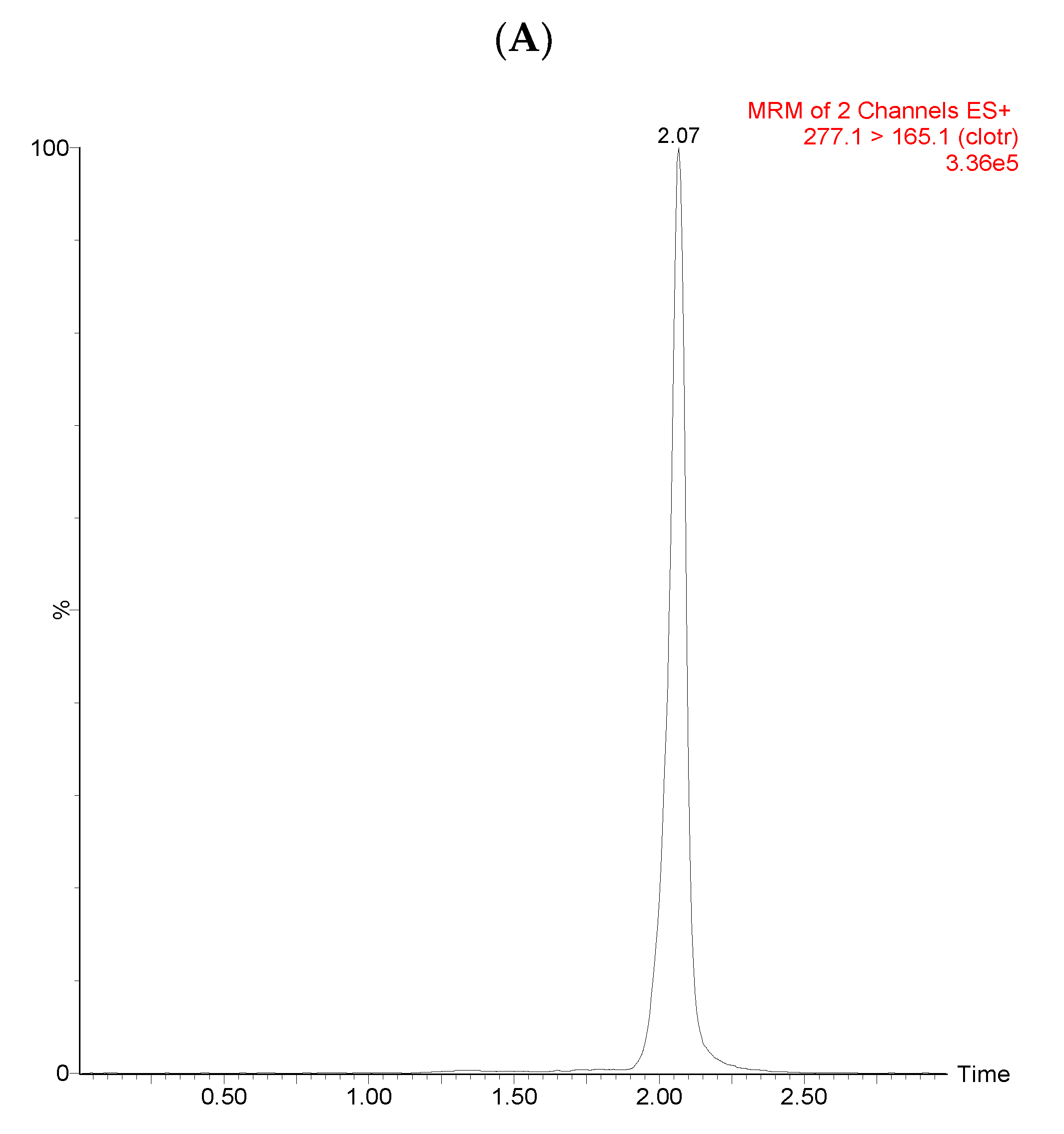
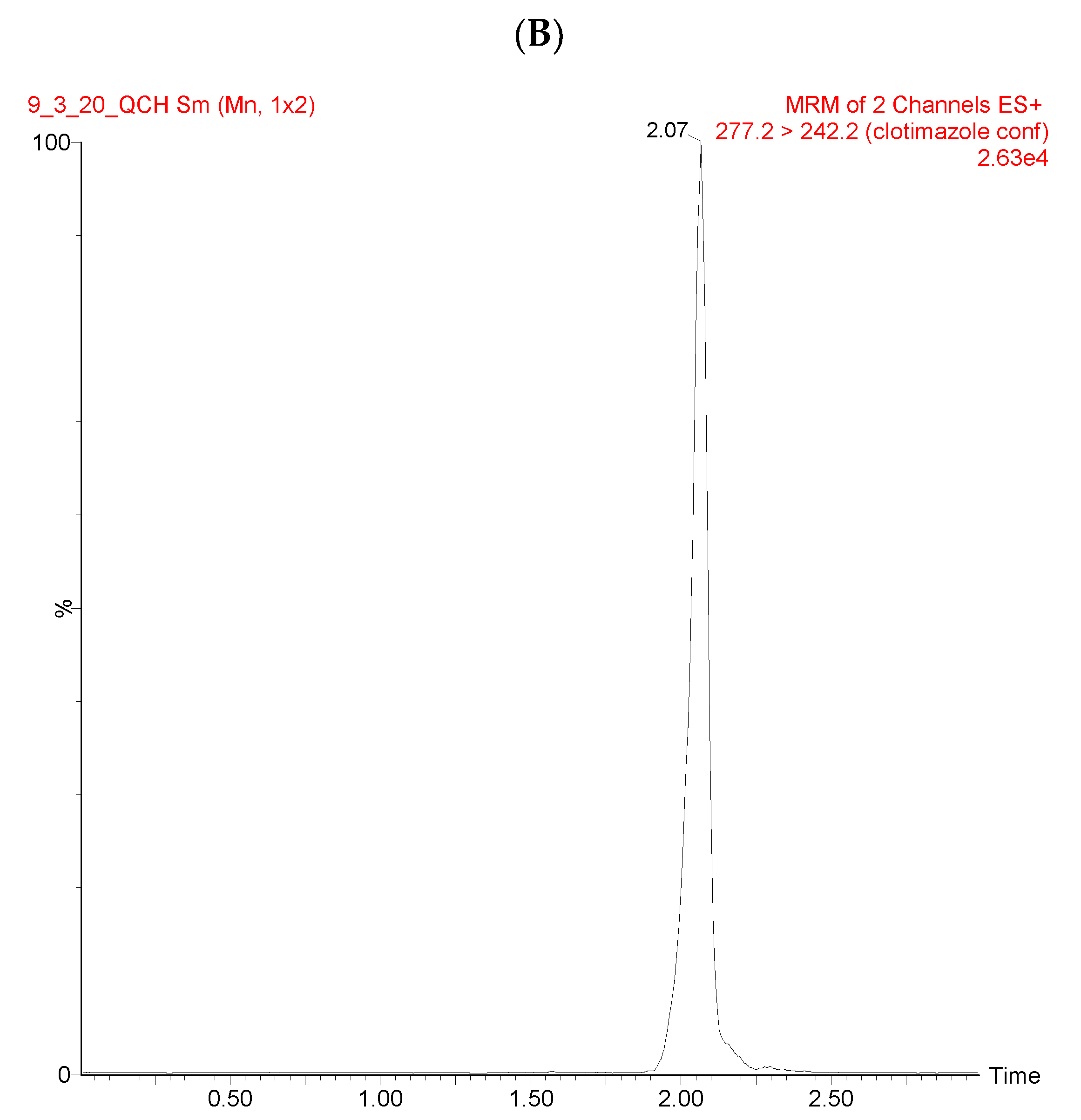
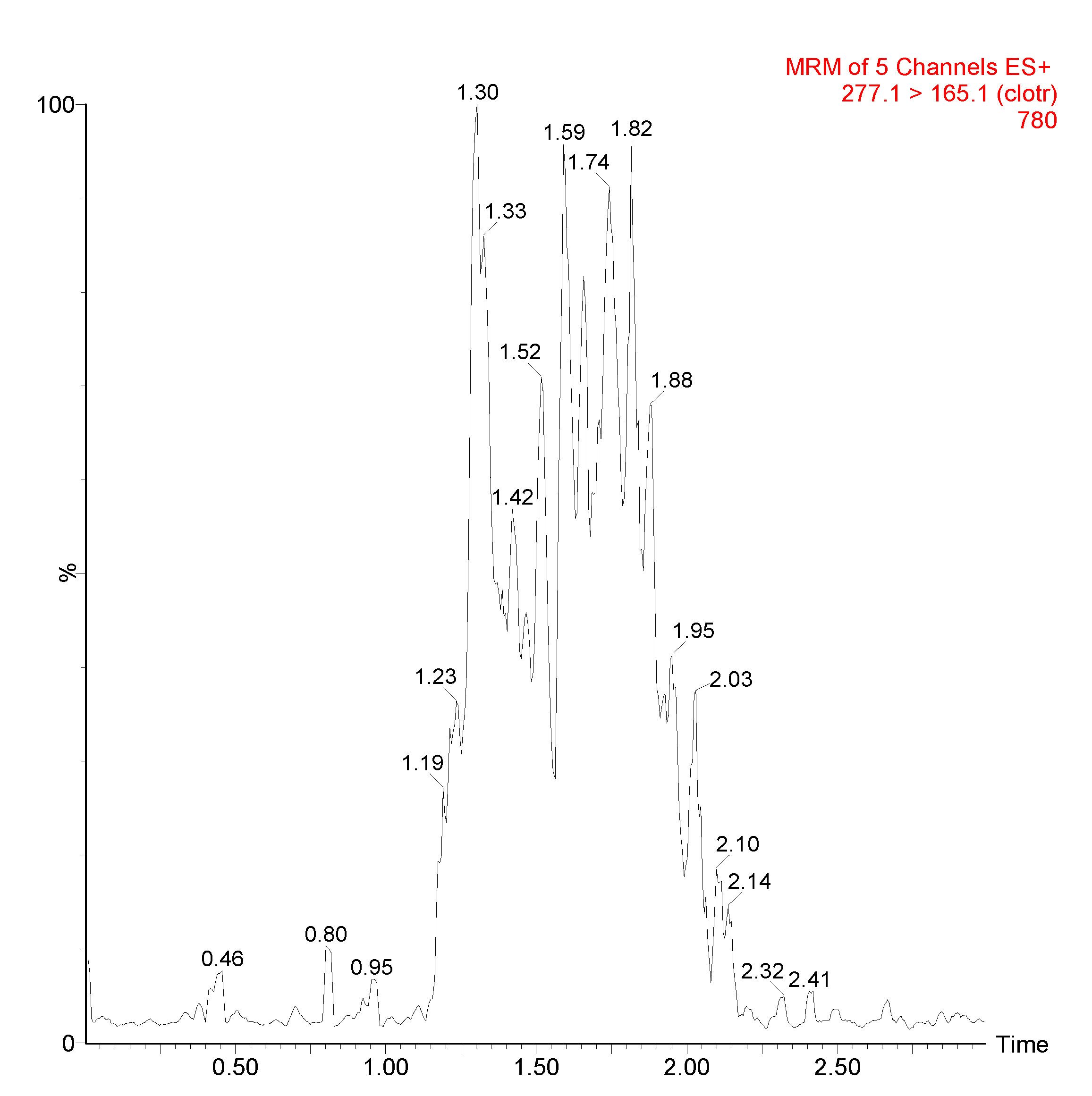

| Source (ESI+) and Analyzer (SI+) and | Settings |
|---|---|
| Capillary voltage (Kv) | 4.0 |
| Cone voltage (V) | 40.0 |
| Extractor (V) | 3.0 |
| Source temperature (°C) | 150 |
| Desolvation temperature (°C) | 250 |
| Cone gas flow (L/h) | 1 |
| Desolvation gas flow (L/h) | 500 |
| Collision energy (eV) | 27.0 |
| Intraday CV | 7.80% |
| Inter-day CV | 2.88% |
| LOD | 0.135 ng/mL |
| LOQ | 0.450 ng/mL |
| Range of linearity | 0.488–250 ng/mL |
| Equation | Y = 0.9436 * X + 1.537 |
| R2 | 0.9903 |
| CV intra-subject | 11.34% |
| Time of Spiked Samples Measure | Nominal Concentration [ng/mL] | [ng/mL] | Mean [ng/mL] ± SD (N) | % Deviation Respect the Nominal Concentration | Mean % Deviation | |
|---|---|---|---|---|---|---|
| After extraction | 250 | 220.2 | 222.9 ± | 4.0 (3) | −11.9 | −10.8 |
| 221.1 | −11.6 | |||||
| 227.5 | −9 | |||||
| 100 | 104.4 | 105.0 ± | 4.5 (3) | 4.4 | 5.5 | |
| 101.7 | 1.7 | |||||
| 110.4 | 10.4 | |||||
| 25 | 27.7 | 28.5 ± | 0.8 (3) | 10.7 | 13.8 | |
| 28.5 | 14.1 | |||||
| 29.2 | 16.7 | |||||
| After keeping at 4 °C | 250 | 200.6 | 205.5 ± | 7.3 (3) | −19.8 | −17.8 |
| 213.8 | −14.5 | |||||
| 202.0 | −19.2 | |||||
| 100 | 99.1 | 105.8 ± | 5.9 (3) | −0.9 | 5.6 | |
| 110.5 | 10.5 | |||||
| 107.1 | 7.1 | |||||
| 25 | 30.9 | 30.1 ± | 0.7 (3) | 23.7 | 20.4 | |
| 29.7 | 18.8 | |||||
| 29.7 | 18.7 | |||||
| After keeping at −20 °C | 250 | 276.1 | 259.1 ± 15.8 (3) | 10.4 | 3.6 | |
| 256.6 | 2.6 | |||||
| 244.7 | −2.1 | |||||
| 100 | 103.1 | 98.9 ± | 6.4 (3) | 3.1 | −1.4 | |
| 101.3 | 1.3 | |||||
| 91.3 | −8.7 | |||||
| 25 | 32.9 | 29.1 ± | 3.4 (3) | 31.8 | 16.3 | |
| 27.5 | 9.9 | |||||
| 26.8 | 7.2 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisi, L.; Ciotti, G.M.P.; Navarra, P. Development of an UPLC-MS/MS Method for Quantitative Analysis of Clotrimazole in Human Plasma Samples. Separations 2020, 7, 62. https://doi.org/10.3390/separations7040062
Lisi L, Ciotti GMP, Navarra P. Development of an UPLC-MS/MS Method for Quantitative Analysis of Clotrimazole in Human Plasma Samples. Separations. 2020; 7(4):62. https://doi.org/10.3390/separations7040062
Chicago/Turabian StyleLisi, Lucia, Gabriella Maria Pia Ciotti, and Pierluigi Navarra. 2020. "Development of an UPLC-MS/MS Method for Quantitative Analysis of Clotrimazole in Human Plasma Samples" Separations 7, no. 4: 62. https://doi.org/10.3390/separations7040062
APA StyleLisi, L., Ciotti, G. M. P., & Navarra, P. (2020). Development of an UPLC-MS/MS Method for Quantitative Analysis of Clotrimazole in Human Plasma Samples. Separations, 7(4), 62. https://doi.org/10.3390/separations7040062




