Insights into the Adsorptive Separation of Ethylene/Ethane in LTA-Type Zeolites
Abstract
1. Introduction
2. Materials and Methods
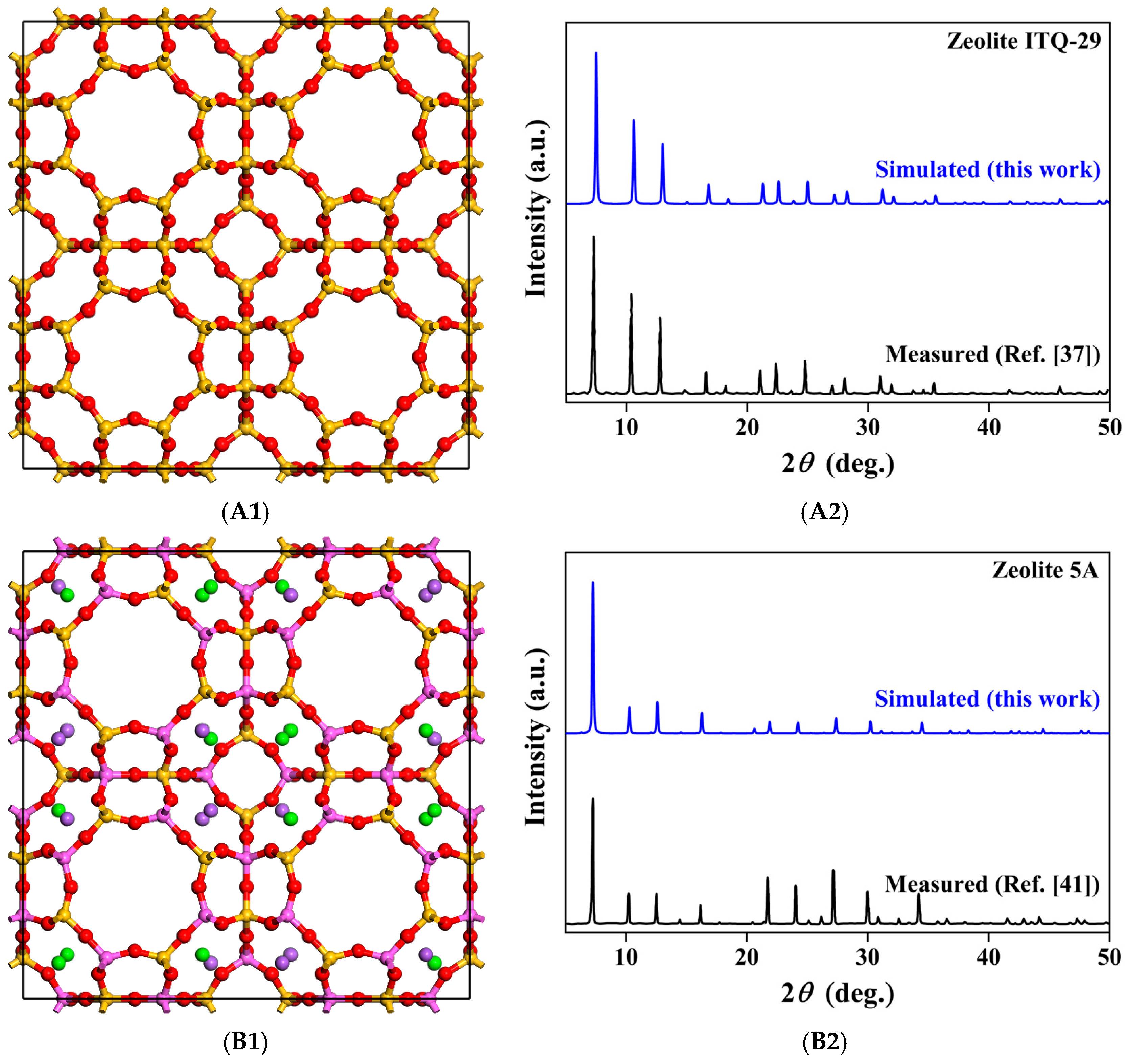
2.1. Structural Models and Density Functional Theory Calculations
2.2. Grand Canonical Monte Carlo Simulations
2.3. Molecular Dynamics Simulations
3. Results and Discussion
3.1. Adsorption Structures and Adsorption Energies
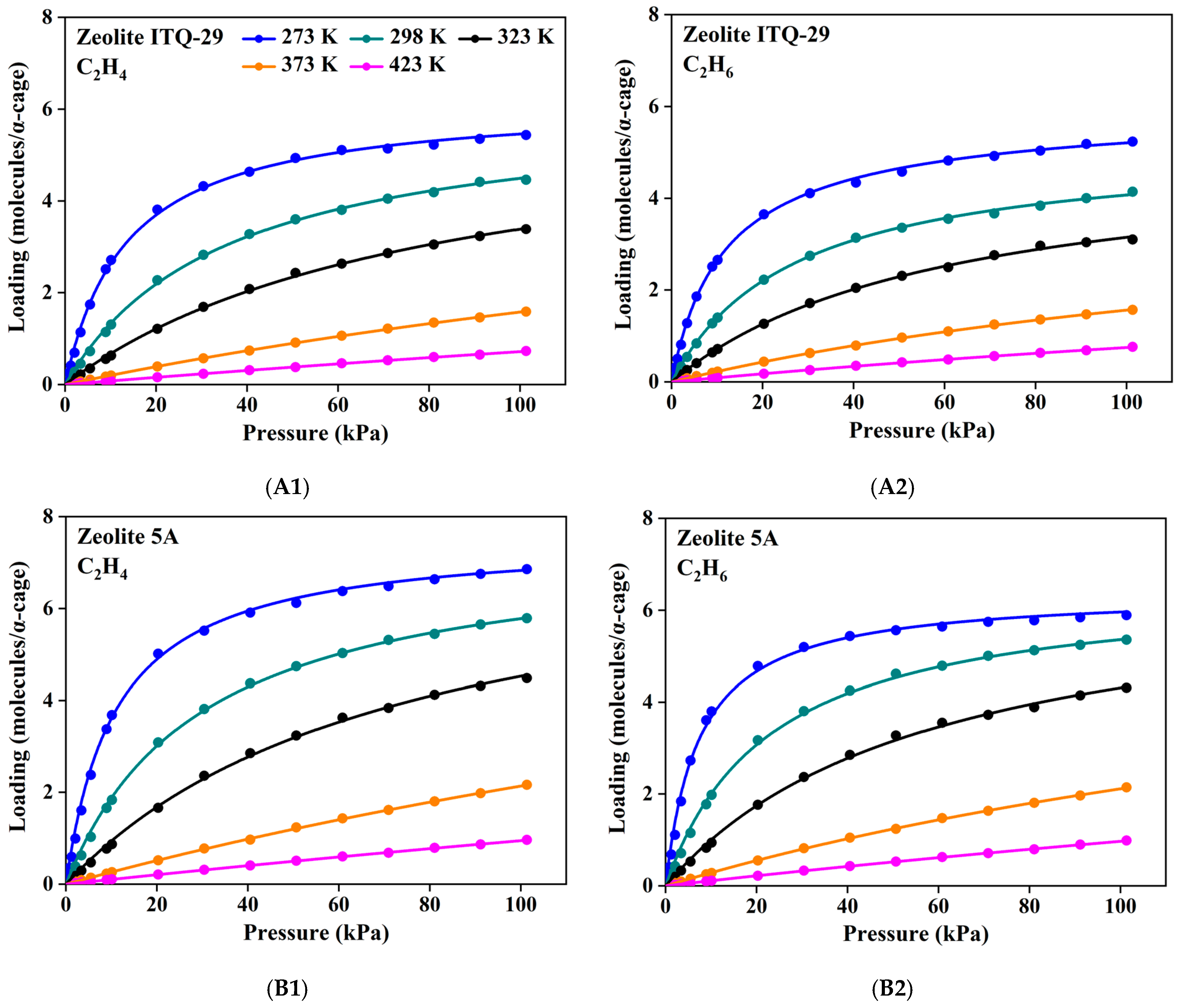
3.2. Adsorption Isotherms
3.3. Diffusion Coefficients and Activation Energies
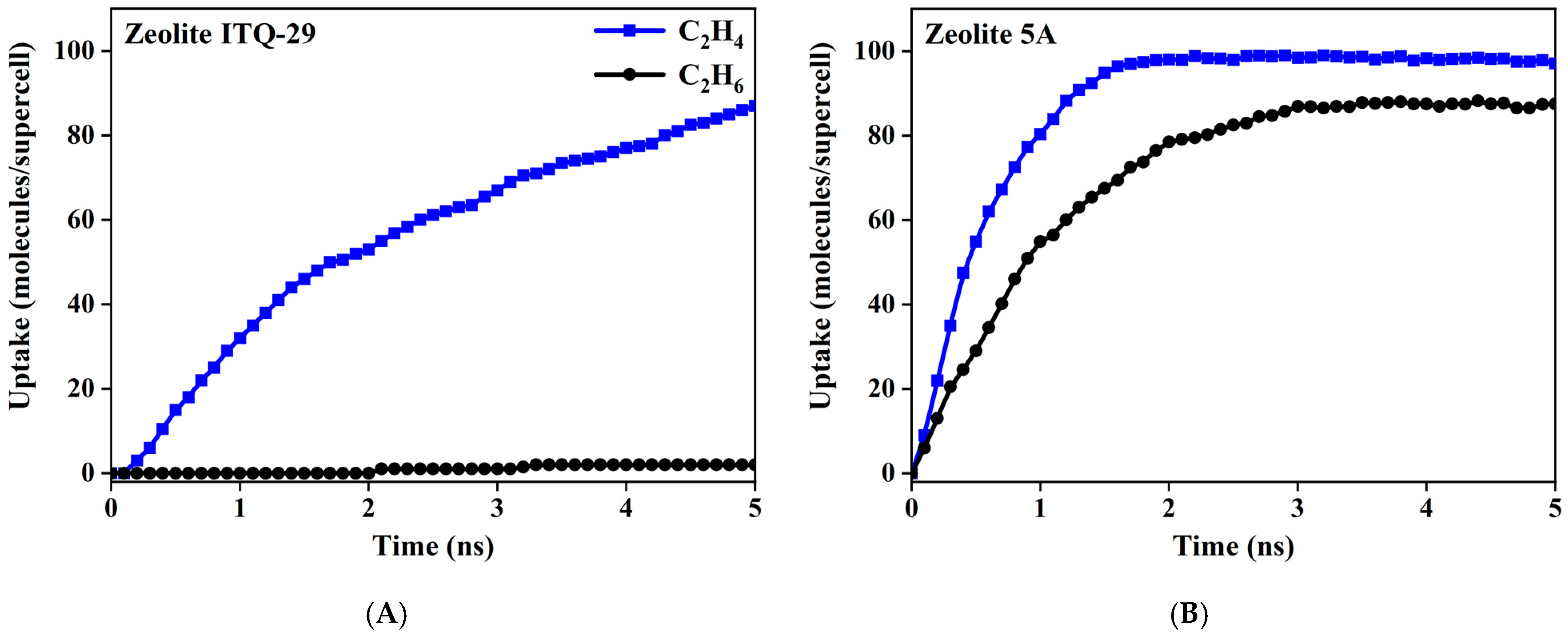
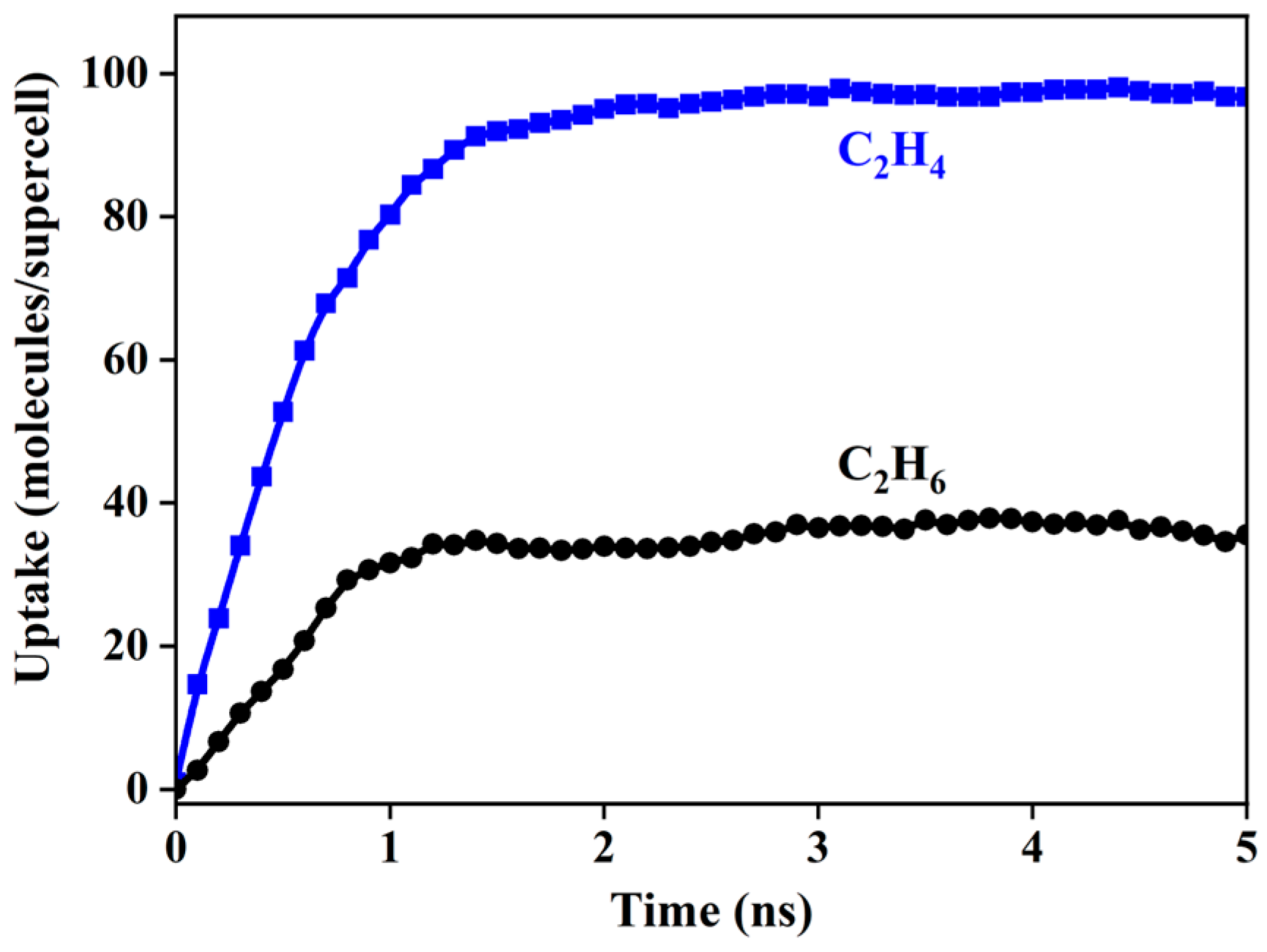
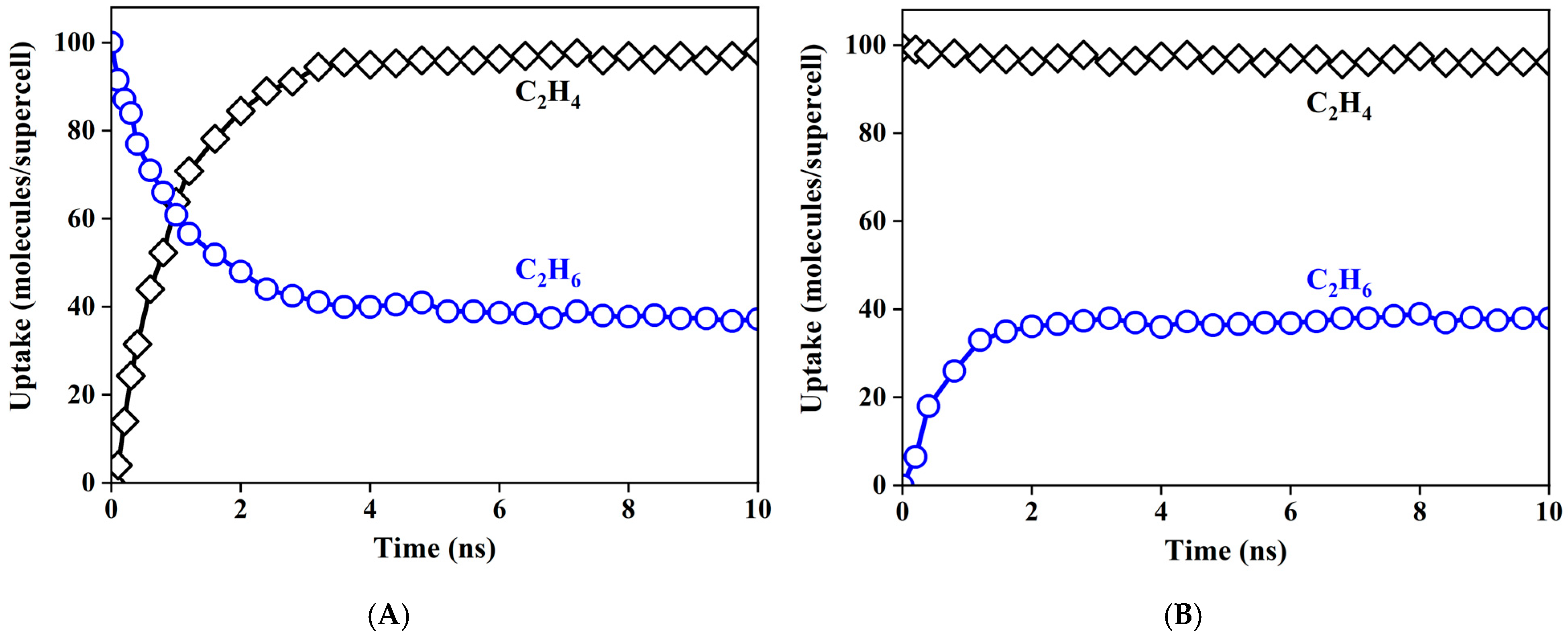
3.4. Adsorption Kinetics
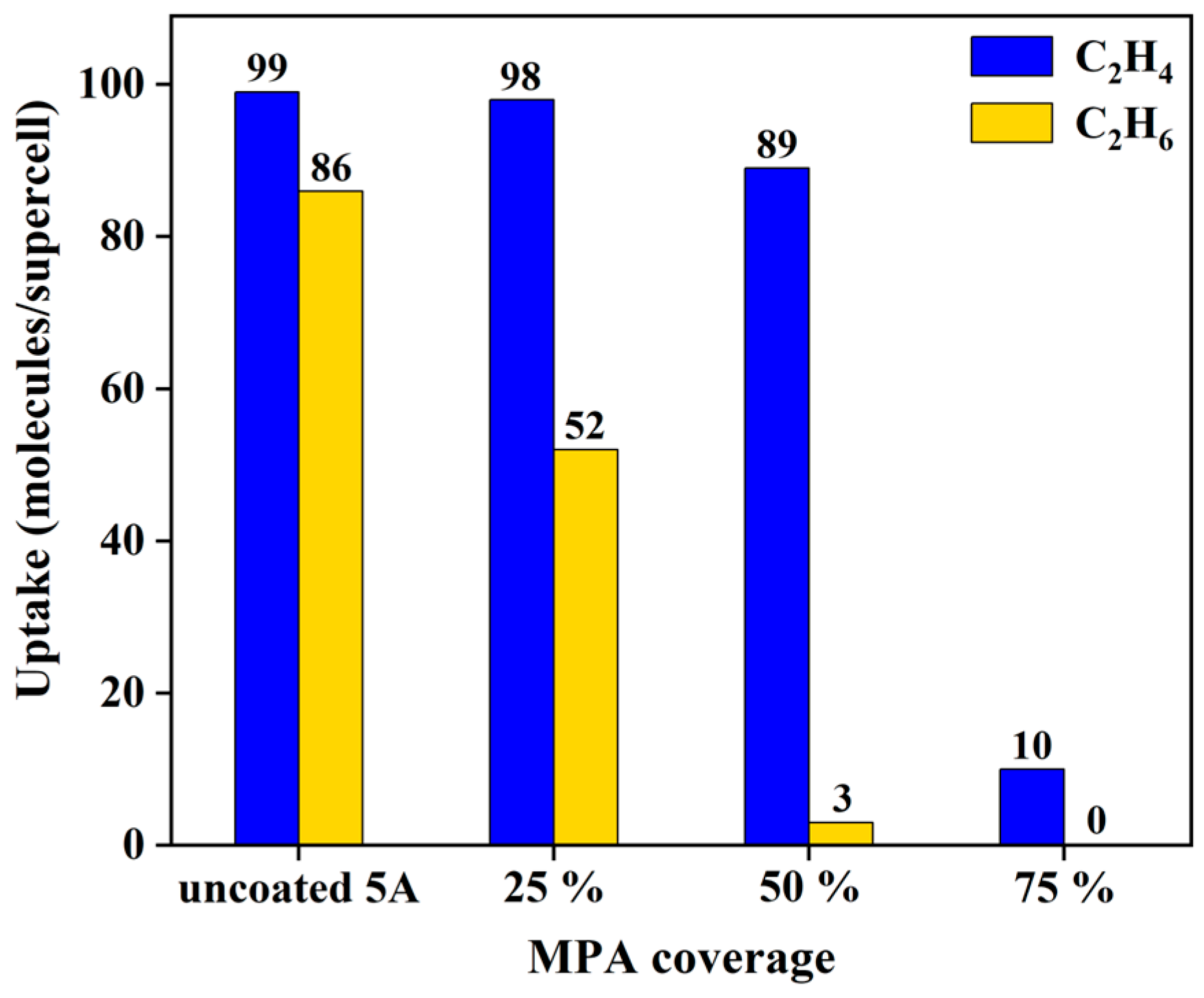
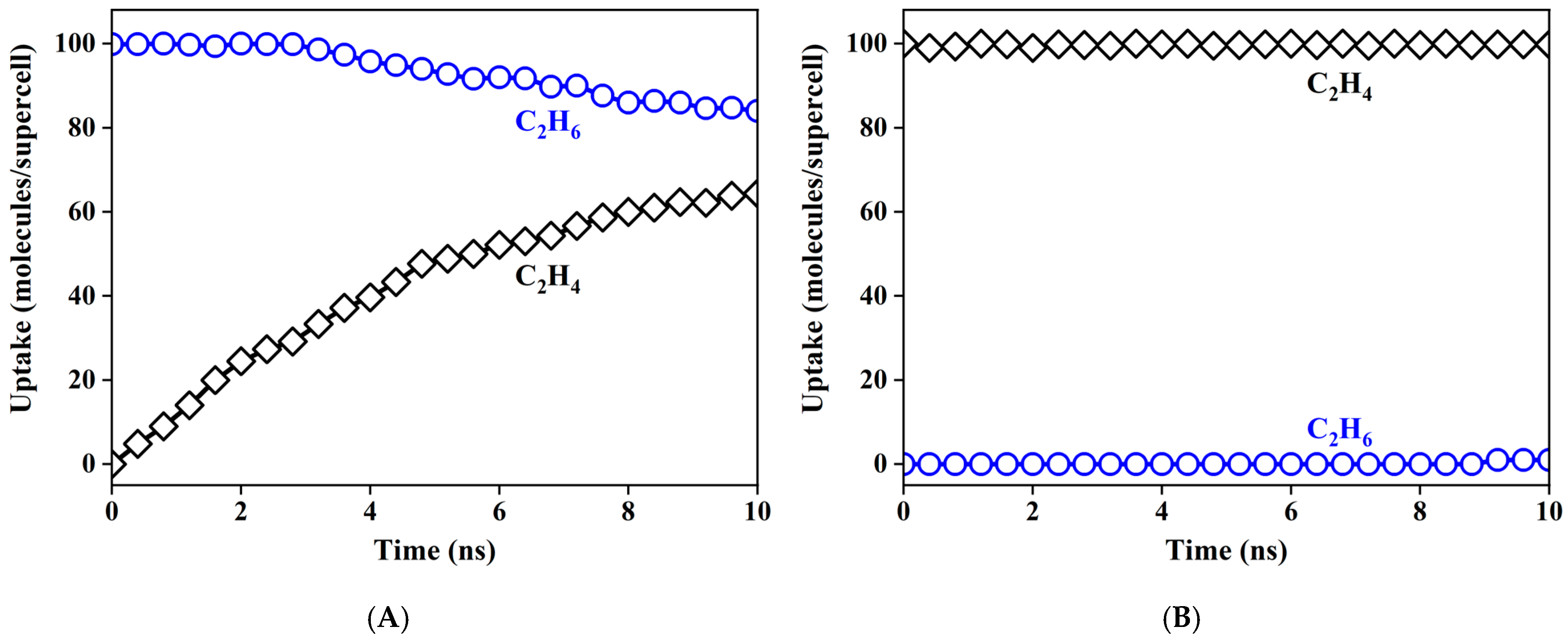
3.5. Surface Modification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| MD | Molecular dynamics |
| MPA | Methyl phosphonic acid |
| D | Diffusion coefficients |
References
- Almuqati, N.S.; Aldawsari, A.M.; Alharbi, K.N.; González-Cortés, S.; Alotibi, M.F.; Alzaidi, F.; Dilworth, J.R.; Edwards, P.P. Catalytic production of light olefins: Perspective and prospective. Fuel 2024, 366, 131270. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-polyethylene (Bio-PE), bio-polypropylene (Bio-PP) and bio-poly (ethylene terephthalate) (Bio-PET): Recent developments in bio-based polymers analogous to petroleum-derived ones for packaging and engineering applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, J.; Wang, F. An economic analysis of twenty light olefin production pathways. J. Energy Chem. 2021, 56, 193–202. [Google Scholar] [CrossRef]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W., Jr.; Kortunov, P.; Ravikovitch, P.I.; et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Reyes, S.C. Adsorptive separation of light olefins from paraffins. Microporous Mesoporous Mater. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- Mersmann, A.; Fill, B.; Hartmann, R.; Maurer, S. The potential of energy saving by gas-phase adsorption processes. Chem. Eng. Technol. 2000, 23, 937–944. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Zhang, H.; Zhang, J.; Pan, Q.; Yan, W. Zeolites for the separation of ethylene, ethane, and ethyne. Front. Chem. Sci. Eng. 2024, 18, 108. [Google Scholar] [CrossRef]
- Sun, Z.; Shu, Q.; Zhang, Q.; Li, S.; Zhu, G.; Wang, C.; Zhang, J.; Li, H.; Huang, Z. A hydrothermal synthesis process of ZSM-5 zeolite for VOCs adsorption using desilication solution. Separations 2024, 11, 39. [Google Scholar] [CrossRef]
- Xing, J.; Jing, F.; Chu, W.; Sun, H.; Yu, L.; Zhang, H.; Luo, S. Improvement of adsorptive separation performance for C2H4/C2H6 mixture by CeO2 promoted CuCl/activated carbon adsorbents. Acta Phys.-Chim. Sin. 2015, 31, 2158–2164. [Google Scholar] [CrossRef]
- Sircai, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework-based splitter for separating propylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xu, Y.; Yao, X.; Wang, C.; Liu, P.; Zhao, H.; Lu, J.; Ju, J. Efficient removal of tetracycline by metal-organic framework ZIF-67 and its mechanism. Separations 2024, 11, 63. [Google Scholar] [CrossRef]
- Kan, X.; Wang, J.; Dong, Y. COFs as porous organic materials for the separation and purification of ethylene from C2 hydrocarbons. Chem. Commun. 2024, 60, 13984–13997. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, Z.; He, Y.; Xue, W.; Huang, H.; Zhong, C. Machine-learning-aided computational study of covalent organic frameworks for reversed C2H6/C2H4 separation. Ind. Eng. Chem. Res. 2022, 61, 11116–11123. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Wang, J.; Wen, H.; Krishna, R.; Wu, H.; Zhou, W.; Chen, Z.; Li, B.; Qin, G.; et al. Selective ethane/ethylene separation in a robust microporous hydrogen-bonded organic framework. J. Am. Chem. Soc. 2020, 142, 633–640. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, L.; Pei, J.; Krishna, R.; Wu, H.; Zhou, W.; Qin, G.; Chen, B.; Li, B. A rod-packing hydrogen-bonded organic framework with suitable pore confinement for benchmark ethane/ethylene separation. Angew. Chem. Int. Ed. 2021, 60, 10304–10310. [Google Scholar] [CrossRef]
- Saha, D.; Kim, M.B.; Robinson, A.J.; Babarao, R.; Thallapally, P.K. Elucidating the mechanisms of paraffin-olefin separations using nanoporous adsorbents: An overview. iScience 2021, 24, 103042. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Feng, C.; E, J.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Foster, M.D. Packing sticky hard spheres into rigid zeolite framework. Microporous Mesoporous Mater. 2009, 118, 106–114. [Google Scholar] [CrossRef]
- Padin, J.; Rege, S.U.; Yang, R.T.; Cheng, L.S. Molecular sieve sorbents for kinetic separation of propane/propylene. Chem. Eng. Sci. 2000, 55, 4525–4535. [Google Scholar] [CrossRef]
- Liu, J.; Goss, J.; Calverley, T.; Liu, Y.; Broomall, C.; Kang, J.; Golombeski, R.; Anaya, D.; Moe, B.; Mabe, K.; et al. Carbon molecular sieve fiber with 3.4–4.9 angstrom effective micropores for propylene/propane and other gas separations. Microporous Mesoporous Mater. 2020, 305, 110341. [Google Scholar] [CrossRef]
- Rege, S.U.; Padin, J.; Yang, R.T. Olefin/paraffin separations by adsorption: π-complexation vs. kinetic separation. AIChE J. 1998, 44, 799–809. [Google Scholar] [CrossRef]
- Sala, A.; Pérez-Botella, E.; Jordá, J.L.; Cantin, A.; Rey, F.; Valencia, S. ITQ-69: A germanium-containing zeolite and its synthesis, structure determination, and adsorption properties. Angew. Chem. Int. Ed. 2021, 60, 11745–11750. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Zhang, B.; Ren, Y.; Chen, J.; Gui, J.; Shi, X.; Yan, W.; Li, J.; Li, L. Enhancing the separation of C2H4/C2H6 in customized MOR zeolites. Inorg. Chem. Front. 2024, 11, 2427–2435. [Google Scholar] [CrossRef]
- Baamran, K.; Moreno, J.D.L.; Rownaghi, A.A.; Rezaei, F. Kinetic assessment of light hydrocarbons separation over Fe-doped 13X composite sorbents under multicomponent feed conditions. Ind. Eng. Chem. Res. 2023, 62, 11917–11929. [Google Scholar] [CrossRef]
- Ellis, L.D.; Parker, S.T.; Hu, J.; Zaccarine, S.F.; Stellato, M.J.; Funke, H.H.; Sievers, C.; Pylypenko, S.; Falconer, J.L.; Medlin, J.W. Tuning gas adsorption selectivity and diffusion rates in zeolites with phosphonic acid monolayers. Cell Rep. Phys. Sci. 2020, 1, 100036. [Google Scholar] [CrossRef]
- Zhou, X.; Falconer, J.L.; Medlin, J.W. Organic-coated zeolites for selective gas adsorption: Effect of functional group identity and coating density. Sep. Purif. Technol. 2025, 363, 132040. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Zhang, H.; Cheng, C.; Doan, H. Applications of molecular simulations for separation and adsorption in zeolites. Microporous Mesoporous Mater. 2017, 242, 294–348. [Google Scholar] [CrossRef]
- Mitra, S.; Sharma, V.K.; Chaplot, S.L.; Mukhopadhyay, R. Diffusion of hydrocarbon in zeolite and effect due to pore topology: Neutron scattering and MD simulation studies. Chem. Phys. 2014, 430, 69–77. [Google Scholar] [CrossRef]
- Guo, C.; Guan, Z.; Zhou, J.; Tang, S.; Zhang, C.; Zhao, K. Molecular insights into improved oxygen adsorption: Ag+ and Ce3+ doping in Li-LSX for air separation. Appl. Surf. Sci. 2024, 663, 160186. [Google Scholar] [CrossRef]
- Liu, S.; Lian, X.; Yue, B.; Xu, S.; Wu, G.; Chai, Y.; Zhang, Y.; Li, L. Control of zeolite local polarity toward efficient xenon/krypton separation. J. Am. Chem. Soc. 2024, 146, 8335–8342. [Google Scholar] [CrossRef]
- Carey, T.; Corma, A.; Rey, F.; Tang, C.C.; Hriljac, J.A.; Anderson, P.A. The effect of extra framework species on the intrinsic negative thermal expansion property of zeolites with the LTA topology. Chem. Commun. 2012, 48, 5829–5831. [Google Scholar] [CrossRef]
- Seff, K.; Shoemaker, D.P. The structures of zeolite sorption complexes. I. The structures of dehydrated zeolite 5A and its iodine sorption complex. Acta Crystallogr. 1967, 22, 162–170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Seminario, J.M. Energetics using DFT: Comparions to precise ab initio and experiment. Chem. Phys. Lett. 1993, 206, 547–554. [Google Scholar] [CrossRef]
- Ghosh, A.; Ma, L.; Gao, C. Zeolite molecular sieve 5A acts as a reinforcing filler, altering the morphological, mechanical, and thermal properties of chitosan. J. Mater. Sci. 2013, 48, 3926–3935. [Google Scholar] [CrossRef]
- Ozturk, Z. Hydrogen storage on lithium modified silica based chabazite type zeolite, A computational study. Int. J. Hydrogen Energy 2018, 43, 22365–22376. [Google Scholar] [CrossRef]
- Nam, G.M.; Jeong, B.M.; Kang, S.H.; Lee, B.K.; Choi, D.K. Equilibrium isotherms of CH4, C2H6, C2H4, N2, and H2 on zeolite 5A using a static volumetric method. J. Chem. Eng. Data 2005, 50, 72–76. [Google Scholar] [CrossRef]
- Gulianta, V.V.; Huth, A.J. Force fields for classical atomistic simulations of small gas molecules in silicalite-1 for energy-related gas separations at high temperatures. J. Porous Mater. 2013, 20, 741–751. [Google Scholar] [CrossRef]
- Luca, G.D.; Pullumbi, P.; Barbieri, G.; Famà, A.D.; Bernardo, P.; Drioli, E. Gusev and Suter calculation of the diffusion coefficients of light gases in silicalite-1 membrane and silica-sodalite zeolite. Sep. Purif. Technol. 2004, 36, 215–228. [Google Scholar] [CrossRef]
- Witte, N.D.; Robijins, S.; Denayer, J.F.M.; Dusselier, M.; Assche, T.R.C.V. Inverse gas chromatography study of n-alkane and 1-alkene adsorption on pure-silica LTA (ITQ-29) and CHA. J. Phys. Chem. C 2023, 127, 24393–24402. [Google Scholar] [CrossRef]
- Derrah, R.I.; Loughlin, K.F.; Ruthven, D.M. Sorption of ethylene, propylene and cyclopropane in 5A zeolite. J. Chem. Soc. 1972, 68, 1947–1955. [Google Scholar] [CrossRef]
- Bläker, C.; Mauer, V.; Pasel, C.; Dreisbach, F.; Bathen, D. Adsorption mechanisms of ethane, ethene and ethyne on calcium exchanged LTA and FAU zeolites. Adsorption 2024, 30, 1547–1564. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Yang, C. Molecule design of effective C2H4/C2H6 separation membranes: From 2D nanoporous graphene to 3D AHT zeolite. J. Membr. Sci. 2020, 604, 118033. [Google Scholar] [CrossRef]
- Foster, M.D.; Rivin, I.; Treacy, M.M.J.; Delgado Friedrichs, O. A geometric solution to the largest-free-sphere problem in zeolite framework. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Golipour, H.; Mokhtarani, B.; Mafi, M.; Moradi, A.; Godini, H.R. Experimental measurement for adsorption of ethylene and ethane gases on copper-exchanged zeolites 13X and 5A. J. Chem. Eng. Data 2020, 65, 3920–3932. [Google Scholar] [CrossRef]
- Zhou, X.; Falconer, J.L.; Medlin, J.W. Competitive adsorption between propylene and propane on zeolite 5A and the influence of organic phosphonic acid coatings. Sep. Purif. Technol. 2024, 346, 127435. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Shang, H.; Liu, J.; Jin, X.; Wang, X.; Wang, Y.; Li, J.; Yang, J. Critical role of zeolite adsorbents in a displacement-adsorption separation process of methane-nitrogen gas. Ind. Eng. Chem. Res. 2025, 64, 2966–2976. [Google Scholar] [CrossRef]
- Zhou, X.; Falconer, J.L.; Medlin, J.W. Mechanism of selectivity control for zeolites modified with organic monolayers. Microporous Mesoporous Mater 2022, 337, 111913. [Google Scholar] [CrossRef]




| Adsorbent | Adsorbate | Adsorption Energy/Enthalpy (kJ/mol) | Source |
|---|---|---|---|
| ITQ-29 | C2H4 | −27.7 | This work |
| ITQ-29 | C2H6 | −21.5 | This work |
| 5A | C2H4 | −45.6 | This work |
| 5A | C2H6 | −32.3 | This work |
| ITQ-29 | C2H4 | −25.7 | [46] |
| ITQ-29 | C2H6 | −23.0 | [46] |
| 5A | C2H4 | −33.5 | [47] |
| 5A | C2H6 | −27.6 | [47] |
| 5A | C2H4 | −59.0 | [48] |
| 5A | C2H6 | −37.0 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhou, S.; Elsayed, M.A.; Chen, Z.; Jiang, C.; Hu, Y.; Manggada, G.W. Insights into the Adsorptive Separation of Ethylene/Ethane in LTA-Type Zeolites. Separations 2025, 12, 146. https://doi.org/10.3390/separations12060146
Zhao X, Zhou S, Elsayed MA, Chen Z, Jiang C, Hu Y, Manggada GW. Insights into the Adsorptive Separation of Ethylene/Ethane in LTA-Type Zeolites. Separations. 2025; 12(6):146. https://doi.org/10.3390/separations12060146
Chicago/Turabian StyleZhao, Xiaohui, Shixue Zhou, Magdy Abdelghany Elsayed, Zhongyuan Chen, Chunhui Jiang, Yongli Hu, and Gumawa Windu Manggada. 2025. "Insights into the Adsorptive Separation of Ethylene/Ethane in LTA-Type Zeolites" Separations 12, no. 6: 146. https://doi.org/10.3390/separations12060146
APA StyleZhao, X., Zhou, S., Elsayed, M. A., Chen, Z., Jiang, C., Hu, Y., & Manggada, G. W. (2025). Insights into the Adsorptive Separation of Ethylene/Ethane in LTA-Type Zeolites. Separations, 12(6), 146. https://doi.org/10.3390/separations12060146






