Abstract
The increasing presence of tetracycline antibiotics (TCs) in water sources poses significant environmental and public health risks, necessitating effective treatment technologies. This study investigates the degradation of three types of TCs in water—Tetracycline (TC), Oxytetracycline (OTC), and Chlortetracycline (CTC)—using nonthermal plasma (NTP) coupled with the persulfate (PS) process. The combined NTP/PS system was optimized for various operational parameters, including PS concentration, pH, and reaction time, to achieve maximum degradation and mineralization efficiency. The results showed that the NTP/PS system achieved over 90% degradation of all TCs under optimal conditions, outperforming plasma alone treatment. The degradation kinetics followed a pseudo-first-order model, indicating a rapid initial breakdown of TCs. The degradation mechanism was elucidated through the identification of intermediate byproducts using liquid chromatography-mass spectrometry (LC-MS/MS). Free radicals, such as sulfate (SO4•−) and hydroxyl (•OH) radicals, were identified as the primary reactive species responsible for TCs degradation. This study demonstrates the potential of the NTP/PS system as an efficient and sustainable solution for the removal of antibiotic contaminants from water. Further research on the scalability and application in real wastewater conditions is recommended.
1. Introduction
The widespread use of antibiotics in human and veterinary medicine and agriculture has led to their persistent presence in the environment [1]. Tetracyclines (TCs) are among the most commonly detected antibiotics in surface water, groundwater, and soil due to their extensive use and limited biodegradability [2,3]. Through their persistent nature and incomplete metabolism, TCs continue to enter the environment through wastewater treatment effluents and other anthropogenic activities, raising ecological and public health concerns [4]. These compounds exhibit high environmental stability, largely due to their complex molecular structures, which make them resistant to conventional biodegradation processes. The persistence of TCs in aquatic environments raises serious concerns, particularly regarding the promotion of antibiotic-resistant bacteria (ARB) and the dissemination of antibiotic resistance genes (ARGs) [5]. In addition to human health risks, TCs can disrupt aquatic ecosystems by altering microbial community structures, inhibiting photosynthetic organisms such as algae [6], and potentially inducing developmental toxicity in aquatic fauna [7]. Recent findings also highlight the phytotoxic effects of TCs on edible crops: a study by Chohra et al. (2025) demonstrated that higher concentrations of TC (25–50 mg/L) inhibited germination and growth in Kimchi cabbage, while lower concentrations (5–10 mg/L) induced a hormetic effect. The study further confirmed TC accumulation in plant tissues, suggesting potential risks to human health through the food chain and underscoring the urgent need for improved environmental control and remediation strategies [8].
Current treatment technologies for TCs removal from water systems primarily rely on conventional biological treatment, adsorption, and membrane filtration. However, these methods face significant limitations that hinder their widespread implementation. Biological treatment processes often prove ineffective due to the antibacterial nature of TCs, while adsorption methods merely transfer the contaminants to another phase without achieving complete degradation. Membrane filtration, although effective in removing TCs, generates concentrated waste streams requiring further treatment. Traditional Advanced Oxidation Processes (AOPs), such as UV/H2O2 and Fenton oxidation, often require significant energy input or chemical additives and may produce toxic transformation products [9,10].
Nonthermal plasma (NTP) has emerged as a promising technique that can produce a rich mixture of reactive species, including hydroxyl radicals (•OH), ozone (O3), hydrogen peroxide (H2O2), and other short-lived radicals, offering the potential to degrade a wide range of pollutants without added chemicals [11,12]. Recent studies have demonstrated that activating persulfate (PS) enhances contaminant removal efficiency by generating sulfate radicals (SO4•–), which are effective oxidants under various environmental conditions [13]. Persulfate activation through NTP has gained considerable attention in environmental remediation due to its ability to generate the powerful SO4•− along with other high-potential oxidants.
Despite the growing body of research on both NTP technology and PS activation, several critical knowledge gaps remain unaddressed. First, the mechanisms of PS activation by NTP-generated species are not fully understood. Second, the transformation pathways of TCs under combined NTP/PS treatment require detailed investigation to ensure the formation of non-toxic end products. Third, the potential synergistic effects between NTP-generated reactive species and activated PS have not been systematically evaluated.
The novelty of this research lies in the innovative coupling of NTP technology type gliding arc discharge with PS activation for TC, OTC, and CTC degradation in water. This combination potentially offers several advantages over conventional treatment methods, including enhanced oxidation and mineralization efficiency, reduced discharge time, and minimal chemical input. The study also addresses the crucial need for developing more effective and sustainable treatment technologies for emerging contaminants in water systems.
The primary objectives of this research are to: (1) investigate the effectiveness of NTP-/PS for TCs degradation in water; (2) elucidate the mechanisms of PS activation by NTP and subsequent TCs degradation pathways; (3) evaluate the influence of key operational parameters on treatment efficiency; and (4) assess the potential formation of transformation products and their estimated toxicity. The scope includes laboratory-scale experiments using a custom-designed NTP reactor, comprehensive analytical methods for monitoring TCs degradation and transformation products, and detailed mechanistic studies using various analytical and spectroscopic techniques. This research aims to provide valuable insights into the development of more efficient and environmentally friendly water treatment technologies for antibiotic removal.
2. Materials and Methods
2.1. Plasma Discharge Setup and Treatment Procedure
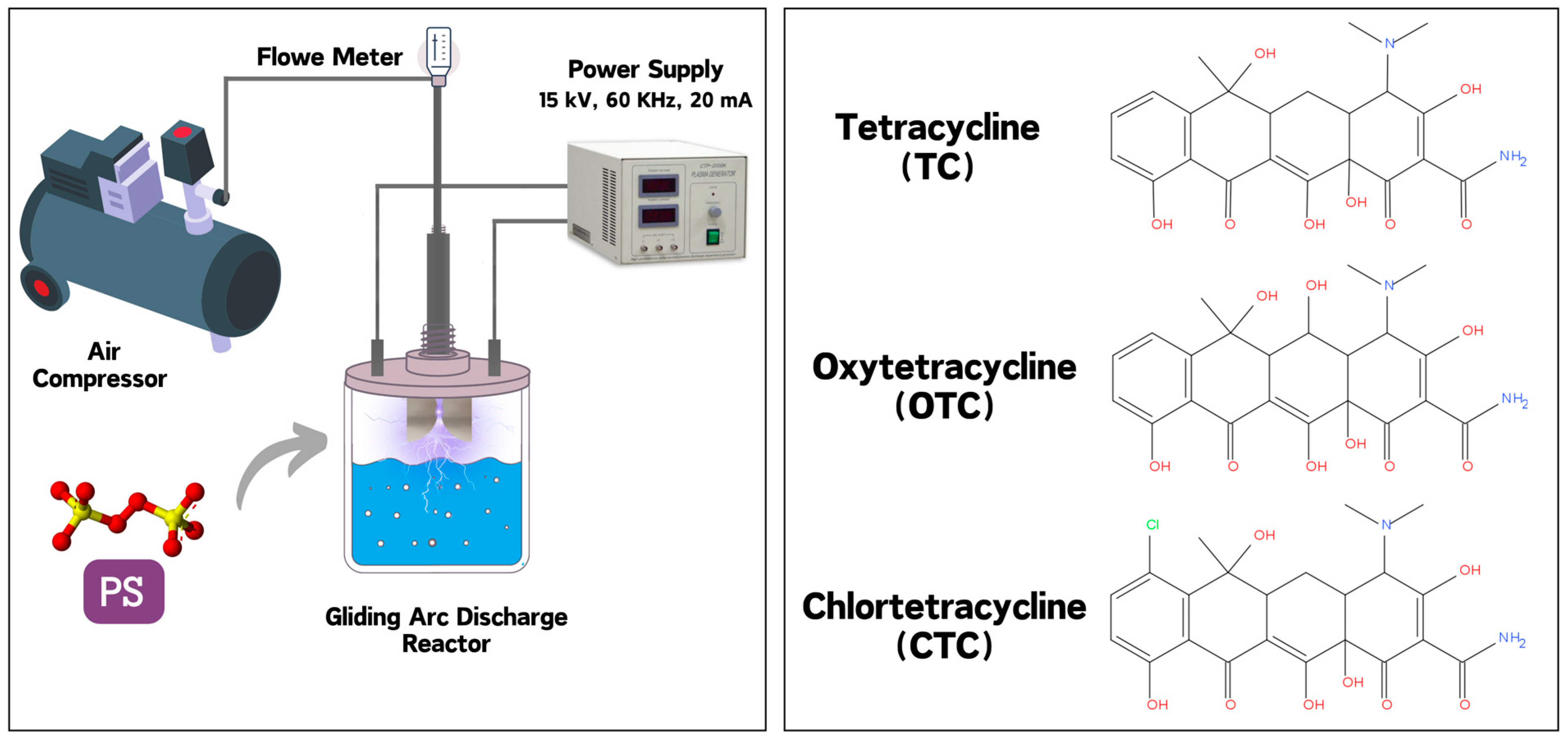
A gliding arc discharge reactor operating at atmospheric pressure was used for the plasma treatment. Air was employed as the working gas. The reactor consisted of two diverging electrodes with a gap of 3 mm at the inlet and 5 cm at the outlet, powered by a high-voltage AC power supply (15 kV). The plasma was generated by applying a voltage of 15 kV, creating a stable gliding arc that ionized the air to produce reactive species. For each experiment, 100 mL of solution at varying initial concentrations was treated. The solutions were continuously stirred during the plasma treatment to ensure uniform exposure. Samples were collected at regular intervals (0, 1, 5, 10, 20, 30, etc. min) for subsequent analysis. The reactor setup and the chemical structures of the target antibiotics are presented in Figure 1.

Figure 1.
Experimental setup and chemical structure of the compounds studied.
2.2. Plasma Persulfate Activation Process
In the combined plasma and activated persulfate NTP/PS treatment system, 100 mL of TCs solution was first subjected to plasma treatment for an optimized time period based on preliminary experiments.
The NTP/PS activation experiments were conducted using the same NTP reactor. Sodium persulfate (Na2S2O8) was added to the solution at a predetermined concentration to enhance radical generation. The solution was continuously stirred to ensure homogeneity. The reaction was carried out for different treatment times with a maximum of 30 min, and liquid samples were collected at regular intervals for analysis. The degradation of the target pollutant was monitored using high-performance liquid chromatography (HPLC), and the mineralization was assessed by a Total Organic Carbon Analyzer. Reactive oxygen species (ROS) and sulfate radical (SO4•−) generation were analyzed using different methods detailed in the Supplementary Files. Additional parameters, including solution pH and conductivity.
2.3. Analytical Methods
The concentrations and the degradation efficiency of TCs were monitored using a HPLC Shimadzu system with a diode array detector and a C18 column. Mobile phase consisted of acetonitrile (phase A) and water at 0.1 M phosphoric acid (phase B), with a flow rate of 1 mL/min and injection volume of 20 μL. The degradation efficiency (η) was calculated using Equation (1):
where C0 is the initial concentration of TCs, and Ct is the concentration (mg/L) at time t.
The mineralization efficiency was evaluated using a Shimadzu Total Organic Carbon (TOC) Analyzer (TOC-L series). The reduction in TOC was used to assess the extent of mineralization, calculated by Equation (2):
where TOC0 is the initial TOC concentration, and TOCt is the TOC concentration at time (t).
These analytical techniques provided comprehensive insights into the efficiency of NTP alone and NTP/PS treatments in removing and mineralizing TCs from aqueous solutions.
The evolution of pH and conductivity throughout the plasma and the NTP/PS treatment is also reported. Although the initial pH of each solution was adjusted using either 1 M NaOH or 1 M HCl, it was observed that the plasma discharge consistently caused a rapid drop in pH within the first few minutes of treatment. As a result, the initial pH conditions could not be maintained throughout the process. Therefore, the results of pH variation experiments are not included in this study.
Quantification of reactive species generated during the plasma and NTP/PS processes was conducted to better understand the underlying degradation mechanisms. The concentrations of ozone (O3), hydrogen peroxide (H2O2), nitrate (NO3−), nitrite (NO2−), and hydroxyl radicals (•OH) were measured using established analytical protocols. Detailed descriptions of these measurement methods are available in Supplementary Materials Text S2. Materials and reagents used in this study are presented in detail in Supplementary Materials Text S1.
3. Results
3.1. Degradation Efficiency and Kinetic Study
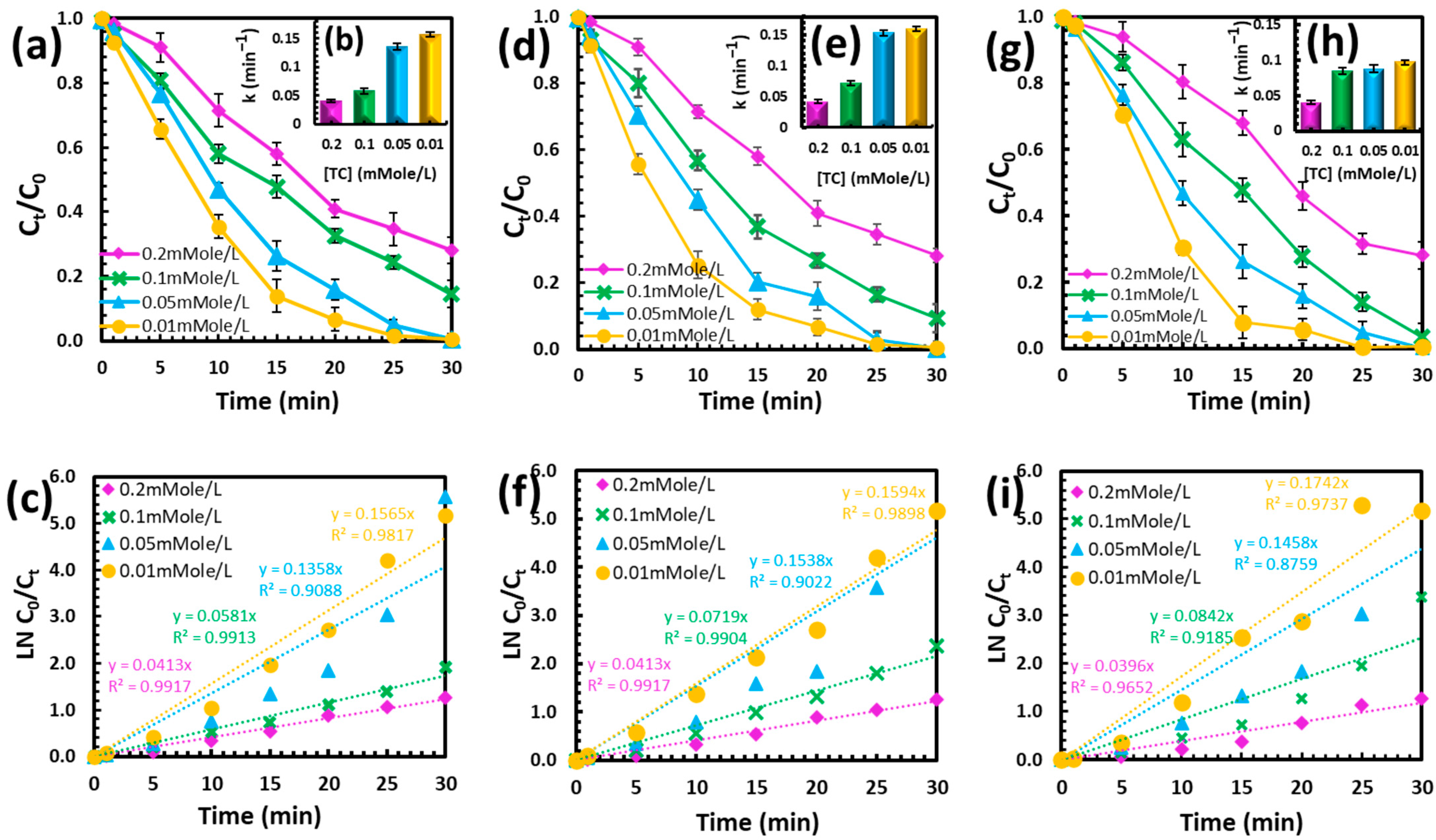
In wastewater treatment, the initial pollutant concentration plays a critical role in determining the efficiency of degradation processes. As shown in Figure 2, the degradation performance of the NTP/PS system was evaluated across varying initial concentrations of TC, OTC, and CTC. A notable decrease in removal efficiency was observed at higher concentrations (0.2 mM). This is likely due to the fixed rate of reactive species generation under constant plasma power. When the pollutant load increases, the available radicals (e.g., •OH and SO4•−) become insufficient to fully degrade all target molecules within the same timeframe [14]. Despite this, the system maintained high overall degradation rates ranging from 70% to 99% for all three TCs and for all initial concentrations after 30 min of treatment. This indicates the strong adaptability of the NTP/PS process for treating different contamination levels in practical applications.

Figure 2.
Effect of initial TC concentration on (a) degradation efficiency, (b) kinetic study, and (c) rate constants for TC; (d–f) corresponding results for OTC; and (g–i) corresponding results for CTC. The blank control (no PS) exhibited no change in degradation efficiency, and the PS-only control is presented in Figure 3.
The rate constants (k) for each initial TCs concentration are represented in Figure 2b, Figure 2f, and Figure 2i for TC, OTC, and CTC, respectively. The observed decrease in rate constants with increasing TCs concentration confirms the inverse relationship between pollutant load and degradation efficiency. Specifically, the rate constants for 0.01 mM and 0.05 mM were significantly higher than those for 0.1 mM and 0.2 mM. These findings underscore the efficiency of the NTP/PS system at lower pollutant concentrations and highlight the need for optimization when treating highly contaminated water.
Figure 2 also provides a linearized representation of the pseudo-first-order kinetics of TCs degradation, showing the natural logarithm of the concentration ratio ln (C0/Ct) plotted against time. The high R2 values (ranging from 0.9088 to 0.9917) confirm that the degradation follows pseudo-first-order kinetics across all concentrations. The slope of each line represents the rate constant (k), with the steepest slope observed for 0.01 mM TC. This linearity indicates that the reaction kinetics are primarily governed by the concentration of reactive oxidative species and their interaction with TCs. While the system is highly effective at lower concentrations, its performance diminishes at higher pollutant loads. To enhance efficiency at higher concentrations, strategies such as increasing the PS dosage may be required.
3.2. Effect of PS: TCs Molar Ratio
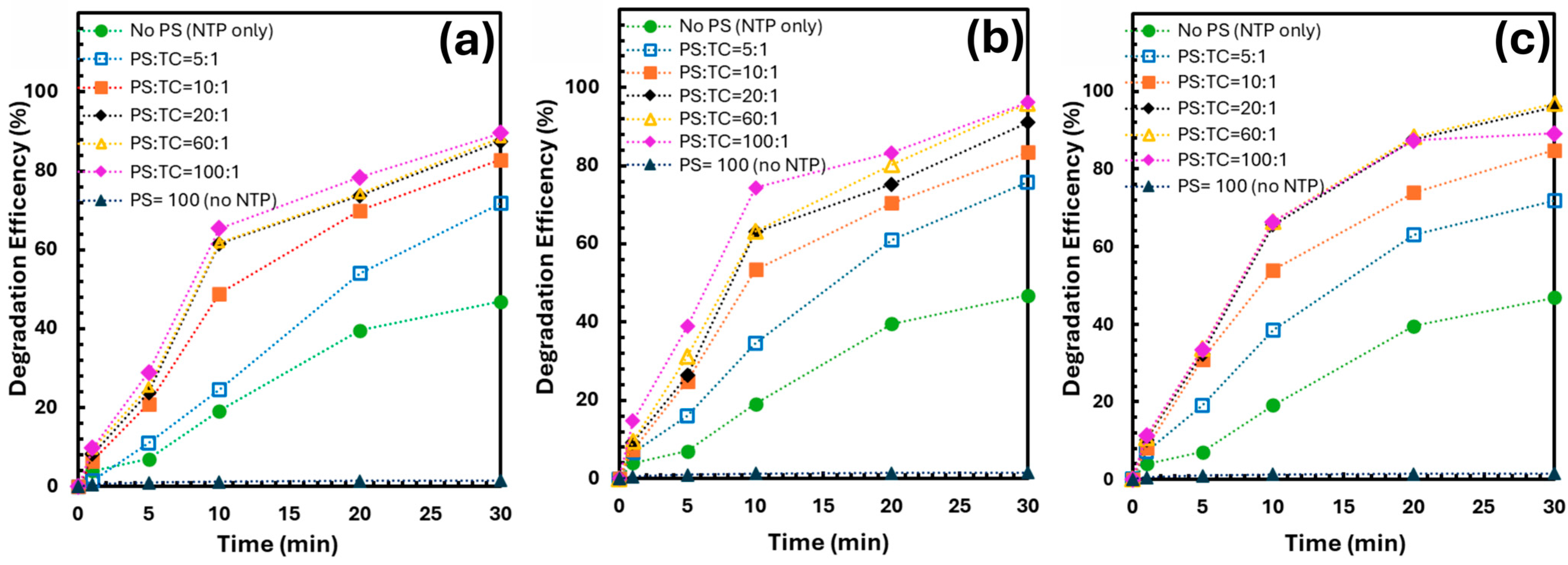
The influence of PS-to-TCs (PS/TCs) molar ratio on degradation efficiency was investigated by varying PS dosages while maintaining a constant initial TCs concentration of 0.2 mM (Figure 3). As the PS/TCs ratio increased from 0 to 100, the removal efficiency of all three TCs improved significantly, attributed to the enhanced generation of oxidative species such as SO4•− and •OH radicals. Relatively, a plateau was observed at a ratio of 20:1 and above, where the efficiencies reached approximately 87.91~92.7% of TCs were removed after 30 min of plasma treatment. Increasing the PS dosage beyond this point yielded only marginal improvement, with a further gain of 2.8% at the highest tested dose.

Figure 3.
Degradation efficiencies of TCs at different PS doses (PS/TCs molar ratio) (a) TC, (b) OTC, and (c) CTC.
While higher PS concentrations promote degradation, the benefits diminish at elevated ratios due to radical saturation and side reactions, which reduce oxidative efficiency [13,15]. Moreover, excessive PS usage may lead to increased solution conductivity and potential secondary pollution, making overuse economically and environmentally unsustainable. These results suggest that an optimal PS/TCs molar ratio exists, beyond which further addition provides minimal benefit and may compromise the overall sustainability of the process.
3.3. Initial pH and Conductivity
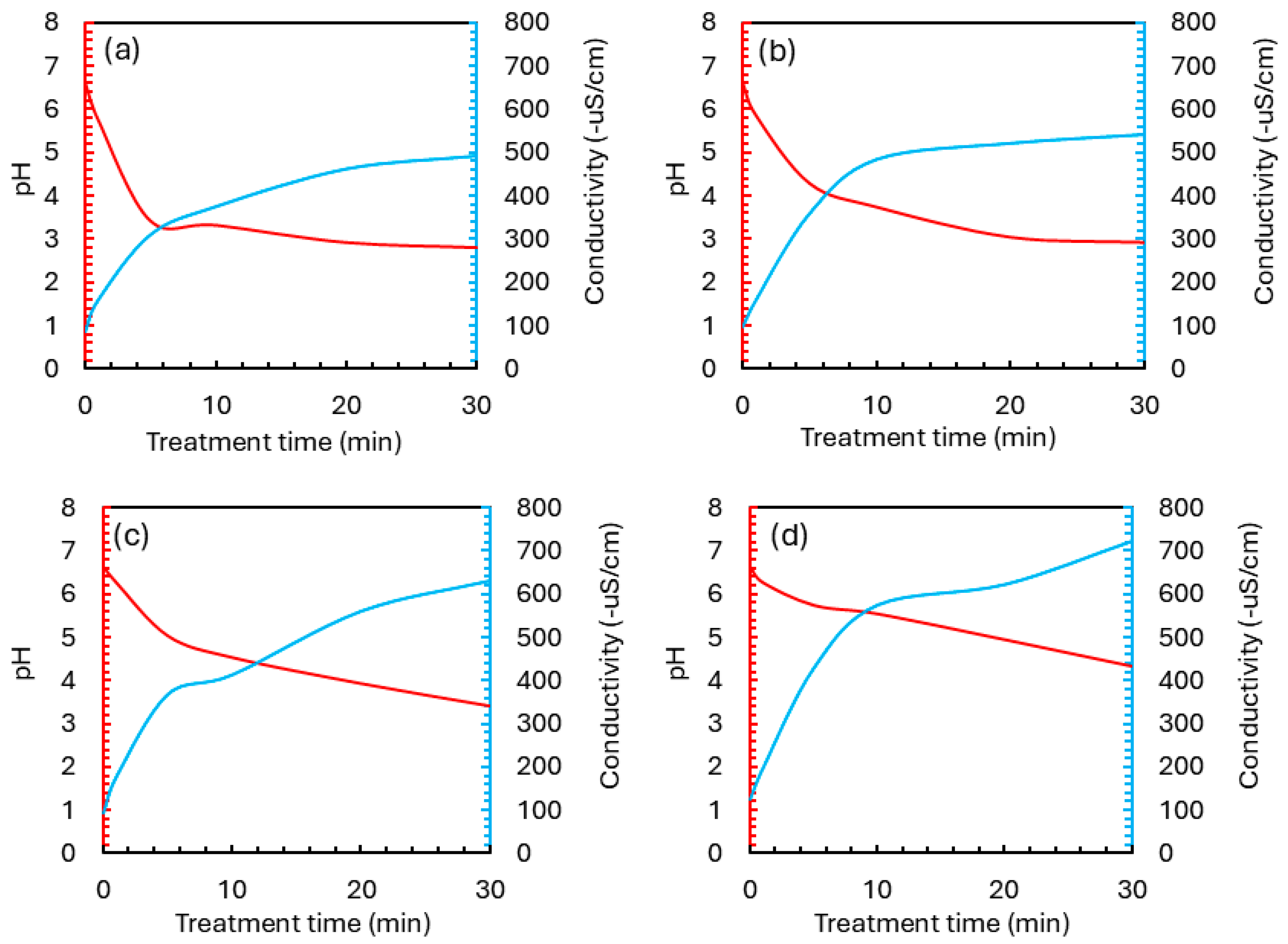
Previous studies have demonstrated that PS activation is significantly influenced by the pH of the reaction medium. SO4•− are predominantly generated under acidic conditions, while under neutral or slightly alkaline environments, PS tends to form sulfuric acid and hydroxyl radicals instead [16,17]. This shift in radical species can impact the efficiency of advanced oxidation processes. However, in our systems, this behavior is altered due to the continuous generation of acidic species such as nitric and nitrous acids from the interaction between air plasma and the aqueous phase [18]. As a result, regardless of the initial pH, the treated solution consistently becomes acidic within the first few minutes of discharge. This pH shift limits the system’s ability to maintain alkaline conditions, even when a base is added initially.
Consequently, our experimental results showed only minor variations in pH in plasma-treated solutions and NTP/PS-treated solutions at different PS doses. Notably, the pH drop was slightly slower in the presence of PS compared to plasma alone (Figure 4), likely due to PS buffering capacity and its participation in radical-mediated reactions [19]. This moderation of pH evolution may enhance system stability during long-term operation. In parallel, conductivity measurements (Figure 4) revealed a gradual increase throughout the reaction, particularly with higher PS dosages. This increase reflects the accumulation of ionic byproducts such as sulfate, nitrate, and nitrite. While elevated conductivity is an expected consequence of oxidation reactions, excessive ionic buildup may pose challenges for downstream applications such as water reuse or discharge into sensitive ecosystems. Therefore, controlling PS dosage is crucial for balancing degradation efficiency and minimizing secondary effects.

Figure 4.
pH and conductivity evolution in NTP/PS systems at different PS doses: (a) plasma alone, PS = 0; (b) 1:5; (c) 1:20; (d) 1:60.
Overall, these findings demonstrate that the NTP/PS system operates effectively, and the natural acidification of the system ensures consistent sulfate radical formation, while PS presence helps regulate the extent of pH decline. This adaptability makes the NTP/PS process suitable for treating waters of varying chemical profiles without requiring tight pH control.
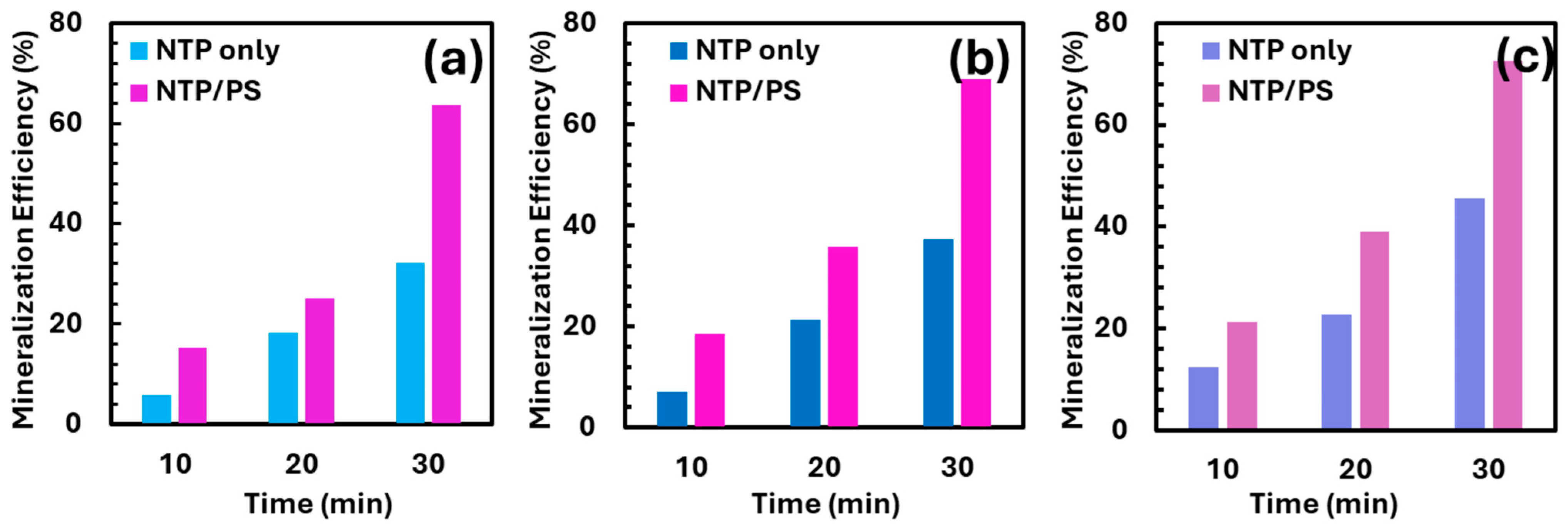
3.4. Evaluation of Mineralization Efficiency
One of the main challenges in advancing AOPs and NTP toward full-scale applications lies in achieving substantial mineralization of organic pollutants. TOC is a critical parameter for assessing the extent of mineralization, as it reflects the reduction in total organic matter rather than just parent compound degradation. In this study, TOC measurements were conducted to evaluate the mineralization efficiency of TCs treated under both plasma-alone and NTP/PS systems at a 1:20 TCs: PS molar ratio and initial concentration of 0.0.5 mM (Figure 5). In all cases, the NTP/PS system showed significantly higher mineralization than plasma alone, particularly at longer treatment times. After 30 min, mineralization efficiencies reached around 65–70% in the NTP/PS system, compared to 35–45% under plasma alone. These results indicate that the combined system not only degrades the parent compounds more effectively but also leads to more complete breakdown into inorganic end products, likely due to the enhanced generation of reactive oxygen species (ROS). The consistent trend across all three antibiotics suggests the broad effectiveness of the NTP/PS system for advanced mineralization of tetracycline-class pollutants.

Figure 5.
Mineralization efficiency of treated solutions by plasma and NTP/PS systems: (a) TC; (b) OTC; (c) CTC. Initial concentration = 0.05 mM. Molar ratio TCs/PS = 1:20.
3.5. Role of Reactive Species
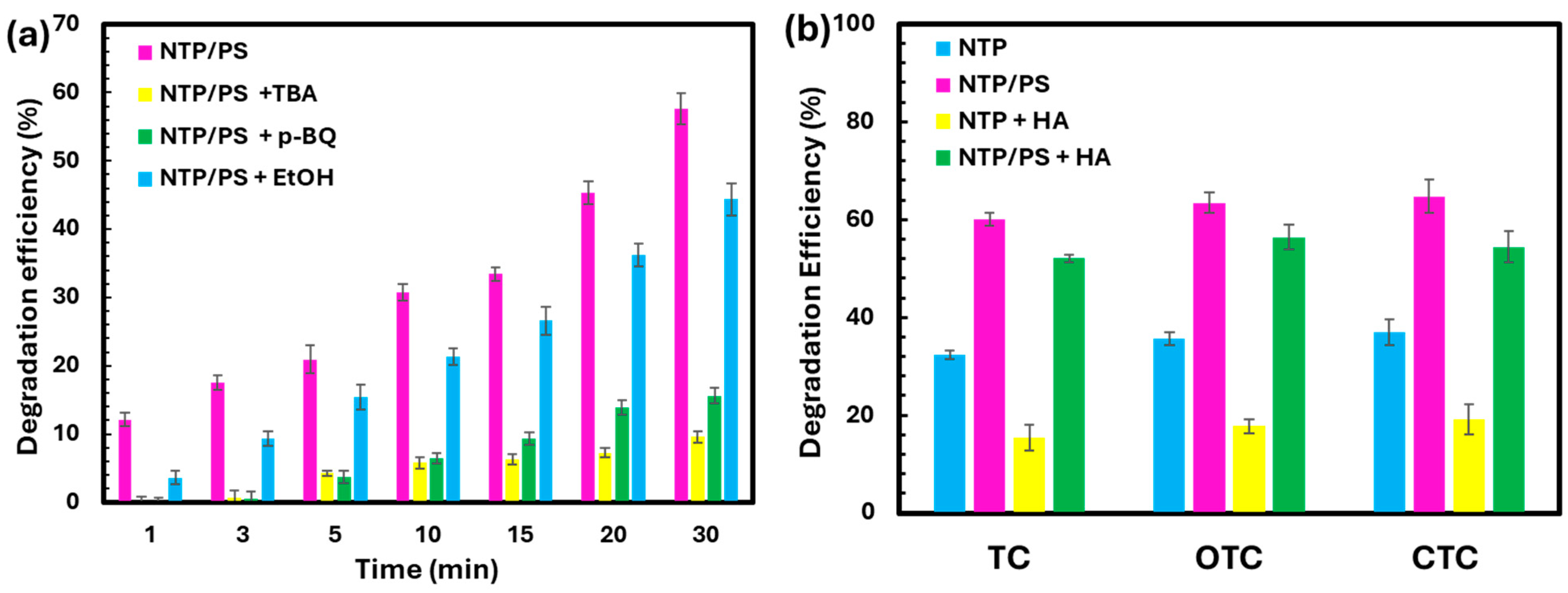
The quantification of key reactive species such as O3, H2O2, NO3−, NO2−, and •OH has been thoroughly investigated in our previous studies using the same plasma reactor configurations [20,21]. Building on that foundation, the present study investigates the roles of dominant reactive species in tetracycline degradation through targeted quenching experiments. Tert-butyl alcohol (TBA), ethanol (EtOH), and p-benzoquinone (p-BQ) were employed as scavengers for •OH, both •OH and SO4•−, free electrons, and superoxide radicals (O2•−), respectively. Since the scavenging results for TC, OTC, and CTC followed similar trends, only the data for TC are presented in Figure 6a, while those for OTC and CTC are provided in the Supplementary Materials (Figure S1). The addition of TBA, which is a selective •OH scavenger that also partially reacts with SO4•−, produced the greatest inhibition, indicating that •OH and SO4•− are the principal oxidants responsible for TC degradation in the NTP/PS system. p-BQ also caused a marked decrease in degradation efficiency, further supporting the central role of O2•− in the oxidation process. In contrast, EtOH had a comparatively smaller impact, suggesting a limited contribution under the tested conditions. These observations are consistent with previous reports identifying •OH and SO4•− as the primary reactive species in plasma-activated persulfate systems [9,22]. Fu et al. (2025) used Electron paramagnetic resonance (EPR) to distinguish the generation of ROS in various species. The strongest signals were found for DMPO-•OH and DMPO-O2•−, which indicated that these two radicals were dominant in that study [23].

Figure 6.
(a) Effect of different scavengers on the degradation efficiency of TC in the NTP/PS system. (b) The effect of organic matter load (Humic Acid) on the degradation efficiency.
The effect of background organic matter on degradation efficiency is shown in Figure 6b. The presence of humic acid (HA, 20 mg L−1) significantly suppressed the degradation of TC, OTC, and CTC compared to the HA-free control. This inhibition is attributed to the strong radical-scavenging capacity of HA, which competes with antibiotics for •OH and SO4•−, as well as its ability to form complexes with target molecules or physically shield them from oxidizing species. These findings indicate that natural organic matter can reduce the efficiency of the NTP/PS process in real water matrices, and that pretreatment or process optimization may be required for practical applications.
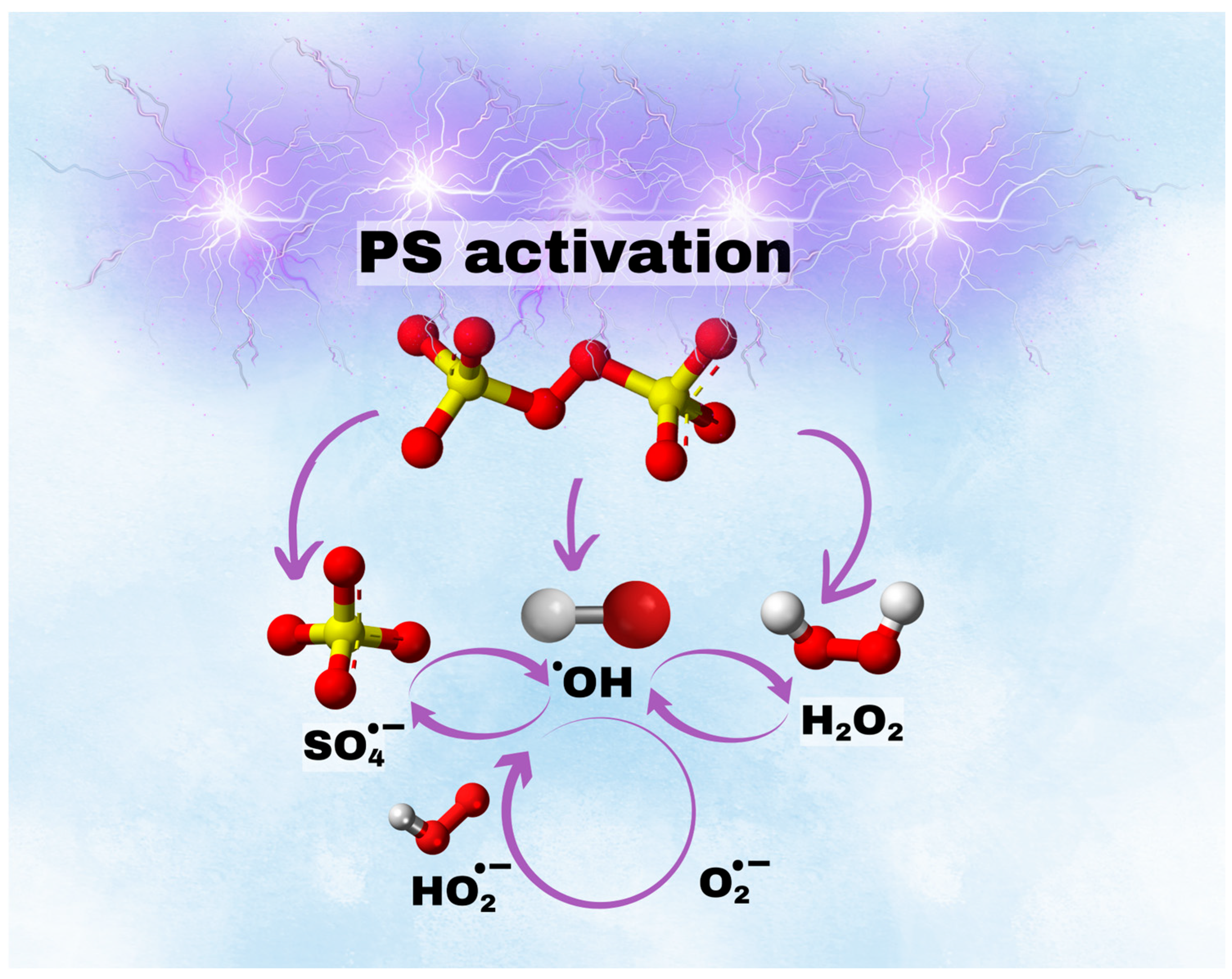
3.6. The Mechanism of Plasma-Activated Persulfate (PS)
The mechanism of NTP/PS degradation involves a cascade of electron transfer reactions and radical generation, leading to the efficient breakdown of organic contaminants [24]. Initially, persulfate ions (S2O82−) interact with plasma-generated electrons and water, producing SO4•−, which are key oxidants in this process (Equation (3)). Additionally, persulfate can undergo single-electron reduction to form S2O8•− (Equation (4)), which further decomposes into SO4•− and SO4− (Equation (5)). The SO4•− then react with water and electrons to form •OH, SO4− ions, and HSO4− (Equation (6)). •OH also contribute to further PS activation, generating additional SO4•− and HO2• (Equation (5)), while HO2• reacts with PS and protons to produce H2O2 and more SO4•− (Equation (8)).
In water, HSO5− decomposes into HSO4− and H2O2 (Equation (7)), further fueling radical generation. H2O2 interacts with •OH, forming hydroperoxyl radicals (Equation (10)), which dissociate into O2•− in acidic conditions (Equation (9)). The interplay of H2O2 and HO2• also generates singlet oxygen, water, hydroxide ions, and electrons (Equation (11)), while additional reactions between O2•− and H2O2 contribute to singlet oxygen formation and further radical cycling (Equations (11) and (12)).
S2O82− + 2e− (plasma) + H2O → 2SO4•− + 2e− + 2H+
S2O82− + e− (plasma) → S2O8•− + 2e−
S2O8•− + e− → SO4•− +SO42−
2SO4•− + H2O + e− → •OH + 2SO42− +HSO4−
•OH + S2O82− + H2O + e− → SO4•− + SO42− + HO2•
HO2• + S2O82− + H+ + e−→ H2O2 + 2SO4•−
H2O2 + •OH → HO2•+ H2O
HO2• → H+ + •O2−
2•O2− + 2H2O→1O2 + H2O2 + 2OH−
•O2− + •OH → 1O2 + OH−
This intricate network of radical transformations and the mechanism of PS activation by plasma are presented in Figure 7.

Figure 7.
Mechanism of NTP-PS activation.
Overall, these results confirm that NTP/PS primarily relies on hydroxyl and sulfate radicals for pollutant degradation, with contributions from other ROS and electron transfer processes. This insight is valuable for optimizing advanced oxidation processes in wastewater treatment and environmental remediation.
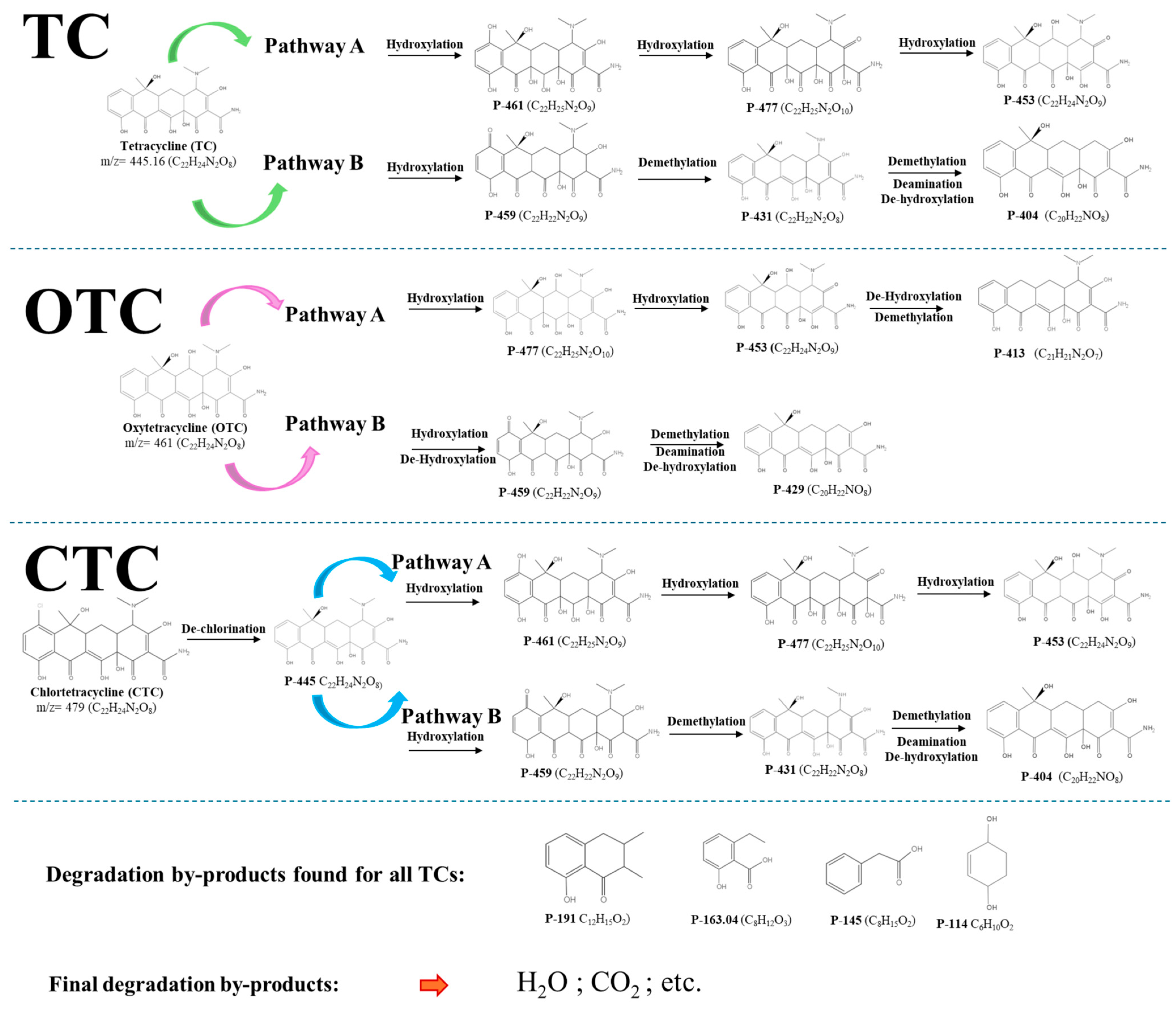
3.7. Degradation Pathway of TC, OTC, and CTC by NTP/PS
The various ROS generated by the NTP/PS system, primarily •OH and SO4•−, initiate oxidative attack on TCs by targeting electron-rich functional groups [25]. The proposed degradation pathways of TC, OTC, and CTC under the NTP/PS system are illustrated in Figure 8, and the assignment of intermediates is based on LC-MS/MS analysis. For TC (m/z 445), two main initial pathways were identified. Pathway A: •OH or SO4•− attack at the phenolic and enolic sites promotes hydroxylation, forming P-461 (m/z +16). Further hydroxylation produces P-477 (m/z +32), which subsequently undergoes oxidative cleavage of the amide-containing ring to yield P-453 (m/z –24 from P-477). Continued oxidation and decarboxylation lead to smaller products such as P-404 (m/z –49). Pathway B: Radical-mediated demethylation of the dimethylamino group yields P-431 (m/z –14), followed by deamination and dehydroxylation to produce P-404 (m/z –27 from P-431). For OTC (m/z 461), two analogous pathways were observed. Pathway A: Hydroxylation generates P-453, followed by dehydroxylation and demethylation to form P-413. Pathway B: Sequential hydroxylation and dehydroxylation yield P-459, while oxidative demethylation and deamination produce P-429.

Figure 8.
Proposed degradation pathways.
For CTC (m/z 479), the first step is dechlorination (likely via reductive pathways involving hydrated electrons or plasma-generated reductants), producing P-445, which corresponds to TC. From this point, CTC degradation follows the same pathways described for TC, explaining why the intermediate profiles for TC and CTC largely overlap.
Across all three antibiotics, further oxidation leads to common low-molecular-weight products detected at m/z 191, 163, 145, and 114. These species are consistent with aromatic ring cleavage products and aliphatic carboxylic acids, indicating progression toward mineralization. The presence of these shared intermediates suggests a converging degradation pathway at later stages, driven by sustained radical attack and oxidative fragmentation [26].
The proposed sequence of transformations, starting with radical attack on susceptible sites, followed by stepwise demethylation, deamination, hydroxylation, and ring opening, aligns with established degradation mechanisms of TCs under advanced oxidation processes [27,28,29]. This provides strong support for the reliability of the pathways presented in Figure 8.
3.8. Toxicity Assessment
The environmental safety of degradation products was evaluated using both estimated toxicity prediction and experimental phytotoxicity assays. The prediction analysis was performed with the Toxicity Estimation Software Tool (T.E.S.T., version 5.1.2) which estimated several key toxicological endpoints for parent TCs and their identified degradation intermediates based on Quantitative Structure Activity Relationships (QSAR) method. The results for all P-m/z are presented in Figure 9a–g).

Figure 9.
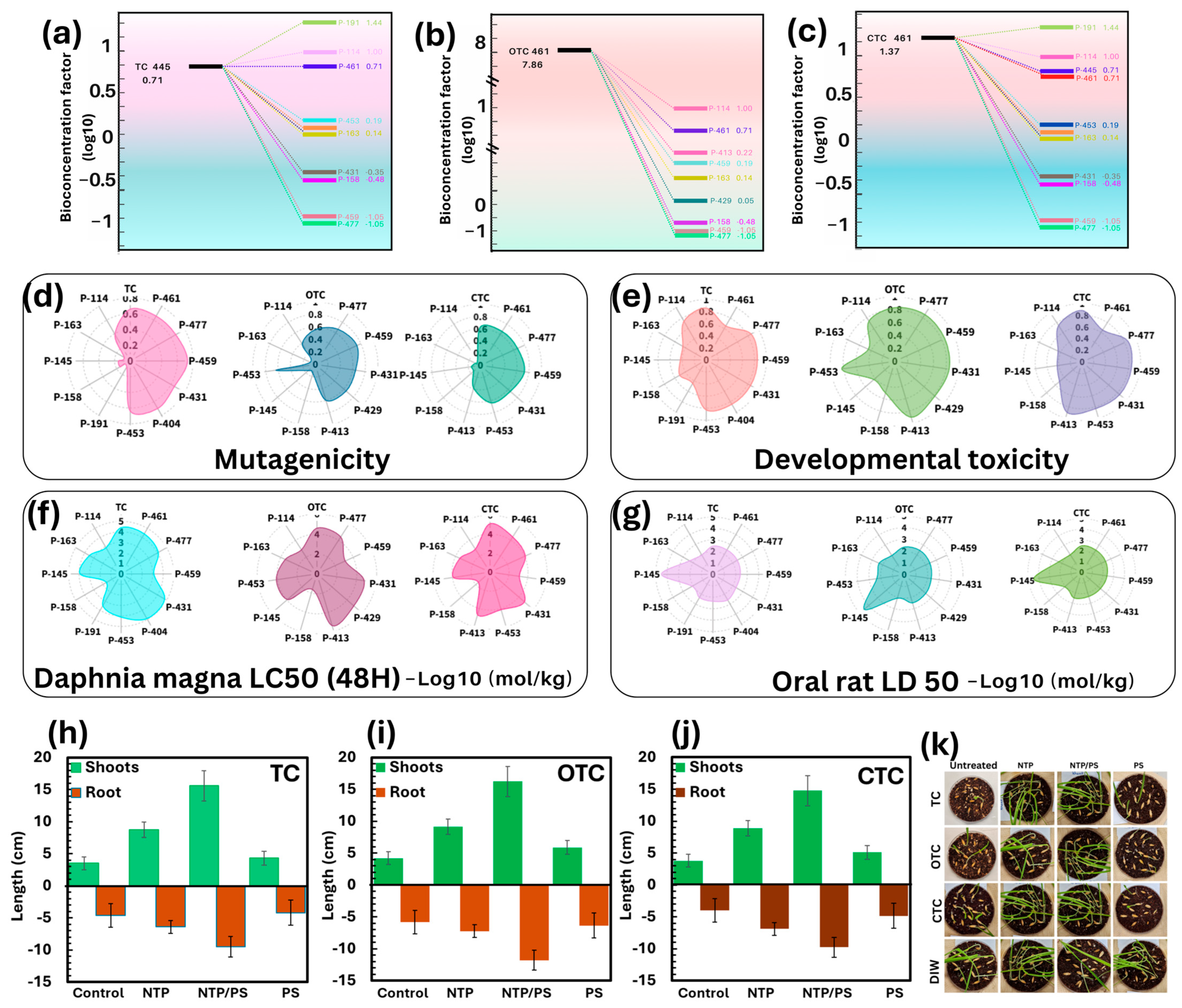
Toxicity assessment of T.E.S.T software: (a) bioconcentration factor of TC and its degradation products; (b) bioconcentration factor of OTC and its degradation products; (c) bioconcentration factor of CTC and its degradation products; (d) Mutagenicity; (e) Developmental toxicity; (f) Daphnia magna; (g) Oral rat. Shoots and roots of germinated seeds after 5 days: (h) TC; (i) OTC; (j) CTC. (k) Seed germination phenotypes of Wheat (Avena Sativa).
3.8.1. Bioconcentration Factor (BCF)
Predicted BCF values (log10 scale; Figure 9a–c) indicate that the parent antibiotics (particularly OTC (log BCF = 7.08) exhibit a high bioaccumulation potential. After NTP/PS treatment, most detected intermediates displayed markedly lower BCF values, generally falling below log BCF = 1, suggesting a substantial reduction in the risk of bioaccumulation in aquatic organisms. This decrease is a favorable indicator of reduced long-term environmental persistence and trophic magnification potential.
3.8.2. Mutagenicity and Developmental Toxicity
The plots for mutagenicity (Figure 9d) and developmental toxicity (Figure 9 e) show that the untreated antibiotics displayed moderate to high predicted risk scores. In contrast, most degradation products exhibited significantly reduced mutagenicity and developmental toxicity scores, indicating a shift toward less genotoxic and teratogenic chemical profiles post-treatment.
3.8.3. Aquatic Toxicity (Daphnia magna LC50)
Acute toxicity to Daphnia magna over 48 h was predicted as log10(mol/kg) values (Figure 9f). Parent TCs exhibited relatively low LC50 values, indicative of higher toxicity, while most degradation intermediates showed higher LC50 values, reflecting decreased acute aquatic toxicity. This is consistent with the reduction in hydrophobicity and bioaccumulation potential observed in the BCF analysis.
3.8.4. Mammalian Toxicity (Oral Rat LD50)
Predicted oral LD50 values (Figure 9g) suggest that the untreated antibiotics pose higher mammalian toxicity risks, whereas most degradation products have higher LD50 values, signifying reduced acute toxicity in mammals.
3.8.5. Phytotoxicity Assays
To complement the toxicity assessment, experimental phytotoxicity tests were conducted using wheat (Avena sativa) seed germination and growth assays. Measurements of shoot and root length (Figure 9h–j) showed that untreated antibiotic solutions caused significant inhibition of germination and growth, particularly in root elongation. Treatment with NTP or NTP/PS substantially mitigated this phytotoxicity, with samples irrigated with NTP/PS-treated solutions showing shoot and root growth statistically comparable to the deionized water (DIW) control. In contrast, PS alone was ineffective in detoxifying the solutions, consistent with its almost no degradation efficiency observed in chemical analyses. Representative seedling images (Figure 9k) visually confirm these findings, illustrating the stark contrast between untreated and treated samples.
Overall, the combined evidence from predictive toxicology and phytotoxicity experiments strongly indicates that NTP/PS treatment not only degrades TCs effectively but also transforms them into products with markedly reduced bioaccumulation potential, mutagenicity, developmental toxicity, and acute toxicity to both aquatic and terrestrial organisms. This dual approach provides a robust environmental safety evaluation and supports the potential application of NTP/PS technology for antibiotic-contaminated water treatment.
3.9. Advantages and Practical Considerations
In this work, PS served as a chemical oxidant activated by plasma-generated reactive species, rather than as a photosensitizer. In the GAD reactor used here, PS activation was achieved predominantly through reactions with •OH and SO4•− radicals, along with other short-lived oxidative species produced in the discharge, rather than through photolytic pathways. This distinction enables operation independently of light sources or weather conditions, in contrast to solar-driven or UV-based processes [30]. Compared with other advanced oxidation processes such as UV/PS, solar/TiO2, and ozonation, the NTP/PS system demonstrated rapid degradation within minutes, high efficiency under near-neutral pH, and the ability to reduce phytotoxicity of treated solutions. Additionally, persulfate’s stability, ease of handling, and compatibility with decentralized setups make this approach attractive for practical water treatment applications.
4. Conclusions
This study demonstrates the high efficiency and environmental promise of the nonthermal plasma-activated persulfate (NTP/PS) system for the degradation and mineralization of tetracycline antibiotics (TC, OTC, and CTC). The process achieved over 97% degradation efficiency, significantly outperforming plasma treatment alone, and resulted in substantial mineralization with the formation of less toxic byproducts. Mechanistic investigations revealed that SO4•− and •OH radicals, along with contributions from superoxide radicals and free electrons, played central roles in driving key degradation pathways such as N-demethylation, hydroxylation, dechlorination, and ring cleavage. Toxicity predictions confirmed that most intermediates exhibited reduced mutagenicity and bioconcentration potential compared to the parent compounds, highlighting the environmental safety of the transformation products. Overall, the NTP/PS system presents a rapid, efficient, and sustainable advanced oxidation process with strong potential for wastewater treatment and environmental remediation. Future work should focus on scaling up the system, optimizing energy use, and testing performance in real wastewater conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12080222/s1, Figure S1: LCMSMS spectra; Table S1: Degradation products toxicities.
Author Contributions
A.O., conceptualization, methodology, formal analysis, data curation, and writing review and editing; B.T.G., formal analysis and data curation. K.-M.L., conceptualization, review, and editing. I.-K.K., funding acquisition, supervision, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Global Joint Research Program grant number [202507050001].
Data Availability Statement
All Data are presented in the manuscript.
Acknowledgments
This work was supported by the Global Joint Research Program funded by Pukyong National University (202507050001).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Kim, H.; Hong, Y.; Park, J.E.; Sharma, V.K.; Cho, S.I. Sulfonamides and tetracyclines in livestock wastewater. Chemosphere 2013, 91, 888–894. [Google Scholar] [CrossRef]
- El Shaer, M.; Eldaly, M.; Heikal, G.; Sharaf, Y.; Diab, H.; Mobasher, M.; Rousseau, A. Antibiotics Degradation and Bacteria Inactivation in Water by Cold Atmospheric Plasma Discharges Above and Below Water Surface. Plasma Chem. Plasma Process. 2020, 40, 971–983. [Google Scholar] [CrossRef]
- Chang, D.; Mao, Y.; Qiu, W.; Wu, Y.; Cai, B. The Source and Distribution of Tetracycline Antibiotics in China: A Review. Toxics 2023, 11, 214. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic effects of tetracycline and its degradation products on freshwater green algae. Ecotoxicol. Environ. Saf. 2018, 147, 43–47. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Chohra, H.; Lee, K.A.; Choe, H.; Cho, J.Y.; Kantharaj, V.; Cheong, M.S.; Kim, Y.-N.; Lee, Y.B. Dose-Dependent Physiological Response to Transient Bioaccumulation of Tetracycline in Kimchi Cabbage (Brassica campestris L.). Antibiotics 2025, 14, 501. [Google Scholar] [CrossRef]
- Lou, J.; An, J.; Wang, X.; Cheng, M.; Cui, Y. A novel DBD/VUV/PMS process for efficient sulfadiazine degradation in wastewater: Singlet oxygen-dominated nonradical oxidation. J. Hazard. Mater. 2023, 461, 132650. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Pham, T.H.; Bui, H.M.; Bui, T.X. Advanced Oxidation Processes for the Removal of Pesticides; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Chen, C.; Ma, C.; Yang, Y.; Yang, X.; Demeestere, K.; Nikiforov, A.; Van Hulle, S. Degradation of micropollutants in secondary wastewater effluent using nonthermal plasma-based AOPs: The roles of free radicals and molecular oxidants. Water Res. 2023, 235, 119881. [Google Scholar] [CrossRef]
- Guo, H.; Pan, S.; Hu, Z.; Wang, Y.; Jiang, W.; Yang, Y.; Wang, Y.; Han, J.; Wu, Y.; Wang, T. Persulfate activated by non-thermal plasma for organic pollutants degradation: A review. Chem. Eng. J. 2023, 470, 144094. [Google Scholar] [CrossRef]
- Song, S.; Wang, H.; Huang, Y.; Ma, Y. Analysis of influencing parameters and reactive substance for enrofloxacin degradation in a dielectric barrier discharge plasma/peroxydisulfate system. Environ. Eng. Res. 2024, 29, 230717. [Google Scholar] [CrossRef]
- Ji, H.; Xu, Y.; Shi, H.; Yang, X. Enhanced activation of persulfate by magnetic NiFe2O4@CuO coupled with ultrasonic for degradation of oxytetracycline: Activation mechanism and degradation pathway. Appl. Surf. Sci. 2024, 652, 159373. [Google Scholar] [CrossRef]
- Liu, X.; Köpke, J.; Akay, C.; Kümmel, S.; Imfeld, G. Sulfamethoxazole Transformation by Heat-Activated Persulfate: Linking Transformation Products Patterns with Carbon and Nitrogen Isotope Fractionation. Environ. Sci. Technol. 2025, 59, 5704–5714. [Google Scholar] [CrossRef]
- Rajabizadeh, A.; Abdipour, H.; Mansoorian, H.J. A new approach for the elimination of Rhodamine B dye using a combination of activated persulfate and dithionite in the presence of magnetic fields. Chem. Eng. Process.-Process Intensif. 2025, 209, 110160. [Google Scholar] [CrossRef]
- Vlad, I.E.; Anghel, S.D. Time stability of water activated by different on-liquid atmospheric pressure plasmas. J. Electrostat. 2017, 87, 284–292. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Guo, H.; Puyang, C.; Han, J.; Li, Y.; Ruan, Y. Mechanism and process of sulfamethoxazole decomposition with persulfate activated by pulse dielectric barrier discharge plasma. Sep. Purif. Technol. 2021, 287, 120540. [Google Scholar] [CrossRef]
- Ouzar, A.; Goutomo, B.T.; Nam, K.; Kim, I.K. Efficient removal of tetracycline antibiotic by nonthermal plasma-catalysis combination process. Environ. Eng. Res. 2025, 30, 240254. [Google Scholar] [CrossRef]
- Ouzar, A.; Goutomo, B.T.; Nam, K.; Kim, I.K. Enhanced removal of malachite green from wastewater using nonthermal plasma gliding arc discharge combined with ferrate oxidation. Desalin. Water Treat. 2024, 320, 100743. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Q. Dielectric barrier discharge plasma activates persulfate to degrade norfloxacin: Mechanism and degradation pathways. Plasma Med. 2018, 8, 321–333. [Google Scholar] [CrossRef]
- Fu, Y.; Chi, J.; Wu, Y.; Li, J.; Tan, M.; Li, C.; Du, H.; Hao, D.; Zhu, H.; Wang, Q.; et al. Synergistic electric fields induced by unilateral doping modulation for enhanced organic pollutant degradation and sterilization. Appl. Surf. Sci. 2025, 692, 162711. [Google Scholar] [CrossRef]
- Chen, W.; Wu, H.; Fan, J.; Fang, Z.; Lin, S. Activated persulfate by DBD plasma and activated carbon for the degradation of acid orange II. Plasma Sci. Technol. 2020, 22, 034009. [Google Scholar] [CrossRef]
- Fang, C.; Wang, S.; Xu, H.; Huang, Q. Degradation of tetracycline by atmospheric-pressure non-thermal plasma: Enhanced performance, degradation mechanism, and toxicity evaluation. Sci. Total Environ. 2022, 812, 152455. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yuan, D.; Rao, Y.; Li, N.; Qi, J.; Cheng, T.; Sun, Z.; Gu, J.; Huang, H. Persulfate activation in gas phase surface discharge plasma for synergetic removal of antibiotic in water. Chem. Eng. J. 2018, 337, 446–454. [Google Scholar] [CrossRef]
- KyereYeboah, K.; Qiao, X.C. Non-thermal plasma activated peroxide and percarbonate for tetracycline and oxytetracycline degradation: Synergistic performance, degradation pathways, and toxicity evaluation. Chemosphere 2023, 336, 139246. [Google Scholar] [CrossRef]
- Wang, C.; Qu, G.; Wang, T.; Deng, F.; Liang, D. Removal of tetracycline antibiotics from wastewater by pulsed corona discharge plasma coupled with natural soil particles. Chem. Eng. J. 2018, 346, 159–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Fernandes, S.M.; Alcobia, T.; Lourenço, M.C.; Oliveira, M.C.; Marques, A.C. Optimization of TiO2 loaded sol-gel derived MICROSCAFS® for enhanced minocycline removal from water and real wastewater. J. Sol-Gel Sci. Technol. 2025, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).