The Application of
Abstract
1. Introduction
2. Materials and Methods
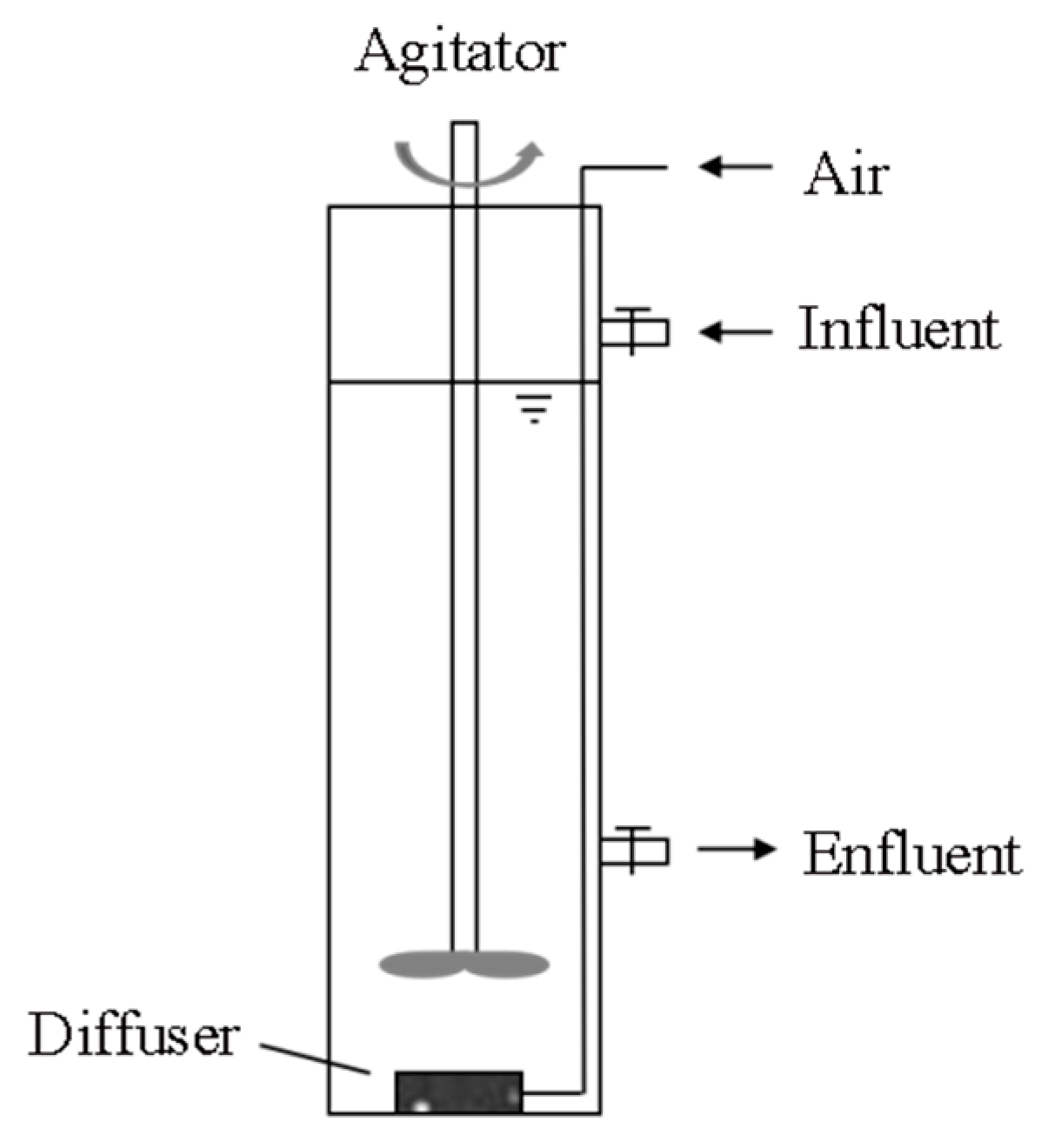
2.1. Reactor System and Operation
2.2. Influent Water Quality
2.3. Polysulfide Solutions
2.4. Selection of Polysulfide Species
- M—actual generation of , in mg/L;
- —theoretical production of when actual removal of entirely due to S2−, in mg/L;
- —theoretical production of when actual removal of entirely due to S0, in mg/L;
- —proportion of the contribution of S2− to the actual removal of , in %;
- —proportion of the contribution of S0 to the actual removal of , in %.
- —theoretical generation of , in mg/L;
- —actual generation of , in mg/L;
- —proportion of the contribution of S2− to the theoretical removal of , in %;
- —proportion of the contribution of S0 to the theoretical removal of , in %;
- —relative utilization rate of S2− to removal, in %;
- —relative utilization rate of S0 to removal, in %.
2.5. Optimization of Polysulfide Dosage
2.6. Research on the Stable Operation of the Polysulfide Autotrophic Denitrification Process
3. Results and Discussion
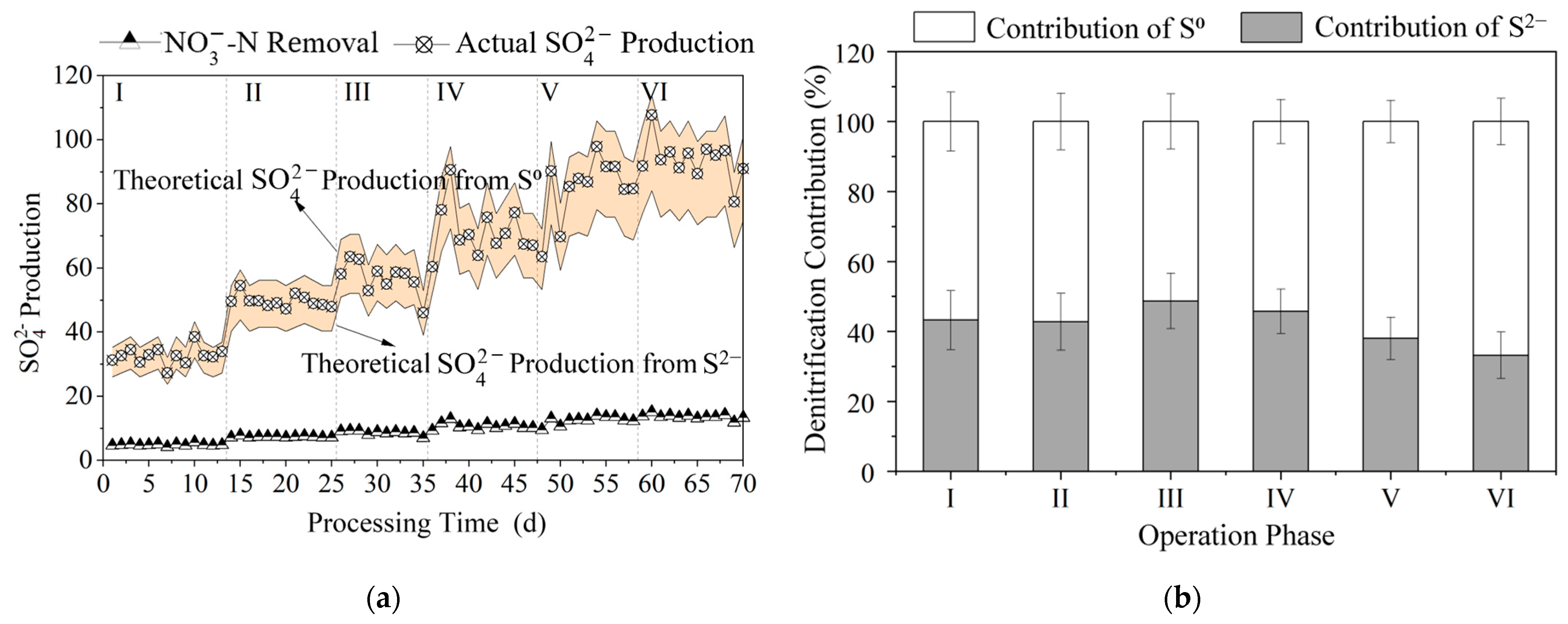
3.1. Influence of Polysulfide Species on the Effectiveness of Autotrophic Denitrification for Nitrogen Removal
3.1.1. Comparison of Nitrogen Removal Performance Among Different Polysulfide Species
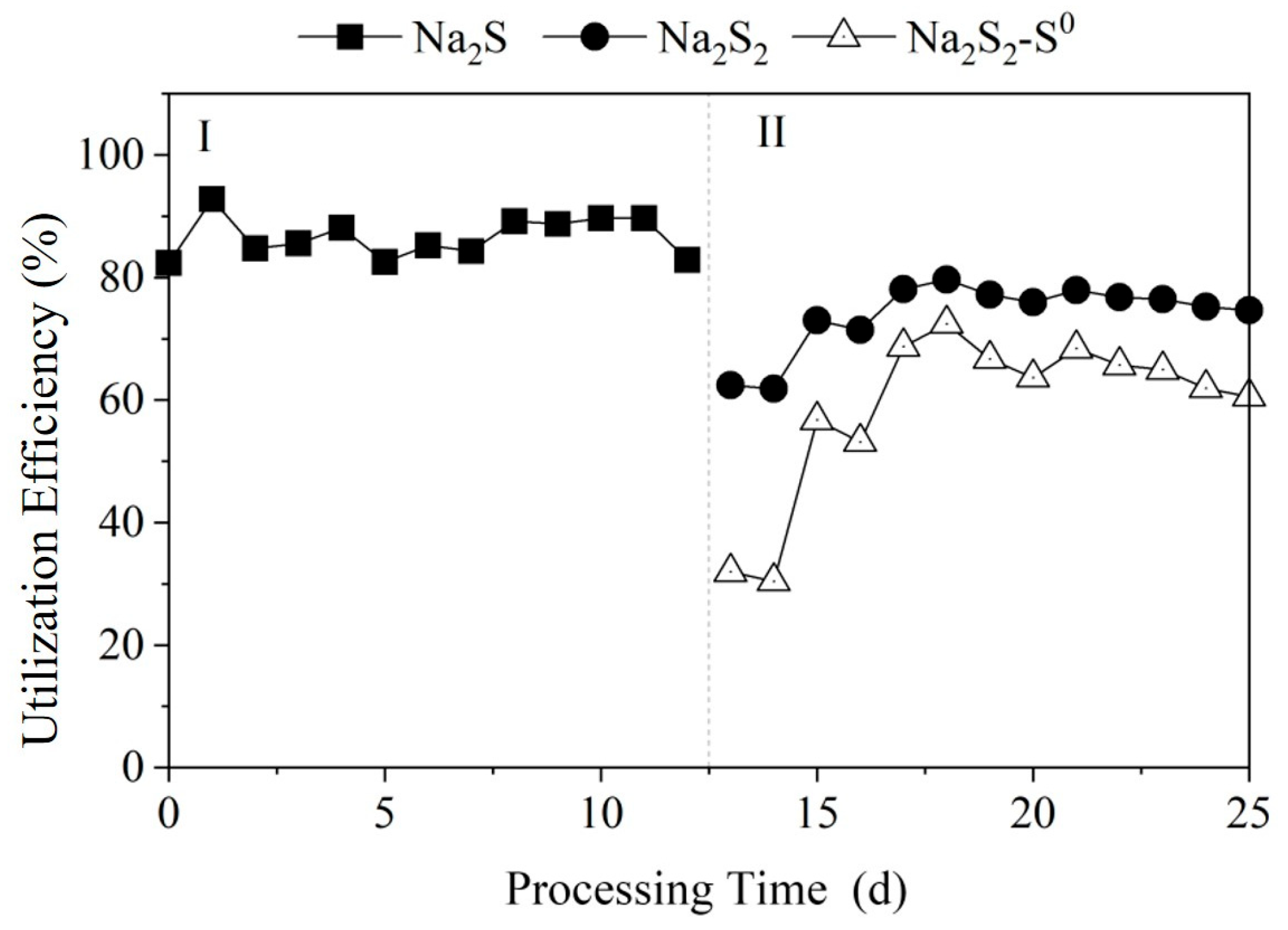
3.1.2. Sulfur Utilization Efficiency Among Different Polysulfide Species
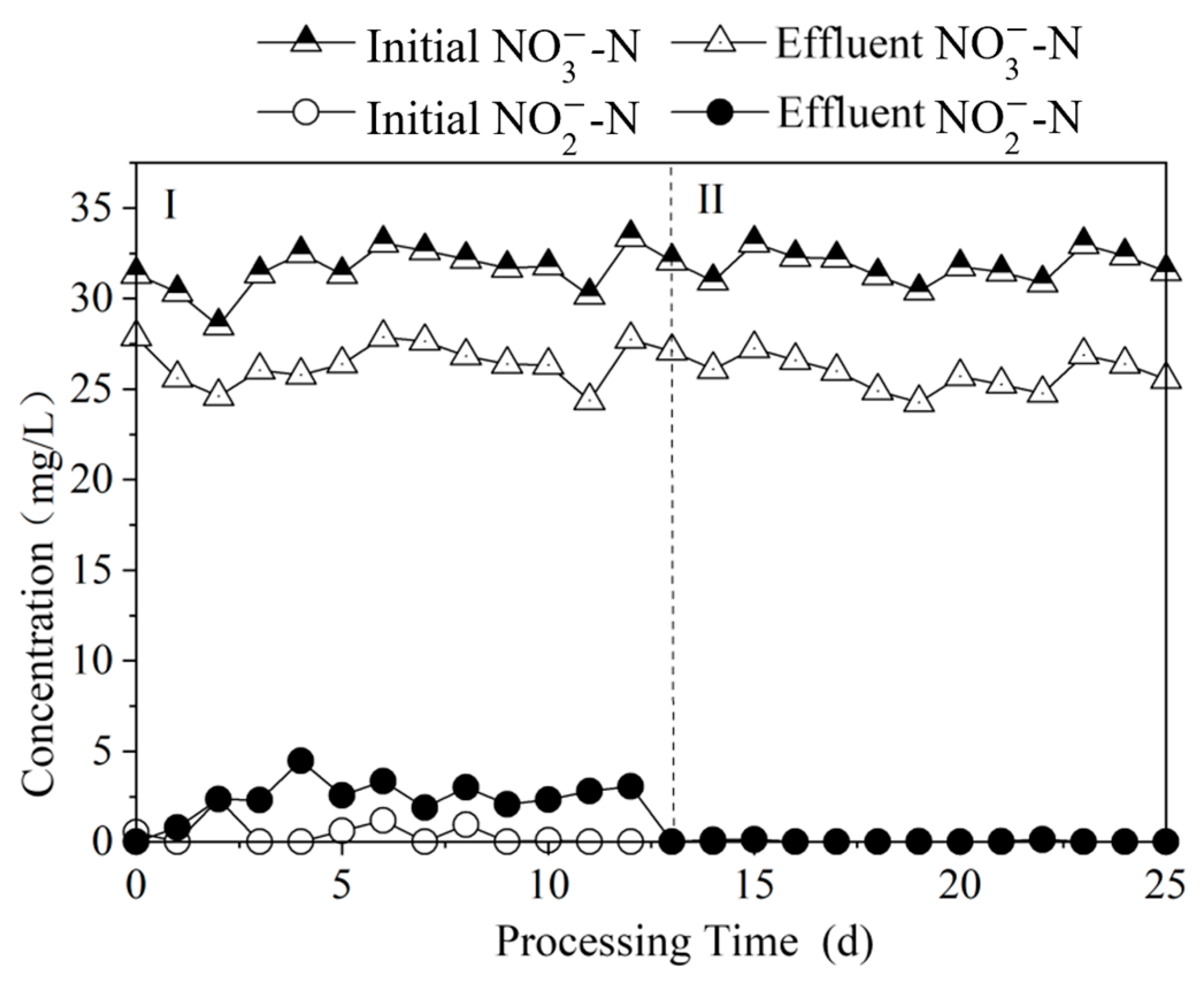
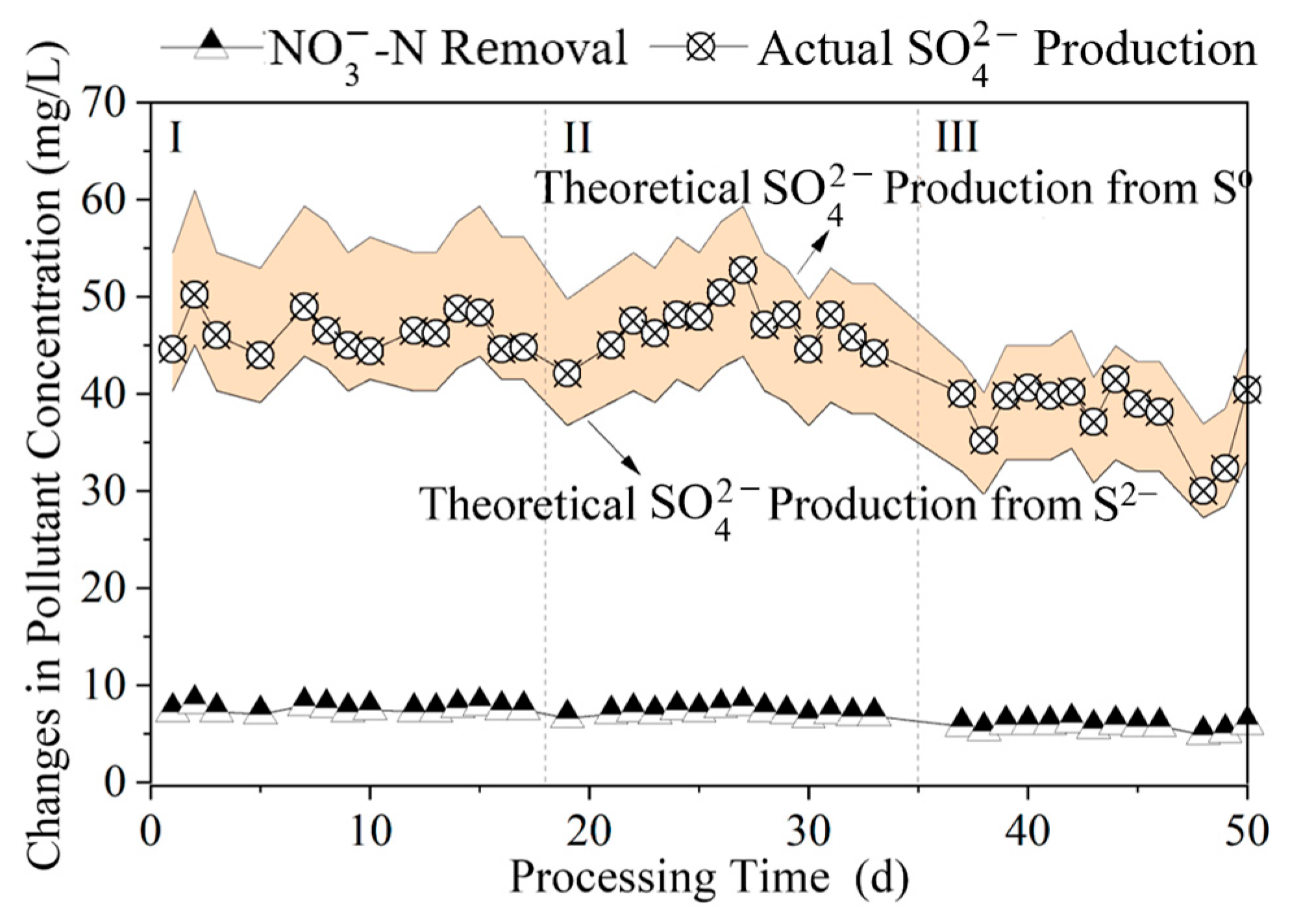
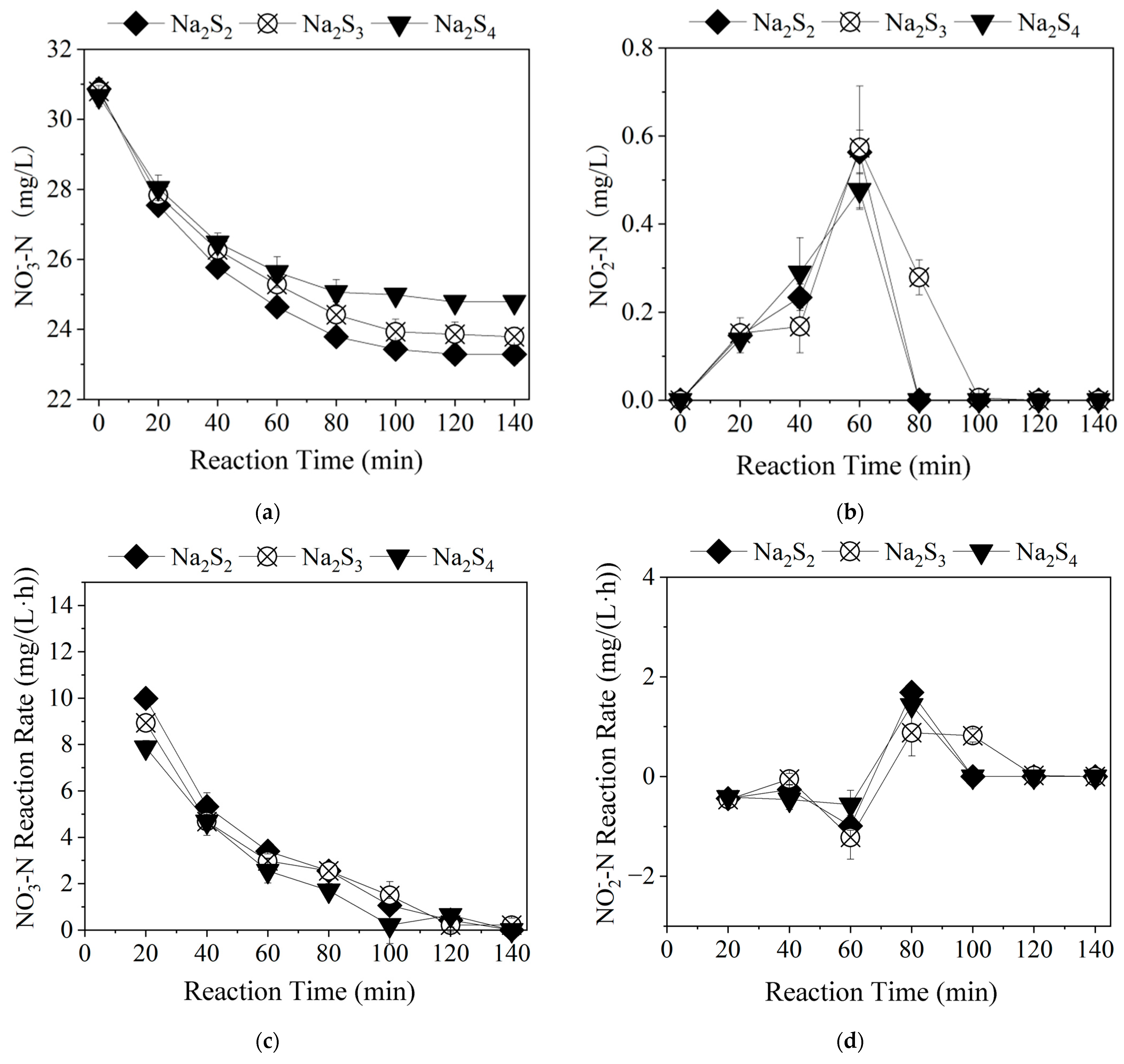
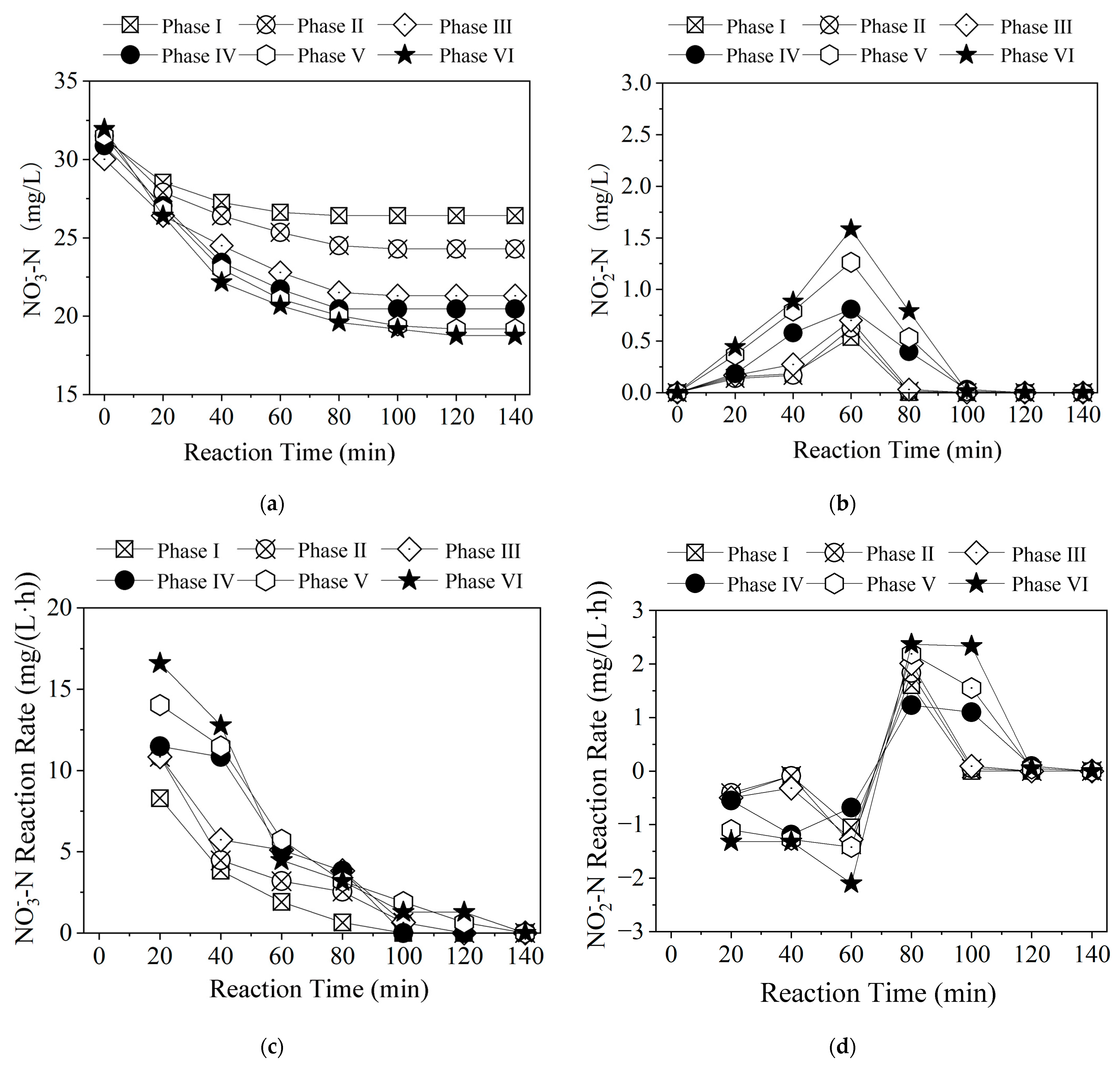
3.1.3. Time-Dependent Changes in Nitrogen Pollutants Among Different Polysulfide Species
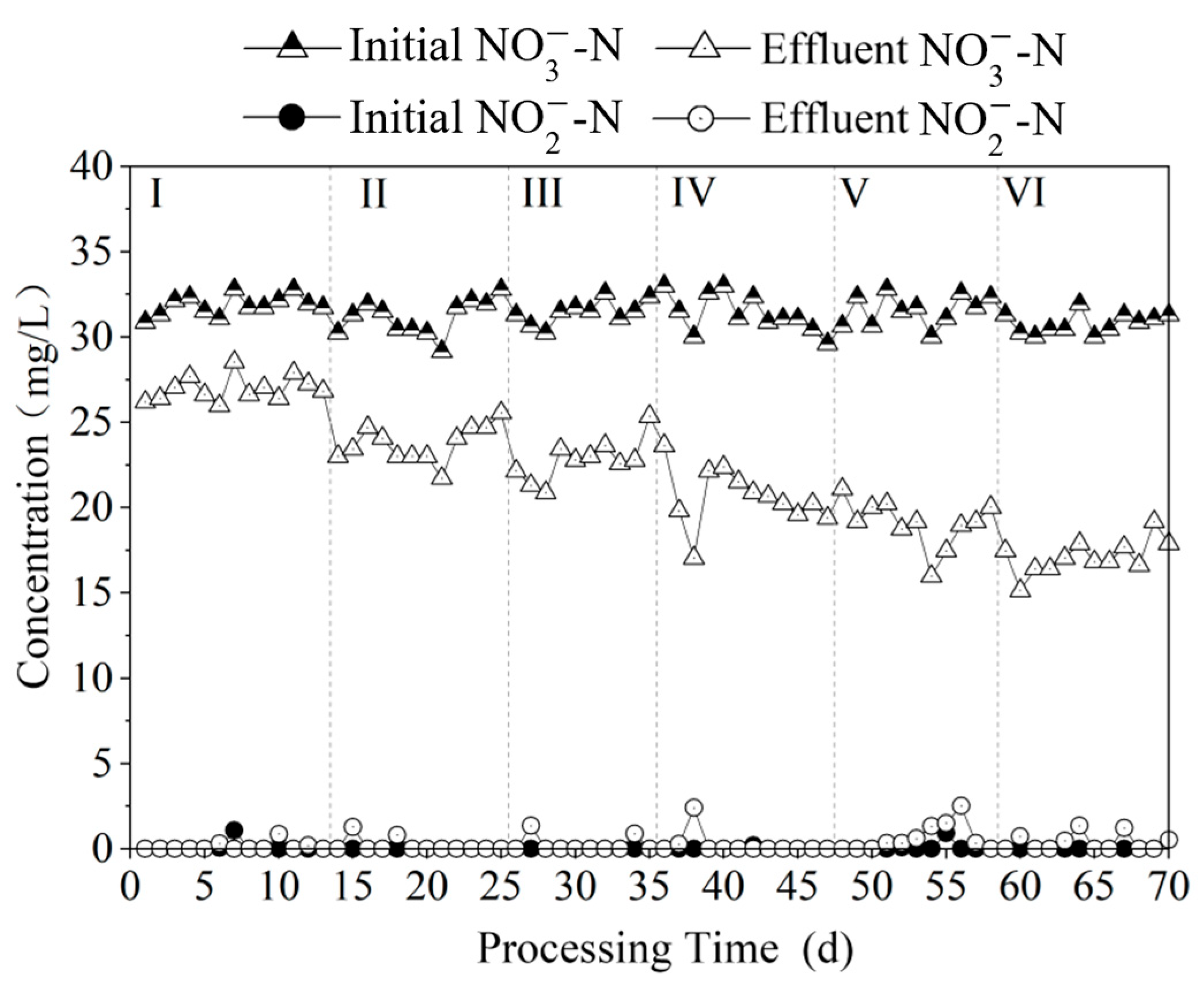
3.2. Influence of Polysulfide Dosage on the Effectiveness of Autotrophic Denitrification for Nitrogen Removal
3.2.1. Comparison of Nitrogen Removal Effect at Different Na2S3 Dosages
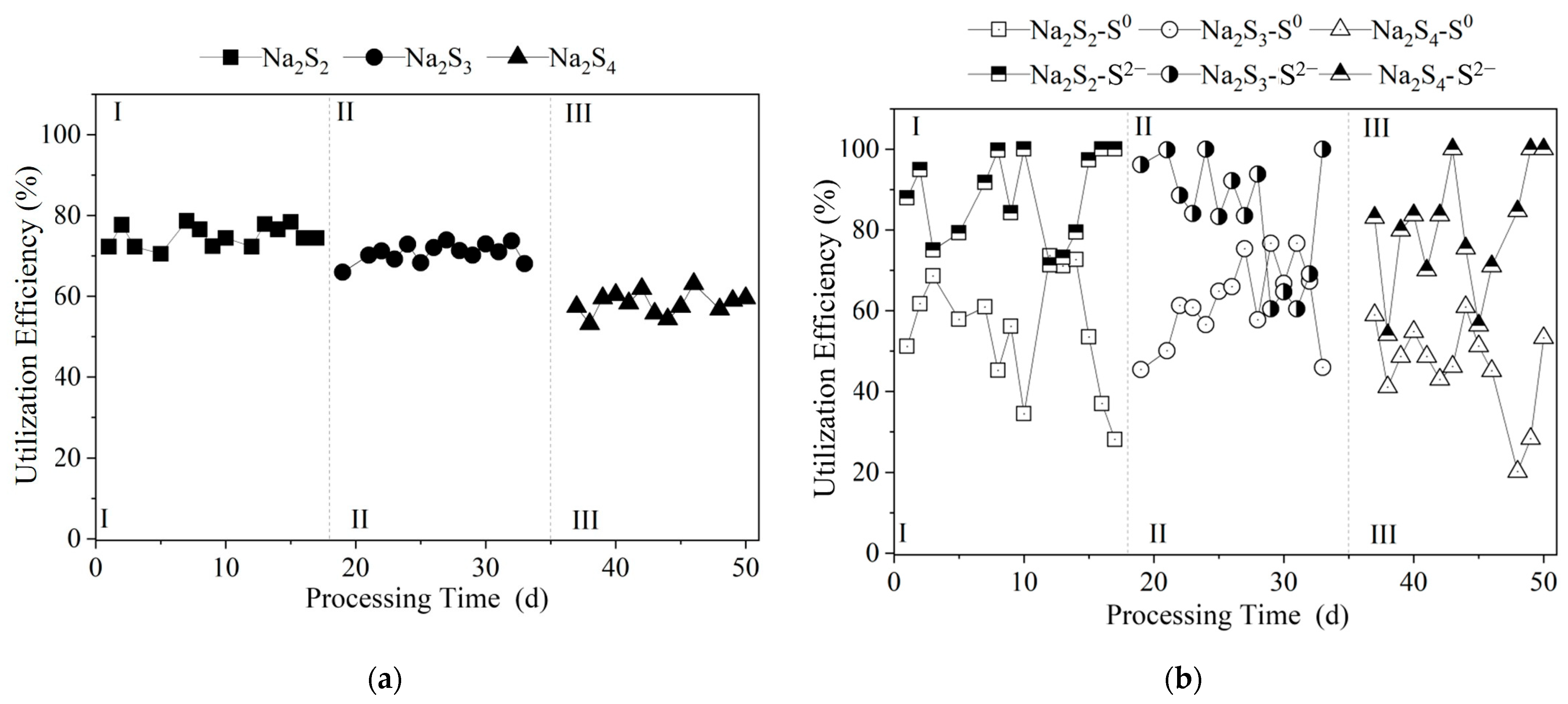
3.2.2. Sulfur Utilization Efficiency at Different Na2S3 Dosages
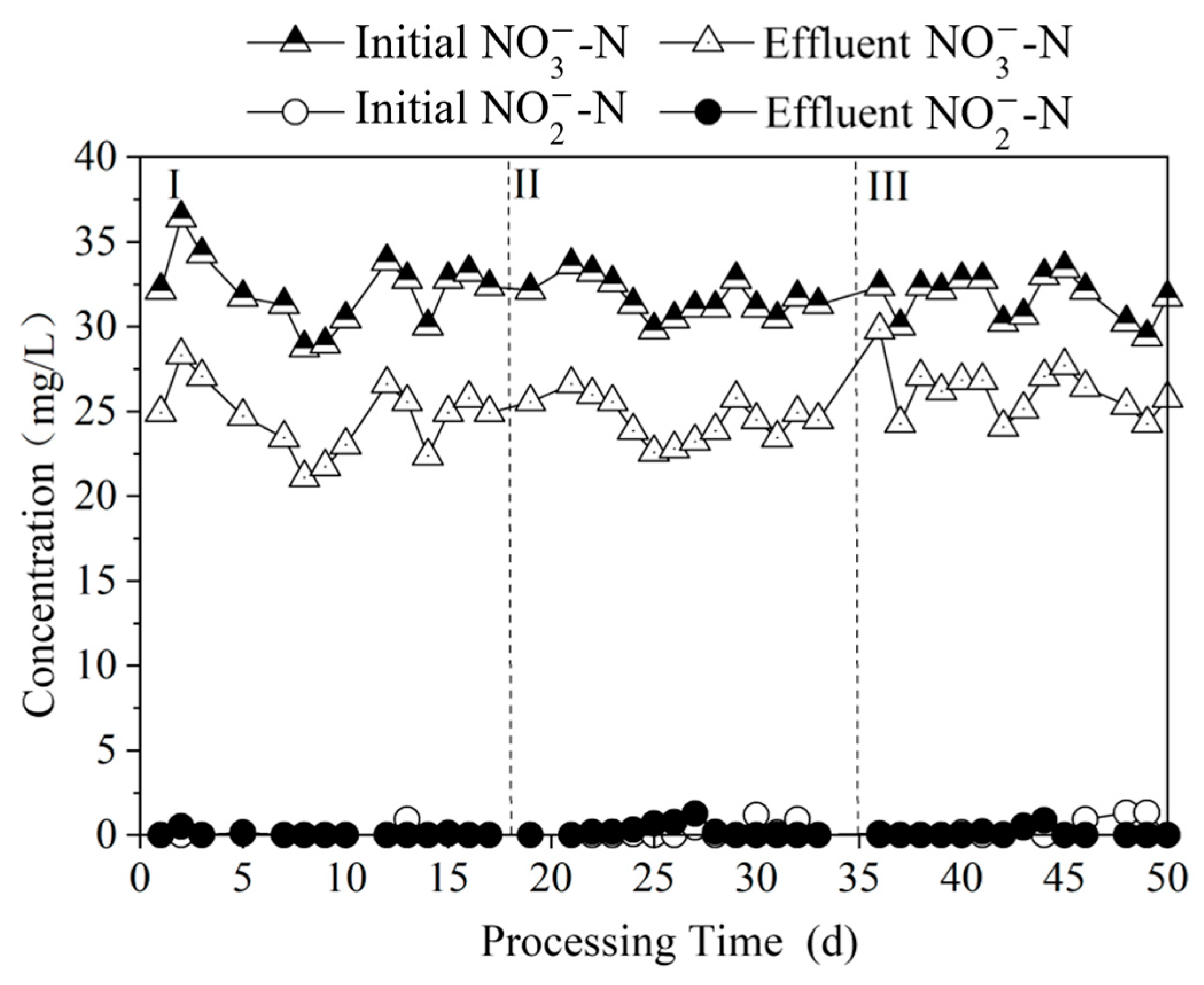
3.2.3. Time-Dependent Changes in Nitrogen Pollutants at Different Na2S3 Dosages
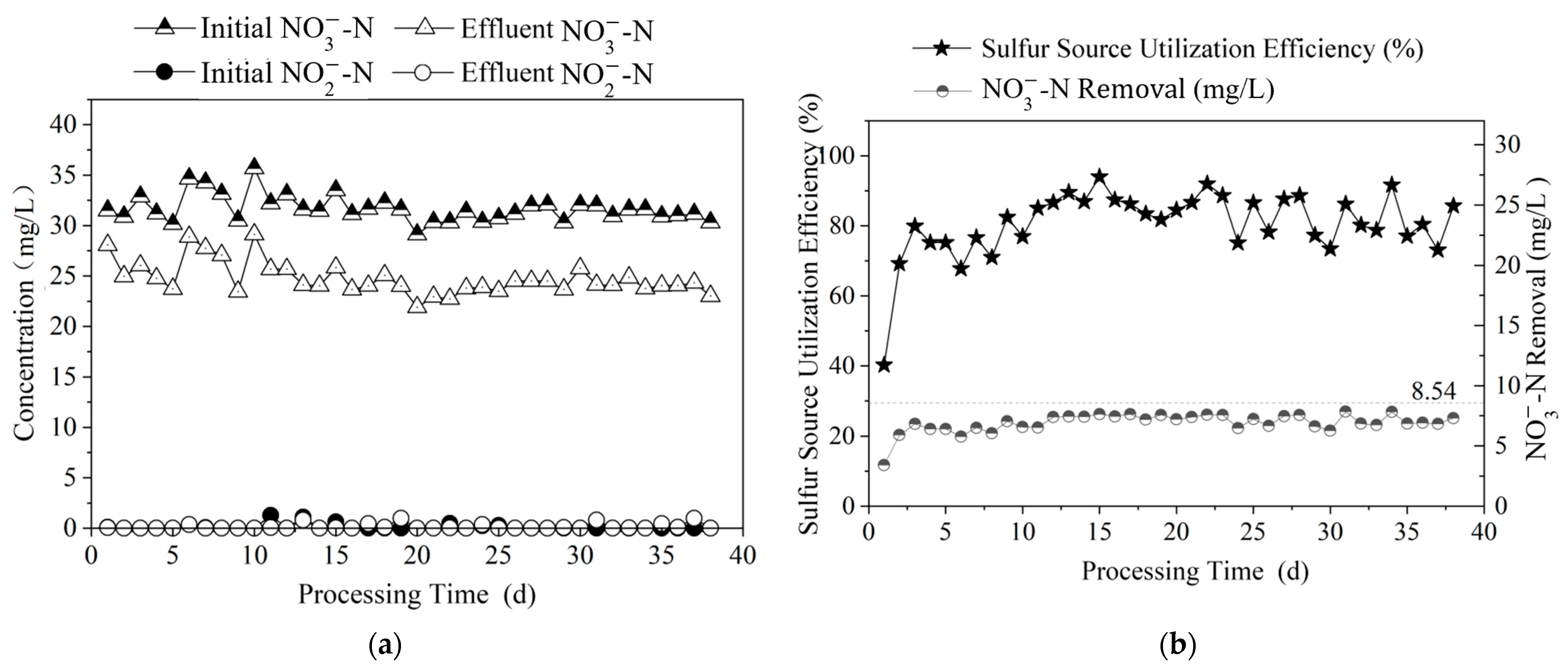
3.3. Study on Stable Operation of Polysulfide Autotrophic Denitrification Process
3.3.1. Effect of Nitrogen Removal
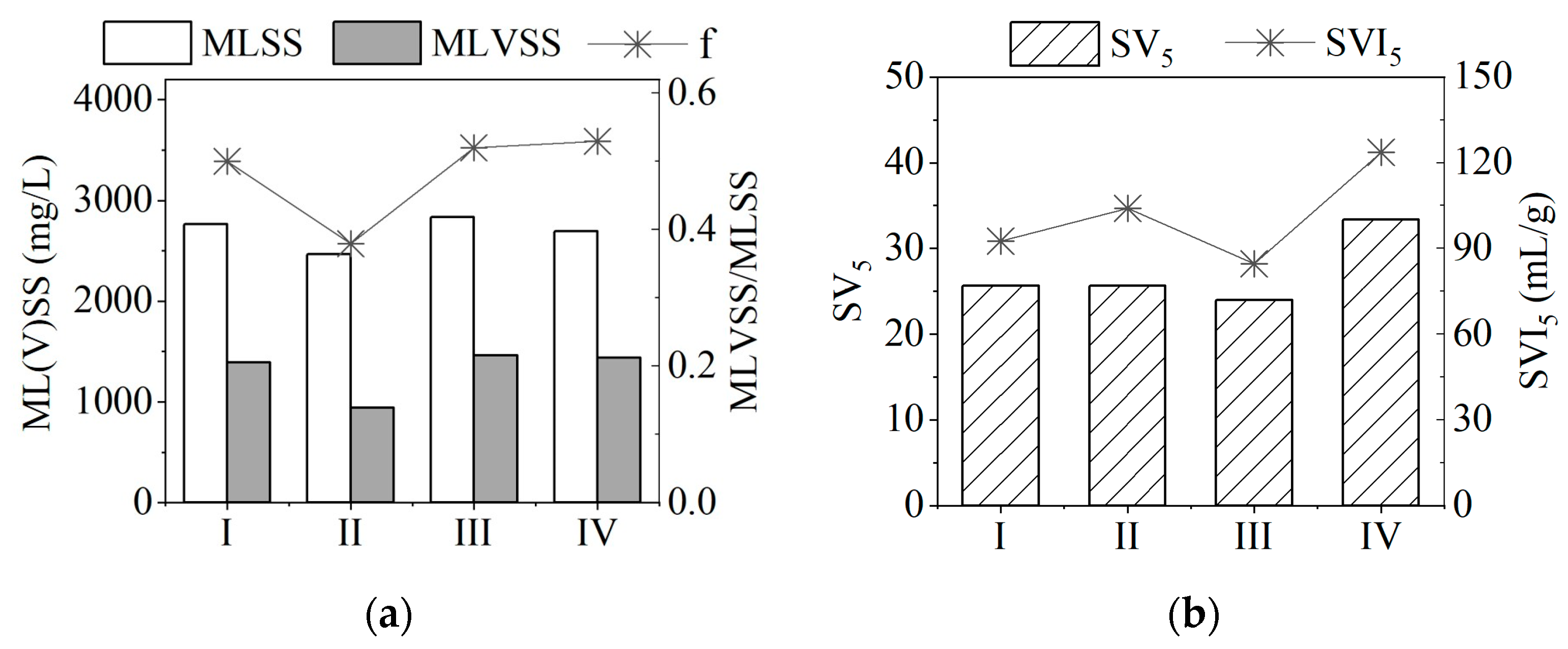
3.3.2. Changes in Sludge Characteristics
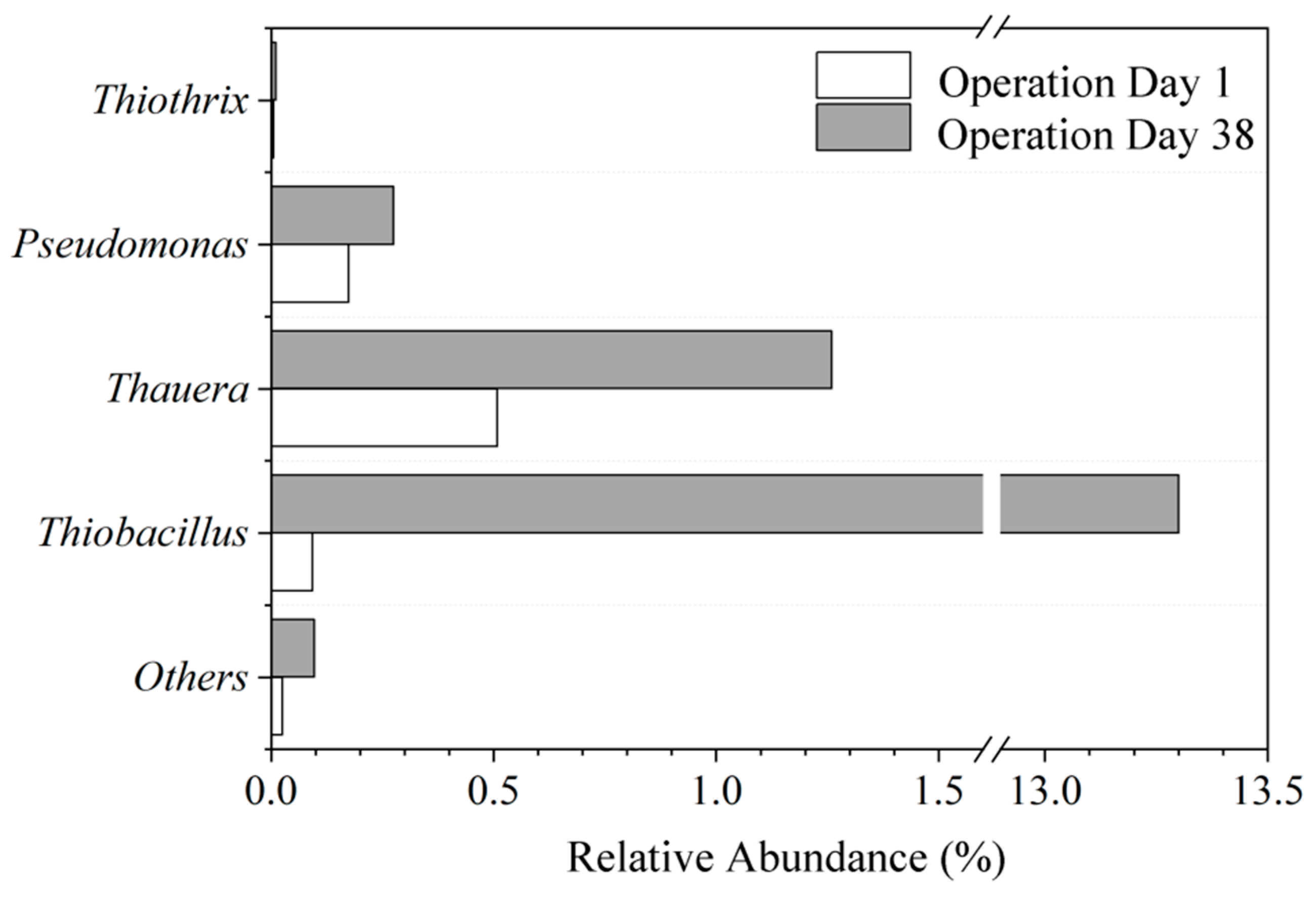
3.3.3. Changes in Microbial Community Structure
3.4. Cost Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, G.X.; Kong, Z.A.; Zhang, Y.F.; Qiu, L.W.; Wang, H.; Yan, Q. Simultaneous ammonia and nitrate removal by novel integrated partial denitrification-anaerobic ammonium oxidation-bioelectrochemical system. Bioresour. Technol. 2024, 396, 130428. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.P.; Li, Q.H.; Meng, C.L.; Han, M.S.; Ma, Y.M.; Brancelj, A. Using a structural equation model to assess the spatiotemporal dynamics and driving factors of phytoplankton in the plateau hongfeng reservoir in southwest china. Aquat. Ecol. 2022, 56, 1297–1313. [Google Scholar] [CrossRef]
- DB4403/T 64-2020; Standards of Water Quality for Wastewater Treatment Plant. Shenzhen Administration for Market Regulation: Shenzhen, China, 2020.
- Liu, S.Q.; Long, Z.Q.; Liang, J.S.; Zhang, J.; Hu, D.F.; Hou, P.F.; Zhang, G.M. Interpretable causal machine learning optimization tool for improving efficiency of internal carbon source-biological denitrification. Bioresour. Technol. 2025, 416, 131787. [Google Scholar] [CrossRef]
- Chen, T.Y.; Yang, X.Y.; Sun, Q.; Hu, A.Y.; Qin, D.; Li, J.W.; Wang, Y.H.; Yu, C.P. Changes in wastewater treatment performance and the microbial community during the bioaugmentation of a denitrifying pseudomonas strain in the low carbon-nitrogen ratio sequencing batch reactor. Water 2022, 14, 540. [Google Scholar] [CrossRef]
- DiCapua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron donors for autotrophic denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Zhai, L.M.; CaiJi, Z.M.; Liu, J.; Wang, H.Y.; Ren, T.Z.; Gai, X.P.; Xi, B.; Liu, H.B. Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol. Fertil. Soils. 2015, 51, 113–122. [Google Scholar] [CrossRef]
- Yang, W.M.; Zhao, Q.; Lu, H.; Ding, Z.; Meng, L.; Chen, G.H. Sulfide-driven autotrophic denitrification significantly reduces n2o emissions. Water Res. 2016, 90, 176–184. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Gao, J.Q.; Liu, L.; Mao, Y.L.; Kang, H.Y.; Song, Z.X.; Cai, M.; Guo, P.C.; Chen, K. Performance and by-product generation in sulfur-siderite/limestone autotrophic denitrification systems: Enhancing nitrogen removal efficiency and operational insights. J. Environ. Manag. 2024, 370, 123042. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Li, B.l. Autotrophic nitrogen removal characteristics of pn-anammox process enhanced by sulfur autotrophic denitrification under mainstream conditions. Bioresour. Technol. 2020, 316, 123926. [Google Scholar] [CrossRef]
- Zhu, T.T.; Ding, J.Z.; Liu, Y.R.; Li, X.F.; Wang, Z.W.; Liu, Y.W. The effect of organic sources on the electron distribution and n2o emission in sulfur-driven autotrophic denitrification biofilters. Sci. Total Environ. 2023, 903, 166126. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Zhang, L.; Mu, X.T.; Li, G.B.; Guan, X.Q.; Hong, J.Y.; Jiang, F. Overlooked pathways of denitrification in a sulfur-based denitrification system with organic supplementation. Water Res. 2020, 169, 115084. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, E.; Toledo-Alarcón, J.; Fernández, E.; Campos, J.L.; Oyarzún, R.; Etchebehere, C.; Cardeña, R.; Cabezas, A.; Koók, L.; Bakonyi, P.; et al. A review of autotrophic denitrification for groundwater remediation: A special focus on bioelectrochemical reactors. J. Environ. Chem. Eng. 2024, 12, 111552. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Z.K.; Xu, Y.F.; Liang, C.Z.; Peng, L. Improved performance of sulfur-driven autotrophic denitrification process by regulating sulfur-based electron donors. Water 2024, 16, 730. [Google Scholar] [CrossRef]
- Li, W.J.; Zhu, L.; Pan, C.; Chen, W.D.; Xu, D.D.; Kang, D.; Guo, L.Y.; Mei, Q.Q.; Zheng, P.; Zhang, M. Insights into the superior bioavailability of biogenic sulfur from the view of its unique properties: The key role of trace organic substances. Environ. Sci. Technol. 2023, 57, 1487–1498. [Google Scholar] [CrossRef]
- Ma, K.; Adams, M.W. Ferredoxin: Nadp oxidoreductase from pyrococcus furiosus. Methods Enzymol. 2001, 334, 40–45. [Google Scholar]
- Sorokin, D.Y.; Rusanov, I.I.; Pimenov, N.V.; Tourova, T.P.; Abbas, B.; Muyzer, G. Sulfidogenesis under extremely haloalkaline conditions in soda lakes of kulunda steppe (Altai, Russia). FEMS Microbiol. Ecol. 2010, 73, 278–290. [Google Scholar] [CrossRef][Green Version]
- Qiu, Y.Y.; Gong, X.Z.; Zhang, L.; Zhou, S.J.; Li, G.B.; Jiang, F. Achieving a novel polysulfide-involved sulfur-based autotrophic denitrification process for high-rate nitrogen removal in elemental sulfur-packed bed reactors. ACS ES&T Eng. 2022, 2, 1504–1513. [Google Scholar]
- Bao, H.X.; Wang, H.L.; Wang, S.T.; Sun, Y.L.; Zhang, X.N.; Cheng, H.Y.; Qian, Z.M.; Wang, A.J. Response of sulfur-metabolizing biofilm to external sulfide in element sulfur-based denitrification packed-bed reactor. Environ. Res. 2023, 231, 116061. [Google Scholar] [CrossRef]
- Wang, F.; Liang, D.D.; He, W.H.; Liu, G.H.; Li, J.N.; Tian, Y.; Feng, Y.J. Insights into sulfur cycling contribution to nitrogen removal in sulfur autotrophic denitrification combined microbial electrochemical denitrification. Chem. Eng. J. 2024, 482, 148882. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zheng, K.; Zhai, S.Y.; Cheng, H.Y.; Qian, Z.M.; Wang, H.C.; Yang, J.X.; Zhang, X.N.; Wang, A.J. A novel pattern of coupling sulfur-based autotrophic disproportionation and denitrification processes for achieving high-rate and precisely adjustable nitrogen removal. Chem. Eng. J. 2023, 476, 146772. [Google Scholar] [CrossRef]
- Qiao, Z.S.; Zhang, Y.G.; Meng, Z.H.; Xie, Q.S.; Lin, L.; Zheng, H.F.; Sa, B.S.; Lin, J.; Wang, L.S.; Peng, D.L. Anchoring polysulfides and accelerating redox reaction enabled by Fe-based compounds in lithium–sulfur batteries. Adv. Funct. Mater. 2021, 31, 2100970. [Google Scholar] [CrossRef]
- Wang, W.; Xi, K.; Li, B.W.; Li, H.J.; Liu, S.; Wang, J.N.; Zhao, H.Y.; Li, H.L.; Abdelkader, A.M.; Gao, X.P.; et al. A sustainable multipurpose separator directed against the shuttle effect of polysulfides for high-performance lithium–sulfur batteries. Adv. Energy Mater. 2022, 12, 2200160. [Google Scholar] [CrossRef]
- Johnston, K.A.K.Y.; van Lankveld, M.; Rink, R.d.; Mol, A.R.; Keesman, K.J.; Buisman, C.J.N. Influence of oxidation-reduction potential and pH on polysulfide concentrations and chain lengths in the biological desulfurization process under haloalkaline conditions. Water Res. 2024, 259, 121795. [Google Scholar] [CrossRef]
- Fu, K.M.; Kang, J.; Zhao, J.; Bian, Y.H.; Li, X.D.; Yang, W.B.; Li, Z.R. Efficient nitrite accumulation in partial sulfide autotrophic denitrification (PSAD) system: Insights of S/N ratio, pH and temperature. Environ. Technol. 2024, 45, 5419–5436. [Google Scholar] [CrossRef]
- Polizzi, C.; Gabriel, D.; Munz, G. Successful sulphide-driven partial denitrification: Efficiency, stability and resilience in srt-controlled conditions. Chemosphere 2022, 295, 133936. [Google Scholar] [CrossRef]
- Zhan, M.J.; Zeng, W.; Wu, C.C.; Chen, G.X.; Meng, Q.A.; Hao, X.J.; Peng, Y.Z. Impact of organic carbon on sulfide-driven autotrophic denitrification: Insights from isotope fractionation and functional genes. Water Res. 2024, 255, 121507. [Google Scholar] [CrossRef]
- Hao, X.D.; Wei, H.Y.; Yu, W.B.; Zhu, Y.M. Analysis of the pros and cons for sulfur autotrophic denitrification technology. Acta Sci. Circumstantiae 2024, 44, 1–10. [Google Scholar]
- Ucar, D.; Yilmaz, T.; Di Capua, F.; Esposito, G.; Sahinkaya, E. Comparison of biogenic and chemical sulfur as electron donors for autotrophic denitrification in sulfur-fed membrane bioreactor (SMBR). Bioresour. Technol. 2020, 299, 122574. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Li, W.; Shi, M.; Zhang, M.; Xu, P.L.; Li, B.L.; Huang, Y. Effects of different reduced sulfur forms as electron donors in the start-up process of short-cut sulfur autotrophic denitrification. Bioresour. Technol. 2022, 354, 127194. [Google Scholar] [CrossRef]
- Wang, S.S.; Cheng, H.Y.; Zhang, H.; Su, S.G.; Sun, Y.L.; Wang, H.c.; Han, J.L.; Wang, A.J.; Guadie, A. Sulfur autotrophic denitrification filter and heterotrophic denitrification filter: Comparison on denitrification performance, hydrodynamic characteristics and operating cost. Environ. Res. 2021, 197, 111029. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Xie, L.Y.; Lyu, C.; Xu, P.L.; Yuan, Y.; Li, X.; Huang, Y.; Li, W.; Zhang, M.; Shi, M. Effects of salinity on nitrite and elemental sulfur accumulation in a double short-cut sulfur autotrophic denitrification process. Bioresour. Technol. 2023, 369, 128432. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Ren, N.Q.; Wang, A.J.; Liu, L.H.; Lee, D.J. Enhanced performance of denitrifying sulfide removal process under micro-aerobic condition. J. Hazard. Mater. 2010, 179, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Yu, J.H.; Wang, H.T.; Jin, C.J.; Zhao, Y.G.; Guo, L. Effect of magnetic powder (Fe3O4) on heterotrophic-sulfur autotrophic denitrification efficiency and electron transport system activity for marine recirculating aquacultural wastewater treatment. J. Environ. Manag. 2024, 370, 122749. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.X.; Wang, D.X.; Chen, G.; Zhao, X.B.; Fan, L.H. Advance in the sulfur-based electron donor autotrophic denitrification for nitrate nitrogen removal from wastewater. World J. Microbiol. Biotechnol. 2023, 40, 7. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.C.; Wang, D.H.; Yang, Y.; Fan, L.J.; Xu, S.J.; Zhuang, X.L. Quorum sensing responses of activated sludge to free nitrous acid: Zoogloea deformation, ahl redistribution, and microbiota acclimatization. Water Res. 2023, 238, 119993. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Hasar, H.; Kaksonen, A.H.; Rittmann, B.E. Performance of a sulfide-oxidizing, sulfur-producing membrane biofilm reactor treating sulfide-containing bioreactor effluent. Environ. Sci. Technol. 2011, 45, 4080–4087. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Q.; Li, Z.L.; Ma, X.D.; Hou, Y.N.; Ren, N.Q.; Wang, A.J. Relationship between functional bacteria in a denitrification desulfurization system under autotrophic, heterotrophic, and mixotrophic conditions. Water Res. 2021, 188, 116526. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, K.; Yang, S.; Li, Z.J.; Wang, Y.Z.; Ma, F.; Chen, P.; Zhu, T. A novel coupling process to replace the traditional multi-stage anammox process-sulfur autotrophic denitrification coupled anammox system. Biodegradation 2024, 35, 565–582. [Google Scholar] [CrossRef]
- Ma, X.R.; Zheng, Z.M.; Bian, W.; Li, J.; Zhou, R.X.; Zhou, X.Y. Study on operation efficiency and microbial community structure of sulfur-based autotrophic denitrification system. China Environ. Sci. 2020, 40, 4335–4341. [Google Scholar]
- Hisaya, K.; Miho, W.; Manabu, F. Sulfuritortus calidifontis gen. Nov., sp. Nov., a sulfur oxidizer isolated from a hot spring microbial mat. Int. J. Syst. Evol. Microbiol. 2017, 67, 1355–1358. [Google Scholar]
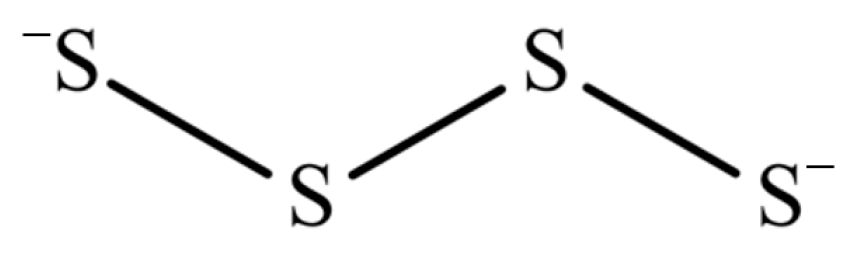


| Filling | Aerobic Phase | Anoxic Phase | Aeration Stripping | Settlement | Draining | Cycle |
|---|---|---|---|---|---|---|
| 10 | 240 | 180 | 5 | 40 | 5 | 480 |
| Components | Concentration (mg/L) | Mean Value (mg/L) | Pharmaceuticals |
|---|---|---|---|
| 20–25 | 22.5 | NH4Cl | |
| TP | 3–5 | 4.0 | KH2PO4 |
| Alkalinity | 240–300 | 270 | NaHCO3 |
| Components | Concentration (g/L) |
|---|---|
| EDTA-2Na | 6.37 |
| FeSO4·7H2O | 9.15 |
| Components | Concentration (g/L) |
|---|---|
| EDTA-2Na | 19.11 |
| ZnSO4·7H2O | 0.43 |
| CoCl2·6H2O | 0.24 |
| MnCl2·4H2O | 0.99 |
| CuSO4·5H2O | 0.25 |
| NaMo4·2H2O | 0.22 |
| NiCl2·6H2O | 0.19 |
| NaSeo4·10H2O | 0.21 |
| H3BO4 | 0.014 |
| Species | Molar Ratio Csodium sulfide:Csulfur powder | |
|---|---|---|
| n = 1 | Na2S | - |
| n = 2 | Na2S2 | 1:1 |
| n = 3 | Na2S3 | 1:2 |
| n = 4 | Na2S4 | 1:3 |
| Stage | Species | Concentration (mol/L) | Dosage (mL) | Dosage of S (mg S/L) |
|---|---|---|---|---|
| I | Na2S | 0.1 | 8.0 | 8.5 |
| II | Na2S2 | 0.1 | 8.0 | 17.1 |
| Stage | Species | Dosage of S (mg S/L) | Content (mg/L) |
|---|---|---|---|
| I | Na2S2 | 21.4 | 10.0 |
| II | Na2S3 | 22.5 | 10.0 |
| III | Na2S4 | 23.1 | 10.0 |
| Stage | Species | Solution (mol/L) | Solution (mg/L) | (mg S/L) | Content (mg/L) |
|---|---|---|---|---|---|
| I | Na2S3 | 0.1 | 4.0 | 12.8 | 5.7 |
| II | Na2S3 | 0.1 | 6.0 | 19.2 | 8.5 |
| III | Na2S3 | 0.1 | 8.0 | 25.6 | 11.4 |
| IV | Na2S3 | 0.1 | 12.0 | 38.4 | 17.1 |
| V | Na2S3 | 0.1 | 16.0 | 51.2 | 22.8 |
| VI | Na2S3 | 0.1 | 20.0 | 64.0 | 28.5 |
| Groups | Sampling Period | Sulfur Concentration in System mg S/L |
|---|---|---|
| I | Day 1 of operation | 19.2 |
| II | Day 12 of operation | 19.2 |
| III | Day 22 of operation | 19.2 |
| IV | Day 38 of operation | 19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, X.; Ye, C.; He, Z.; Wang, H.; Li, J.
The Application of
Sun Y, Zhang X, Ye C, He Z, Wang H, Li J.
The Application of
Sun, Yingxue, Xiaolei Zhang, Chenli Ye, Ziying He, Hongjie Wang, and Ji Li.
2025. "The Application of
Sun, Y., Zhang, X., Ye, C., He, Z., Wang, H., & Li, J.
(2025). The Application of







