Automation of µ-SPE (Smart-SPE) and Liquid-Liquid Extraction Applied for the Analysis of Chemical Warfare Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Analytes
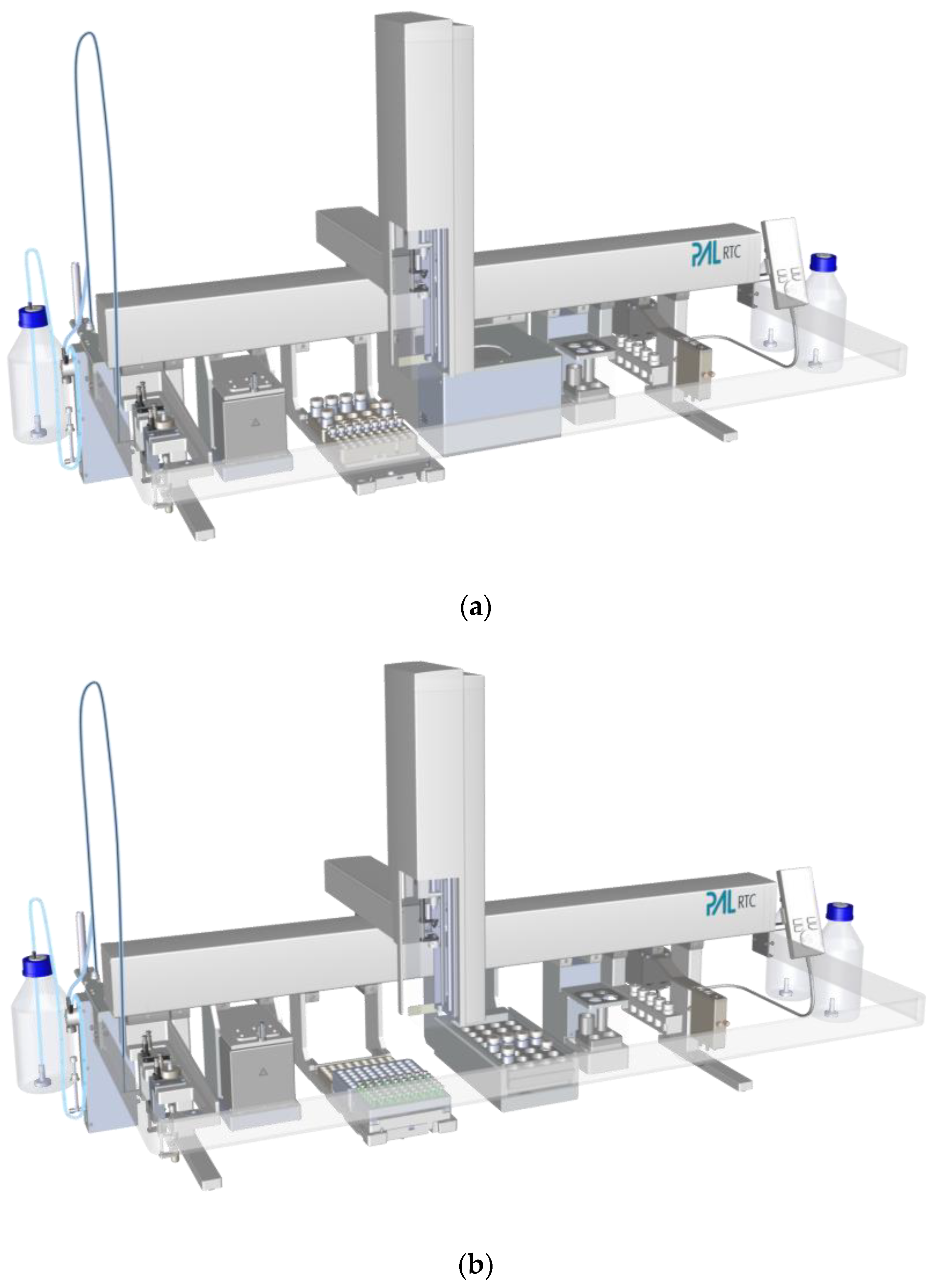
2.2. Hardware Equipment
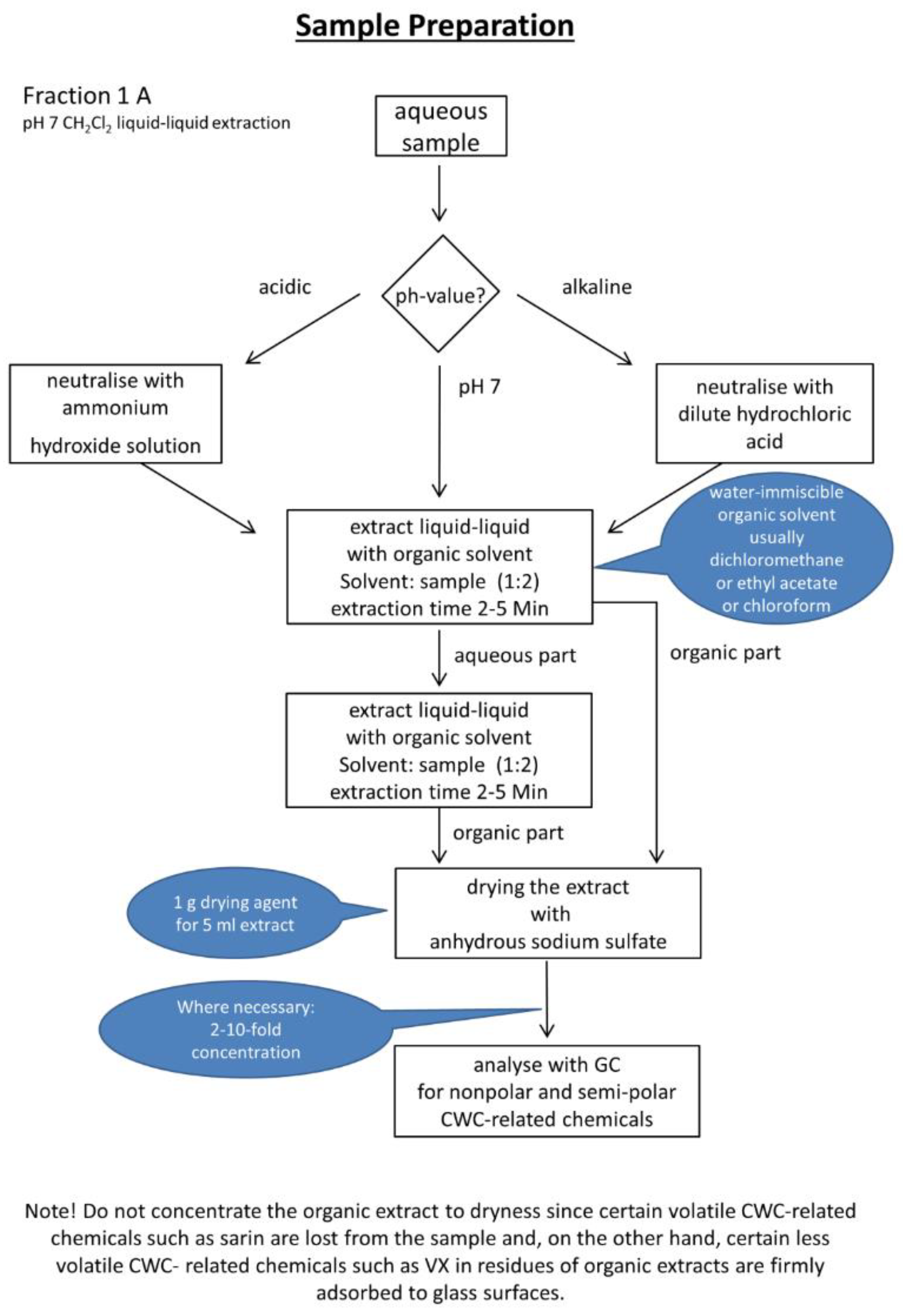
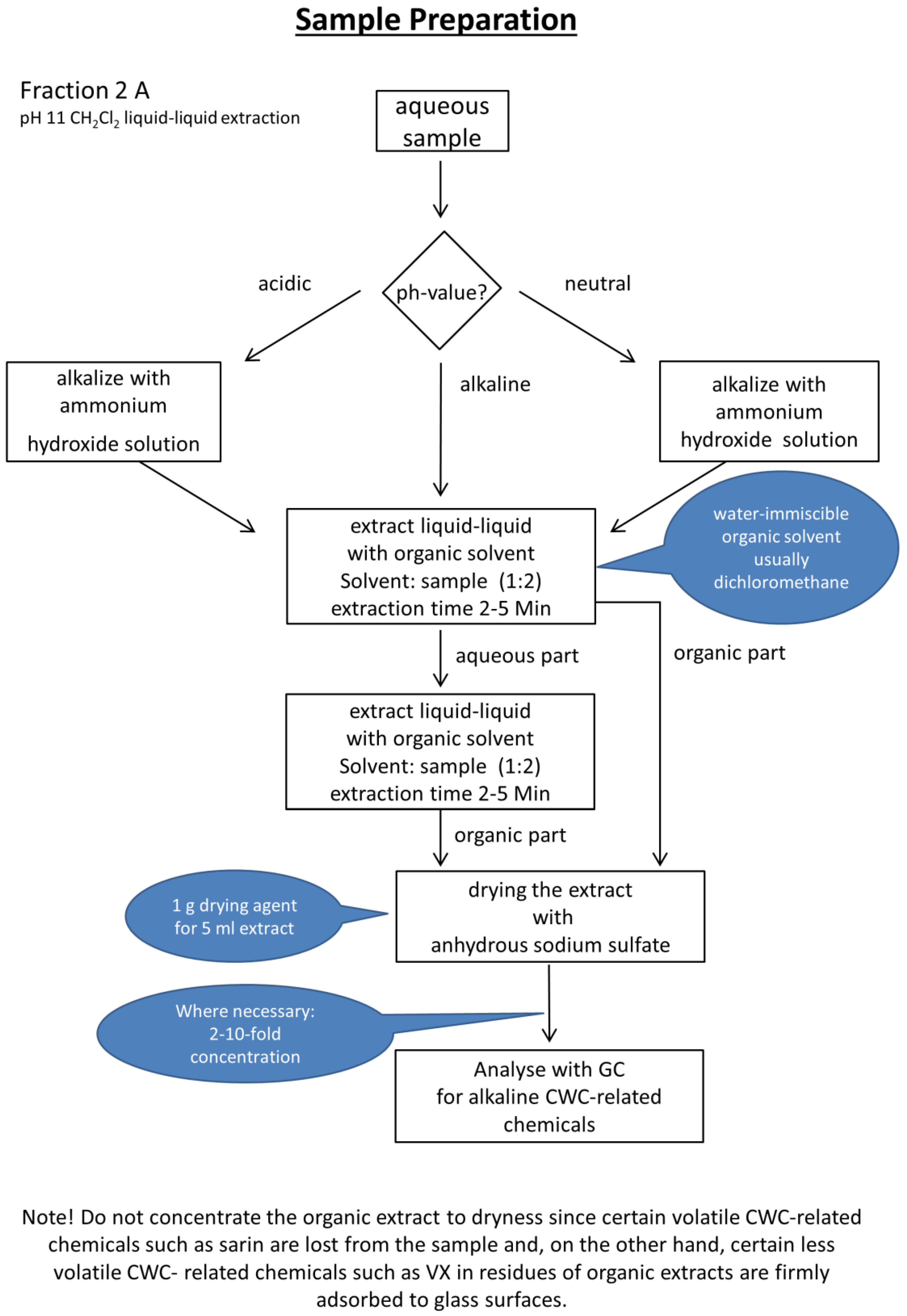
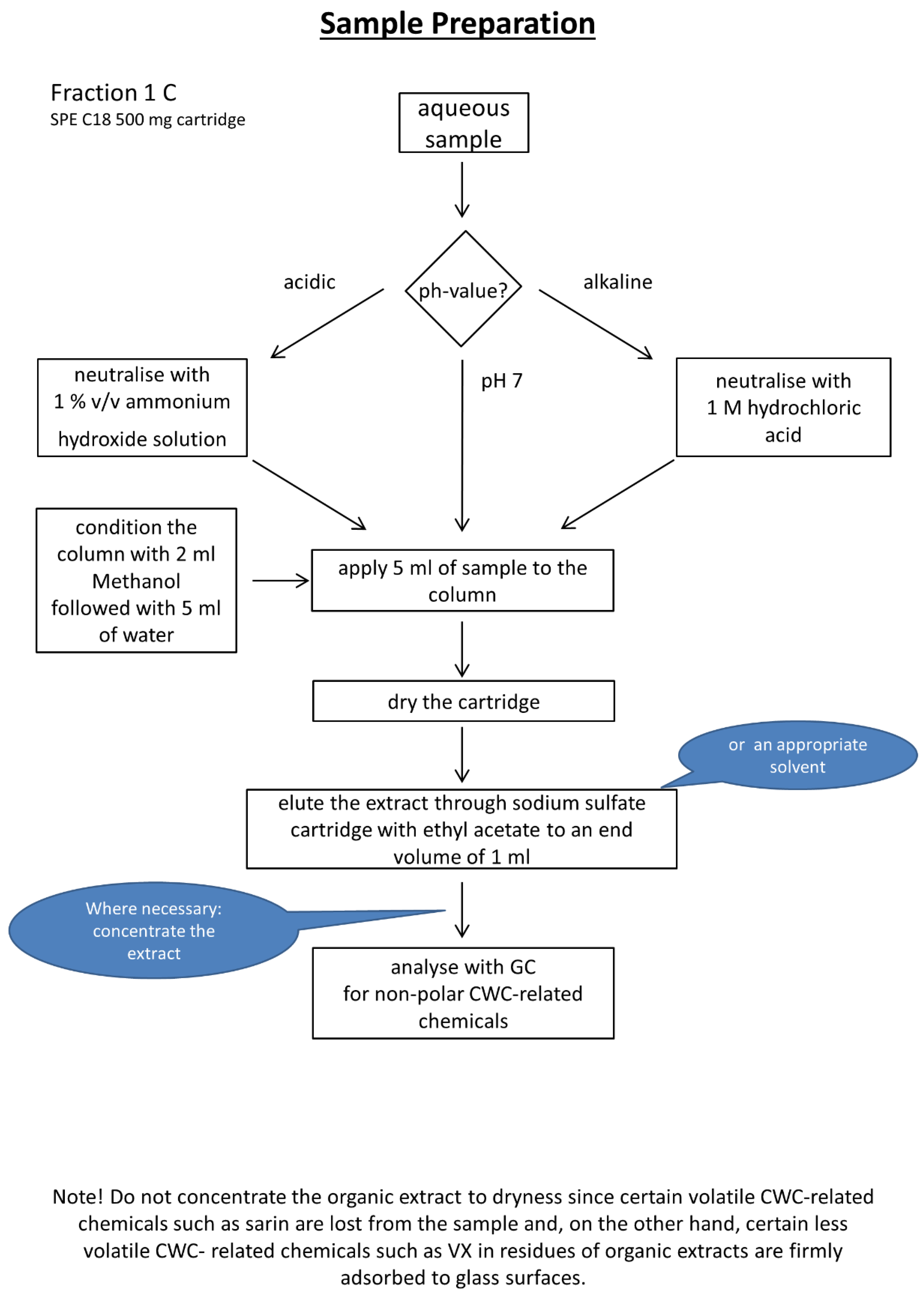
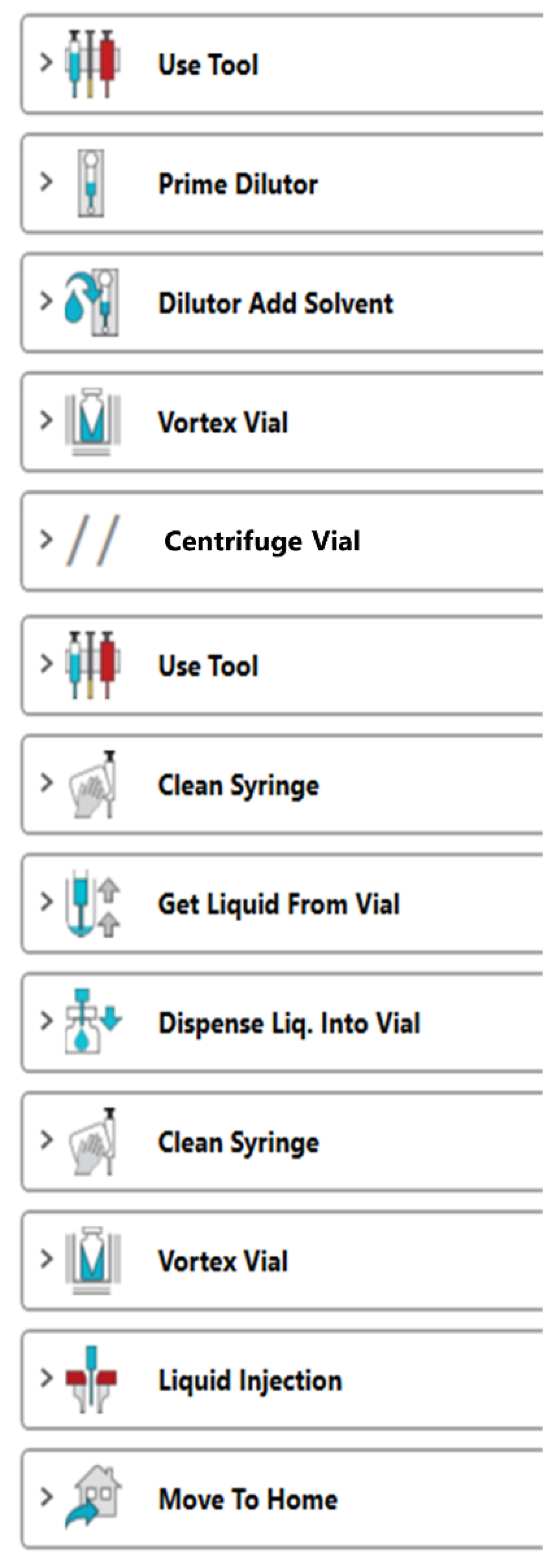
2.3. Experimental Workflows
3. Results and Discussion
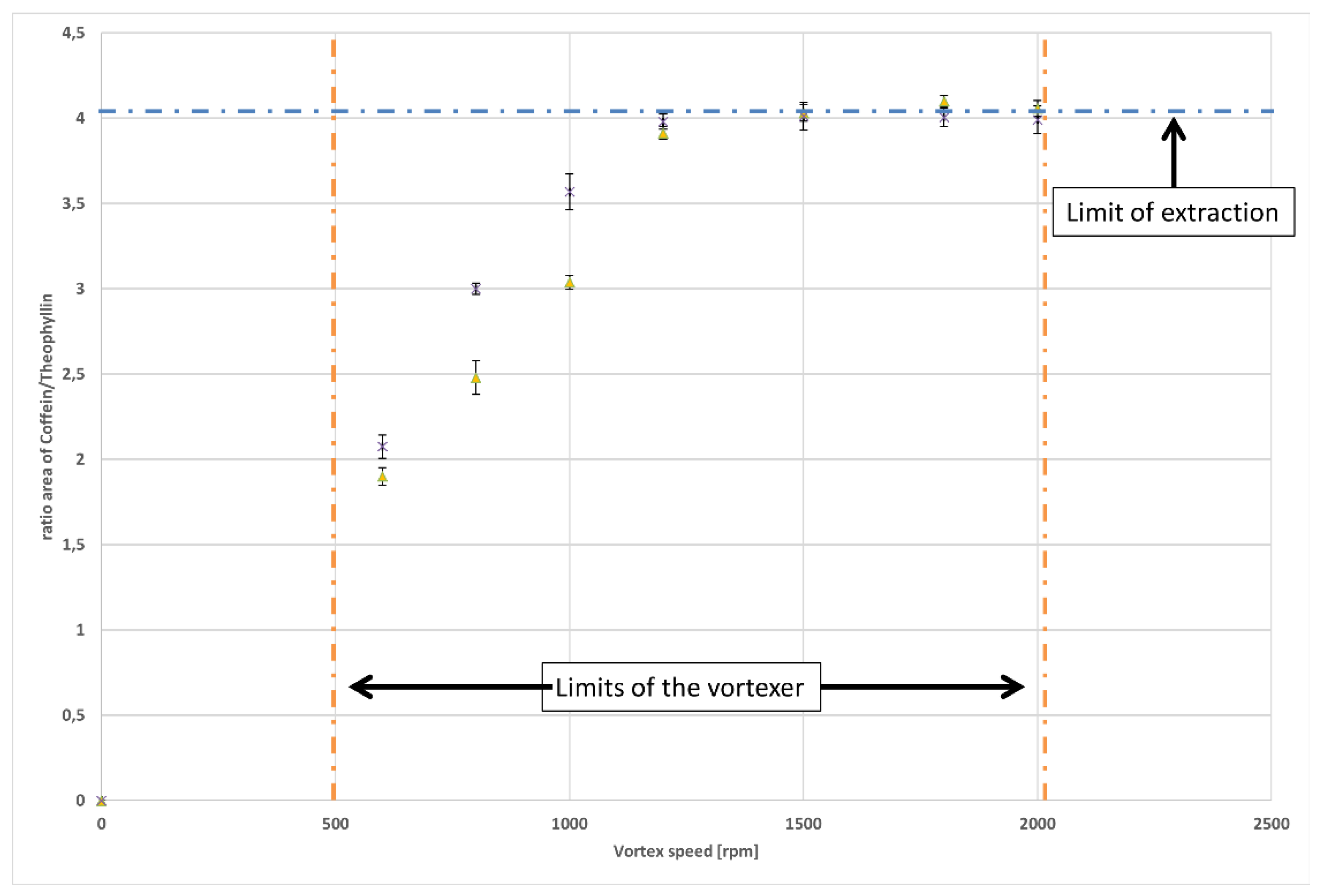
3.1. Automated Liquid-Liquid Extraction
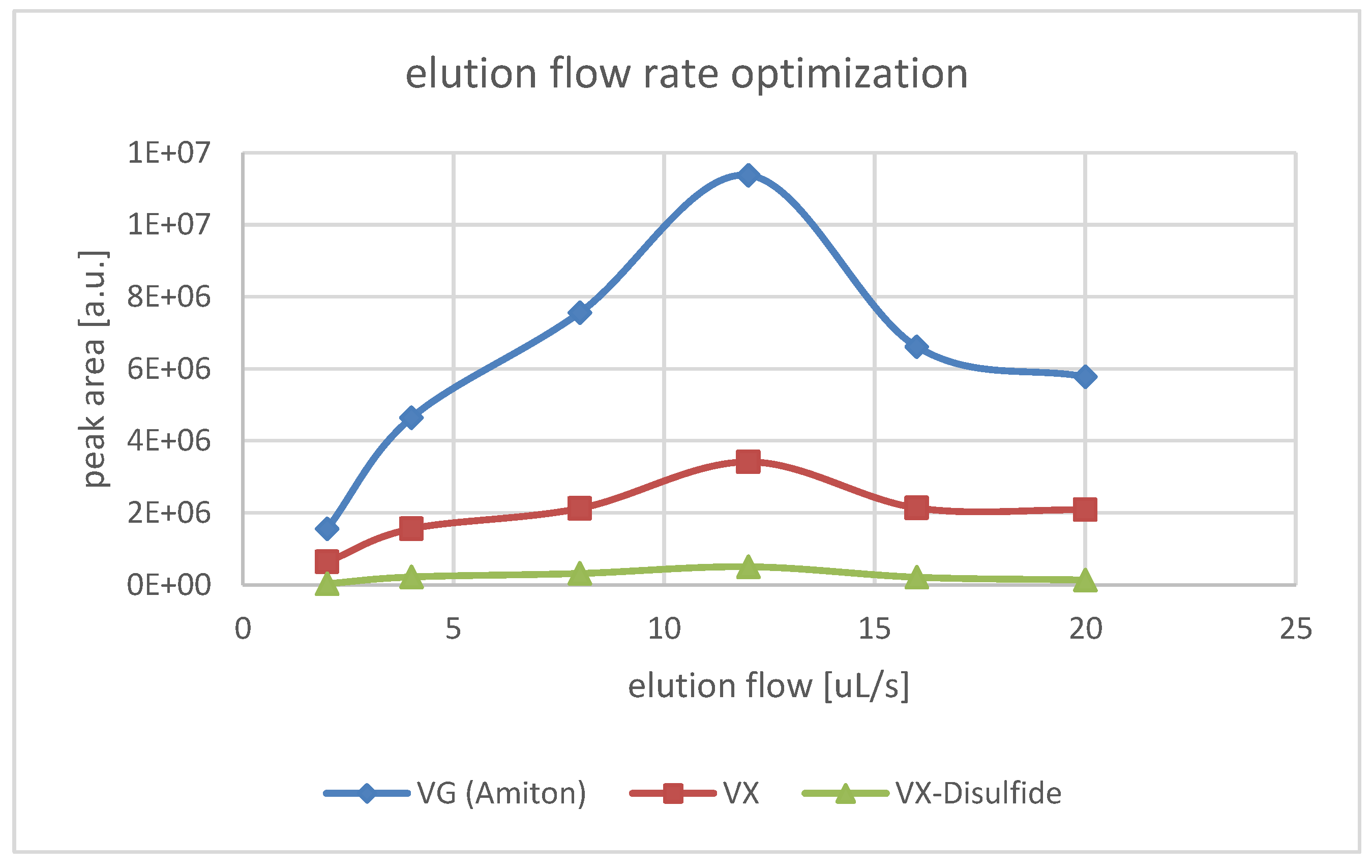
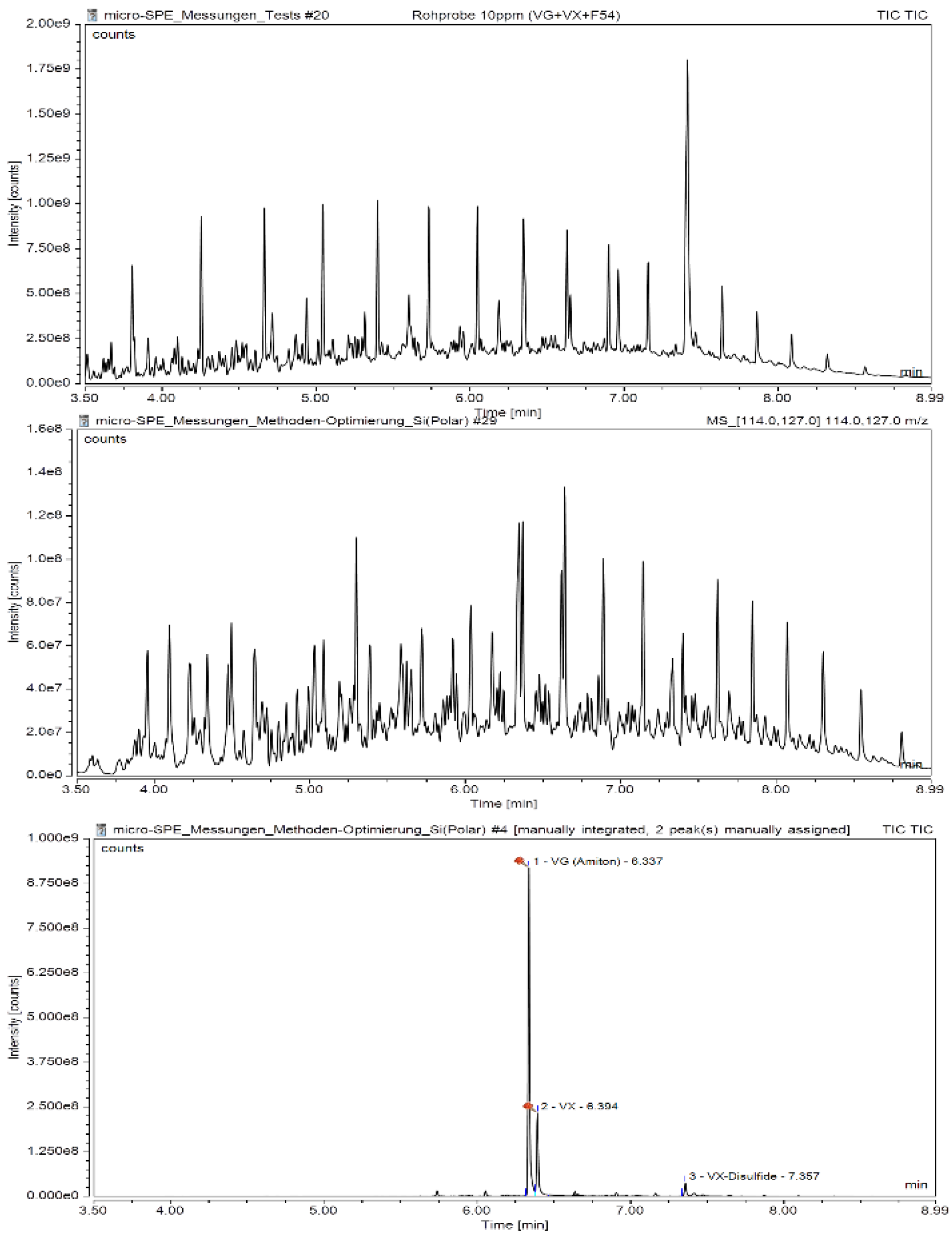
3.2. Automated µ-SPE
4. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pawliszyn, J.; Lord, H.L. Handbook of Sample Preparation; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chui, M.; Manyika, J.; Miremadi, M. Where Machines Could Replace Humans and Where They Can‘t (Yet)? Available online: http://www.oregon4biz.com/assets/e-lib/Workforce/MachReplaceHumans.pdf (accessed on 25 September 2019).
- Armbruster, D.A.; Overcash, D.R.; Reyes, J. Clinical Chemistry Laboratory Automation in the 21st Century—Amat Victoria curam (Victory loves careful preparation). Clin. Biochem. Rev. 2014, 35, 143–153. [Google Scholar]
- Vanninen, P. Recommended Operating Procedures for Analysis in the Verification of Chemical Disarmament; University of Helsinki: Helsinki, Finnland, 2017. [Google Scholar]
- Wittsiepe, J.; Nestola, M.; Kohne, M.; Zinn, P.; Wilhelm, M. Determination of polychlorinated biphenyls and organochlorine pesticides in small volumes of human blood by high-throughput on-line SPE-LVI-GC-HRMS. J. Chromatogr. B 2014, 945–946, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.L. Sample Preparation for Analysis of Chemicals Related to the Chemical Weapons Convention in an Off-site Laboratory. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Häkkinen, V.M.A. Analysis of chemical warfare agents in water by solid phase extraction and two-channel capillary gas chromatography. J. Sep. Sci. 1991, 14, 811–815. [Google Scholar] [CrossRef]
- Bae, S.Y.; Winemiller, M.D. Quantification of VX Nerve Agent in Various Food Matrices by Solid-Phase Extraction Ultra-Performance Liquid Chromatography–Time-of-Flight Mass Spectrometry; Edgewood Chemical Biological Center: Edgewood, WA, USA, 2016; p. 26. [Google Scholar]
- Sinha Roy, K.; Goud, D.R.; Chandra, B.; Dubey, D.K. Efficient Extraction of Sulfur and Nitrogen Mustards from Nonpolar Matrix and an Investigation on Their Sorption Behavior on Silica. Anal. Chem. 2018, 90, 8295–8299. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Liu, S.L.; Xi, H.L.; Yu, H.L.; Zhou, S.K.; Huang, G.L.; Liang, L.H.; Liu, J.Q. Simultaneous quantification of four metabolites of sulfur mustard in urine samples by ultra-high performance liquid chromatography-tandem mass spectrometry after solid phase extraction. J. Chromatogr. A 2017, 1492, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sellström, A. Report of the United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic on the Alleged Use of Chemical Weapons in the Ghouta Area of Damascus on 21 August 2013. Available online: https://www.un.org/zh/focus/northafrica/cwinvestigation.pdf (accessed on 26 September 2019).
- Althoff, M.A.; Bertsch, A.; Metzulat, M.; Kalthoff, O.; Karaghiosoff, K. New Aspects of the Detection and Analysis of Organo(thio)phosphates related to the Chemical Weapons Convention. Phosphorus Sulfur 2016, 192, 149–156. [Google Scholar] [CrossRef]
- Trinkwasseranlayse der Stadt Sonthofen; Stadt Sonthofen: Sonthofen, Germany, 2018; Volume 2018, pp. 1–2.
- ITSP Solutions Inc. Available online: https://www.itspsolutions.com/ (accessed on 22 May 2019).
- PAL Method Composer. Available online: https://www.palsystem.com/index.php?id=850 (accessed on 27 July 2019).
- TriPlus™ RSH™ Sampling Workflow Editor Software. Available online: https://www.thermofisher.com/order/catalog/product/1R77010-1200 (accessed on 27 July 2019).
- BIP Gasreinigungstechnologie. Available online: http://www.tig.de/produkte/bip-gasreinigungs-technologie.html (accessed on 25 September 2019).
- Althoff, M.A.; Bertsch, A.; Metzulat, M.; Klapötke, T.M.; Karaghiosoff, K.L. Application of Headspace and Direct Immersion Solid-Phase Microextraction in the Analysis of Organothiophosphates related to the Chemical Weapons Convention from Water and Complex Matrices. Talanta 2017, 174, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Costa, R. Newly Introduced Sample Preparation Techniques: Towards Miniaturization. Crit. Rev. Anal. Chem. 2014, 44, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: Hoboken, NJ, USA, 2011. [Google Scholar]
- Solid Phase Extraction Specifications. Available online: http://www.gerstel.com/pdf/SPE_Spec_en.pdf (accessed on 26 September 2019).
- Jaffuel, A.; Huteau, A.; Moreau, S. Direct Liquid Chromatography Tandem Mass Spectrometry Analysis of Glyphosate, AMPA, Glufosinate, and MPPA in Water Without Derivatization. Column 2018, 14, 36–40. [Google Scholar]

| Instrument Parameter | Value |
|---|---|
| GC | Thermo Fisher Scientific Trace 1310 with PTV Injector |
| Data System | Chromeleon 7.2 SR 4 |
| Analytical column | Agilent J&W GC-column (CP-Sil 8 CB Low Bleed/MS, 30 m 0.25 µm) |
| Oven conditions: Temperature program | 70(1) −290 °C at 30 °C min−1, post temperature 290 °C (1) |
| Carrier gas | Helium BIP® [17] |
| Injection conditions: Injector temperature | 250 °C |
| Injection type Purge flow Liner type | liquid injection: splitless, injection volume 1 µL 1.20 mL/min 130 mm × 2.0 mm ID glass liner |
| MS | Thermo Fisher Scientific TSQ Duo, Triple Quadrupole scan time: 0.2 s |
| Transfer line temperature | 280 °C |
| Ion source temperature | 280 °C |
| Ionization mode | EI |
| FID | Thermo Fisher Scientific FID-Module temperature: 250 °C H2-flow: 35 mL N2-flow: 40 mL synthetic air-flow: 350 mL |
| Matrix 1 | Recovery Rate ± RSD [%] | ||
|---|---|---|---|
| VX | VG | HD | |
| tap water (pH 7) | 64.1 ± 9.6 | 79.8 ± 8.3 | 47.3 ± 3.8 |
| tap water (pH 11) | 88.0 ± 3.4 | 85.9 ± 5.1 | 22.1 ± 2.7 |
| surface water (pH 7) | 96.0 ± 3.4 | 99.9 ± 3.6 | 51.5 ± 4.0 |
| surface water (pH 11) | 98.7 ± 1.4 | 99.9 ± 1.8 | 50.7 ± 1.4 |
| soil | 66.2 ± 2.7 | 88.2 ± 4.0 | 46.8 ± 4.4 |
| wipe | 65.6 ± 3.1 | 58.4 ± 2.3 | 51.2 ± 1.4 |
| Matrix (Cartridge) | Recovery ± RSD [%] | |||
|---|---|---|---|---|
| VX | VX-Disulfide | VG | HD | |
| water (C18-EC) | 56.5 ± 0.5 | 57.9 ± 2.2 | 65.8 ± 0.1 | 64.8 ± 1.9 |
| water samples [7] | 24 ± 10 | –1 | –1 | 32 ± 7 |
| diesel fuel (silica) | 76.3 ± 1.3 | 77.4 ± 0.2 | 98.2 ± 1.5 | –2 |
| Parameter | µ-SPE (This Work) | SPE (Gerstel MPS [21]) | SPE (Laboratory) |
|---|---|---|---|
| sorbent volume | 10–45 mg | 0.1–2 g | 0.1 g – bulk |
| solvent volume | 125 µL | ≤10 mL | 3 mL |
| processing time | 10 min | unknown | 15 min |
| flow rate | 1–100 µL/s | 10–250 µL/s | ca. 1 drop/s |
| enrichment factor | ≤200 | 5–10 | Indefinite |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althoff, M.A.; Bertsch, A.; Metzulat, M. Automation of µ-SPE (Smart-SPE) and Liquid-Liquid Extraction Applied for the Analysis of Chemical Warfare Agents. Separations 2019, 6, 49. https://doi.org/10.3390/separations6040049
Althoff MA, Bertsch A, Metzulat M. Automation of µ-SPE (Smart-SPE) and Liquid-Liquid Extraction Applied for the Analysis of Chemical Warfare Agents. Separations. 2019; 6(4):49. https://doi.org/10.3390/separations6040049
Chicago/Turabian StyleAlthoff, Marc André, Andreas Bertsch, and Manfred Metzulat. 2019. "Automation of µ-SPE (Smart-SPE) and Liquid-Liquid Extraction Applied for the Analysis of Chemical Warfare Agents" Separations 6, no. 4: 49. https://doi.org/10.3390/separations6040049
APA StyleAlthoff, M. A., Bertsch, A., & Metzulat, M. (2019). Automation of µ-SPE (Smart-SPE) and Liquid-Liquid Extraction Applied for the Analysis of Chemical Warfare Agents. Separations, 6(4), 49. https://doi.org/10.3390/separations6040049




