Electrochemistry–Mass Spectrometry for Generation and Identification of Metabolites of Selected Drugs from Different Therapeutic Groups in Comparison with In Vitro and In Vivo Approaches
Abstract
1. Introduction
2. Experimental
2.1. Apparatus
2.2. Reagents
2.3. In Silico Metabolite Prediction
2.4. In Vitro Studies Using Liver Microsomal Fraction Enzymes
2.5. EC-LC-MS/MS Experiments
2.6. Real Samples from Patients
2.7. Microextraction in Packed Syringe Procedure
2.8. Method Validation
3. Results and Discussion
3.1. Selection of Mass Spectrometer Operating Parameters
3.2. Application of the Central Composition Plan
3.3. In Silico Prediction of Metabolism
3.4. Electrochemical Simulation of the Metabolism of Selected Drugs
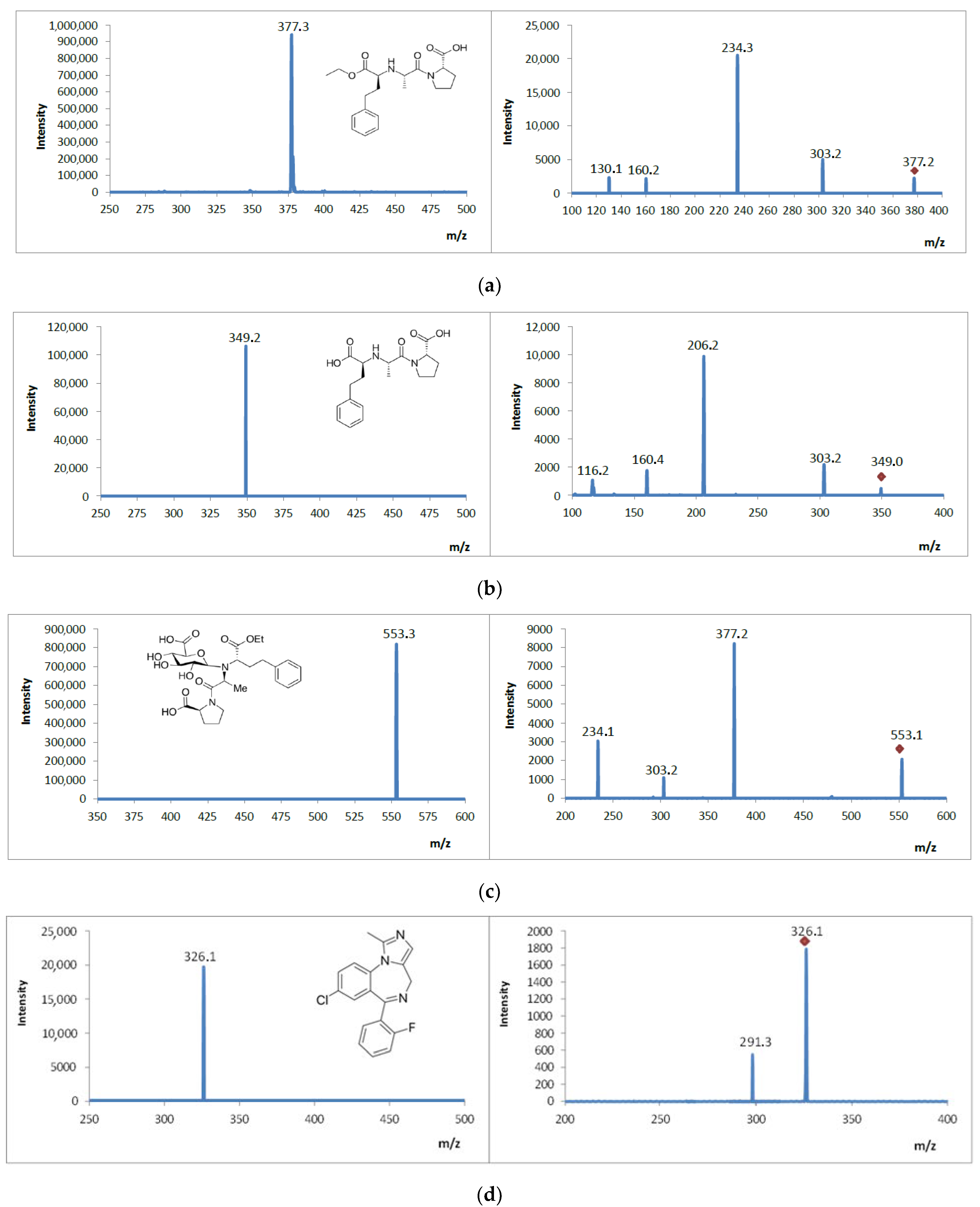
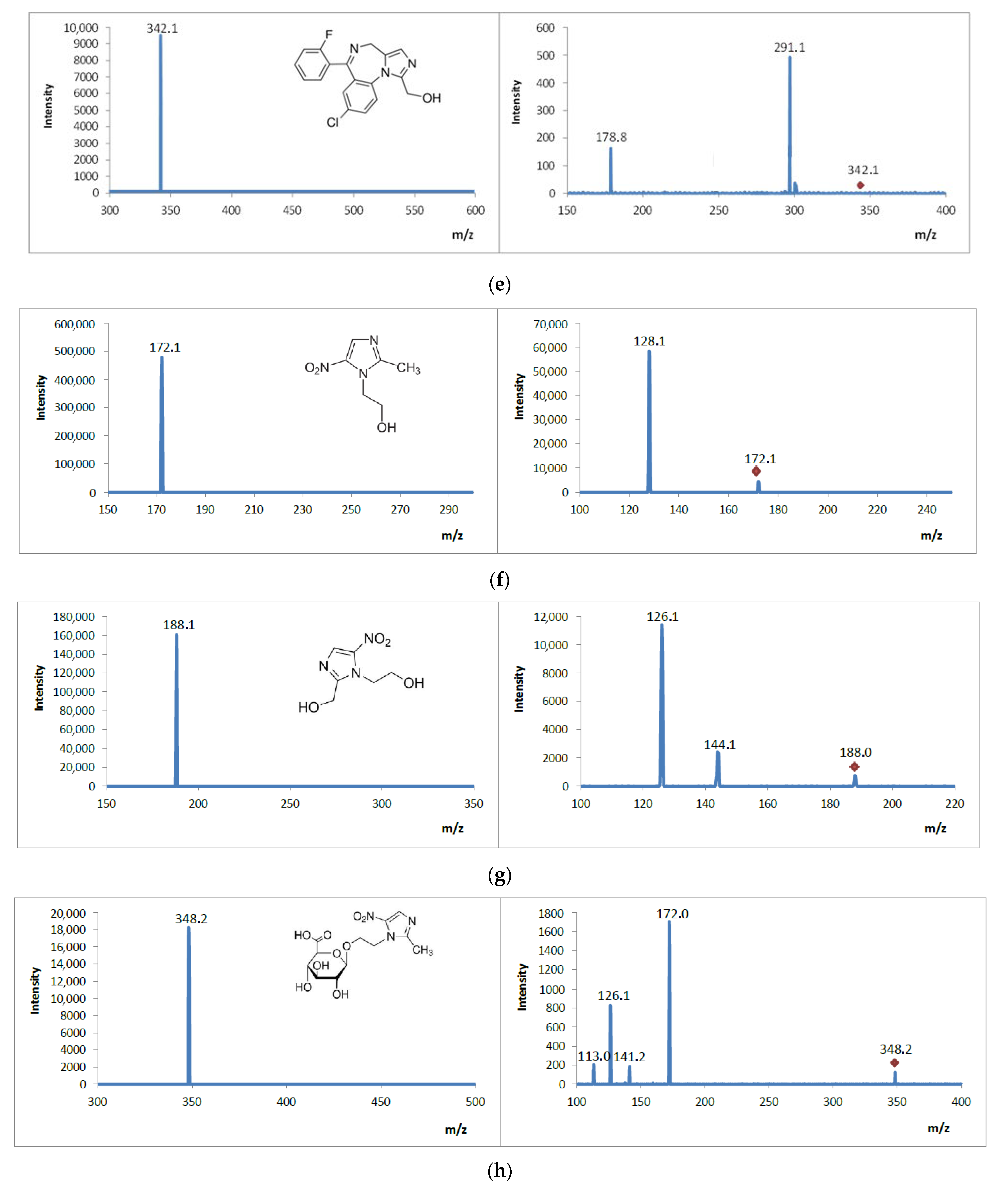
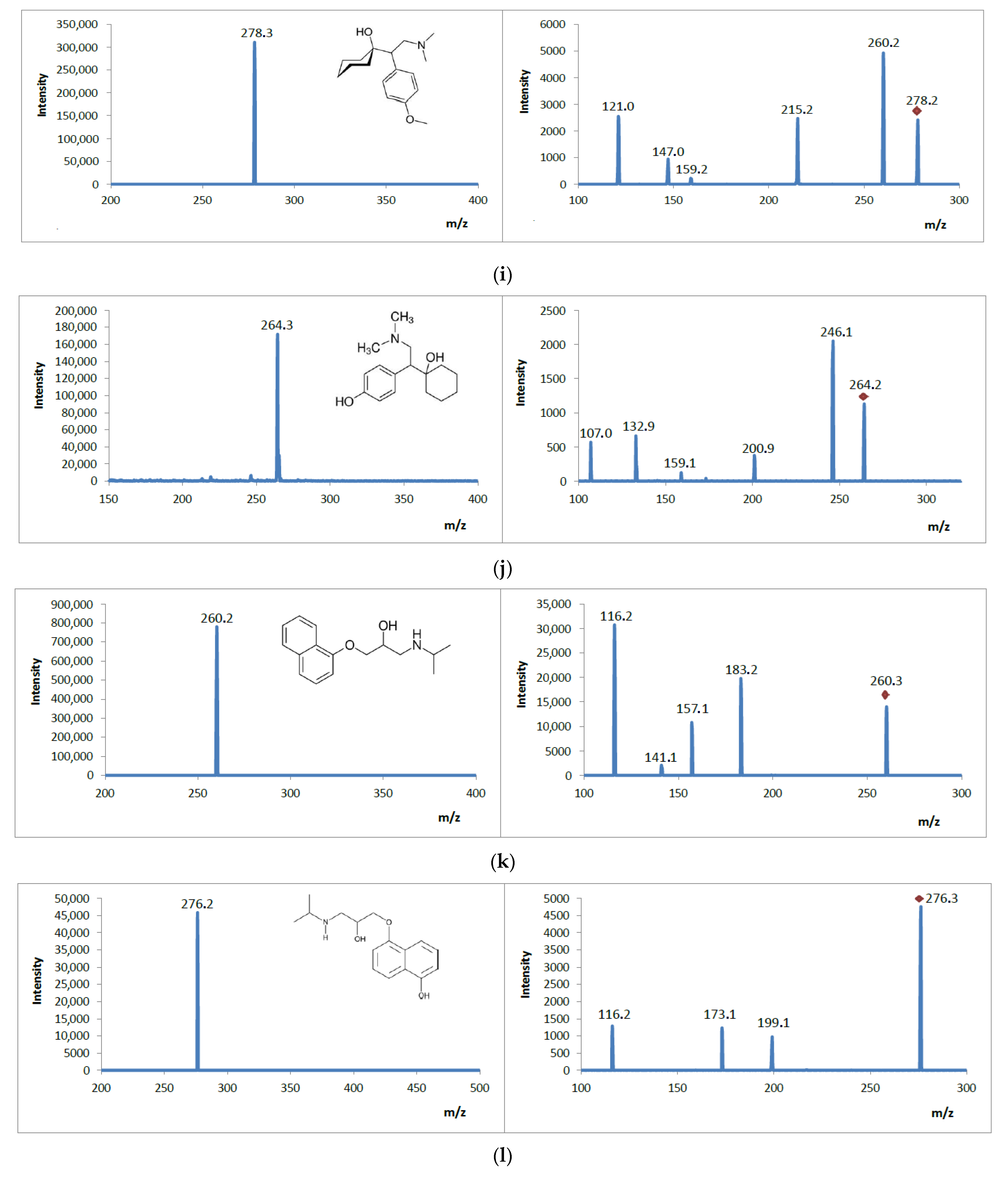
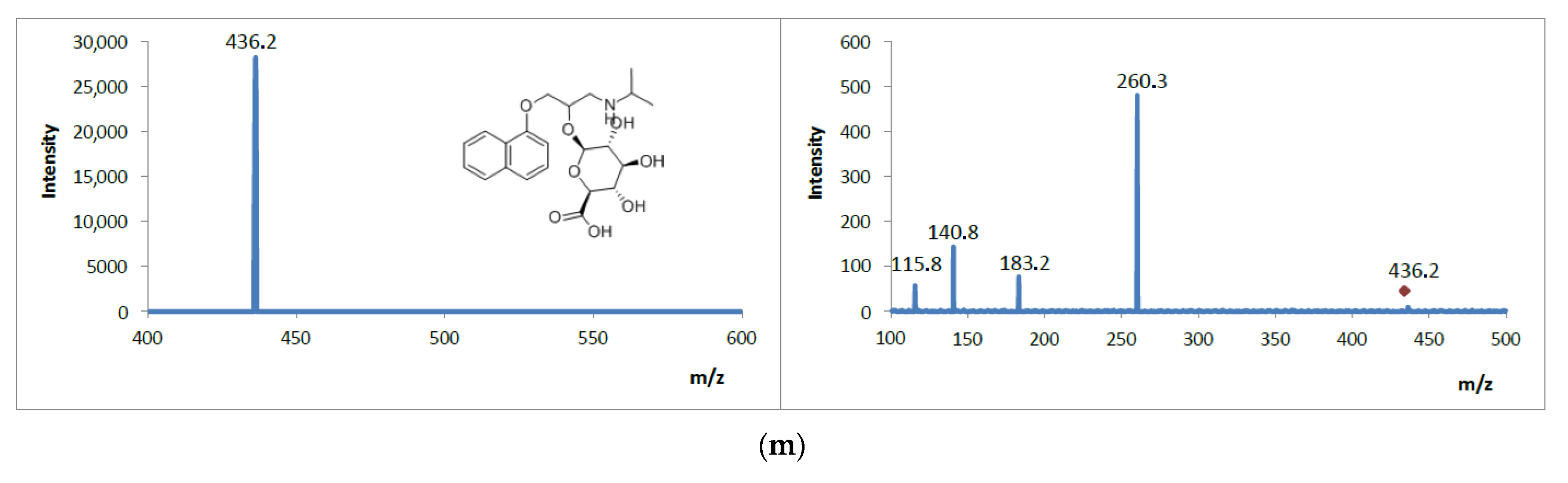
3.5. Analysis of Mass Spectrometric Fragmentation Patterns of Selected Drugs and Their Pharmacologically Active Metabolites for EC/ESI-MS/MS Experiments
3.6. Comparison of the Obtained Results
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bussy, U.; Boisseau, R.; Thobie-Gautier, C.; Boujtita, M. Electrochemistry-mass spectrometry to study reactive drug metabolites and CYP450 simulations. Trends Anal. Chem. 2015, 70, 67–73. [Google Scholar] [CrossRef]
- Karst, U. Electrochemistry/Mass Spectrometry (EC/MS)-A New Tool To Study Drug Metabolism and Reaction Mechanisms. Angew. Chem. 2004, 43, 2476–2478. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, W.; Baumann, A.; Karst, U. Electrochemistry and LC-MS for Metabolite Generation and Identification: Tools, Technologies and Trends. LC-GC N. Am. 2010, 28, 470–478. [Google Scholar]
- Permentier, H.P.; Bruins, A.P.; Bischoff, R. Electrochemistry-mass spectrometry in drug metabolism and protein research. Mini-Rev. Med. Chem. 2008, 8, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chipiso, K.; Simoyi, R.H. Electrochemistry-coupled to mass spectrometry in simulation of metabolic oxidation of methimazole: Identification and characterization of metabolites. J. Electroanal. Chem. 2016, 761, 131–140. [Google Scholar] [CrossRef]
- Tabacovaa, S.A.; Kimmelb, C.A. Enalapril: Pharmacokinetic/dynamic inferences for comparative developmental toxicity. A review. Reprod. Toxic. 2001, 15, 467–478. [Google Scholar]
- Gu, Q.; Chen, X.; Zhong, D.; Wang, Y. Simultaneous determination of enalapril and enalaprilat in human plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2004, 813, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lamp, K.; Lacy, M.K.; Freeman, C. Metronidazole, Clindamycin and Streptogramin Pharmacodynamics. In Antimicrobial Pharmacodynamics in Theory and Clinical Practice; CRC Press: Boca Raton, FL, USA, 2001; pp. 221–227. [Google Scholar] [CrossRef]
- Kathriarachchi, U.L. Development of a LC-MS method for simultaneous determination of amoxicillin and metronidazole in human serum using hydrophilic interaction chromatography (HILIC). J. Chromatogr. B 2018, 1089, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Marquet, P.; Baudin, O.; Gaulier, J.M.; Lacassie, E.; Dupuy, J.L.; Francois, B.; Lachatre, G. Sensitive and specific determination of midazolam and 1-hydroxymidazolam in human serum by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B 1999, 734, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Svanström, C.; Hansson, G.P.; Svensson, L.D.; Sennbro, C.J. Development and validation of a method using supported liquid extraction for the simultaneous determination of midazolam and 1-hydroxy-midazolam in human plasma by liquid chromatography with tandem mass spectrometry detection. J. Pharm. Biomed. Anal. 2012, 58, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Trobeca, K.C.; Trontelj, J.; Springer, J.; Lainscak, M.; Kos, M.K. Liquid chromatography–tandem mass spectrometry method for simultaneous quantification of bisoprolol, ramiprilat, propranolol and midazolam in rat dried blood spots. J. Chromatogr. B 2014, 958, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Steenen, S.A.; van Wijk, A.J.; van der Heijden, G.; van Westrhenen, R.; de Lande, J.; de Jongh, A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J. Psychopharm. 2016, 30, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Barman Balfour, J.A.; Jarvis, B. Venlafaxine Extended-Release. CNS Drugs 2000, 14, 483–503. [Google Scholar] [CrossRef]
- Bhatt, J.; Jangid, A.; Venkatesh, G.; Subbaiah, G.; Singh, S. Liquid chromatography–tandem mass spectrometry (LC–MS–MS) method for simultaneous determination of venlafaxine and its active metabolite O-desmethyl venlafaxine in human plasma. J. Chromatogr. B 2005, 829, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gu, G. Validation of an LC-MS/MS method for simultaneous quantification of venlafaxine and its five metabolites in rat plasma and its application in a pharmacokinetic study. J. Chromatogr. B 2018, 1087–1088, 29–35. [Google Scholar] [CrossRef] [PubMed]
- King, R.S. Biotransformations in Drug Metabolism. In Drug Metabolism Handbook: Concepts and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 17–21. [Google Scholar]
- Oellerich, M.; Armstrong, V.W. Prodrug Metabolites: Implications for Therapeutic Drug Monitoring. Clin. Chem. 2001, 47, 805–806. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn Kops, C.; Šícho, M.; Mazzolari, A.; Kirchmair, J. GLORYx: Prediction of the Metabolites Resulting from Phase 1 and Phase 2 Biotransformations of Xenobiotics. Chem. Res. Toxicol. 2021, 34, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou-Feunang, Y.; Fiamoncini, J.; Gil-de-la-Fuente, A.; Greiner, R.; Manach, C.; Wishart, D.S. BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification. J. Cheminform. 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Structural Formula  | Molecular Formula | Molar Mass [g/mol] | Application |
|---|---|---|---|---|
| Enalapril | 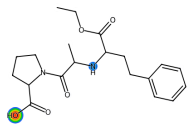 | C20H28N2O5 | 376.45 | angiotensin converting enzyme inhibitor |
| Enalaprilat (pharmacologically active metabolite) | C18H24N2O5 | 348.40 | angiotensin converting enzyme inhibitor | |
| Enalapril glucuronide (metabolite) | C26H36N2O11 | 552.58 | angiotensin converting enzyme inhibitor | |
| Metronidazole | 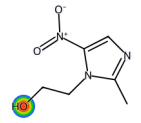 | C6H9N3O3 | 171.16 | antibiotic |
| Hydroxymetronidazole (pharmacologically active metabolite) | C6H9N3O4 | 187.15 | antibiotic | |
| Metronidazole glucuronide (metabolite) | C12H17N3O9 | 347.28 | antibiotic | |
| Midazolam | 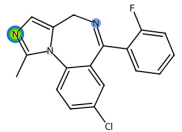 | C18H13ClFN3 | 325.78 | anesthetic |
| Hydroxymidazolam (pharmacologically active metabolite) | C18H13ClFN3O | 341.77 | anesthetic | |
| Propranolol |  | C16H21NO2 | 259.34 | β-blocker |
| Hydroxypropranol (pharmacologically active metabolite) | C16H21NO3 | 275.35 | β-blocker | |
| Propranolol glucuronide (metabolite) | C22H29NO8 | 435.47 | β-blocker | |
| Venlafaxine | 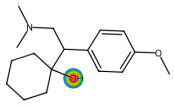 | C17H27NO2 | 277.40 | antidepressant |
| Desmethylvenlafaxine (pharmacologically active metabolite) | C16H25NO2 | 263.38 | antidepressant |
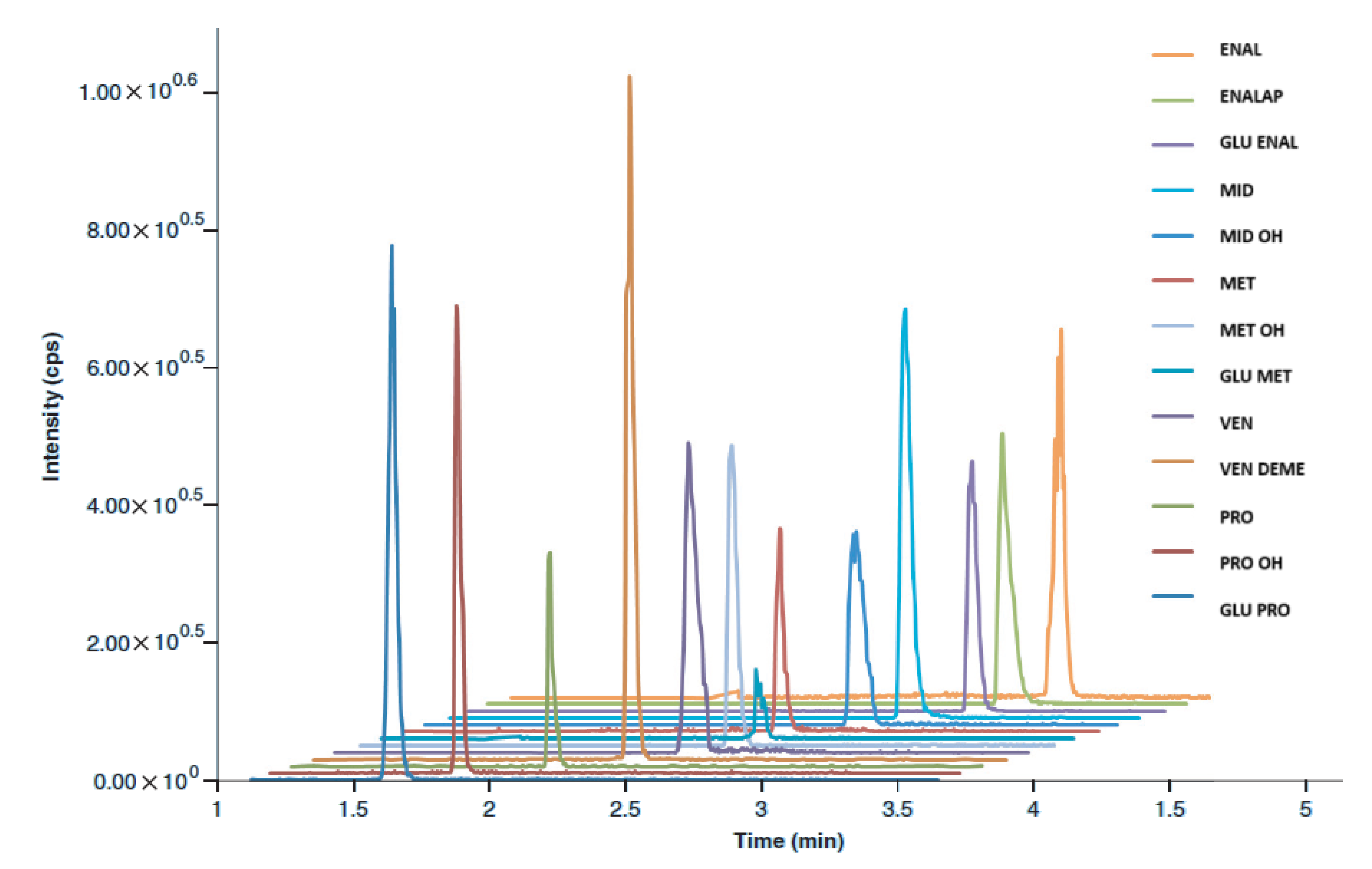
| Drug/Metabolite (Abbreviation) | Parent Ion [M+H]+ | Product Ion— Qualifier Q1 | Product Ion— Quantifier Q3 | Drying Gas Temperature [°C] | Fragmentor Voltage [V] |
|---|---|---|---|---|---|
| ENAL | 377 | 234 | 303 | 320 | 70 |
| ENALAP | 349 | 206 | 117 | 320 | 70 |
| GLU ENAL | 553 | 377 | 234 | 320 | 70 |
| MID | 326 | 248 | 291 | 320 | 110 |
| MID OH | 342 | 248 | 288 | 320 | 110 |
| MET | 172 | 128 | 111 | 320 | 70 |
| MET OH | 188 | 126 | 144 | 320 | 70 |
| GLU MET | 348 | 172 | 126 | 320 | 70 |
| VEN | 278 | 260 | 121 | 290 | 110 |
| VEN DEME | 264 | 246 | 107 | 290 | 110 |
| PRO | 260 | 116 | 183 | 290 | 150 |
| PRO OH | 276 | 116 | 199 | 290 | 150 |
| GLU PRO | 436 | 260 | 116 | 290 | 150 |
| Input Variables | Levels | ||
|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |
| (X1) Fragmentor voltage [V] | 70 | 110 | 150 |
| (X2) Drying gas temperature [°C] | 290 | 320 | 350 |
| Experiment number | X1 | X2 | |
| 5 | 290 (−1) | 110 (+1) | |
| 1 | 350 (+1) | 110 (−1) | |
| 4 | 320 (0) | 110 (0) | |
| 8 | 290 (0) | 150 (+1) | |
| 3 | 290 (0) | 70 (−1) | |
| 7 | 350 (−1) | 150 (−1) | |
| 9 | 320 (−1) | 150 (0) | |
| 2 | 320 (+1) | 70 (0) | |
| 6 | 350 (+1) | 70 (+1) | |
| Compound | Ion ID | Experimental m/z | Chemical Formula |
|---|---|---|---|
| Enalapril Product ions | D1 | 377 | C20H29N2O5 |
| P1 | 303 | C17H26N2O3 | |
| P2 | 234 | C13H16NO3 | |
| P3 | 160 | C7H14NO3 | |
| P4 | 130 | C5H8NO3 | |
| Enalaprilat Product ions | M1 | 349 | C18H25N2O5 |
| M1P1 | 303 | C17H26N2O3 | |
| M1P2 | 206 | C11H12NO3 | |
| M1P3 | 160 | C7H14NO3 | |
| M1P4 | 116 | C4H4NO3 | |
| Enalapril glucuronide Product ions | M2 | 553 | C26H36N2O11 |
| M2P1 | 377 | C20H29N2O5 | |
| M2P2 | 303 | C17H26N2O3 | |
| M2P3 | 234 | C13H16NO3 | |
| Midazolam Product ions | D2 | 326 | C18H14ClFN3 |
| P1 | 291 | C18H14FN3 | |
| Hydroxymidazolam Product ions | M1 | 342 | C18H14ClFN3O |
| M1P1 | 291 | C18H14FN3 | |
| M1P2 | 178 | C11H18N2 | |
| Metronidazole Product ions | D3 | 172 | C6H10N3O3 |
| P1 | 128 | C4H6N3O2 | |
| Hydroxymetronidazole Product ions | M1 | 188 | C6H10N3O4 |
| M1P1 | 144 | C4H6N3O3 | |
| M1P2 | 126 | C4H4N3O2 | |
| Metronidazole glucuronide Product ions | M2 | 348 | C12H18N3O9 |
| M2P1 | 172 | C6H10N3O3 | |
| M2P2 | 141 | C4H3N3O3 | |
| M2P3 | 126 | C4H4N3O2 | |
| M2P4 | 113 | C3H3N3O2 | |
| Venlafaxine Product ions | D4 | 278 | C17H28NO2 |
| P1 | 260 | C17H26NO | |
| P2 | 215 | C15H19O | |
| P3 | 159 | C11H11O | |
| P4 | 147 | C10H11O | |
| P5 | 121 | C8H7O | |
| Desmethylvenlafaxine Product ions | M1 | 264 | C16H26NO2 |
| M1P1 | 246 | C16H24NO | |
| M1P2 | 201 | C14H16O | |
| M1P3 | 159 | C10H11O | |
| M1P4 | 133 | C9H9O | |
| M1P4 | 107 | C7H7O | |
| Propranolol Product ions | D5 | 260 | C16H22NO2 |
| P1 | 183 | C13H11O | |
| P2 | 157 | C11H9O | |
| P3 | 141 | C10H5O | |
| P4 | 116 | C8H4O | |
| Hydroxypropranolol Product ions | M1 | 276 | C16H22NO3 |
| M1P1 | 199 | C13H11O2 | |
| M1P2 | 173 | C11H9O2 | |
| M1P3 | 116 | C8H4O | |
| Propranolol glucuronide Product ions | M2 | 436 | C22H30NO8 |
| M2P1 | 260 | C16H22NO2 | |
| M2P2 | 183 | C13H11O | |
| M2P3 | 141 | C10H5O | |
| M2P4 | 116 | C8H4O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szultka-Młyńska, M. Electrochemistry–Mass Spectrometry for Generation and Identification of Metabolites of Selected Drugs from Different Therapeutic Groups in Comparison with In Vitro and In Vivo Approaches. Separations 2025, 12, 243. https://doi.org/10.3390/separations12090243
Szultka-Młyńska M. Electrochemistry–Mass Spectrometry for Generation and Identification of Metabolites of Selected Drugs from Different Therapeutic Groups in Comparison with In Vitro and In Vivo Approaches. Separations. 2025; 12(9):243. https://doi.org/10.3390/separations12090243
Chicago/Turabian StyleSzultka-Młyńska, Małgorzata. 2025. "Electrochemistry–Mass Spectrometry for Generation and Identification of Metabolites of Selected Drugs from Different Therapeutic Groups in Comparison with In Vitro and In Vivo Approaches" Separations 12, no. 9: 243. https://doi.org/10.3390/separations12090243
APA StyleSzultka-Młyńska, M. (2025). Electrochemistry–Mass Spectrometry for Generation and Identification of Metabolites of Selected Drugs from Different Therapeutic Groups in Comparison with In Vitro and In Vivo Approaches. Separations, 12(9), 243. https://doi.org/10.3390/separations12090243





