Development and Testing of a 4-Columns Periodic Counter-Current Chromatography System Based on Membrane Adsorbers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Protein Purification
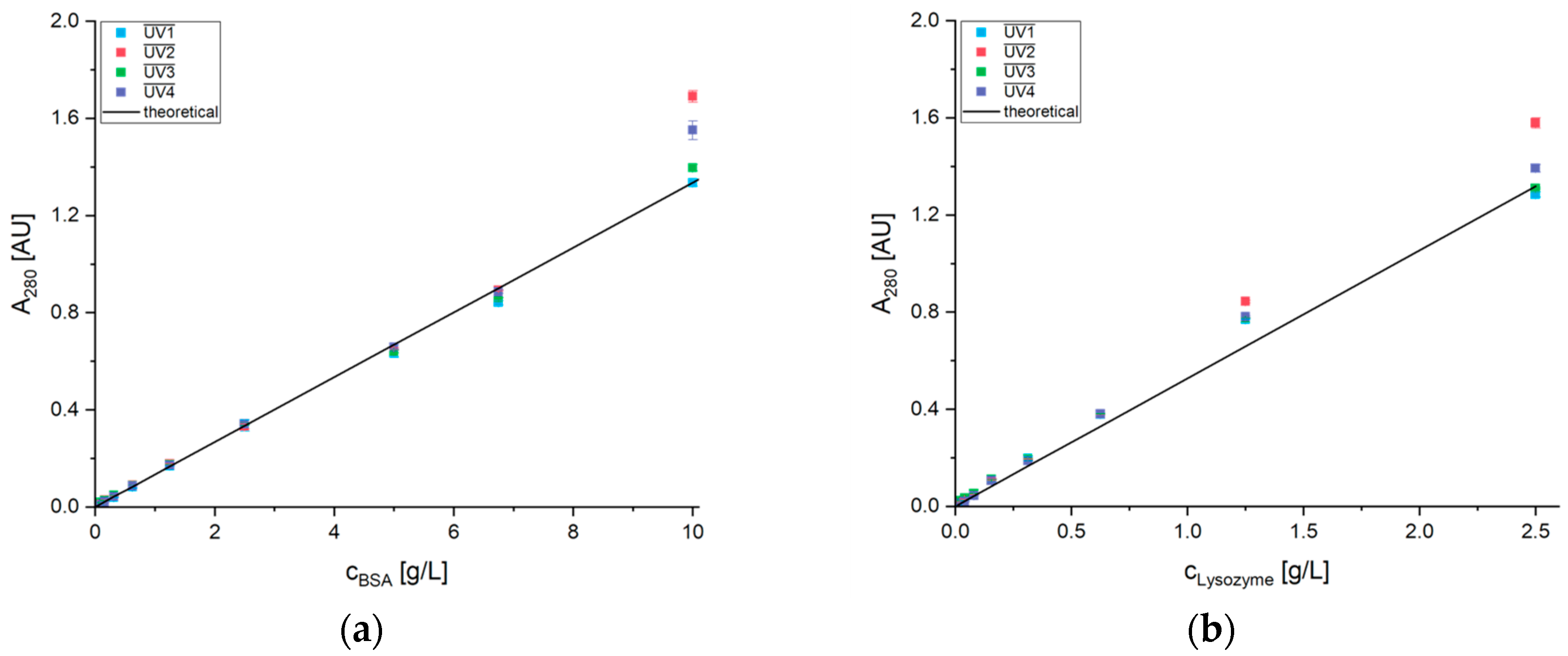
2.2.2. Protein Quantification
2.2.3. Qualitative Analysis Using SDS-PAGE
3. Results
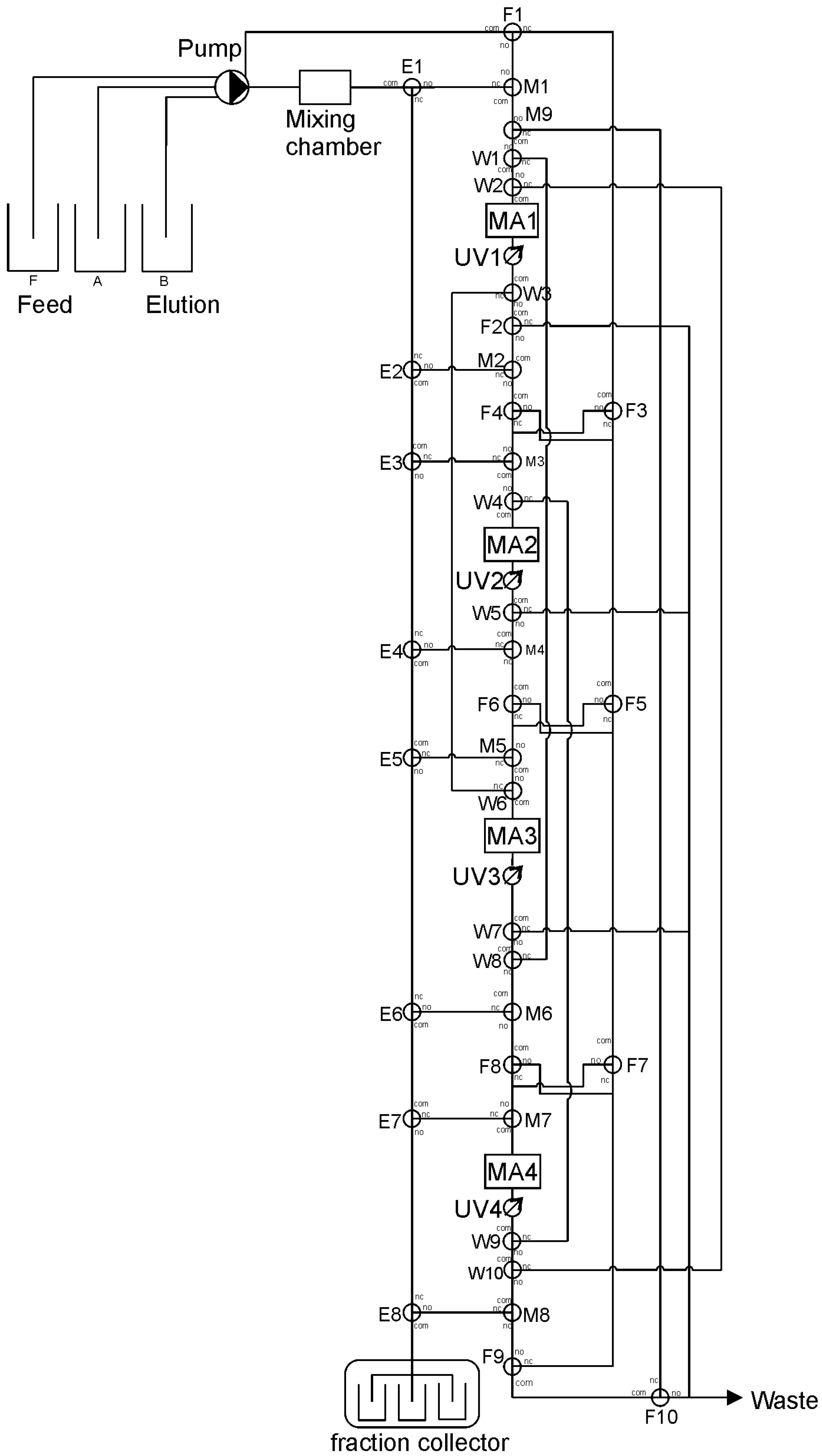
3.1. System Setup
3.2. Integration of SiLA2 and Blockly
3.3. Continuous Chromatography with Model Proteins
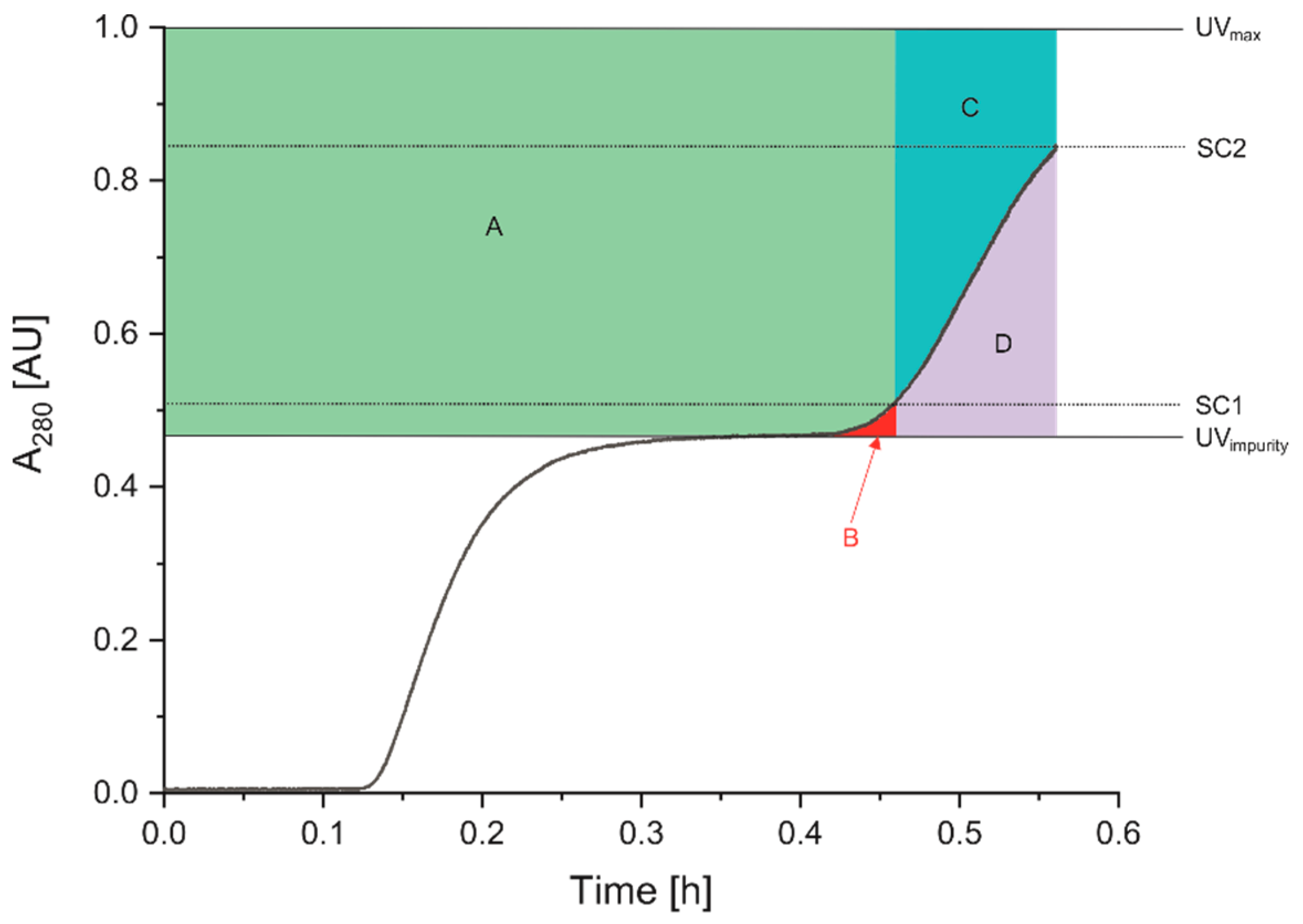
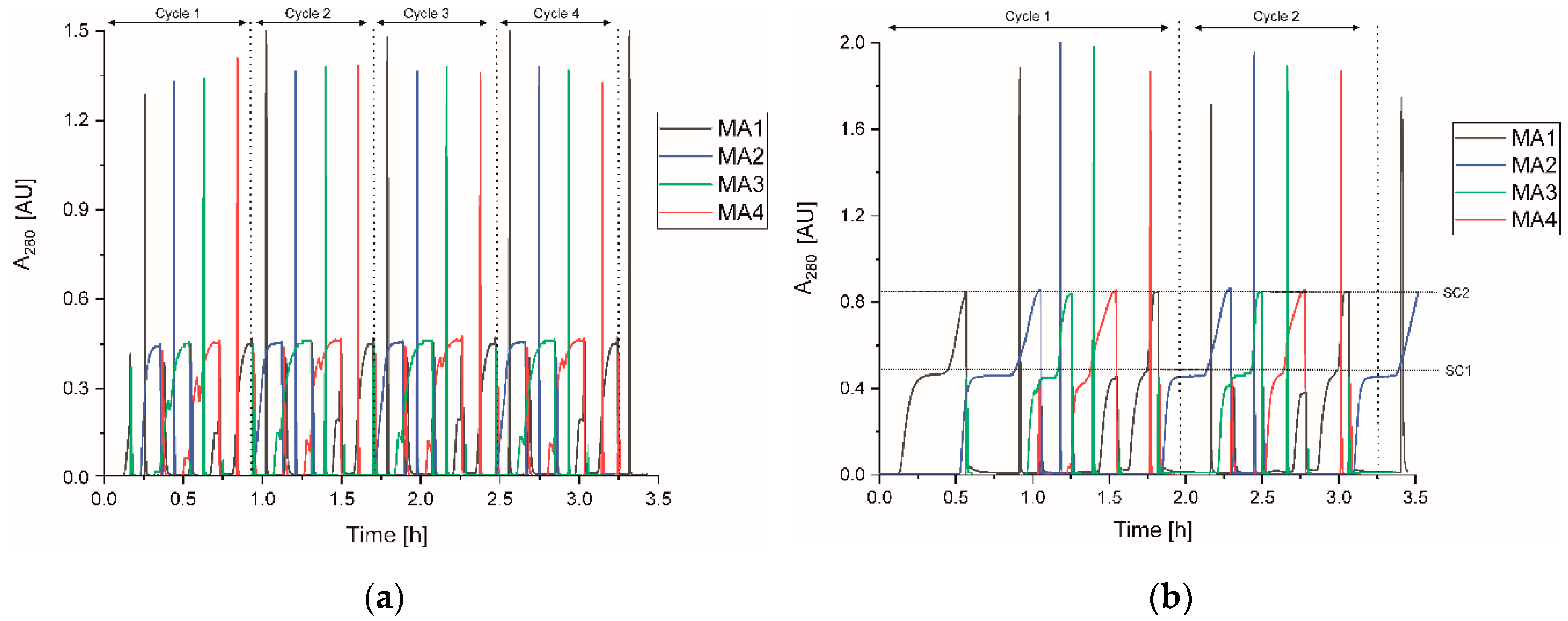
3.3.1. Time-Controlled Process (Static Control)
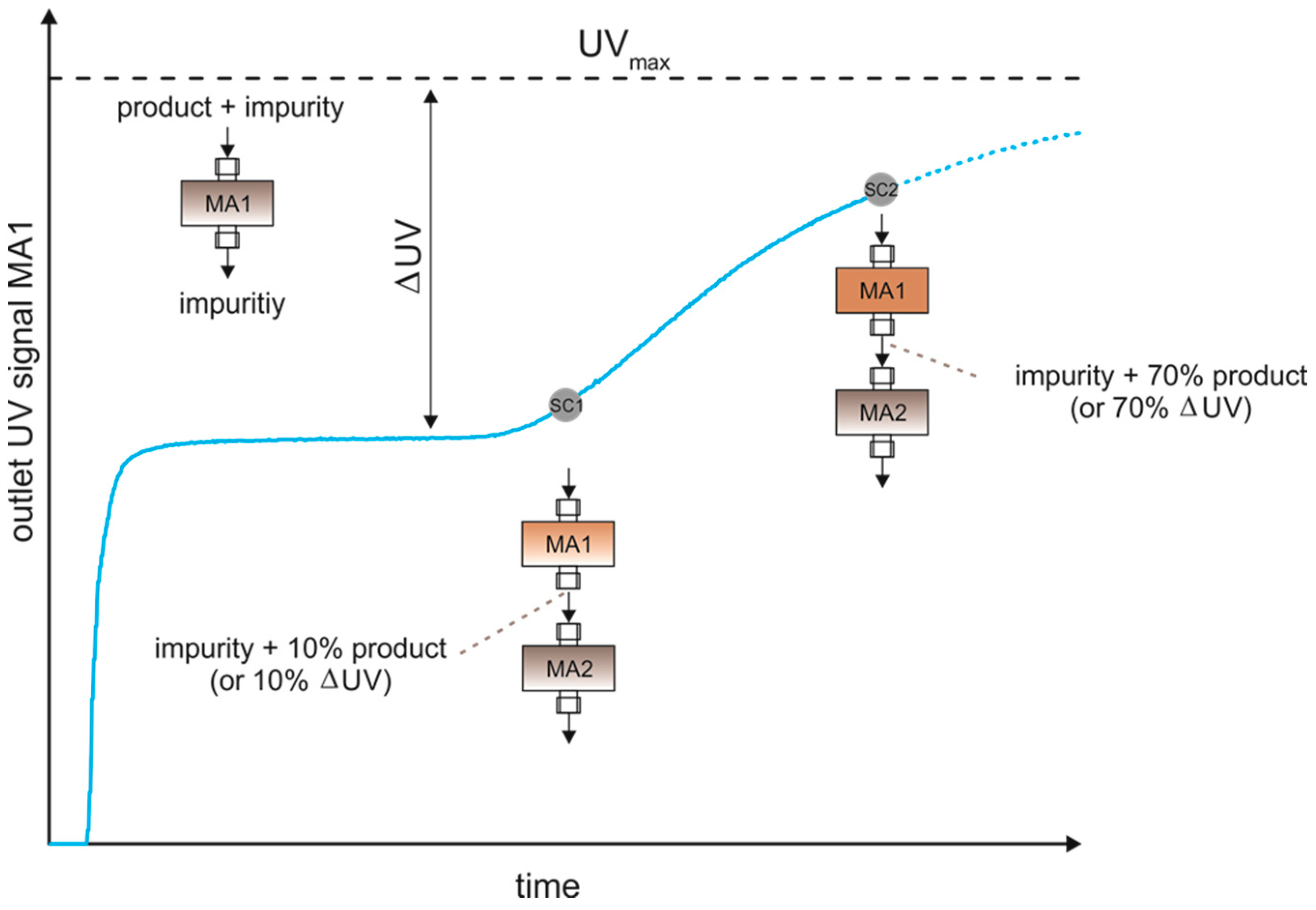
3.3.2. Dynamic Control with UV-Signal
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ozturk, S.S. Opportunities and Challenges for the Implementation of Continuous Processing in Biomanufacturing. Contin. Process. Pharm. Manuf. 2014, 457–478. [Google Scholar] [CrossRef]
- Brämer, C.; Schreiber, S.; Scheper, T.; Beutel, S. Continuous purification of Candida antarctica lipase B using 3-membrane adsorber periodic counter-current chromatography. Eng. Life Sci. 2018, 18, 414–424. [Google Scholar] [CrossRef]
- Brämer, C.; Ekramzadeh, K.; Lammers, F.; Scheper, T.; Beutel, S. Optimization of continuous purification of recombinant patchoulol synthase from Escherichia coli with membrane adsorbers. Biotechnol. Prog. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Warikoo, V.; Godawat, R.; Brower, K.; Jain, S.; Cummings, D.; Simons, E.; Johnson, T.; Walther, J.; Yu, M.; Wright, B.; et al. Integrated continuous production of recombinant therapeutic proteins. Biotechnol. Bioeng. 2012, 109, 3018–3029. [Google Scholar] [CrossRef]
- Heeter, G.A.; Liapis, A.I. Perfusion chromatography: Performance of periodic countercurrent column operation and its comparison with fixed-bed operation. J. Chromatogr. A 1995, 711, 3–21. [Google Scholar] [CrossRef]
- Pathak, M.; Ma, G.; Bracewell, D.G.; Rathore, A.S. Re-use of protein A resin: Fouling and economics. BioPharm Int. 2015, 28, 28–33. [Google Scholar]
- Wolfgang, J.; Prior, A. Continuous Annular Chromatography. Mod. Adv. Chromatogr. 2002, 76, 233–255. [Google Scholar]
- Godawat, R.; Brower, K.; Jain, S.; Konstantinov, K.; Riske, F.; Warikoo, V. Periodic counter-current chromatography–design and operational considerations for integrated and continuous purification of proteins. Biotechnol. J. 2012, 7, 1496–1508. [Google Scholar] [CrossRef]
- Janson, J.-C. Protein Purification: Principles, High Resolution Methods, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 151. [Google Scholar]
- Rajendran, A.; Paredes, G.; Mazzotti, M. Simulated moving bed chromatography for the separation of enantiomers. J. Chromatogr. A 2009, 1216, 709–738. [Google Scholar] [CrossRef]
- Whitaker, S.C.; Francis, R.; Siegel, R.C. Validation of Continuously Perfused Cell Culture Processes for Production of Monoclonal Antibodies. In Validation of Biopharmaceutical Manufacturing Processes; American Chemical Society: Washington, DC, USA, 1998; Volume 698, pp. 3–28. [Google Scholar]
- Bisschops, M. Bio SMB TM Technology: Continuous Countercurrent Chromatography Enabling a Fully Disposable Process. Biopharm. Prod. Technol. 2012, 1, 769–791. [Google Scholar]
- Steinebach, F.; Müller-Späth, T.; Morbidelli, M. Continuous counter-current chromatography for capture and polishing steps in biopharmaceutical production. Biotechnol. J. 2016, 11, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Skoglar, H.; Blom, H.; Mathiasson, L.; Akerblom, A.; Łącki, K. The use of dynamic control in periodic counter-current chromatography. Bioprocess Int. 2015, 13, 29150261. [Google Scholar]
- Heuer, C.; Küsters, E.; Plattner, T.; Seidel-Morgenstern, A. Design of the simulated moving bed process based on adsorption isotherm measurements using a perturbation method. J. Chromatogr. A 1998, 827, 175–191. [Google Scholar] [CrossRef]
- Mathiasson, L.; Skoglar, H.; Berg, M.; Sichting, M.; Chmielowski, R.; Forma, E.; Nordvarg, H. Continuous Chromatography beyond Affinity Capture of Monoclonal Antibodies. 2017. Available online: https://dc.engconfintl.org/biomanufact_iii/2/ (accessed on 21 November 2019).
- Baur, D.; Angarita, M.; Müller-Späth, T.; Steinebach, F.; Morbidelli, M. Comparison of batch and continuous multi-column protein A capture processes by optimal design. Biotechnol. J. 2016, 11, 920–931. [Google Scholar] [CrossRef]
- Pollock, J.; Bolton, G.; Coffman, J.; Ho, S.V.; Bracewell, D.G.; Farid, S.S. Optimising the design and operation of semi-continuous affinity chromatography for clinical and commercial manufacture. J. Chromatogr. A 2013, 1284, 17–27. [Google Scholar] [CrossRef] [PubMed]
- El-Sabbahy, H.; Fagan, L.; Nancollis, V. Factors Affecting the Productivity of 4-Column Periodic Counter Current Chromatography (4C-PCC). 2015. Available online: https://dc.engconfintl.org/biomanufact_ii/113/ (accessed on 21 November 2019).
- Rathore, A.S.; Kateja, N.; Agarwal, H. Continuous Downstream Processing for Production of Biotech Therapeutics. Contin. Biomanuf. Innov. Technol. 2017, 261–288. [Google Scholar] [CrossRef]
- Knudsen, H.L.; Fahrner, R.L.; Xu, Y.; Norling, L.A.; Blank, G.S. Membrane ion-exchange chromatography for process-scale antibody purification. J. Chromatogr. A 2001, 907, 145–154. [Google Scholar] [CrossRef]
- Gebauer, K.H.; Thömmes, J.; Kula, M.R. Breakthrough performance of high-capacity membrane adsorbers in protein chromatography. Chem. Eng. Sci. 1997, 52, 405–419. [Google Scholar] [CrossRef]
- Demmer, W.; Nussbaumer, D. Large-scale membrane adsorbers. J. Chromatogr. A 1999, 852, 73–81. [Google Scholar] [CrossRef]
- Mothes, B.; Pezzini, J.; Schroeder-Tittmann, K.; Villain, L. Accelerated, seamless antibody purification. Bioprocess Int. 2016, 14, 5. [Google Scholar]
- Zobel-Roos, S.; Stein, D.; Strube, J. Evaluation of Continuous Membrane Chromatography Concepts with an Enhanced Process Simulation Approach. Antibodies 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, E.; George, A.; Wolk, B. Improving affinity chromatography resin efficiency using semi-continuous chromatography. J. Chromatogr. A 2012, 1227, 154–162. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brämer, C.; Lammers, F.; Scheper, T.; Beutel, S. Development and Testing of a 4-Columns Periodic Counter-Current Chromatography System Based on Membrane Adsorbers. Separations 2019, 6, 55. https://doi.org/10.3390/separations6040055
Brämer C, Lammers F, Scheper T, Beutel S. Development and Testing of a 4-Columns Periodic Counter-Current Chromatography System Based on Membrane Adsorbers. Separations. 2019; 6(4):55. https://doi.org/10.3390/separations6040055
Chicago/Turabian StyleBrämer, Chantal, Frank Lammers, Thomas Scheper, and Sascha Beutel. 2019. "Development and Testing of a 4-Columns Periodic Counter-Current Chromatography System Based on Membrane Adsorbers" Separations 6, no. 4: 55. https://doi.org/10.3390/separations6040055
APA StyleBrämer, C., Lammers, F., Scheper, T., & Beutel, S. (2019). Development and Testing of a 4-Columns Periodic Counter-Current Chromatography System Based on Membrane Adsorbers. Separations, 6(4), 55. https://doi.org/10.3390/separations6040055




