2. In Vivo SPME Extraction of Mammal Tissues
One of the major problems associated to tissue sampling is related to the adverse effects that can be produced during its collection. The use of miniaturized devices for in vivo SPME sampling can minimize these effects, allowing the direct analysis of tissues located in different body areas.
In this context, interesting devices have been recently developed for extraction of neurotransmitters performing in vivo brain sampling. Lendor et al. have developed a SPME-based miniaturized probe, having hydrophilic lipophilic balanced particles with strong cation exchange groups (HLB-SCX) SPME coating and a layer of biocompatible PAN, for the chemical biopsy of rat brain [
17]. Brain sampling is assisted by a software-controlled driving system equipped with a needle able to promote probe penetration. The presence of a guiding cannula preserves the coating from contamination until the probe reaches the brain area to be sampled. The developed solid-phase microextraction-liquid chromatography-tandem mass spectrometry (SPME-LC-MS/MS) protocol in a surrogate brain matrix proved to be suitable for determining neurochemicals at physiological levels. Finally, the proof-of concept in vivo application was performed on macaque brain, extracting several neurochemicals simultaneously from three brain areas. Very recently, in another study reliable measurements of different neuromodulators like glutamate, dopamine, acetylcholine and choline have been simultaneously made by in vivo SPME sampling within the frontal cortex and striatum of macaque monkeys during goal-directed behavior [
28].
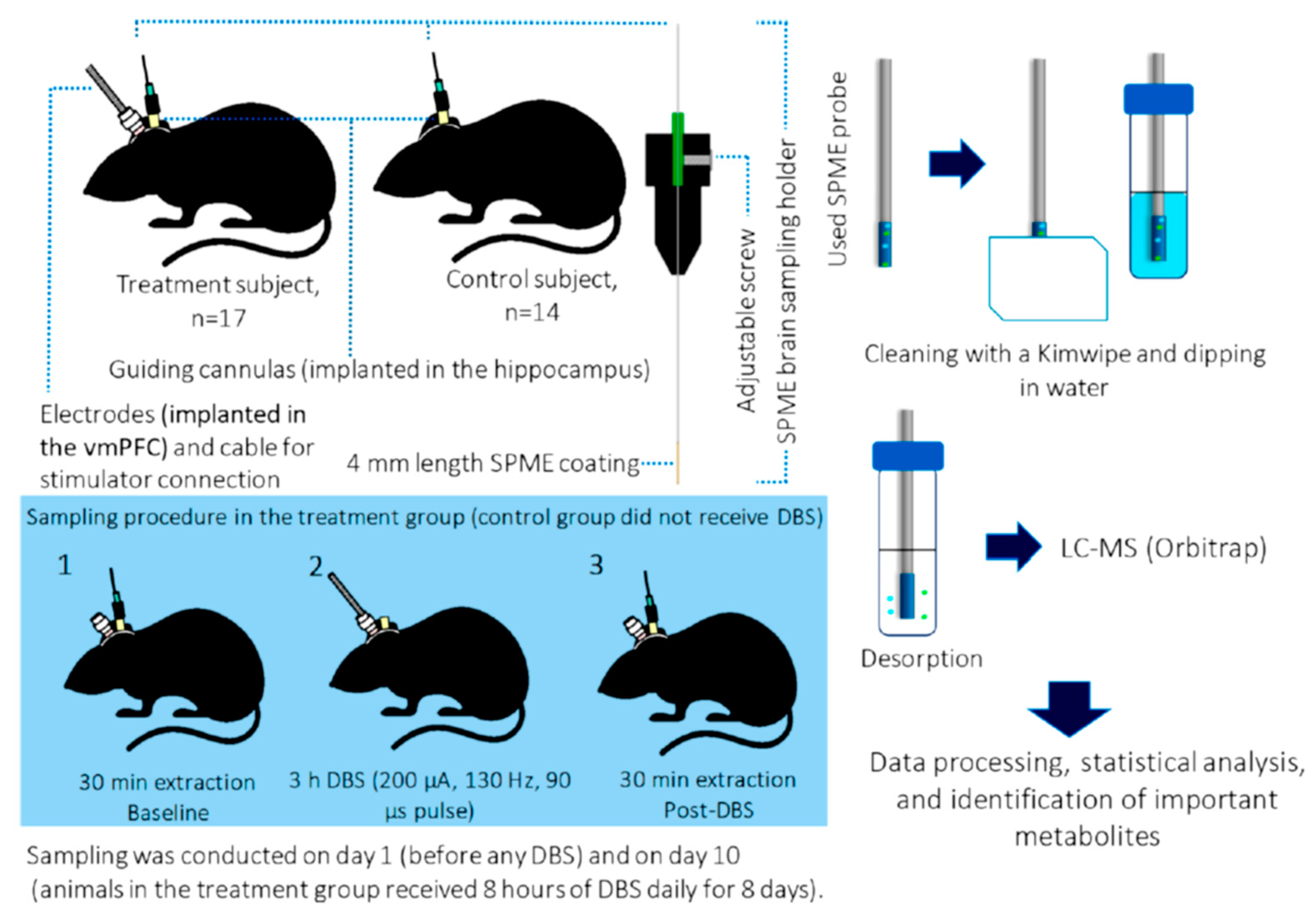
In comparison to microdialysis-mass spectrometry, which is the most common method for determining multiple neuromodulators in awake behaving animals, the proposed in vivo SPME sampling combines the high enrichment and does not destroy the tissue during the positioning of both the probe and the guiding cannula, thus avoiding the damage-induced release of neuromodulators (
Figure 1). Finally, an additional advantage of the proposed approach relies on the highest affinity of the used SPME coating towards hydrophobic compounds. The developed SPME-LC-MS/MS protocol allowed the effective determination of the neuromodulators at different concentration levels, thus being useful for a better understanding of the neuromodulatory system. In vivo brain sampling using the same SPME probe has been also carried out by Reyes-Garces et al. [
29] with the aim of performing the untargeted in vivo analysis of rodent’s brain after deep brain stimulation (DBS). Metabolite changes occurring in brain hippocampi after 3 h of DBS and after 8 days of daily DBS therapy were evaluated. Findings demonstrated that acute DBS was able to produce changes in the levels of several metabolites like citrulline, phosphor- and glycosphingolipids, whose concentration levels increased after the therapy. By contrast, after chronic DBS, a decrease in the corticosterone levels were observed.
Taking into account that liver is the main metabolic organ of mammals, in vivo SPME has been applied also for the selective capture of luteolin and its metabolites in rat liver [
15]. In vivo sampling is proposed as valid alternative to the use of liver microsomes and homogenates since these approaches can cause serious damages or animal death due to the high invasiveness. Owing to the presence of cavities having high affinity for specific analytes, the use of MIP-based coatings has been proposed to enhance SPME selectivity with respect to commercial fibers. In this study, a molecularly imprinted polymer-solid-phase microextraction (MIP-SPME) coating was devised and applied for the LC-MS/MS analysis of the target analytes. The MIP-SPME fiber was prepared using luteolin, acrylamide and ethylene glycol dimethacrylate as template, functional monomer and cross-linker, respectively. As for luteolin metabolites, three compounds were identified, i.e., apigenin, chrysoeriol and diosmetin, thus proposing in vivo sampling as a promising tool for assessing the real metabolic pathway of target compounds. Since MIPs are usually incompatible with macromolecules, requiring a preliminary clean-up, a restricted molecularly imprinted solid-phase microextraction (RAMIPs-SPME) coating has been proposed by Wang and coworkers for the selective determination of hesperetin and its metabolites in rat livers [
30]. Being able of selectively adsorbing analytes from complex matrices and eliminating macromolecules, the use of RAMIP-based material offered the advantages of both MIPs and restricted access materials. The compounds extracted by in vivo RAMIPs-SPME were analyzed by ultra-performance-liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), showing the highest affinity and enrichment capability of the developed coating with respect to both other MIPs and commercially available PDMS and DVB fibers.
Since the determination of the concentration of anticancer drugs could improve the efficacy of chemotherapic treatments reducing adverse effects on patients, recently Roszkowska et al. have developed an in vivo SPME-LC-MS/MS protocol for the quantitation of doxorubicin in lung tissue [
31]. Preliminary experiments carried out using the ex vivo SPME approach allowed to optimize the operative conditions for doxorubicin extraction using lamb lungs as surrogate matrix. Validation proved the reliability of the developed protocol, obtaining a quantitation limit (LOQ) of 2.5 µg/g, a good precision with relative standard deviation (RSD) < 14.7% and a good linearity in the 2.5–50 µg/g range. Then, in vivo SPME sampling was performed on real pig lung samples to detect doxorubicin after in vivo lung perfusion, proving the capability of in vivo SPME for therapeutic drug monitoring in clinical studies, opening the possibility to perform sampling several times in different areas of the organ, without the need of biopsies.
In the field of cancer research, untargeted metabolite analysis on resected tumors could be used as a new diagnostic approach able to characterize endogenous metabolites, thus being potentially useful for biomarker discovery. Performing ex vivo SPME sampling on tumors allows sample availability for additional analyses like histological testing, thus obtaining a complete pattern of information about a specific disease. A very interesting study has been proposed by Zhang and coworkers, who developed a disposable handheld device, the MasSpec Pen, for the real time sampling of tissues [
32].
The device was characterized by a pen-sized handheld device directly integrated into a laboratory-built MS interface. The biocompatible sampling probe allowed the time and volume-controlled extraction of molecules from tissue by means of water droplets. After a few seconds of extraction, the droplets were transported from the MasSpec Pen to the mass spectrometer for molecular analysis. Preliminary experiments were performed by ex vivo sampling on tissue sections of more than 200 patients, then the MasSpec Pen was tested for in vivo tissue analysis in mice using a murine model of human breast cancer. Under anesthesia, the skin overlying the tumors was removed, and several tissue regions, i.e., multiple positions of the top of the tumor, the core of the tumor after partial tumor resection, and adjacent normal soft connective tissue, were analyzed. A distinctive molecular profile was observed between adjacent normal soft connective tissue regions and tumor regions. No observable macroscopic or microscopic damages of the tissue regions analyzed were detected. Furthermore, no apparent effects to the health of the animals were observed caused by the MasSpec Pen analysis during surgery, thus suggesting the effectiveness of the device for in vivo molecular evaluation and cancer diagnosis.
An overview of the discussed applications is reported in
Table 1.
3. In Vivo SPME Extraction of Fish Tissues
In vivo analysis of fish samples is an emerging strategy for monitoring the health of aquatic ecosystems and water contamination from both natural sources and anthropogenic pollutants [
33]. In fact, fish could absorb contaminants through their gills, skin, or gastrointestinal tract [
33,
34,
35]. In vivo tissue monitoring of pharmaceuticals [
36,
37,
38,
39,
40,
41], persistent pollutants [
42,
43,
44], pesticides [
45] and antibacterial agents [
46] provides valuable information regarding their concentration in the ecosystem, bioaccumulation levels and metabolism. In particular, lipophilic pollutants can easily diffuse through biological membranes and accumulate in fish tissues and organs [
47,
48,
49,
50]. The increased concentration levels of toxic compounds and the possibility of additive effects related to the accumulation of multiple contaminants can result in physiological disorders and alteration of the normal metabolism, affecting reproductive, immune, nervous, cardiovascular and endocrine systems [
33,
34,
35,
51]. In addition, compounds accumulated in fish tissues could be transferred to other consumers in the food chain, leading to biomagnification phenomena [
35,
52,
53,
54]. Standard methods used for the assessment of the bioconcentration, metabolism and elimination of targeted compounds in fish and for environmental monitoring require sampling of tens of individuals; moreover, analyses are usually performed from ex vivo tissues [
55].
Due to the high complexity of the matrix, extraction has to be carried out in order to remove the possible interfering species and to concentrate the target compounds. To this purpose, several sample preparation techniques have been proposed among which SPME, liquid extraction (LE), Soxhlet extraction, pressurized liquid extraction, microwave-assisted extraction and Quick Easy Cheap Effective Rugged Safe extraction (QuEChERS) [
56,
57,
58]. Sampled tissues are usually homogenized and processed using organic solvents, leading to a challenging identification of compounds naturally present in the living systems, due to phenomena such as cellular degradation, chemical alterations, enzymatic activity and aggregation processes [
59,
60].
In vivo SPME, integrating sampling, extraction, and enrichment of the analytes into a single step is able to overcome these limitations and therefore has been proposed for both target and untargeted analysis of chemical compounds in fish tissues [
2]. Usually the sampling is performed by inserting the fiber in the dorsal-epaxial muscle of the anesthetized or immobilized fish to a depth of about 2 cm (
Figure 2). This approach proved to be the best solution since the sampling of the dorsal-epaxial muscle reflects the overall composition of the tissue due to the uniform distribution of the analytes [
61]. Sampling duration ranges between 1 and 20 min, thus resulting in the possibility to both observe short-term events occurring in dynamic biological systems and capture highly reactive compounds in living organisms. In addition, long-time processes such as compounds uptake, metabolism and elimination can be monitored onto the individual test subject, strongly reducing sample size and allowing for the calculation of inter-sample variability with the possibility of highlighting inflammation and disease states of abnormal samples [
51,
60].
In vivo SPME target analysis has been focused on tracing the uptake and elimination of persistent organic pollutants (POPs), personal care products (PCPs) and pharmaceuticals used in aquaculture. Xu et al. [
45] proposed a simple and time-efficient method to trace the uptake and elimination kinetics of both organochlorine (OCPs) and organophosphorus (OPPs) pesticides from the dorsal-epaxial muscle of tilapias (
Oreochromis mossambicus) and pomfrets (
Piaractus brachypomus). The extraction was performed using homemade PDMS-coated fibers. The uptake and elimination of the pesticides were monitored via gas chromatography-mass spectrometry (GC-MS) targeting the unaltered pesticides. The use of in vivo SPME allowed to monitor the fast elimination processes of OPPs, guaranteeing an accurate determination of fast changing species without the enzyme quenching steps required in the ex vivo process. These analyses resulted in the calculation of the elimination kinetics (k
e) and the bioconcentration factors (BCFs), which were consistent with those reported in United States Environmental Protection Agency (US EPA) databases. Subsequently, the metabolism of fenthion was monitored via solid phase microextraction-liquid chromatography-mass spectrometry (SPME-LC-MS) using thicker PDMS fibers, leading to the identification of fenthion sulfoxide, fenthoxon and fenthoxon sulfoxide. The elimination kinetics of fenthion and its metabolites was monitored along the time, assessing a different persistence of the species in the muscle compartment.
Trace analysis of PCPs was performed on rainbow trout (
Oncorhynchus mykiss) [
43]. The extraction from the dorsal-epaxial muscle was performed using C
18 SPME fiber probes, followed by GC-MS analysis to detect targeted UV filters and polycyclic aromatic musks. Method calibration was performed in vivo, whereas validation parameters, i.e., extraction time profile, detection limits (LODs), LOQs, linearity and repeatability were obtained ex vivo due to the inter-fish variation. Analyte time profile and sampling rates presented significant differences between the in vivo and in vitro applications due to the physiological activity of the living organisms. The use of C
18 SPME fiber allowed the extraction of small molecules able to penetrate and be adsorbed onto the embedded particles, thus excluding the large matrix-derived biomolecules and strongly reducing interfering phenomena. Uptake and elimination of synthetic musks (SMs) in tilapias were also studied by Chen et al. [
44] using sampling-rate calibration for the quantification of the analytes [
62]. In particular, musk xylene (MX), musk ketone (MK), galaxolide™ (HHCB), and tonalide™ (AHTN) were investigated. The analytes presented different uptake and bioconcentration factors: nitro musks, hypothesized as potential carcinogenic substances, showed greater bioaccumulation potential (BCFs 989 ± 56 and 241 ± 73 for MX and MK, respectively) than polycyclic musks (67 ± 15 and 99 ± 28 for HHCB and AHTN). Elimination kinetics presented the same trend present in Ocaña-Rios [
43] for all the analyzed compounds, with an initial rapid elimination of the SMs, followed by a slower concentration decrease.
In-muscle detection of pharmaceuticals used in aquaculture was investigated by Huang [
36] and Tang [
46], targeting anesthetics and fluoroquinolones respectively. Both studies deal with health safety, focusing on the elimination of potentially harmful pharmaceuticals from edible fish. Huang proposed the use of in vivo SPME for a rapid in situ sampling of anesthetics in living fish, since they could be metabolized or degraded during fish samples transportation to the lab [
36]. Due to the rapid metabolism of anesthetics, in order to obtain a better estimation of anesthetics concentrations, SPME was proposed as valid alternative to standard methods requiring multiple sample preparation steps, i.e., fish euthanasia, tissues removal and homogenization. Method development and validation were performed ex vivo analyzing homogenized fish muscle, whereas elimination study was performed in vivo. The concentration of five anesthetics was monitored for 5 h by exposing for 10 min homemade PDMS fibers in the dorsal-epaxial muscle of tilapias. The study demonstrated a rapid metabolism of the anesthetics, with half-life times ranging between 44 and 150 min and a decline of 10% of their concentration within the first 10 min. The concentration decline profile was demonstrated to be different compared to that obtained by spiking the analytes in homogenized tissue samples due the physiological activity. The proposed method exhibited lower LODs (in the 1.7–9.4 ng/g range) and reduced analysis times compared to traditional ex vivo methods, strongly simplifying sample preparation step.
Tang [
46] focused the study on target analysis of 5 fluoroquinolones (FQs) on cultured pufferfish (
Takifugu obscurus). Due to the high economic value of pufferfish and its major consumption in East-Asia, FQs are often used in its cultivation to prevent pathogenic infection. Despite their intense use in veterinary disease treatment, these antibacterial agents are known to accumulate in fish tissues and are considered a potential health risk for human for seafood consumption. In the study, an in vivo SPME-LC-MS/MS method was developed and validated to assess the concentration levels of FQs in the dorsal-epaxial muscle of immature pufferfish using homemade biocompatible SPME fibers coated by PAN-C
18. PAN is usually chosen as binder for stationary phase immobilization due to its biocompatibility, high chemical and mechanical stability [
1]. The octadecyl silica was selected because of its good extraction efficiency toward a wide range of compounds. The developed coating proved to be characterized by higher extraction capabilities compared to PDMS (9–31 times) and comparable efficiency to commercial C
18 fibers. Method optimization and validation were performed using spiked water samples. Good linearity and LOQs lower than those reported in the standard LE method were obtained. No significant difference was observed when the in vivo SPME-LC-MS/MS method was compared with the official liquid extraction-liquid chromatography-tandem mass spectrometry (LE-LC-MS/MS) method.
In order to obtain a more efficient extraction of the analytes from the fish matrix, researchers are developing new biocompatible coatings designed to provide enhanced enrichment of target compounds, while eliminating possible interferences. Additional features of these materials rely on the improved wettability, high surface area, presence of specific functional groups able to provide stable chemical interactions, tunable pore sizes [
1]. The developed phases are based on the use of new functionalized polymeric fiber coatings [
14,
63], polymeric-coated nanoparticles [
37,
38], functionalized nanotubes [
39], metal organic frameworks (MOFs) [
40] and polyelectrolyte dispersed in microcapsules [
41]. Biocompatible polystyrene-polydopamine-glutaraldehyde (PS@PDA-GA) coating has been developed for sampling analytes in semisolid tissues and accelerate sampling kinetics thanks to the increased surface area [
14,
63]. The coating of the device is designed in order to expel the air trapped in the wettable 3D-interconnected pores in presence of the tissue fluid, thus allowing the full accessibility of the whole surface. By the use of this SPME coating, sampling equilibrium is reached within few minutes, boosting the speed of the analysis and simplifying method calibration. This coating was tested for the extraction of tetrodotoxin (TTX) from pufferfish [
63] and pharmaceuticals from tilapias muscle [
14].
Chen et al. [
63] developed a rapid in vivo SPME-LC-MS/MS method for the detection of TTX in pufferfish muscle using electrospun (PS@PDA-GA) fibers. Electrospinning was proposed since it allowed a fine control of fiber diameter, morphology and orientation of the micro/nanofibers, obtaining fully exposed surface active sites and high permeability [
64]. The developed coating was characterized by higher extraction capabilities compared to commercially available PDMS (nearly 120 times) and PA (about 20 times) fibers. Spiked water solutions were used for the optimization of the extraction and desorption conditions, whereas method validation was performed on homogenized pufferfish muscle spiked with TTX. The developed SPME-LC-MS/MS method exhibited a wide linearity range, good repeatability (RSDs < 12.1%) and lower LOQ (7.3 ng/g) compared to the official method (LOQ: 50 ng/g). Animal studies were performed by applying the in vivo sampling on fish divided in a control group and five different sample groups fed with increasing amount of TTX (1–80 MU/g·body mass/day). Method accuracy was assessed by comparing the concentration of two groups obtained by using the developed method with those of the official method, showing no significant difference. Backwards, when the analysis was performed on fish cultured with lower dosage of TTX, only the in vivo SPME-LC-MS/MS method was able to detect the target toxin, proving its superior detection capabilities compared to the official LE-based method.
The PS@PDA-GA coated-fibers were also tested for the extraction of pharmaceuticals from immature tilapias [
14]. The coating was deposited onto electrospun fibers inserted inside a syringe needle, which was withdrawn during fiber exposure. PS@PDA-GA coated-fibers extraction efficiency of pharmaceuticals was compared in vitro with those of electrospun PS and PDMS coated fibers at equilibrium, resulting in signals at least 4.4 and 26 times higher respectively. In addition, the hydrophobic surface of the PS@PDA-GA coated-fibers proved to circumvent the adhesion of biological macromolecules. LODs for the target pharmaceuticals were in the 1.1–8.9 ng/g range, whereas RSDs for inter-fiber repeatability were in the 5.9–14.3% range. Finally, the developed fibers were tested for extracting pharmaceuticals from tilapia dorsal-epaxial muscle with a 10 min extraction, resulting in satisfactory sensitivity and accuracy. Fish exposed to fluoxetine and norfluoxetine were analyzed by both the SPME-LC-MS/MS and the LE-LC-MS/MS resulting in comparable mean concentration levels.
Qiu has proposed different coatings deposited onto electrospun fibers for the extraction of pharmaceuticals from fish tissues [
37,
38,
39]. Quartz fiber (QF) coated by gluing poly(diallyldimethilammonium chloride) (PDDA) assembled graphene oxide (GO)-coated C
18 composite particles (C
18@GO@PDDA) with polyaniline (PANI) and modified with polynorepinephrine (pNE) were used (
Figure 3) [
37]. This biocompatible coating was devised in order to avoid both the absorption of biomacromolecules and rejection reactions when the fiber is exposed inside the living tissue. C
18 silica nanoparticles were chosen for extracting hydrophobic compounds, whereas GO was proposed due to the large surface area and the possible π-π interactions with the aromatic compounds as well as the high extraction capabilities towards hydrophilic molecules. PDDA is a cationic polyelectrolyte agent able to interact with acidic compounds. PANI and pNE were selected since they allow coating of any surface creating a bioinspired layer. The developed SPME fibers were tested for the extraction of 10 acidic pharmaceuticals from tilapia muscle. The extraction capabilities were compared ex vivo with commercially available fibers resulting in extraction 8.0–81.7 and 2.7–23.3 times higher respectively for PDMS and PA. LODs were in the 0.13–8.44 ng/g range. Linearity was demonstrated up to a range of 1–5000 ng/g and an inter-fiber repeatability in the 2.6–11.5 ng/g range was proved. The in vivo analysis was performed on living tilapias monitoring the uptake of the target pharmaceuticals along a period of 96 h.
For monitoring neutral, acidic and basic pharmaceuticals in living fish and vegetables, biocompatible copolymer poly(lactic acid-caprolactone) (PLCL) containing sulfonated γ-Al
2O
3 nanoparticles, sheathed with norepinephrine has been proposed [
38] (sulfonated Al
2O
3@PLCL/pNE SPME fiber—
Figure 4). The PLCL copolymer was chosen to be fully biocompatible, while maintaining a sufficient mechanical strength to be used for target monitoring of immature tilapias muscle and spinach (
Basella alba L.) stem. Sulfonation allowed for the interaction between the fiber coating and the amino groups of basic sulphanilamide pharmaceuticals, thus improving their extraction from the living systems. The extraction capabilities of the developed fibers were 36–85 and 4–15 times higher than the PDMS fibers for the basic and the acidic/neutral pharmaceuticals, respectively. The fibers were tested in vivo obtaining LODs at nanogram of pharmaceuticals per gram of tissue level. Accuracy of the SPME-LC-MS/MS method was compared with that of the standard LE-LC-MS/MS method, demonstrating no significant difference between the obtained results, except for the sulfafurazole, which was detected only by SPME extraction.
A coating obtained by functionalization of carbon nanotubes (CNT) with polypyrrole (PPY) and pNE has been proposed for the electrosorption-enhanced extraction of ionized acidic pharmaceutical from fish muscle [
39]. PPY was chosen to obtain a biocompatible coating and to increase the conductivity of the extractive phase. CNT were used because of their 3D interconnected structure and the possibility to establish π-π and hydrophobic interactions with the target compounds. The advantage of the proposed method relies on the electrosorption-enhanced extraction, requiring the application of a low-power electric field to move the ionized analytes toward the coating via electrophoresis and complementary charge attraction, significantly improving the extraction efficiency and speed. The sampling device consists of a conductive SPME fiber connected with the working electrode, four opposite parallel stainless steel electrodes surrounding the fiber and connected with the counter electrode, and another uncoated electrospun needle connected with the reference electrode (
Figure 5). The three-electrode system provided a stable electric field, resulting in enhanced extraction kinetics and accelerating the diffusion rates of the ionized analytes. The sampling time is reduced to 1 min compared to the 20 min required by the common SPME process, obtaining comparable or better extraction efficiencies for the target pharmaceuticals in deionized water. LODs in the 0.12–0.25 ng/g range and RSDs < 9.50% for inter-fiber repeatability were obtained. The proposed system was finally tested for the extraction of ionized pharmaceuticals from mature tilapias. The concentration of the ionized pharmaceuticals was monitored over a period of 360 h, allowing repeated long-time monitoring on living fish. The BCF values, in the 1.84–16.18 range, reached stability after 168 h of exposure.
MOFs have been also proposed as SPME coating for the in vivo monitoring of antibiotics (ABs) in fish muscle [
40]. The monitoring of ABs in aquatic ecosystem is of paramount importance due to their worldwide massive use and the related spreading of antibiotic resistant bacteria and allergic sensitization. MOFs are particularly appealing due to their high surface area, tunable pore size, and adjustable internal surface properties obtained by changing both the metal ions center and organic ligand repeating units. In this study, a MIL-101(Cr)-NH
2 was proposed as SPME coating because of the large porosity and the excellent stability in both water and organic solvents. SPME extraction was optimized in terms of extraction and desorption time on six model ABs in water solution. LODs at ng/L level were obtained with a satisfactory repeatability (RSDs < 10%). The performance of developed fibers was compared with commercially available PDMS, PDMS/DVB, C
18 and PA fibers, showing enhanced extraction capabilities for all the analytes except in the case of tilmicosin (C
18 presented higher enhanced normalized peak area) and trimethoprim (no significant difference compared with PDMS/DVB). The ABs were monitored in living tilapias dorsal-epaxial muscle, obtaining low inter-fiber RSDs (<14.5%) and LODs in the 0.18–1.12 ng/g. The sampling rates (in the 11–69 ng/min range) were used for calculating the concentrations of ABs in the fish tissue, achieving results comparable with those obtained by using the reference LE method applied onto homogenized tissue samples, thus demonstrating the suitability of the developed SPME-LC-MS/MS method for the ABs in vivo monitoring.
Polyelectrolyte microcapsules dispersed in silicone rubber were tested for SPME monitoring of antidepressants, i.e., fluoxetine and its metabolite norfluoxetine, in brains of living tilapias [
41]. Differently from conventional fibers, extracting only neutral species, this coating was able to interact with both neutral and protonated analytes, thus increasing sampling efficiency. In addition, the use of microcapsules boosted the kinetic of sampling. Thanks to these features, it was possible to miniaturize the fiber dimension, allowing the sampling of immature fish brain. The competition effect between the two ionized analytes was negligible at biological concentration levels, whereas salting-out effect was significant in phosphate buffer solution. The two target analytes were monitored in vivo in both fish muscle and brain at environmentally relevant concentrations. Sampling rate calibration was used to determine the concentration of the antidepressants in the fish tissue. The results were comparable with those obtained by the LE method, demonstrating a high inter-sample variability. As expected, the concentration of the analytes in brain were from 4.4 to 9.2 times higher than those in the dorsal-epaxial muscle.
Untargeted analysis of exposome has shifted the focus from a bottom-up to a top-down approach in order to understand the impact of multiple and simultaneous exposures to environmental contaminants and detect modifications in the profile of endogenous compounds [
65]. Direct in vivo SPME extraction has been proposed for monitoring fish tissues in a nonlethal manner, using coatings able to extract a wide range of low-molecular-weight compounds [
10,
50,
51,
60,
66]. In addition, the use of SPME for in vivo monitoring has allowed the sampling of very labile and highly reactive species, which could be degraded during tissue samples transportation, storage and handling [
60]. Since both endogenous and exogenous compounds can be detected, chemometric techniques have to be applied to highlight different contribution and detect abnormal samples [
50,
51,
60,
66,
67].
Roszkowska [
66] studied the metabolome profile associated to benzo[α]pyrene (BaP) exposition using in vivo solid-phase microextraction-liquid chromatography-high resolution mass spectrometry (SPME-LC-HRMS) analysis. It is recognized that the presence of BaP and its metabolites cause modification of cellular metabolism with carcinogenic, mutagenic, and cytotoxic effects, exacerbated by its bioaccumulation in biological tissues [
68]. In the study, the dorsal-epaxial muscle of rainbow trout exposed to sublethal doses of BaP (1 and 10 ng/L) was sampled using commercially available biocompatible SPME mixed-mode probes in order to detect dose and time dependent alteration in tissue metabolome by case-control model. Being BaP able to affect several metabolic pathways, mainly related to cellular osmotic regulation, energy and lipid metabolism, the results achieved in the study indicated that BaP exposition was able to decrease the number of detected compounds. A different amino acid composition was observed between the three groups: several amino acids were detected free in the control group, whereas only free tryptophan and proline were present in the high-dose exposed group, with a concentration of tryptophan 10 times higher than the control level. Labile compounds, such as arachidonoyl serinol, an endocannabinoid metabolite, were also detected by in vivo SPME: this compound was detected in the high-dose group, probably indicating that BaP affects also the endocannabinoid system. In fact, endocannabinoids are reactive neuroprotective agents, whose dysregulation in the signaling pathway is associated with the inability of the biota to adapt to environmental contaminants exposure. Interestingly, fish exposed to the lowest concentration level of BaP presented significant metabolic pattern changes over the monitored period: the first days they showed features similar to the high-dose exposed group, whereas, after 14 days, similarities with the control groups were observed. This may imply the presence of an adaptation mechanism of the metabolism in response to the presence of pollutants in the surrounding environment.
Bessonneau [
60] and Roszkowska [
50] have studied the exposome via in vivo SPME monitoring in white sucker (
Catostomus commersonii) tissues with the final aim of proving that in vivo SPME from fish tissues is suitable for detecting alterations in biota pathways. More precisely, Bessonneau [
60] has examined the differences between metabolites detected by ex vivo and in vivo SPME using molecular networking analysis. The in vivo extraction was performed for 20 min by inserting into the dorsal-epaxial muscle of the fish a stainless steel blade coated by 5 µm C
18 particles, immobilized using polyacrylonitrile-C
18 (PAN-C
18). Ex vivo analysis followed the same procedure sampling non-homogenized frozen tissues from euthanized fish. Analyte identification and networking were performed by ultra performance-liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) and UPLC-MS/MS using molecular networking software. Although most of the identified molecules were endogenous substances, four analytes, namely 4-methoxycinnamic acid, 1-hydroxybenzotriazole, diethylphthalate, and phenoxybenzamine were recognized as exogenous compounds, used in formulation of cleaning products, cosmetics and pharmaceuticals. Globally, 16% of the nodes were only detected by in vivo SPME sampling, whereas 21% were obtained only using ex vivo SPME sampling. The compounds detected only by in vivo SPME were both endogenous and exogenous substances, characterized by chemical instability and/or reactivity: for example, the detected cinnamic acids are prone to auto-oxidation during sample transportation and storage. The species observed only by ex vivo extraction are mainly produced by bacterial and enzymatic metabolism of the degraded tissue. Therefore, in vivo SPME allows the monitoring of labile species, avoiding the detection of biodegradation products.
Roszkowska has applied in vivo SPME to perform non-lethal sampling of white sucker to monitor the response to the exposition of contaminants from sites upstream, adjacent and downstream a development region of oil sands [
50]. PAN-C
18 coated blades were used for SPME in order to extract both hydrophilic and hydrophobic compounds from fish tissues. The UPLC-HRMS analysis resulted in the identification of several classes of toxic compounds present in fish muscles, including pesticides, aliphatic and aromatic hydrocarbons, phthalates, mycotoxins, plasticizers, pharmaceuticals and organometallic compounds. Overall, the results demonstrated that fish collected in the sites closely associated with oil sands production and downstream exhibited altered metabolic profiles compared with the control group, collected upstream from the production site. The uptake of toxic compounds resulted in several alteration of lipid metabolism, including glycerophospholipid metabolism, fatty acid activation and de novo lipid biosynthesis, phosphorylation, energy dysregulation and inflammatory states. The in vivo PAN-C
18 SPME extraction allowed also the monitoring of labile and unstable compounds, present at trace levels in fish tissues, such as endocannabinoid metabolites. A parallel study has been performed to examine whether tissue exposome monitored in vivo could detect significant induction of CYP1A1, a gene involved in the biotransformation of toxic compound into more polar substances via oxygenation [
51]. The study investigated small molecules associated with CYP1A1 induction to identify both contaminants inducers of the gene and explore the induction mechanism. The in vivo SPME was proposed in order both to capture unstable and labile compounds like highly reactive oxygen species (ROS), antioxidants and oxygenated derivatives of cholesterols, and to rapidly stabilize short-living metabolites, preventing their oxidation during storage.
Multivariate data analysis (partial least squares-discriminant analysis) and false rate discovery were applied to process data acquired by SPME-UPLC-HRMS. The metabolome changes associated with CPY1A1 induction highlighted that 182 features were significant in female suckers, whereas only 119 were significant in male fish. The results demonstrated that 12 metabolic pathways were involved in fish response to CYP1A1 activation. Since this gene is involved in the oxygenation of toxic compounds to facilitate their elimination, modification in the concentration levels of ROS and anti-oxidants was expected. In female white sucker, increases in the levels of antioxidants and depletion of short-lived oxygenated metabolites were observed: the endogenous antioxidant cellular content was increased as a response to the production of ROS in order to maintain cell redox status and homeostasis. In male suckers, a significant oxidative stress was detected with a depletion of the cellular pool of antioxidants and a decrease in the levels of endogenous pro-oxidants oxysterols. The differences reported between male and female fish exposome could be or sex-specific or related to variability in terms of chemical exposures. Several exogenous compounds were also identified in fish tissues, but strong inter-individual variability was present in cases fish, possibly due to a different exposure in the ecosystem. Among the different omics sciences, lipidomics is gaining increasing interest with the identification of hundreds of novel lipids involved biological transformations: apart from their role as structural components and energy reservoirs, lipids are also intermediates of cellular pathways [
10,
69].
Roszkowska has developed an in vivo SPME-UPLC-HRMS method using biocompatible SPME mixed-mode probes for untargeted lipidomics profiling of muscle tissue in living rainbow trout [
59]. Samples collected from the same sampling group were stored at –80 °C for 1 year and analyzed by both SPME-LC-HRMS and solid-liquid-extraction-liquid chromatography-high resolution mass spectrometry (SLE-LC-HRMS). By in vivo SPME, 845 features were detected in the 100–500
m/z range, 30% of which were annotated as lipid species, divided in three main groups: fatty acids, sterol lipids and glycerolipids. The ex vivo SPME analysis of stored muscle samples showed remarkable differences in lipid composition compared to the in vivo analysis, revealing lipidome profile alterations. The SPME analysis of ex vivo samples resulted in a general 10-fold decrease in the number of detected features and the appearance of higher molecular mass lipids, formed by binding reaction of the previously unbound lipids during sample storage. Spectra obtained by using SLE-UPLC-HRMS method from homogenized fish muscle samples were characterized by the presence of lipid compounds with high molecular masses (up to 900
m/
z). These compounds were identified as high-abundant lipids, such as sterols and glycerophospholipids, released from both cellular membrane and intracellular environments due to the use of organic solvent mixtures. Therefore, they did not represent the unbound fraction of lipids present in living system, which could be detected only by in vivo SPME.
Deng and coworkers [
21,
70] have used biocompatible surface-coated probe nanoelectrospray-high resolution mass spectrometry (BSCP-nanoESI-HRMS) for in vivo lipidomics study of widely used model organisms in ecotoxicology like
Daphnia magna, zebrafish (
Danio rerio), zebrafish eggs and eukaryotic cells. The main advantage of this approach relies on the rapid, microscale and in situ analysis of complex biological samples. The biocompatible microsampling probe, presenting a probe-end diameter of few micrometers, was obtained by coating a tungsten probe with n-octadecyldimethyl[3-(trimethoxysilyl)propyl]-ammonium chloride (DMOAP) and chitosan. The stationary phase was chosen to interact with both the major lipid species (hydrophobic chain of glycerolipids, glycerophospholipids, sphingolipids and fatty acyls) by the C
18 group and with the phosphate/hydroxyl/carboxyl groups by the quaternary ammonium ion via ion-exchange adsorption. Chitosan was used since chitosan-based materials are nontoxic, highly biocompatible, non-immunogenic and suitable for in vivo investigations. In addition, it was possible to prevent the absorption of large biomolecules onto the SPME surface. The optimization of the extraction parameters was performed by in vivo lipid analysis of zebrafish. Analysis was performed in positive ion mode since most of the lipids were detected as [M + Na]
+ and/or [M + K]
+ ions, whereas only a few signals from fatty acids were obtained in negative ion mode. The developed BSCP-nanoESI-HRMS method was compared with conventional direct infusion shotgun MS lipidomics by analyzing tissue extracts. A similar lipid profile was obtained, even though differences in the relative signal intensities were observed. The effectiveness of the coating extraction was demonstrated by sampling zebrafish tissues using uncoated probes, resulting in weaker MS signals. Coating stability was assessed by performing the extraction multiple times using the same probe. No decrease in peak intensity was observed and carryover effect of the coated fiber resulted negligible. The lipid species detected by BSCP-nanoESI-HRMS method were identified via LIPID MAPS structure database. Analysis of zebrafish dorsal-epaxial muscle led to the identification of 137 lipid species excluding isomers, i.e., 57 triradylglycerol (TAG) species, 33 phosphatidylcholine (PC) lipids, 28 phosphoglycerols (PGs), 10 ceramide-1-phosphates (CerPs), 3 fatty acids, (FAs), 2 diradylglycerols (DAGs), 2 cholesteryl esters (CEs), 1 monogalactosyldiacylglycerol (MGDG), and 1 phosphtatidylinositols-ceramide (PI-Cer). The lipid profile of single zebrafish eggs was also obtained: the most intense ions were identified as PC(16:0/18:1) and PC(16:0/22:6) lipids, whereas the signal intensities of many TAGs were much lower compared with those of zebrafish muscle. Principal component analysis (PCA) analysis was applied, showing that zebrafishes and their egg cells were clearly separated into two clusters and the major loadings were attributed to TAGs. Despite the developed method proved to be suitable for the detection of lipid species in living tissues, the accurate location of the C=C bond within the fatty acid chains could not be unambiguously assigned. In addition, isomers could not be differentiated due to the lack of a chromatographic separation. To solve the discrimination of C=C lipid isomers, online Paternò-Büchi (PB) reaction has been proposed [
70]. Benzophenone was used as reagent to obtain high reaction efficiency with the methanol/chloroform solvent mixtures. In addition, the use of benzophenone compared to other PB reactants such as acetone results in 182 Da mass increased product ions, which could be easily discriminated from the original ions in the MS spectrum. In order to promote PB reaction, UV irradiation was performed before spray formation (
Figure 6).
The identification of C=C lipid isomers was obtained by collision induced dissociation (CID): when CID was performed to the PB ions, C=C diagnostic ion pairs, namely [M + O]+ and [M + C13H10]+, having a mass difference of 150 Da, were produced. The identification of the C=C position alongside the fatty acid chain was based on the m/z shift of the CID induced PB product ions compared to the original [PB + M + H]+ parent ion. The proposed technique allowed a relative quantitation of C=C location isomers. Finally, in vivo analysis of lipidome of zebrafish was performed to identify C=C isomers, obtaining a detailed lipid profile.
One of the major drawbacks of the traditional SPME fiber for in vivo applications is the flexibility and the fragility of the silica core, limiting direct sampling of animal tissues. Poole et al. [
42] developed a miniaturized device able to directly puncture robust sample matrices, such as the skin of top-level predator fish, by applying the sorbent coating onto a recession of a stainless steel support, thus protecting the edges of the coating during both puncture and recession steps. The device presents maximum surface area of sorbent per unit of puncture hole diameter, thus maximizing the extraction phase surface area (2.9 times more surface area than traditional nitinol-based bio-SPME fiber). The recessing coating was made by HLB particles suspended in a PAN glue, selected because of their biocompatibility and high extraction capabilities towards a wide variety of polar and nonpolar compounds. In order to demonstrate its robustness and facilitate sampling, the SPME recessed coating needle was also incorporated into custom projectiles, fired by unmodified airsoft guns. The device was tested to extract xenobiotic compounds from wild muskellunge (
Esox masquinongy). The use of SPME projectiles demonstrated that the rapid puncture of fish scales provided a uniform and more repetitively successful sampler administration, reaching the underlying muscle tissue. This test demonstrated that the developed device was able to extract bioaccumulated and bioconcentrated anthropogenic compounds in a top-level predator. SPME-LC-HRMS analysis resulted in the tentative identification of 35 compounds belonging to pesticides, drugs, phytochemicals, lipids and metabolites.
Another major difficulty in untargeted LC-MS analysis is the presence of redundancy peaks, namely co-eluted peaks, multi-charge ions, adducts, neutral loss, isotopologues, and fragments ions that could result in statistical bias with multiple comparisons. Yu has proposed [
67] the GlobalStd algorithm based on paired mass distances (PMD) to remove redundancy peaks from raw LC-MS data and to select independent peaks for further structure/reaction directed analysis. PMD are defined as distance between two masses or mass to charge ratios and this data analysis is based on the identification of unique defect values between analytes in order to identify homologous series or substitution reactions, clustering the compounds that present a similar structure or reactivity. Unknown PMD transitions could be obtained relying on the statistical properties of the LC-MS peak profile. By using this approach, both known and unknown compounds belonging to adducts, neutral loss, the same homologous series, or biochemistry reaction relationship could be identified. The filtered data can be subsequently studied for semi-qualitative or quantitative statistical analyses considering the compounds as a group, thus bypassing the need for identification of each peak present in the raw LC-MS data. The developed method was applied for the in vivo SPME-LC-MS analysis of wild rainbow trout. A total of 1459 peaks was obtained as raw dataset, reduced by the GlobalStd algorithm to only 277 independent peaks, which were imported for subsequent structure directed analysis.
All these studies evidenced that in vivo SPME is a valuable analytical tool for the real time monitoring of target analytes and metabolome profiling of living organisms. This approach was successfully applied for monitoring the uptake, metabolism and elimination of xenobiotic compounds from different fish species. In addition, it allowed the study of exposome directly from living organisms, resulting in the sampling of labile and reactive species, while avoiding the detection of compounds related to tissue sample degradation processes.
An overview of the discussed applications is reported in
Table 2.
4. In Vivo SPME Extraction of Plant Tissues
The conventional analytical process for the determination of a variety of analytes in plant tissues involves several steps due to the general complexity of the matrix [
71]: in fact, solvent extraction and clean-up are usually required for the elimination of interfering compounds prior analysis [
72]. Ex vivo analytical methods might introduce variability, potentially induce degradation of compounds of interest during sample preparation and alter both the spatial distribution and the natural content of investigated compounds in biota. These two aspects lead to an overall reduction of representativeness of analytical outcome [
73], making metabolomics and tracing studies in different plant compartments hard to accomplish accurately.
The need for representative information regarding the actual composition and the dynamics of xenobiotics and biomolecules in plants has led to the development of a variety of in vivo analytical and extraction methods. Compared with other sampling techniques like microdialysis, in vivo tissue collection and biosensors, SPME offers the advantage of a low cost and simple device [
44,
71,
73,
74,
75]. This technique allows the efficient and non-exhaustive extraction of the analytes by inserting the fiber directly in plant tissues with minimum disturbance of the living system. As a further advantage, this technique can be easily hyphenated with chromatography [
74]. Nowadays, in vivo SPME is widely applied to study the uptake kinetics of agrochemicals, pesticides, pharmaceuticals and other emerging contaminants (e.g., synthetic musks) and their bioconcentration in plant compartments, defined as the absorption of xenobiotics from the surrounding environment [
76]. Therefore, it can be used to perform food safety assessment by comparing detected concentrations with minimum residue limits.
Very recently, Shi et al. [
74] have used in vivo SPME coupled with GC-MS for the quantitation of two fungicides, i.e., Y13149 and Y12196, in mung bean (
Vigna radiata) sprouts. For this purpose, a polystyrene/graphene@silica (PS/G@SiO
2) fiber was fabricated via electrospinning and calcination. The coating materials were chosen due to their dimensional structure, rugged surface, superior mechanical and thermal stability. The method was optimized and validated by analyzing water spiked with analytes, demonstrating linearity in the 0.3–100 μg/L range, LODs in the low μg/L range and good precision with RSDs within 12.1%. The average recoveries were 99% and 71% for Y13149 and Y12196, respectively. PS/G@SiO
2 SPME fiber was compared with PS/G fiber and with two commercially available fibers, i.e., 85 μm carboxen/polydimethylsiloxane (CAR/PDMS) and 7 μm PDMS in terms of extraction performance, showing higher extraction efficiency toward Y12196, whereas Y13149 resulted to be better extracted using 7 μm PDMS fibers. Fungicides bioaccumulation was assessed directly in mung bean sprouts by inserting the PS/G@SiO
2 SPME fiber using a cannula into the stems at 2.0 cm depth and kept in place for 10 min at room temperature. Afterwards, the fiber was extracted and rinsed with water, dried and placed in the GC injector for the analysis. Results showed that the concentration of both analytes increased until day 9, thereafter a decrease was observed. Different plant compartments were also sampled to assess whether there was any difference in spatial distribution of xenobiotics. As a result, fungicides concentration was higher at 3 cm from the roots when compared to 7 cm distance, due to the higher lipid content.
In vivo tracing of other classes of xenobiotics was extensively studied by Chen and Qiu’s group mainly using homemade PDMS-coated stainless steel fibers [
12,
44,
77,
78].
OCPs and OPPs have been investigated in different compartments of malabar spinach (
Basella alba) through in vivo SPME [
78]. A sampling rate-SPME (SR-SPME) coupled with the GC-MS method was successfully validated and applied for the in vivo tracing of OCPs (namely, endosulfan, hexachlorobenzene, dichlorodiphenyltrichloroethane, aldrin and mirex) and OPPs (namely, parathion-methyl, propetamphos, fenthion, quinalphos and profenofos). In particular, uptake and elimination kinetics were studied, and several parameters including BCFs, distribution concentration factors (DCFs) and transpiration stream concentration factors (TSCFs) were calculated based on in vivo analytical outcome. Spinach plants were grown hydroponically with nutrient solution spiked with the proper mixture of analytes for the uptake study and, right after, with clean nutrient solution for the elimination study. In vivo sampling was carried out by piercing the interested plant organ with a needle and then inserting the fiber at 1.5 cm depth (
Figure 7); fiber was held in place for 20 min, cleaned with water and addressed for GC-MS analysis.
OPPs were accumulated faster, while OCPs resulted to be more persistent and less inclined to be eliminated from B. alba roots. In stems and foliage similar uptake kinetics and persistence were observed for both classes of analytes. A positive correlation between calculated BCFs and hydrophobicity was demonstrated for all the analytes except fenthion. Overall, the accumulated concentration halved within 4 days after the uptake experiment was concluded. From DCFs it was possible to infer that these two classes of pesticides tend to be more accumulative in roots due to the high lipid concentration. Lastly, hydrophobicity and solubility did not show any clear relationship with TSCFs, suggesting that more complex physiological phenomena are involved.
OPPs have been traced with an in vivo sampling rate-solid phase microextraction-gas chromatography-mass spectrometry (SR-SPME-GC-MS) approach in cabbage (
Brassica parachinensis) and aloe (
Aloe barbadensis) [
12]. Moreover, in vivo SPME coupled with LC-MS/MS was used for the investigation of fenthion metabolites. Pesticides were administrated to
B. parachinensis through a nutrient solution via hydroponic cultivation.
A. barbadensis was cultivated in soil and OPPs were directly sprayed on aloe foliage. The in vivo sampling was performed as reported previously for the tracing study. The validated method reported LODs in the 0.07–2.07 μg/kg and 0.40–1.80 μg/kg range for aloe and cabbage, respectively. Regarding the uptake and the elimination of OPPs from cabbage, analytes were traced in leaves: within 7 days, the concentration of propetamphos, quinalphos and profenofos reached a maximum, whereas a similar behavior was not observed for parathion-methyl and fenthion. For the elimination experiment, the half-lives of the analytes were found to be within the 1.3–5.7 day-range. The accumulation of OPPs in aloe leaves peaked in 8 or 12 h depending on the analyte and a fast elimination was observed after 60 h after the exposure to polluted water. For the investigation of fenthion metabolism in aloe, fibers were desorbed in methanol under agitation and, after the addition of an internal standard, the extract was addressed to LC-MS/MS analysis. In both plants, fenthion resulted to be barely accumulated, whereas its metabolite fenthion-sulfoxide continued to accumulate until 100 h after the exposure.
In vivo SPME has been used also to evaluate whether multi walled carbon nanotubes (MWCNTs) affect the accumulation/elimination process of environmental contaminants i.e., OCPs, OPPs, pyrethroid insecticides and PCPs in mustard (
Brassica juncea) leaves [
77]. For this study, a SR-SPME(PDMS)-GC-MS method was used. Three groups of mustard plants were grown under controlled conditions and watered with tap water spiked with a mixture of all the investigated analytes: different amounts of MWCNTs were added to assess their effect on the uptake and elimination of contaminants (0; 1 and 10 mg/L). It was found that the groups exposed to 1 and 10 mg/L MWCNTs accumulated 10–30% and 20–160% respectively more contaminants compared to the control group. On the other hand, whereas a positive correlation was found between the amount of MWCNTs added in nutrient solution and the analyte concentration in plant tissues, it was observed that as the amount of MWCNTs increased, the accumulation rate constants (k
a) decreased. This behavior was explained taking into account that a longer period of time is needed for the in vivo contaminant concentration to reach a plateau: after the adsorption of the analytes onto MWCNTs, analytes are slowly released. Regarding the depuration capability of
B. juncea, no differences were observed in the elimination of accumulated analytes between the MWCNTs groups and the control group.
Synthetic musks are an emerging class of xenobiotics which caused severe concern in health and environmental safety since they are able to interact with the endocrine system. This class of analytes has been monitored via in vivo SPME in both tilapia and aloe (
Aloe chinensis Baker) without any fish or plant sacrifice [
44]. Regarding the aloe study, SPME sampling was carried out in both leaves and roots at a depth of 1.4 cm and with a sampling time of 10 min. The concentration of all the investigated compounds in aloe leaves plateaued after the third day of exposure, whereas for roots it took only one day. A rapid clearance was observed in both plant compartments: for aloe foliage, the half-lives for all the compounds of interest were between 0.6–0.7 days, whereas for roots the concentration of absorbed SMs halved in less than 12 h after exposure. BCFs calculated for aloe roots were two orders of magnitude higher compared to those obtained for the leaves for all the analytes. This was consistent with the average lipid content: SMs were found to be more likely to be accumulated in high-lipid plant compartments such as roots. Since chemicals in plants needs to reach the leaves from the root cortex, bioaccumulation in aloe leaf is less efficient, with BCFs at least three orders of magnitude lower than those found for tilapias.
The same research group successfully quantitated different pharmaceuticals in
B. alba stems and in tilapia (
O. mossambicus) by using a custom made sulfonated Al
2O
3@PLCL/pNE SPME fiber [
38]. The validated SPME-LC-MS/MS method provided LODs in the 0.02–8.02 μg/kg range for
B. alba, demonstrating linearity within 2–3 orders of magnitude up to 5 mg/kg. Both intra- and inter-fiber precision were also satisfactory, with RSDs within 9.9% and 15.6%, respectively. The extraction from
B. alba was carried out by piercing the stems and holding the fiber in place for 10 min. The fiber was then desorbed in methanol under agitation; thereafter, internal standard was added before addressing the solution to LC-MS/MS analysis. In order to test method accuracy, all the analytes were quantified performing a classical LE from ex vivo samples. No differences were found between the concentration determined with both SPME-LC-MS/MS and LE-LC-MS/MS methods in tilapia and
B. alba. Regarding
B. alba, sulfafurazole could only be detected using the in vivo SR-SPME technique, thus demonstrating the best performances of the in vivo method with respect to the ex vivo one.
Along with xenobiotics, phytohormones and carbohydrates represent other two important classes of analytes of biological interest. Such endogenous compounds are involved in a variety of regulation and pathological processes [
72,
75].
In this context, Fang et al. [
72] have developed a proper SPME coating for the extraction of plant regulators (salicylic acid and three of its derivatives—SAs), i.e., acetylsalicylic acid, 4-methyl salicylic acid and 3-methyl salicylic acid, in aloe leaves with the final aim of monitoring stress levels in plants. For this purpose, C
18@GO@PDDA coated quartz fibers were fabricated. C
18@GO@PDDA were dispersed in DMF with PAN to form a slurry and coated with a pNE layer to ensure biocompatibility. The sampling rate-solid phase microextraction-liquid chromatography-photodiode array detector (SR-SPME-LC-PAD) method was optimized by analyzing homogenized aloe leaves spiked with the analytes and investigating the effect of extraction and desorption time, pH and desorption agitation speed. The extraction performance of C
18@GO@PDDA SPME fiber was compared with those achieved by the commercial 85 μm PA and 65 μm PDMS/DVB, showing higher extraction efficiency toward all the investigated analytes. The validated method provided LODs in the 1.8–2.8 μg/L range and linearity within 3 orders of magnitude. Good intra- and inter-fiber precision were obtained, with RSDs within 8.4% and 9.3%, respectively. The four salicylic acids were also quantified using a classical LE-LC-PAD method: no difference (α = 0.01) was found between the analytical outcomes, thus ensuring the accuracy of the in vivo SR-SPME-LC-PAD method. The method was used to monitor the investigated compounds over time in aloe plants in order to evaluate whether the presence of cadmium, used as stress agent, produced detectable variation in salicylic acids profile over time. As a result, SAs concentration in Cd-stressed group peaked within 16 h. The control group was used to assess the impact of the injuries on plant leaves, made by inserting the SPME fiber: the concentration for all the investigated analytes increased within 6 h but less dramatically in respect to the Cd-stressed group. Thereafter, SAs levels decreased slowly and returned normal within 12 h.
Thin core-shealth electrospun nanofibers with pure PANI sheath and polystyrene core (PS-PANI CSEF) compressed into tiny bars have been applied for in vivo extraction of phytohormones gibberellin A3 (GA3), gibberellin A7 (GA7), jasmonic acid (JA), abscisic acid (ABA) and p-hydroxycinnamic acid (p-HCA) in aloe by LC-MS/MS [
73]. The proposed extraction material was biocompatible, characterized by a fast mass transfer rate and large extraction capacity to ensure fast equilibrium extraction and high sensitivity. In vivo extraction was carried out by inserting the PS-PANI CSEF bars in aloe leaves at 3 mm depth for 20 min. Method validation provided LODs in the 0.06–3.1 μg/L range, demonstrating linearity within three orders of magnitude. Good precision and accuracy were obtained, with RSDs always below 13% and recovery rates (RR%) in the 98–110% range. In order to assess suitability of the developed method for the quantitation of acidic phytohormones in aloe foliage, both in vivo and ex vivo methods were applied. GA3 was the only undetected compound using the in vivo extraction, whereas GA3 and GA7 resulted undetectable with the classic ex vivo LE-LC-MS/MS method, thus demonstrating the clear advantages of the developed in vivo approach. Moreover, the in vivo method allowed the detection of the analytes with a spatial resolution of about 3–8 mm
3, whereas the LE method consumed about 60 mg of leaf tissue, without providing any spatial resolution.
Carbohydrate detection in aqueous media, biofluids and tissues is challenging due to the high affinity of these compounds toward water. Owing to its capability of interacting with diols in a rapid and reversible way, phenylboronic acid functionalized MWCNTs, using PAN as binder has been proposed by Chen et al. to develop a custom-made SPME probe (
Figure 8) for carbohydrate detection in biofluids and semisolid biotissues [
75]. Preliminary tests in phosphate buffer solution showed the specificity of the developed probe toward glucose, chosen as a model compound, even in presence of other interfering compounds normally present in biological samples. The developed probe was used for the extraction of carbohydrates from
B. alba by inserting it at an average depth of 1.5 cm with the aid of a cannula needle. Thereafter, the probe was withdrawn and desorbed in acetic acid. Finally, the extracted solution was derivatized for GC-MS analysis. Glucose, mannose, galactose and rhamnose were successfully detected in
B. alba and aloe in 12 h. Finally, MALDI-TOF-MS analyses proved the absence of macromolecules absorbed onto the probe.
Further studies have been conducted by the same research group on the specific monitoring of glucose using a boronate affinity-molecularly imprinted polymer (BA-MIP) biocompatible probe [
79]. The presence of boronate acid monomer in the proposed coating played a pivotal role in increasing SPME selectivity due to the unique pre-self-assembly between glucose and boronic acid creating glucose specific memory cavities. A reduced interference from mannose and galactose was observed, whereas commercially available C
18 and PDMS fibers were not able to extract glucose. Finally, glucose was successfully in vivo extracted from aloe foliage. Again, MALDI-TOF-MS analysis proved the absence of other interfering macromolecules.
Besides targeted analytical chemistry, in vivo extraction techniques play a fundamental role also in untargeted analytical chemistry. In metabolomics, in which the structure and the abundance of a plethora of compounds found in living organisms are accurately investigated, it is extremely important to avoid analyte degradation or the generation of misleading artifacts, which mainly occur during the sample preparation step [
80]. Considering also that in plants gene expression is mainly connected with endogenous compounds, DI-SPME might be the technique of choice for investigating plant metabolites directly in vivo [
81].
In 2016, Musteata et al. successfully used SPME for the in vivo investigation of nonvolatile metabolites in Amazonian plants using UPLC-MS/MS analysis [
71]. In this study, a custom-made SPME coating was tested: SPME devices were prepared by coating stainless steel wires with commercially available silica i.e., reverse phase (RP)-amide-C
16 and pentafluorophenyl-propyl-bonded phase (HS-F5), respectively. Both the coatings were chosen owing to their mechanical resistance and fast equilibration with the sample due to the high surface area of the material. (RP)-amide-C
16 is characterized by a stable amide embedded polar group able to enhance both the extraction of polar compounds and wettability. HS-F5 coating is able to interact with a wide range of analytes via dispersive, dipole-dipole, π-π and charge transfer interactions, thus being suitable for the extraction of polar and basic compounds. Preliminary investigations on a smaller sample of plants in Hawaii (
Psychotria viridis,
Diplopterys cabrerana and
Banisteriopsis caapi) proved the superior extraction capability of HS-F5 for untargeted analysis. In vivo sampling on Amazonian plants was performed by inserting the probes under the bark of each plant and holding them in place for 30 min. Ex vivo LE-UPLC-MS/MS analyses were also performed with the aim of comparing the number of unique signals obtained by using both extraction approaches. Although the total number of compounds extracted by the in vivo procedure was lower than that obtained by using the ex vivo extraction, SPME-UPLC-MS/MS provided 27% exclusive signals. PCA on data obtained by in vivo extraction revealed significant differences among species belonging to the same genus, despite the investigated organisms were similar according to DNA analysis. Finally, considering the 30 min in vivo extraction time compared to the several hours required to prepare the samples via ex vivo approach, in vivo SPME-UPLC-MS/MS method was unequivocally less time-consuming with respect to LE method.
In the same year, apple (
Malus domestica fruit) metabolome has been investigated by Risticevic et al. [
81] with the aim of evaluating the changes in the metabolic fingerprints in response to fruit maturation. A PDMS overcoated commercial 50/30 μm DVB/CAR/PDMS fiber coupled with two-dimensional gas chromatography-time of flight mass spectrometry (GC×GC-TOF-MS) was devised. As for fiber coating, the use of PDMS overcoating allowed reducing fiber fouling damage of DVB-based coatings when placed in direct contact with complex matrices, thus guaranteeing matrix-compatibility while retaining the original coating sensitivity toward the analytes of interest. Sample collection was carried out by inserting three probes perpendicularly respect to the fruit stem at a 3 cm depth with two different sampling configurations. The results achieved after 60 min sampling, revealed significant differences in terms of method precision: in the case of sampling design 1, RSDs of about 22.0% were obtained considering 357 metabolites, whereas with sampling design 2, RSDs of 37.1% were calculated on 111 metabolites. A negative correlation was found between metabolite molecular weight and precision: larger RSDs were attributed to high molecular weight compounds. Precision of the two sampling designs was also evaluated using a homologous series of esters as model compounds: sampling design 1 provided poor precision regarding the determination of high molecular weight compounds, whereas with sampling design 2 lower RSDs values were obtained. Using a total of 40 compounds ex vivo HS-SPME was also performed for comparison purposes obtaining a median RSDs of 13.6%; however in vivo sampling addressed issues related to the stability of labile and hydrophobic metabolites encountered in HS-SPME. Furthermore, PCA on data obtained from in vivo analysis of apples at two different maturation stages showed a good separation between the groups, thus demonstrating the capability of the in vivo DI-SPME-GC×GC-TOF-MS metabolomic platform to provide representative data useful for staging the maturation level of fruits.
An overview of the discussed applications is reported in
Table 3.