Abstract
A sensitive and selective ultra-high performance liquid chromatography-tandem mass spectrometric method was developed for the quantification of temozolomide (TMZ) in nonhuman primate (NHP) plasma, cerebrospinal fluid (CSF), and brain extracellular fluid (ECF) following microdialysis. Ethyl acetate was used to extract the plasma and CSF samples, using theophylline as the internal standard (IS). ECF samples were diluted with acetonitrile prior to analysis. TMZ was separated on a Waters UPLC® BEH C18 column with an isocratic mobile phase of ammonium acetate (10 mM)-0.1% formic acid/acetonitrile (30:70, v/v) in a positive-ion multiple reaction monitoring mode (m/z 195.5→137.6 for TMZ; m/z 181.5→124.2 for IS). The retention time of TMZ and theophylline was 0.45 min with a total run time of 2.5 min. The method was validated over the range from 5–2000 ng/mL in NHP plasma, CSF, and ECF with respect to linearity, accuracy, precision, selectivity, and stability. This method was successfully applied toward the measurement of pharmacokinetic samples following various routes of drug administration.
1. Introduction
Temozolomide (TMZ) is a prodrug that is ultimately metabolized to an alkylating agent. Upon exposure to physiological pH, TMZ is spontaneously hydrolyzed to the intermediate 5-(3-methyl-triazen-1-yl) imidazole-4-carboxamide (MTIC). MTIC further fragments to 5-amino-4-imidazolecarboxamide and the extremely reactive methyldiazonium cation, which methylates DNA at several nucleophilic sites.
N7-guanine, N3-adenine, and O6-guanine [1]. Although the formation of O6-methylguanine accounts for a minority of DNA adducts formed, it is particularly cytotoxic and is correlated with TMZ-mediated cytotoxicity, resulting in mispairing during replication and subsequent strand breakage and tumor cell death. TMZ is FDA approved as a first-line treatment for patients with newly diagnosed glioblastoma multiforme alongside radiotherapy and as a second-line treatment for refractory anaplastic astrocytoma [2].
Although some drugs have shown modest efficacy, there is continued interest in studying the brain penetration of certain anti-glioma agents to improve therapeutic efficacy and clinical outcome. Intranasal delivery is one route being explored as a means to bypass the blood-brain barrier, as agents can undergo direct transport along the olfactory and trigeminal nerves. Studies are ongoing at the National Cancer Institute (NCI) using rhesus macaques as a nonhuman primate (NHP) model administering several agents in a variety of routes (intranasal, intravenous, oral) to understand the extent of drug penetration into different areas of the brain, and if intranasal administration improves brain penetration vs. systemic delivery. TMZ is being studied as one such agent, since existing research demonstrated relatively high brain penetration (20%–40%) [3,4].
Nonhuman primates are an advantageous model for studying the pharmacokinetics (PK) and brain penetration of neuro-oncology agents, as the pharmacology has been predictive of that in humans [5,6]. As more researchers wade into this field, there is a lack of robust published LC-MS/MS assays for measuring TMZ in NHP fluids (plasma, CSF) as well as extracellular fluid (ECF) resulting from microdialysis (MD). MD is a technique that allows the opportunity to measure TMZ concentrations directly in the ECF of the tissue or tumor site sampled, as previously described [7]. It has previously been used to study if TMZ brain penetration is enhanced with bevacizumab [8] and cediranib in rats [9], but there is no published study for measuring TMZ from microdialysis in NHP. Several bioanalytical assays measuring TMZ have been published using LC-MS/MS instrumentation; however, these were all based on human [10] or rodent [8,11,12] biological matrices. Several additional studies have used less selective and sensitive instrumentation as well to study TMZ pharmacokinetics in rodents [13] and humans [3,14,15,16,17,18]. Only one study on TMZ pharmacokinetics used the NHP model, and plasma and CSF levels were measured using a less sensitive HPLC-UV assay (lower limit of quantification (LLOQ):19 ng/mL) [6]. The purpose of this report is to describe a sensitive LC-MS/MS assay that can be used to quantify TMZ concentrations in NHP plasma, CSF, and brain tissue or tumor ECF fluid in situ following MD.
2. Experimental Section
2.1. Materials
Temozolomide (>98% purity), theophylline (>99% purity), hydrochloric acid, formic acid, and ammonium acetate were purchased from Sigma (St. Louis, MO, USA). Optima (LC-MS) grade methanol and acetonitrile were purchased from Fisher Scientific (Pittsburgh, PA, USA). NHP plasma and CSF was purchased from Bioreclamation IVT (Baltimore, MD, USA). CNS perfusion fluid was purchased from MDialysis (North Chelmsford, MA, USA). Ultra-pure deionized water was produced from a Hydro-Reverse Osmosis system (Durham, NC, USA) connected to a Mili-Q UV Plus purifying system (Billerica, MA, USA).
2.2. Preparation of Stock Solutions
To prepare 1 mg/mL stocks for TMZ, aqueous ammonium acetate solution (pH 3.5; 10 mM)-methanol (20:80, v/v) (henceforth referred to as “acidic methanol”) was prepared and used to dissolve TMZ. Subsequent serial dilutions of this stock were prepared in acidic methanol as working stocks for final concentrations in each matrix of 5, 10, 25, 100, 250, 500, 1000 and 2000 ng/mL. These same working stocks were used to prepare quality control (QC) standards for both plasma and CSF at low (15 ng/mL), mid (800 ng/mL), high (1600 ng/mL), and a 50-fold dilution (50,000 ng/mL diluted to 1000 ng/mL) level for plasma. ECF fluid (from MD) had the same low, mid, and high QCs, but used a 4-fold dilution QC up to 5000 ng/mL. The internal standard theophylline was prepared in a 1 mg/mL stock in methanol. This stock was diluted into a 1000 ng/mL solution in ethyl acetate for extractions.
2.3. Sample Preparation
2.3.1. Plasma and CSF
Calibration standards (in duplicate), QC standards (in quintuplet) and study samples were extracted from 100 µL of plasma or CSF. Because plasma and CSF samples acidified with 10% of 1 normal (N) HCl solution to prevent degradation of TMZ [15], all standards were acidified with 10% 1 N HCl to match study samples. A liquid-liquid extraction using ethyl acetate (containing 1000 ng/mL theophylline) was performed, with samples vortexed, centrifuged, and the supernatent (extract) dried before reconstitution in 100 µL of water-acetonitrile-formic acid (50:50:0.1, v/v/v).
2.3.2. MD Extracellular Fluid
Extracellular brain fluid was drawn from various locations using MD technique, as previously described [7], that typically only produces ~10–20 µL of ECF per draw (depending on collection time [over several hours] and pump flow rate). Further, ECF itself can vary in its salinity based on sampling site. To account for such low volumes of high salinity (that suppresses ion signals in the mass spectrometer) and acidity (that can non-uniformly affect compound ionization), while maintaining a lower limit of quantification (LLOQ) of 5 ng/mL, a new sample preparation procedure was developed. Drug free perfusion fluid was acidified with 1 N HCl before use in preparation of calibration and QC standards. A volume of 20 µL was needed to perform this procedure, thus if study samples were below this mark, acidified perfusion fluid (same as that used to make standards) was used to dilute study samples up to the 20 µL mark. Acetonitrile (1 mL) was added to each sample to aid in aqueous evaporation via the formation of an azeotropic mixture. The leftover dried residue contains drug and salts that is then reconstituted in 100 µL of ACN, vortexed, and centrifuged to form a pellet of precipitated salts, thereby avoiding injection from the UHPLC system. Injection volumes were 20 µL. No IS in the initial preparation of MD samples due to the non-uniform ionization that occurred from varying levels of sample acidity, which was difficult to control for, and for the sake of simplicity the IS was left out.
2.4. Instrument Conditions
A Waters ACQUITY UPLC® (Waters Corp, Milford, MA, USA) was used to chromatographically separate TMZ and the IS from unwanted biological remnants post extraction on a Waters UPLC® BEH C18 (2.1 × 50 mm, 1.7 µm) column (Waters Corp, Milford, MA, USA) using an isocratic composition of 10 mM ammonium acetate (10 mM)-acetonitrile (ACN)-formic acid (30:70:0.1, v/v/v) delivered at a flow rate of 0.3 mL/min. Retention times were 0.45 min for both TMZ and the IS. The column eluent was directed into a Micromass (Waters) Quattro Micro® triple quadrupole mass spectrometer (Waters Corp, Milford, MA, USA) with an electrospray ionization source operated in the positive ion mode. Mass spectrometer conditions were: capillary voltage 3000 V, cone voltage 35 V, RF lens 5, extractor 5, source temperature 120 °C, desolvation temperature 350 °C, cone gas flow 30 L/h, desolvation gas flow 900 L/h. TMZ and theophylline (IS) were both selectively identified based on their multiple reaction monitoring (MRM) transitions: m/z 195.5→137.6 (TMZ); m/z 181.5→124.2 (IS) (Figure 1). Both MRM transitions had a dwell time of 200 ms and collision energy of 15 eV.
2.5. Validation
2.5.1. Linearity
This assay was validated according to FDA recommended guidelines for bioanalytical validation [19] for linearity, accuracy, precision, selectivity, and various stability tests. The calibration range in all three matrices (NHP plasma, CSF, MD perfusion fluid) was 5–2000 ng/mL, with dilution capabilities up to 50,000 ng/mL in plasma and CSF, and up to 5000 ng/mL in MD perfusion fluid. A least squares linear regression model was used to assess the calibration linearity, with a weighting factor of 1/x2, with x being the ratio of peak areas for analyte/IS. Fresh calibration standards were prepared daily in drug-free matrices in duplicate. A linear regression correlation of at least r2 > 0.99 was deemed acceptable.
2.5.2. Accuracy and Precision
Accuracy and precision were evaluated by determining TMZ at four different concentrations of QC samples (low-range, mid-range, high-range, 50-fold dilution for plasma; 4-fold dilution for MD perfusion fluid), in five replicates analyzed over 3–4 different days, depending on scarcity of matrix. Each run consisted of blank matrix extracts, internal standard only extracts, calibration standards in duplicate, and QC samples in replicates of five. Accuracy (% DEV) was defined as the percent mean observed concentration relative to the nominal concentration. Assay repeatability, or precision, was determined by both the within-run precision (WRP) and between-run (BRP), as calculated below.
WRP = 100 × [(MSWIT)0.5/GM]
BRP = 100 × [({MSBET − MSWIT}/n)0.5/GM]
GM represents the grand mean, MSWIT represents within-group mean squared, MSBET represents between-group mean squared, and n represents the number of repetitions. FDA guidelines for bioanalytical validation were followed, with ±15% variability in accuracy and precision allowed [19].
2.5.3. Stability
Stability tests were performed to examine the potential for degradation of TMZ in plasma and CSF during freeze/thaw cycles. Samples were assayed at three concentrations (15, 800, 1600 ng/mL) in quintuplet in each matrix. The samples were subjected to three freeze/thaw cycles at −80 °C (two cycles for CSF due to scarcity of matrix), with each freeze cycle lasting at least 12 h. The analyte concentration after each storage period was compared to the concentration of freshly prepared samples in the same analytical run. Freeze/thaw stability was not assessed in MD perfusion fluid, as these study samples were immediately processed and analyzed the same day they were drawn, and due to low collection volumes, there was no opportunity for freezing and re-running.
2.5.4. Extraction Recovery and Matrix Effects
For the calculation of the extraction recovery and matrix effects, three sets were prepared. Set 1 was composed of stock solutions of TMZ spiked into the reconstitution solution ([50/50/0.1, v/v/v] water/ACN/formic acid) at low (25 ng/mL) and high (1000 ng/mL) concentrations in triplicate; set 2 was composed of stock solutions of TMZ spiked into NHP plasma at low (25 ng/mL) and high (1000 ng/mL) concentrations after extraction in triplicate; set 3 was composed of stock solutions of TMZ spiked into NHP plasma at low (25 ng/mL) and high (1000 ng/mL) concentrations before extraction in triplicate. Only the peak area of the analyte was taken into consideration for the calculation of each evaluation. The extraction recovery and matrix effects of TMZ in NHP plasma were calculated as shown below.
% Extraction Recovery = A/B × 100
| A = mean area count of Set 3 for one concentration level |
| B = mean area count of Set 2 for one concentration level |
% Matrix Effect = (slope of calibration curve from standards prepared via Set 2 − standards prepared via Set 1)/standards prepared via Set 1 × 100
2.6. Application
This validated analytical method was tested for applicability by analyzing plasma and CSF from a male rhesus macaque given a 20 mg dose of TMZ via intranasal injection (in a 1 mL solution; ~1.75 mg/kg). Blood (eventually processed into 1 mL of plasma acidified with 100 μL HCl) and CSF (300 μL acidified with 30 μL HCl) were collected at the following times in order to capture the full in vivo disposition: pre-dose, 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, and 24 h post-dose. Acidification of study samples and standards was based on previous data regarding the instability of TMZ in human plasma [17]. Additionally, the same animal was later dosed with a second dose (10 mg/kg) of TMZ administered via 1-h intravenous infusion, with plasma and CSF sampling occurring at pre-dose, 30 min, 1 h (end of infusion), 1.25, 1.5, 2, 3, and 4 h post infusion-start. Microdialysis probes were placed in the animal’s cortex and pons, and brain perfusion dialysate was collected in HCl-coated tubes from each area for approximately 3.5 h and 4 h, respectively. The data presented here were simply for assay applicability and were not meant to represent a comprehensive pharmacokinetic analysis.
3. Results and Discussion
3.1. Selectivity
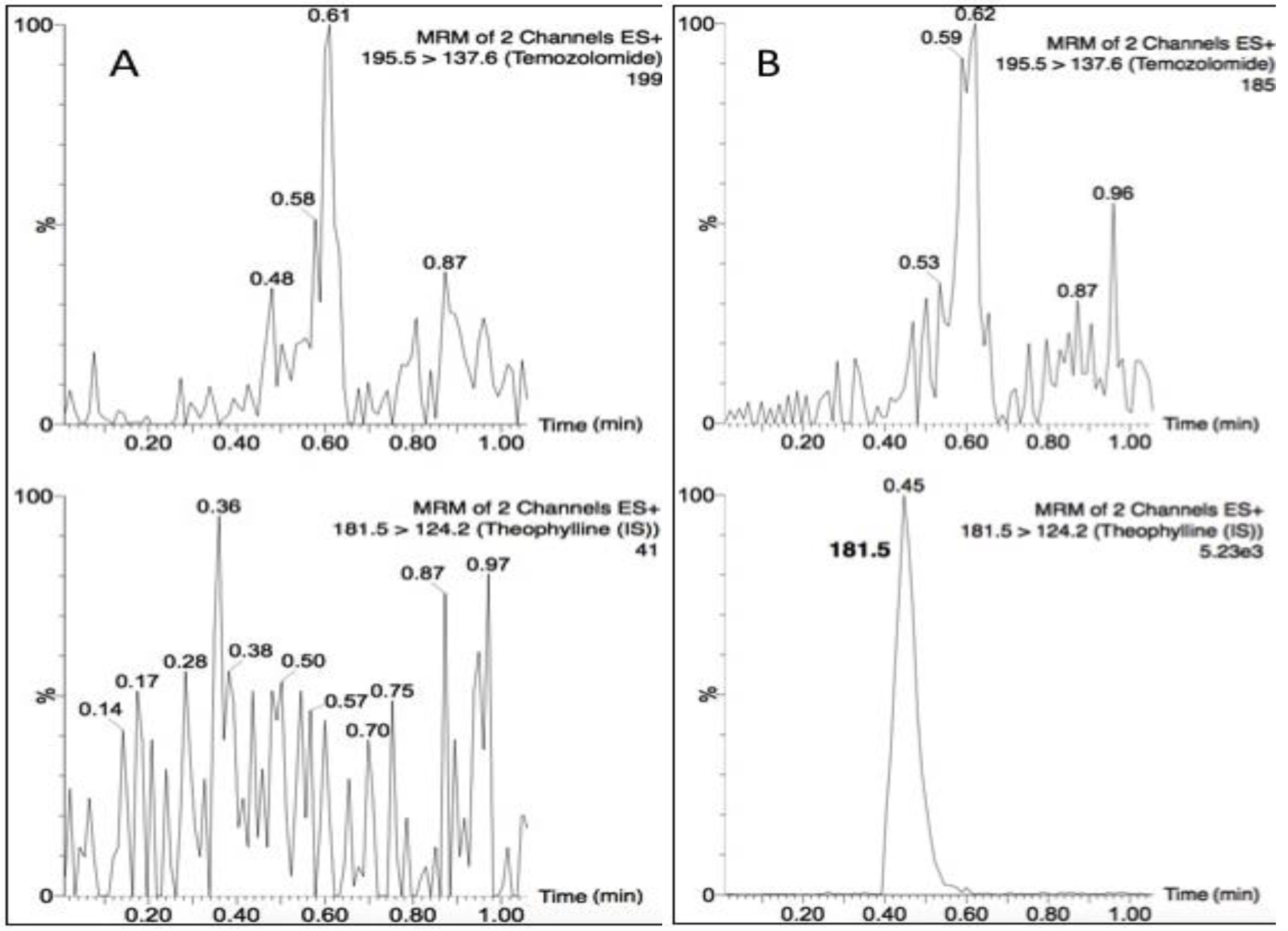
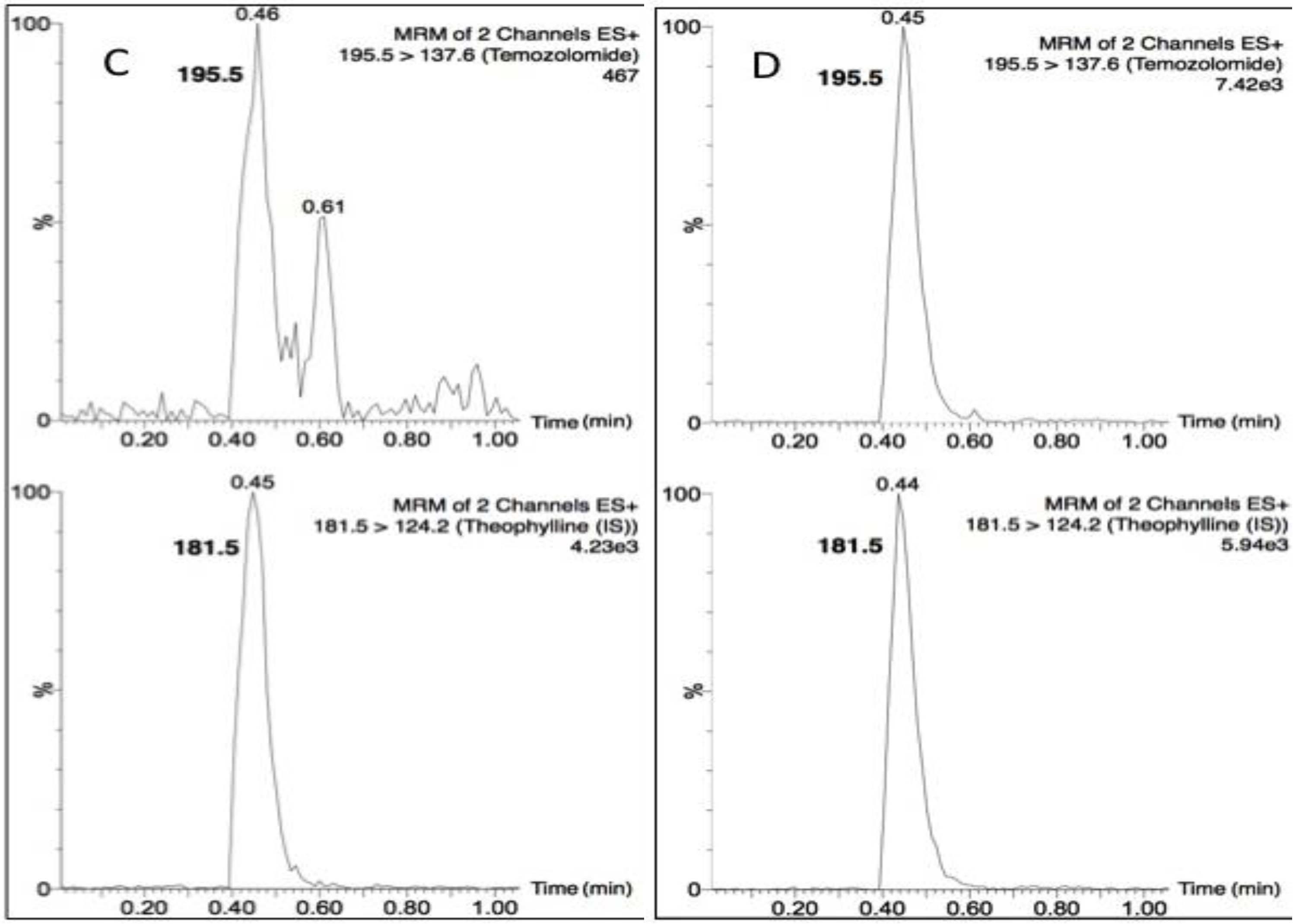
Temozolomide and theophylline (Figure 1) eluted at the same retention time of 0.45 min, however due to their differing precursor and product ion masses, this LC-MS/MS assay was able to identify each compound based on its fragmentation. Figure 2 depicts the chromatograms for drug-free plasma extract (A), with internal standard (B) showing no cross-talk to the analyte transition, the LLOQ of 5 ng/mL (C) and a study sample (D). There was a minor plasma contaminant that eluted near (0.6 min) the analyte retention time, but did not interfere with peak area estimation or quantification.
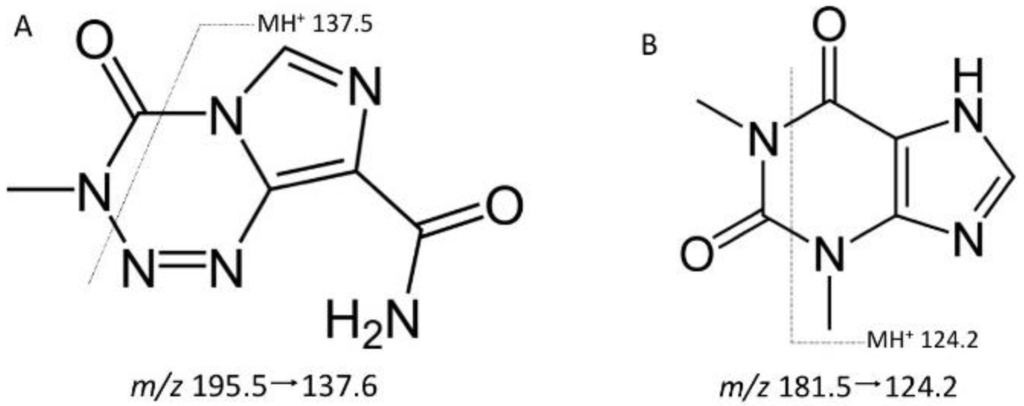
Figure 1.
Structures of temozolomide (TMZ) (A) and the internal standard theophylline (B), with proposed mass spectral fragmentation patterns.
Figure 1.
Structures of temozolomide (TMZ) (A) and the internal standard theophylline (B), with proposed mass spectral fragmentation patterns.
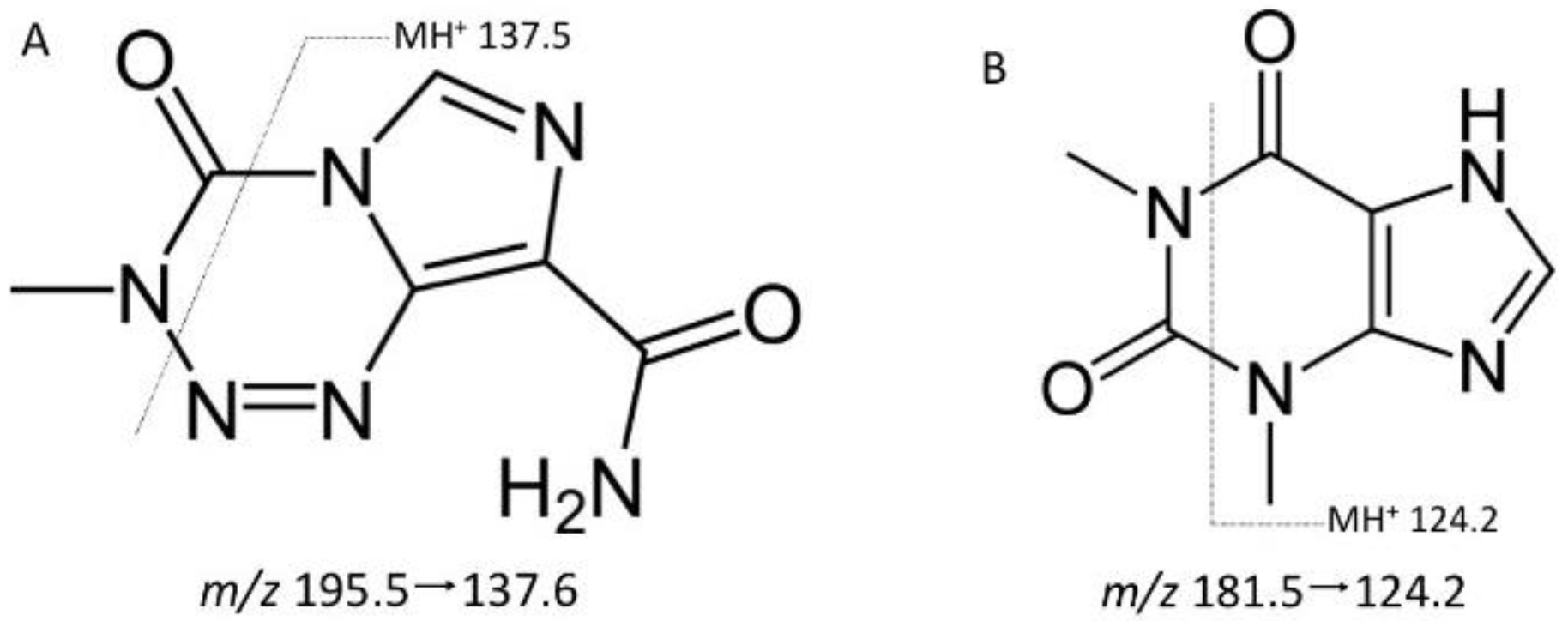
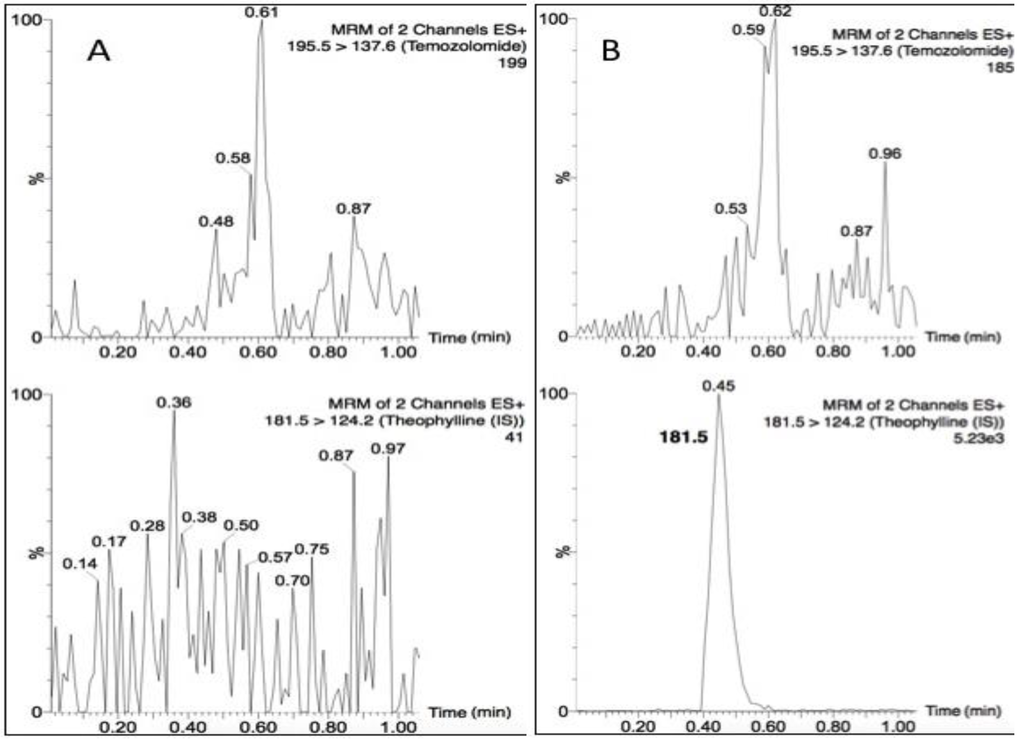
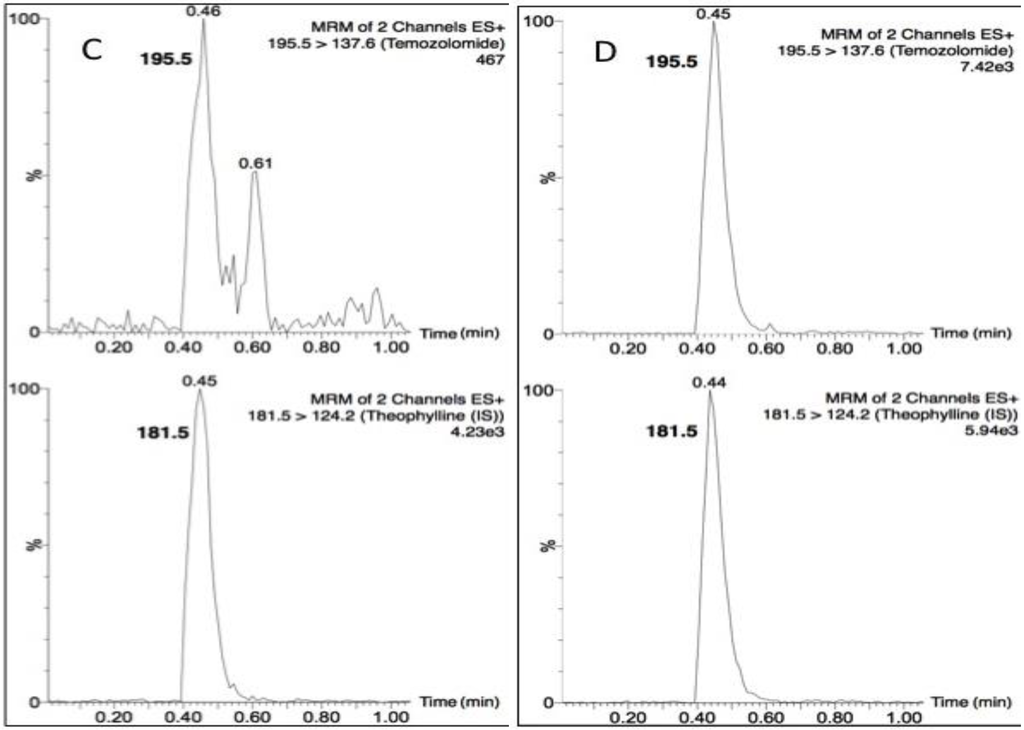
Figure 2.
Chromatograms of (A) a blank plasma extract; (B) internal standard only extract; (C) the lower limit of quantification (LLOQ); and (D) a single plasma sample from a nonhuman primate (NHP) measured 15 min following a 20 mg dose.
Figure 2.
Chromatograms of (A) a blank plasma extract; (B) internal standard only extract; (C) the lower limit of quantification (LLOQ); and (D) a single plasma sample from a nonhuman primate (NHP) measured 15 min following a 20 mg dose.
3.2. Validation
The calibration standards, ranging from 5–2000 ng/mL, were prepared in duplicate on each of four days (n = 8), except in CSF (three days, n = 6). The eight calibration standards proved accurate and precise in each matrix with a mean (±SD) calibration curve linear correlation (r2) of 0.9984 ± 0.0006 (n = 4) in plasma, 0.9975 ± 0.0007 (n = 3) in CSF, and 0.9949 ± 0.0019 (n = 4) in ECF (Figure 3).
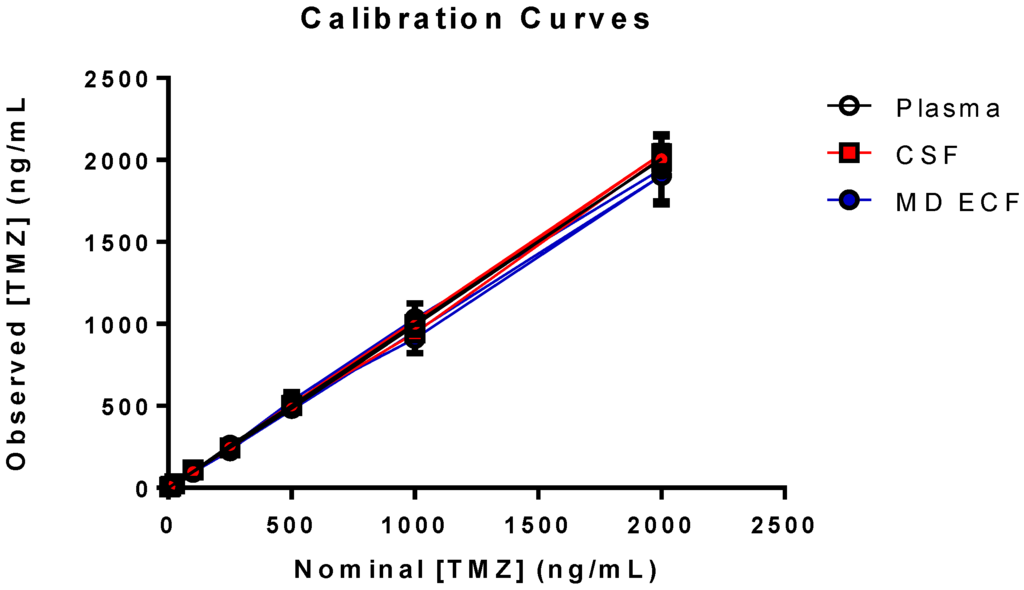
Figure 3.
Calibration curves in NHP plasma, cerebrospinal fluid (CSF), and microdialysis extracellular fluid over the range of 5–2000 ng/mL.
Figure 3.
Calibration curves in NHP plasma, cerebrospinal fluid (CSF), and microdialysis extracellular fluid over the range of 5–2000 ng/mL.
Four quality control (QC) samples, at low (15 ng/mL), mid (800 ng/mL), high (1600 ng/mL), and 50-fold diluted (50,000 ng/mL diluted to 1000 ng/mL) concentrations, were run in quintuplet daily over four days (n = 20) in plasma and ECF, three days (n = 15) in CSF. All QC levels proved accurate and precise, within the required ≤15% (Table 1, Table 2 and Table 3).

Table 1.
Accuracy and Precision in Nonhuman Primate Plasma.
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | Accuracy (%) | WRP (%) | BRP (%) | n |
|---|---|---|---|---|---|---|
| 15 | 15.5 | 2.9 | 103 | 13.7 | 14.7 | 20 |
| 800 | 795 | 45.2 | 99.4 | 5.2 | 2.6 | 20 |
| 1600 | 1593 | 76.3 | 99.5 | 4.5 | 1.9 | 20 |
| 50,000 | 50,325 | 5,150 | 101. | 7.0 | 8.4 | 20 |

Table 2.
Accuracy and Precision in Nonhuman Primate cerebrospinal fluid (CSF).
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | Accuracy (%) | WRP (%) | BRP (%) | n |
|---|---|---|---|---|---|---|
| 15 | 13.3 | 1.4 | 88.6 | 10.0 | 3.1 | 15 |
| 800 | 809 | 54.4 | 101 | 4.2 | 6.2 | 15 |
| 1600 | 1583 | 102 | 99 | 6.2 | 1.8 | 15 |
| 50,000 | 51,580 | 5373 | 96.8 | 9.6 | 4.8 | 15 |

Table 3.
Accuracy and Precision in Nonhuman Primate microdialysis (MD) Perfusion Fluid.
| Nominal (ng/mL) | GM (ng/mL) | SD (ng/mL) | Accuracy (%) | WRP (%) | BRP (%) | n |
|---|---|---|---|---|---|---|
| 15 | 15.1 | 1.8 | 101 | 8.0 | 8.6 | 20 |
| 800 | 8109 | 59.4 | 101 | 6.8 | 2.3 | 20 |
| 1600 | 1646 | 149 | 103 | 8.7 | 3.9 | 20 |
| 5000 | 5289 | 331 | 106 | 4.9 | 4.2 | 20 |
Note: GM, grand mean; SD, standard deviation; DEV (%) relative deviation from nominal value; WRP, within-run precision; n, number of replicate observations within each validation run.
3.3. Stability
Temozolomide was stable through at least two freeze/thaw cycles in NHP CSF and three cycles in plasma, where changes in measured values at each of the three concentrations after each freeze/thaw cycle were below 5% in plasma and 11% in CSF, indicating no significant degradation. The 24-h post-preparative stability measurements were consistent with the initial run (<10% change), allowing samples to be reanalyzed on the following day when necessary without significant degradation.
3.4. Extraction Recovery and Matrix Effects
The extraction recoveries for the low (25 ng/mL) and high (1000 ng/mL) concentrations were 88.6% and 89.4%, respectively. Matrix effects induced a 47% decrease in signal due to ion suppression. These data suggest the ethyl acetate liquid extraction is adequate for sample cleanup for sensitive, quantitative measurements.
3.5. Clinical Application
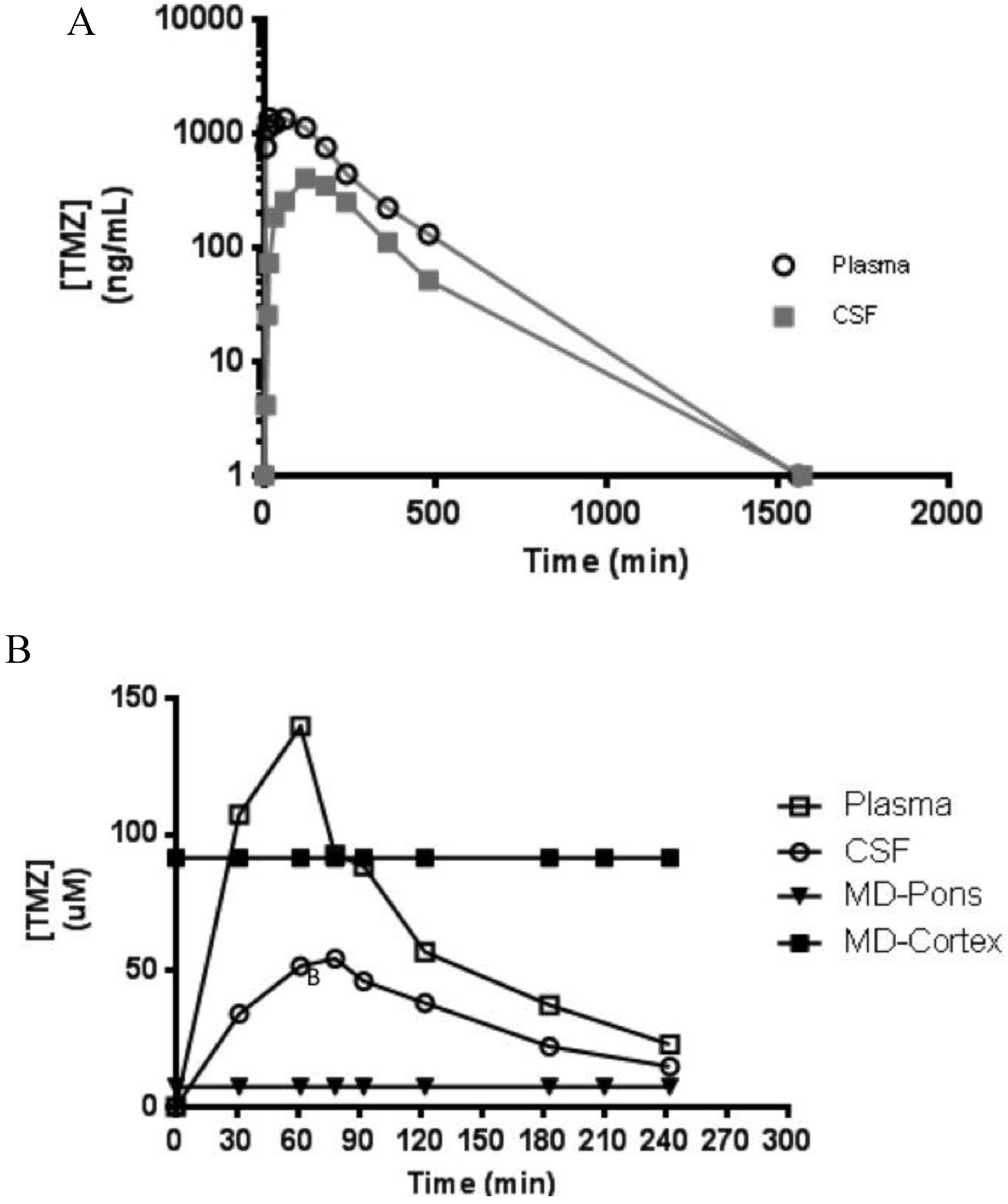
Following a single intranasal bolus delivery of 20 mg in a 1 mL solution (~1.75 mg/kg), both plasma and CSF concentrations of TMZ decreased in a mono-exponential manner over a 24-h sampling period, with CSF penetrance of approximately 40% in this individual specimen (Figure 4A). Additionally, this assay was capable of measuring TMZ from in situ microdialysate in the pons and cortex ECF following a 1-h intravenous infusion (10 mg/kg) (Figure 4B).
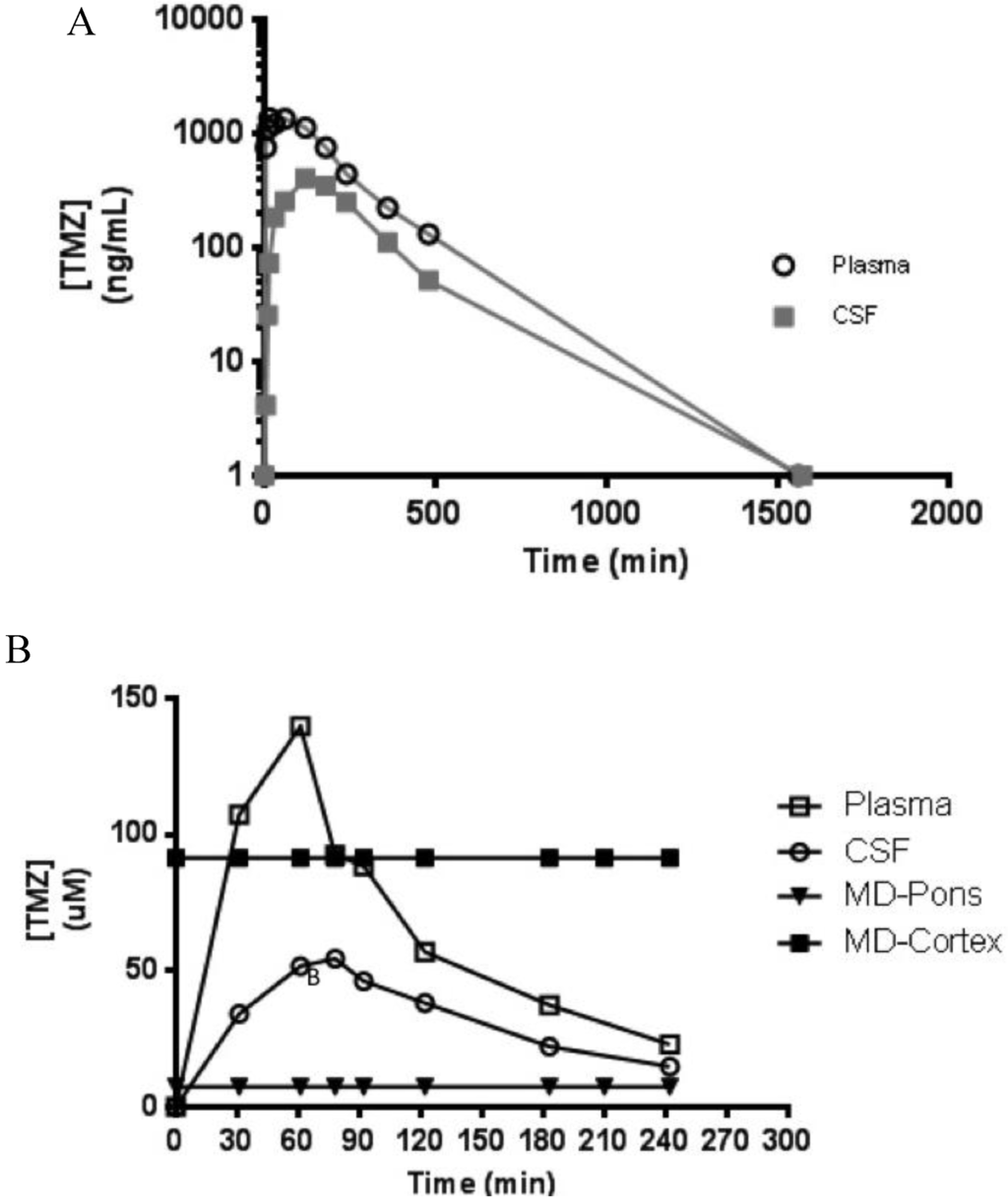
Figure 4.
TMZ plasma and cerebrospinal fluid (CSF) concentration vs time curve from a single NHP dosed with 20 mg intranasally with 24 h sampling (A); and TMZ plasma, CSF, and microdialysis (MD) sampling of extracellular fluid (ECF) from the cortex (over a 3.5 h sampling period) and pons (4-h sampling period) following a 1-h continuous intravenous infusion of 10 mg/kg (B).
Figure 4.
TMZ plasma and cerebrospinal fluid (CSF) concentration vs time curve from a single NHP dosed with 20 mg intranasally with 24 h sampling (A); and TMZ plasma, CSF, and microdialysis (MD) sampling of extracellular fluid (ECF) from the cortex (over a 3.5 h sampling period) and pons (4-h sampling period) following a 1-h continuous intravenous infusion of 10 mg/kg (B).
4. Conclusions
This paper describes an ultra HPLC-MS/MS method designed for the quantitative measurement of TMZ in nonhuman primate plasma, CSF, and ECF taken directly from designated areas in the brain inserted with MD probes. This method proved sensitive (LLOQ 5 ng/mL), selective, accurate, precise, and efficient at recovering drug from NHP plasma (~90% recovery). TMZ was found to be stable through multiple freeze/thaw cycles in both NHP plasma and CSF and post-preparation after 24-h. Ultimately, this concentration data can be used to describe the PK in each matrix, as well as brain penetration analysis and direct drug brain concentration measurement in situ.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US Government. The views in this manuscript are those of the authors and may not necessarily reflect NIH policy. No official endorsement is intended nor should be inferred.
Acknowledgments
The authors would like to acknowledge the contributions of John Bacher and Marvin T. Thomas (Division of Research Services, NCI) for completing the MD surgical procedures, and Rafael Cruz (Pediatric Oncology Branch) for collecting and processing all animal samples. Funding for this work was supported through the Intramural Research Program within the NCI.
Author Contributions
Cody Peer and Lukas Ronner designed the assay; Cynthia McCully and Katherine Warren designed the animal experiments; Lukas Ronner, Louis Rodgers, and Cynthia McCully performed the experiments; Cody Peer, Lukas Ronner, and Louis Rodgers analyzed the data; Cody Peer, Louis Rodgers, and William D. Figg wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UHPLC | ultra-high performance liquid chromatography |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| QC | Quality control |
| LLOQ | Lower limit of quantification |
| NHP | nonhuman primates |
| CSF | cerebrospinal fluid |
| MD | microdialysis |
| PK | pharmacokinetics |
| ECF | extracellular fluid |
References
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.; Tsang, L.L.; Slack, J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [PubMed]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [PubMed]
- Newlands, E.S.; Stevens, M.F.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [PubMed]
- McCully, C.L.; Balis, F.M.; Bacher, J.; Phillips, J.; Poplack, D.G. A rhesus monkey model for continuous infusion of drugs into cerebrospinal fluid. Lab. Anim. Sci. 1990, 40, 520–525. [Google Scholar] [PubMed]
- Patel, M.; McCully, C.; Godwin, K.; Balis, F.M. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J. Neurooncol. 2003, 61, 203–207. [Google Scholar] [PubMed]
- McCully, C.M.; Pastakia, D.; Bacher, J.; Thomas, M.L., 3rd; Steffen-Smith, E.A.; Saleem, K.; Murphy, R.F.; Walbridge, S.; Brinster, L.; Widemann, B.C.; et al. Model for concomitant microdialysis sampling of the pons and cerebral cortex in rhesus macaques (Macaca mulatta). Comp. Med. 2013, 63, 355–360. [Google Scholar] [PubMed]
- Grossman, R.; Rudek, M.A.; Brastianos, H.; Zadnik, P.; Brem, H.; Tyler, B.; Blakeley, J.O. The impact of bevacizumab on temozolomide concentrations in intracranial U87 gliomas. Cancer Chemother. Pharmacol. 2012, 70, 129–139. [Google Scholar] [PubMed]
- Grossman, R.; Tyler, B.; Rudek, M.A.; Kim, E.; Zadnik, P.; Khan, U.; Blakeley, J.O.; Pathak, A.P.; Brem, H. Microdialysis measurement of intratumoral temozolomide concentration after cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, in a U87 glioma model. Cancer Chemother. Pharmacol. 2013, 72, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Meany, H.J.; Warren, K.E.; Fox, E.; Cole, D.E.; Aikin, A.A.; Balis, F.M. Pharmacokinetics of temozolomide administered in combination with O6-benzylguanine in children and adolescents with refractory solid tumors. Cancer Chemother. Pharmacol. 2009, 65, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Goldwirt, L.; Zahr, N.; Farinotti, R.; Fernandez, C. Development of a new UPLC-MSMS method for the determination of temozolomide in mice: Application to plasma pharmacokinetics and brain distribution study. Biomed. Chromatogr. 2013, 27, 889–893. [Google Scholar] [PubMed]
- Chowdhury, S.K.; Laudicina, D.; Blumenkrantz, N.; Wirth, M.; Alton, K.B. An LC/MS/MS method for the quantitation of MTIC (5-(3-N-methyltriazen-1-yl)-imidazole-4-carboxamide), a bioconversion product of temozolomide, in rat and dog plasma. J. Pharm. Biomed. Anal. 1999, 19, 659–668. [Google Scholar] [PubMed]
- Reyderman, L.; Statkevich, P.; Thonoor, C.M.; Patrick, J.; Batra, V.K.; Wirth, M. Disposition and pharmacokinetics of temozolomide in rat. Xenobiotica 2004, 34, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Athawale, R.; Bajaj, A.; Shrikhande, S. Double-salting out assisted liquid-liquid extraction (SALLE) HPLC method for estimation of temozolomide from biological samples. J. Chromatogr. B 2014, 970, 86–94. [Google Scholar]
- Gilant, E.; Kaza, M.; Szlagowska, A.; Serafin-Byczak, K.; Rudzki, P.J. Validated HPLC method for determination of temozolomide in human plasma. Acta Pol. Pharm. 2012, 69, 1347–1355. [Google Scholar] [PubMed]
- Panetta, J.C.; Kirstein, M.N.; Gajjar, A.; Nair, G.; Fouladi, M.; Heideman, R.L.; Wilkinson, M.; Stewart, C.F. Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer Chemother. Pharmacol. 2003, 52, 435–441. [Google Scholar] [PubMed]
- Kim, H.; Likhari, P.; Parker, D.; Statkevich, P.; Marco, A.; Lin, C.C.; Nomeir, A.A. High-performance liquid chromatographic analysis and stability of anti-tumor agent temozolomide in human plasma. J. Pharm. Biomed. Anal. 2001, 24, 461–468. [Google Scholar] [PubMed]
- Shen, F.; Decosterd, L.A.; Gander, M.; Leyvraz, S.; Biollaz, J.; Lejeuneb, F. Determination of temozolomide in human plasma and urine by high-performance liquid chromatography after solid-phase extraction. J. Chromatogr. B 1995, 667, 291–300. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2015.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
