Efficient Separation of Lu from Yb Using Rext-P350@Resin: A Promising Route for No-Carrier-Added 177Lu Production
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Instrumentation and Analysis Methods
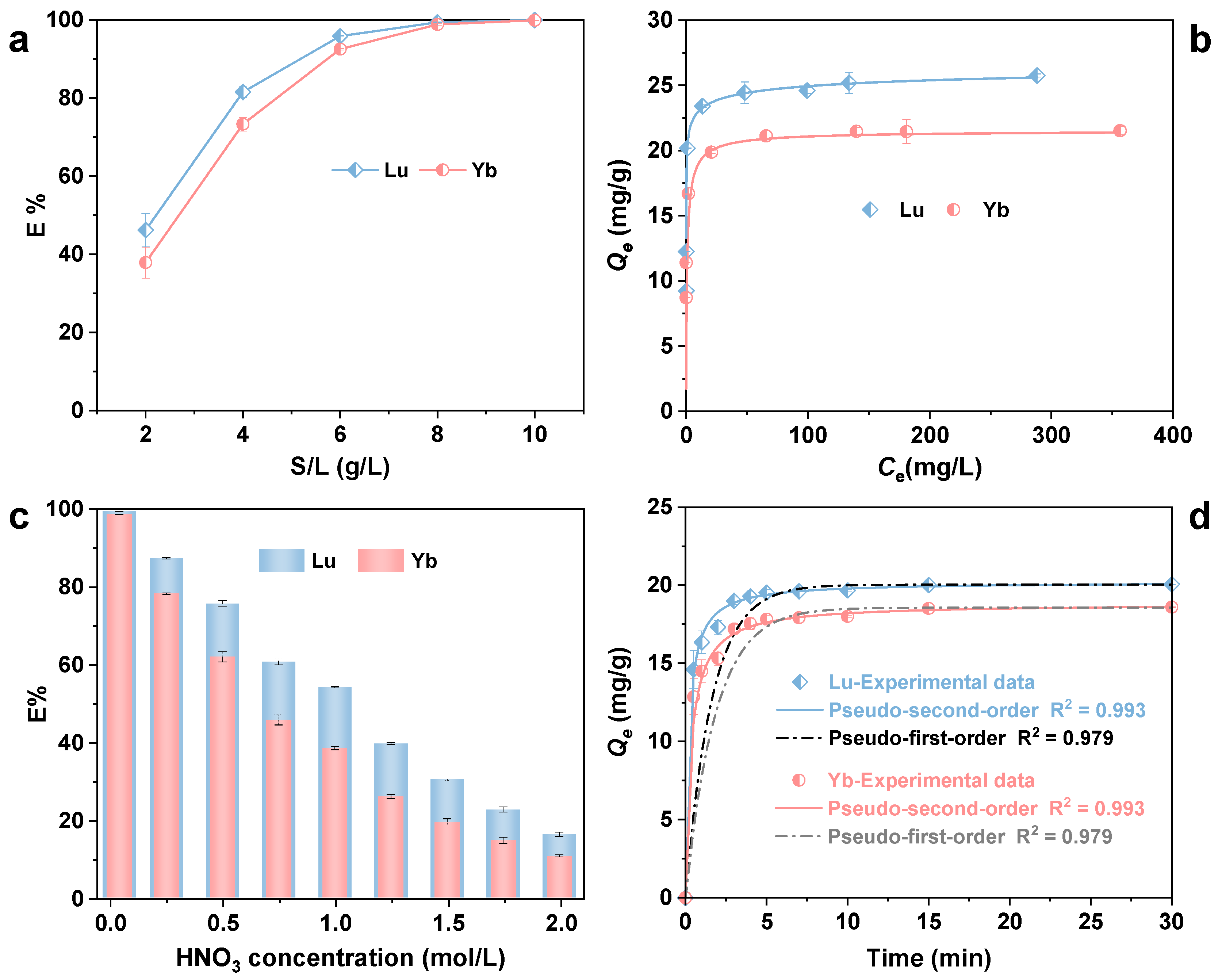
2.3. Batch Sorption Experiments
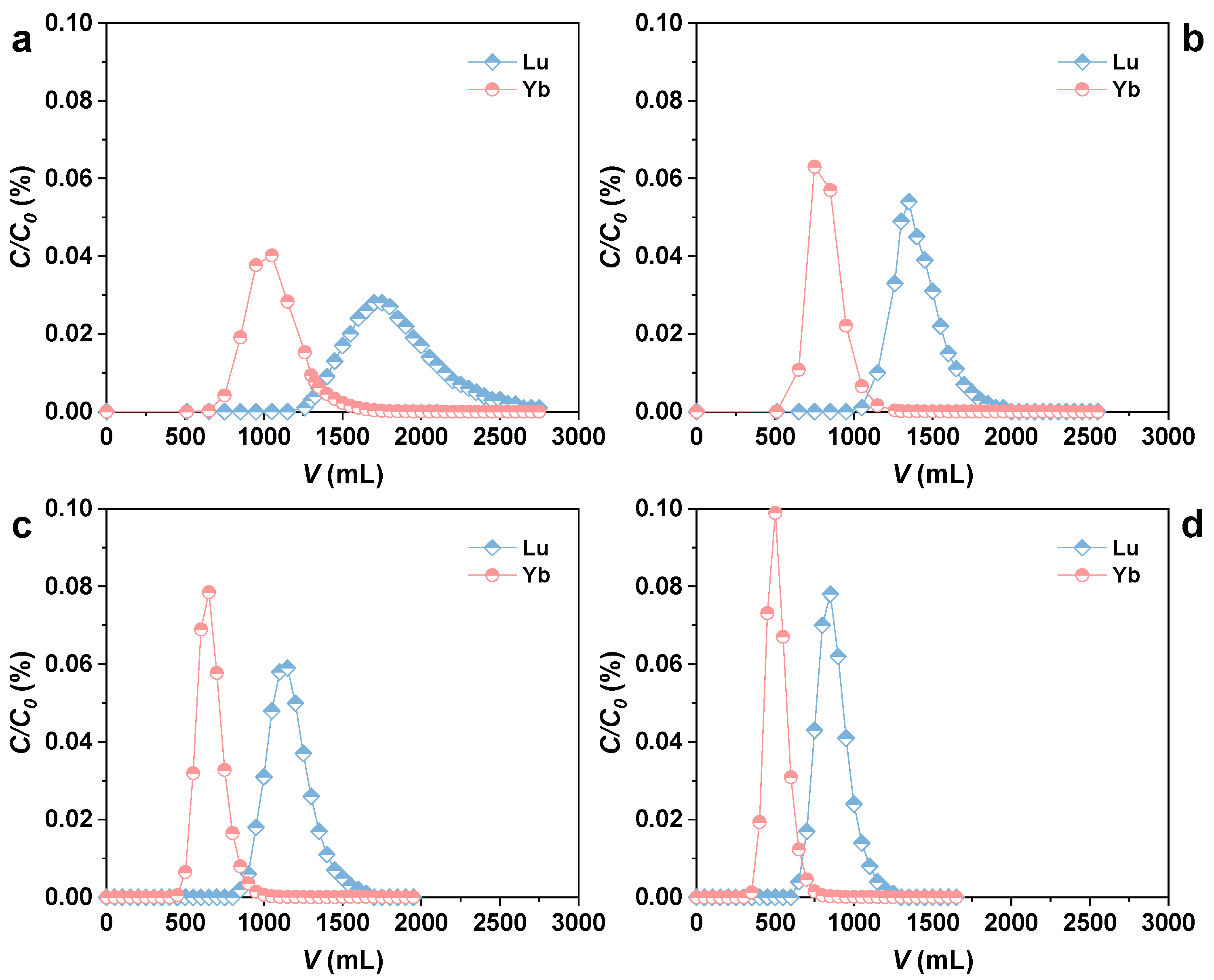
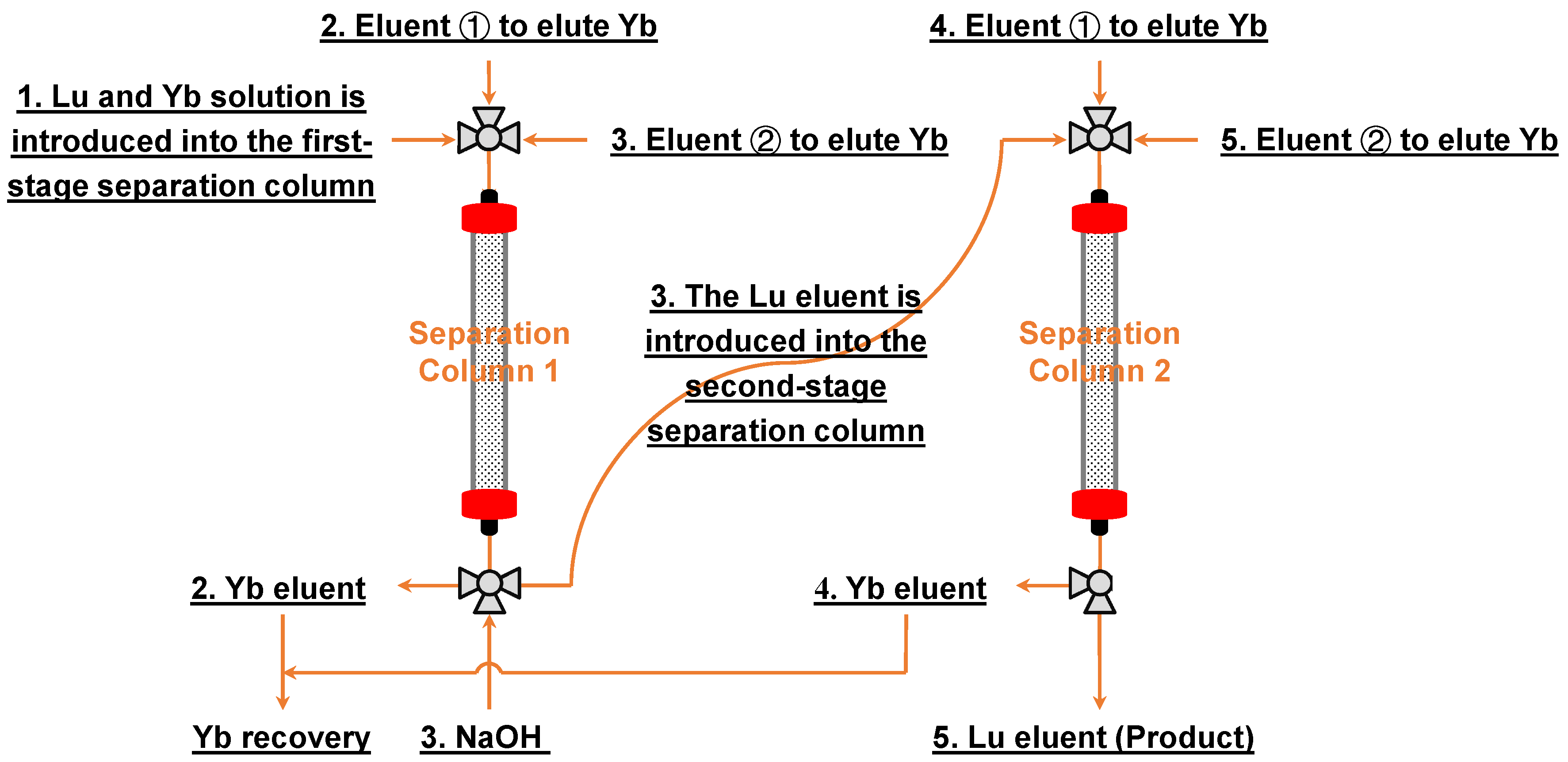
2.4. Lu/Yb Chromatography Separation Experiment
3. Results and Discussion
3.1. Adsorption Properties of Rext-P350@Resin Toward Lu and Yb
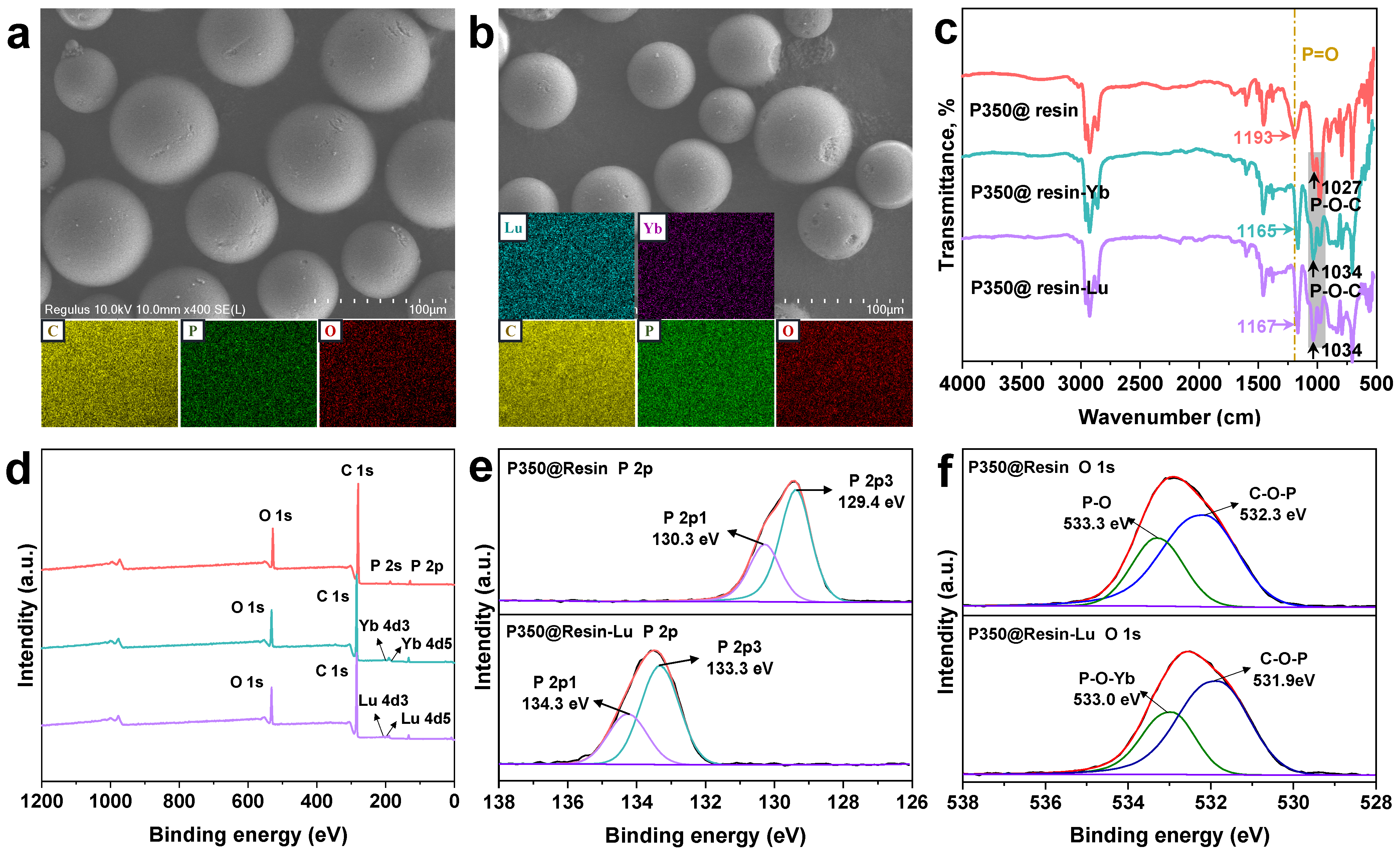
3.2. Characterization of Rext-P350@Resin Before and After Adsorption of Lu and Yb
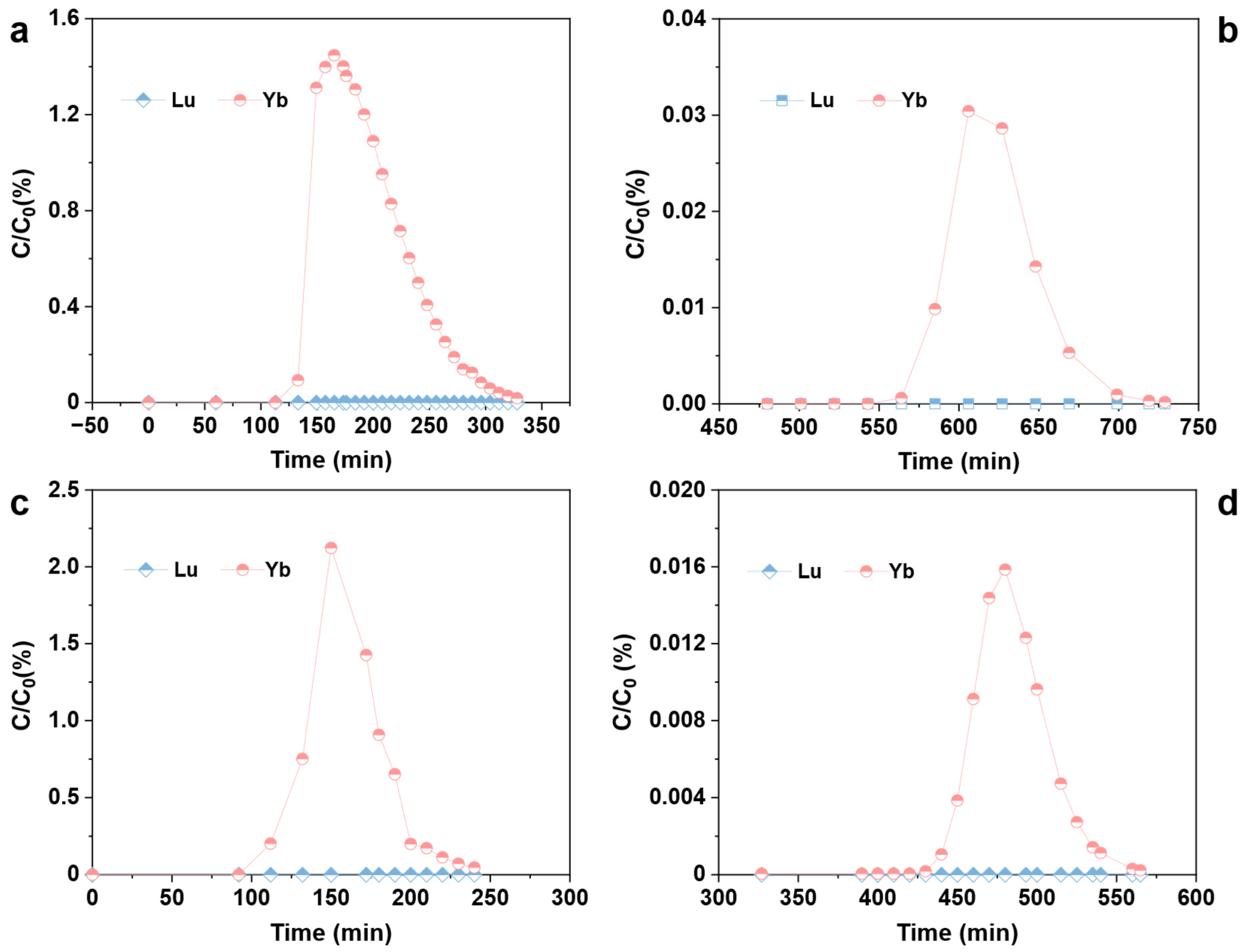
3.3. Dynamic Elution Separation of Lu/Yb by Rext-P350@Resin
3.4. Lu/Yb Continuous Chromatographic Separation Process by Rext-P350@Resin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Kang, F.; Chen, Y.; Zhu, Z.; Wang, F.; Qin, C.; Du, J.; Lan, X.; Wang, J. Landscape of nuclear medicine in china and its progress on theranostics. J. Nucl. Med. 2024, 65, 29S–37S. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Zeglis, B.M.; Lapi, S.E.; Scott, P.J.; Windhorst, A.D.; Abdel-Wahab, M.; Giammarile, F.; Paez, D.; Jalilian, A.; Knoll, P. Trends in nuclear medicine and the radiopharmaceutical sciences in oncology: Workforce challenges and training in the age of theranostics. Lancet Oncol. 2024, 25, e250–e259. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Pillai, M.R.A.; Knapp, F.F. Production of 177Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Liu, C.; Qi, J.; Xiao, C. Lutetium-177 radioisotope and therapeutic radiopharmaceuticals: Production, separation and purification, chelation labeling and clinical application. Chin. Sci. Bull. 2024, 69, 3383–3403. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.A.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Keam, S.J. Lutetium Lu 177 vipivotide tetraxetan: First approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Trikalinos, N.A.; Kim, H.; Vijayan, A.; Amurao, M.; Prasad, V. Use of approved Lu-177 radiopharmaceuticals in patients with end-stage renal disease: A review of the literature and proposed treatment algorithm. J. Neuroendocrinol. 2024, 36, e13393. [Google Scholar] [CrossRef]
- Chen, X.; Tan, F.; Liang, R.; Liao, J.; Yang, J.; Lan, T.; Yang, Y.; Liu, N.; Li, F. A proof-of-concept study on the theranostic potential of 177Lu-labeled biocompatible covalent polymer nanoparticles for cancer targeted radionuclide therapy. Chem. A Eur. J. 2024, 30, e202303298. [Google Scholar] [CrossRef]
- Nanabala, R.; Pillai, M.R.A.; Gopal, B. Preparation of patient doses of [177Lu]Lu-DOTATATE and [177Lu]Lu-PSMA-617 with carrier added (CA) and no carrier added (NCA) 177Lu. Nucl. Med. Mol. Imaging 2022, 56, 313–322. [Google Scholar] [CrossRef]
- Hunt, W.W.; Long, M.; Kamil, U.; Kellapatha, S.; Noonan, W.; Roselt, P.D.; Emmerson, B.; Hofman, M.S.; Haskali, M.B. A scalable protocol for the radiosynthesis of clinical grade lutetium-177-labeled theranostic agents. Nat. Protoc. 2025. [Google Scholar] [CrossRef]
- Park, U.J.; Lee, J.S.; Choi, K.H.; Nam, S.S.; Yu, K.H. Lu-177 preparation for radiotherapy application. Appl. Radiat. Isot. 2016, 115, 8–12. [Google Scholar] [CrossRef]
- Tarasov, V.A.; Andreev, O.I.; Romanov, E.G.; Kuznetsov, R.A.; Kupriyanov, V.V.; Tselishchev, I.V. Production of no-carrier added lutetium-177 by irradiation of enriched ytterbium-176. Curr. Radiopharm. 2015, 8, 95–106. [Google Scholar] [CrossRef]
- Pillai, M.; Chakraborty, S.; Das, T.; Venkatesh, M.; Ramamoorthy, N. Production logistics of 177Lu for radionuclide therapy. Appl. Radiat. Isot. 2003, 59, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S. A review of advances in the last decade on targeted cancer therapy using 177Lu: Focusing on 177Lu produced by the direct neutron activation route. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 443. [Google Scholar] [PubMed]
- Cheisson, T.; Schelter, E.J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 2019, 363, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Development course of separating rare earths with acid phosphorus extractants: A critical review. J. Rare Earths 2019, 37, 468–486. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Lebedev, N.A.; Novgorodov, A.F.; Misiak, R.; Brockmann, J.; Rösch, F. Radiochemical separation of no-carrier-added 177Lu as produced via the 176Ybn, γ177Yb → 177Lu process. Appl. Radiat. Isot. 2000, 53, 421–425. [Google Scholar] [CrossRef]
- Demidov, V.A.; Ushakov, I.A.; Zukau, V.V.; Nesterov, E.A.; Vidyaev, D.G.; Timchenko, S.N. Automated system of carrier-free Lu-177 separation from ytterbium target. Russ. Phys. J. 2024, 67, 188–192. [Google Scholar] [CrossRef]
- Dash, A.; Chakravarty, R.; Knapp, F.F.; MR Pillai, A. Indirect production of no carrier added (NCA) 177Lu from irradiation of enriched 176Yb: Options for ytterbium/lutetium separation. Curr. Radiopharm. 2015, 8, 107–118. [Google Scholar] [CrossRef]
- Bilewicz, A.; Żuchowska, K.; Bartoś, B. Separation of Yb as YbSO4 from the 176Yb target for production of 177Lu via the 176Yb(n, γ)177Yb→177Lu process. J. Radioanal. Nucl. Chem. 2009, 280, 167–169. [Google Scholar] [CrossRef]
- Chakravarty, R.; Das, T.; Dash, A.; Venkatesh, M. An electro-amalgamation approach to isolate no-carrier-added 177Lu from neutron irradiated Yb for biomedical applications. Nucl. Med. Biol. 2010, 37, 811–820. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Cha, K.W.; Park, K.W.; Hong, S.W. Separation and recovery of rare earths by ion exchange chromatography. J. Korean Chem. Soc. 1997, 41, 612–638. [Google Scholar]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Starý, J. Separation of transplutonium elements. Talanta 1966, 13, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Grimes, T.S.; Nash, K.L. Acid dissociation constants and rare earth stability constants for DTPA. J. Solut. Chem. 2014, 43, 298–313. [Google Scholar] [CrossRef]
- Polido Legaria, E.; Samouhos, M.; Kessler, V.G.; Seisenbaeva, G.A. Toward molecular recognition of REEs: Comparative analysis of hybrid nanoadsorbents with the different complexonate ligands EDTA, DTPA, and TTHA. Inorg. Chem. 2017, 56, 13938–13948. [Google Scholar] [CrossRef]
- Bo, L.; Yingjiang, H.; Jianrong, W.; Lei, W.; Yunming, C.; Jinsong, Z.; Ning, L. No-carrier-added lutetium-177 separation technology status. J. Isot. 2024, 37, 185–194. [Google Scholar]
- Philip Horwitz, E.; McAlister, D.R.; Dietz, M.L. Extraction chromatography versus solvent extraction: How similar are they? Sep. Sci. Technol. 2006, 41, 2163–2182. [Google Scholar] [CrossRef]
- Dietz, M.L.; Horwitz, E.P.; Bond, A.H. Extraction chromatography: Progress and opportunities. In Metal-Ion Separation and Preconcentration; ACS Publications: Washington, DC, USA, 1999; Volume 716, pp. 234–250. [Google Scholar]
- Kifle, D. Development and optimization of high-performance extraction chromatography method for separation of rare earth elements. J. Chromatogr. A 2024, 1730, 465120. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Labb, S.A.; Despotopulos, J.D.; Kmak, K.N.; Hoffman, D.R. Extraction and separation of rare earth elements using LN resins in hydrochloric acid. J. Radioanal. Nucl. Chem. 2024, 333, 6525–6531. [Google Scholar] [CrossRef]
- Happel, S. An overview over some new extraction chromatographic resins and their application in radiopharmacy. In Proceedings of the TrisKem International, Goldschmidt2021, Virtual, 4–9 July 2021; Available online: https://www.triskem-international.com/scripts/files/60c4a63e7a4475.44279512/tki_rp_210612.pdf (accessed on 20 June 2025).
- Horwitz, E.; McAlister, D.; Bond, A.; Barrans, R.; Williamson, J. A process for the separation of 177Lu from neutron irradiated 176Yb targets. Appl. Radiat. Isot. 2005, 63, 23–36. [Google Scholar] [CrossRef]
- Zhuo, L.; Yang, Y.; Yue, H.; Xiong, X.; Wang, G.; Wang, H.; Yang, L.; Lin, Q.; Chen, Q.; Tu, J. Effective lutetium/ytterbium separation for no-carrier added lutetium-177 production. J. Radioanal. Nucl. Chem. 2022, 331, 5719–5727. [Google Scholar] [CrossRef]
- Ma, G.; Peng, H.; Fan, X.; Guo, Y.; Li, Y.; Gao, J.; Hu, Y.; Li, B.; Yang, Y.; Zhang, J. Noncovalently functionalized carbon microspheres for separating no-carrier added 177Lu from real neutron-irradiated 176Yb target. Chem. Eng. J. 2025, 507, 160805. [Google Scholar] [CrossRef]
- Ma, G.; Peng, H.; Fan, X.; Li, Y.; Gao, J.; Hu, Y.; Li, B.; Yang, Y.; Zhang, J.; Ma, L. Shape-persistent COF-derived functional carbon microspheres for No-carrier added 177Lu separation. Carbon 2024, 225, 119035. [Google Scholar] [CrossRef]
- Fan, X.; Ma, G.; Peng, H.; Wang, J.; Li, Y.; Liao, J.; Yang, Y.; Ma, L.; Liu, N.; Li, F. Functional core–shell SiO2@ COF: A potential chromatography material for separating no-carrier-added 177Lu. Sep. Purif. Technol. 2025, 11, 134313. [Google Scholar] [CrossRef]
| Resin | Elution | Fractions Collected (mL) | RYb (%) | RLu (%) | DF |
|---|---|---|---|---|---|
| P507 extraction resin | 1.5 M HNO3 | 1400–2500 | 2.95 | 94.7 | 32.0 |
| Rext-P350@Resin | 1.5 M HNO3 | 1150–2100 | 0.50 | 94.4 | 190.5 |
| Rext-P350@Resin | 1.6 M HNO3 | 900–1800 | 1.24 | 97.9 | 79.2 |
| Rext-P350@Resin | 1.8 M HNO3 | 650–1300 | 2.58 | 96.9 | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Chen, Q.; Liu, C.; Xiao, C.; Ni, S. Efficient Separation of Lu from Yb Using Rext-P350@Resin: A Promising Route for No-Carrier-Added 177Lu Production. Separations 2025, 12, 215. https://doi.org/10.3390/separations12080215
Qi J, Chen Q, Liu C, Xiao C, Ni S. Efficient Separation of Lu from Yb Using Rext-P350@Resin: A Promising Route for No-Carrier-Added 177Lu Production. Separations. 2025; 12(8):215. https://doi.org/10.3390/separations12080215
Chicago/Turabian StyleQi, Jiuquan, Qianwen Chen, Chuanying Liu, Chengliang Xiao, and Shuainan Ni. 2025. "Efficient Separation of Lu from Yb Using Rext-P350@Resin: A Promising Route for No-Carrier-Added 177Lu Production" Separations 12, no. 8: 215. https://doi.org/10.3390/separations12080215
APA StyleQi, J., Chen, Q., Liu, C., Xiao, C., & Ni, S. (2025). Efficient Separation of Lu from Yb Using Rext-P350@Resin: A Promising Route for No-Carrier-Added 177Lu Production. Separations, 12(8), 215. https://doi.org/10.3390/separations12080215






