Simulation of Water Vapor Sorption Profiles on Activated Carbons in the Context of the Nuclear Industry
Abstract
1. Introduction
2. Methodology for the Establishment of a Model for Breakthrough Curve Prediction
2.1. Mass Balance Equation
2.2. Adsorption Kinetics
- -
- The tortuosity (τ): given the lack of experimental measurements, a value of 3 was used, corresponding to a good order of magnitude in similar adsorption systems [33].
- -
- The molecular diffusivity (Dm): a value of 2.6 m2 s−1 from a previous work [34] was used.
- -
- The Knudsen diffusivity (DK), was calculated using Equation (10)).
- -
- The surface diffusivity (DS) was estimated using the Sladek et al. [35] correlation, seen in Equation (11).
2.3. Numerical Resolution
3. Experimental Methods
3.1. Tested Adsorbents and Presentation of Their Main Properties
- a non-impregnated activated carbon denoted as AC nI;
- an AC impregnated by KI (5 wt.%) denoted as AC 5KI;
- a co-impregnated AC with a composition similar to the employed one for a nuclear grade AC: 1 wt.% of KI and 5 wt.% of TEDA. This adsorbent is designated in the following by AC Nuclear.
3.2. Determination of H2O Adsorption Isotherms
3.2.1. Experimental Part
3.2.2. Data Processing
- Henry model: applied only for a RH < 40%;
- Klotz equation (Equation (15)): developed in a previous work related to the interaction between proteins and particles [45]. This equation is applied to our study to mathematically consider the sigmoidal nature of the obtained isotherms. This equation was applied in the whole RH range (5–95%).
- molar fraction of the species in the gas (-);H2O adsorbed quantity in a monolayer (g g−1);K constant of the adsorption equilibrium in the Klotz equation (-);Ck constant linked to the first association between the surface and the adsorbent in the Klotz equation (-);maximum molecules association number in the clusters in the Klotz equation (-).
3.3. Determination of H2O Breakthrough Curves
3.3.1. Experimental Protocol and Data Processing
- Humid air generator
- Oven and sample holder
- Humidity captors
3.3.2. Investigated Experimental Conditions
4. Results
4.1. H2O Adsorption Isotherms
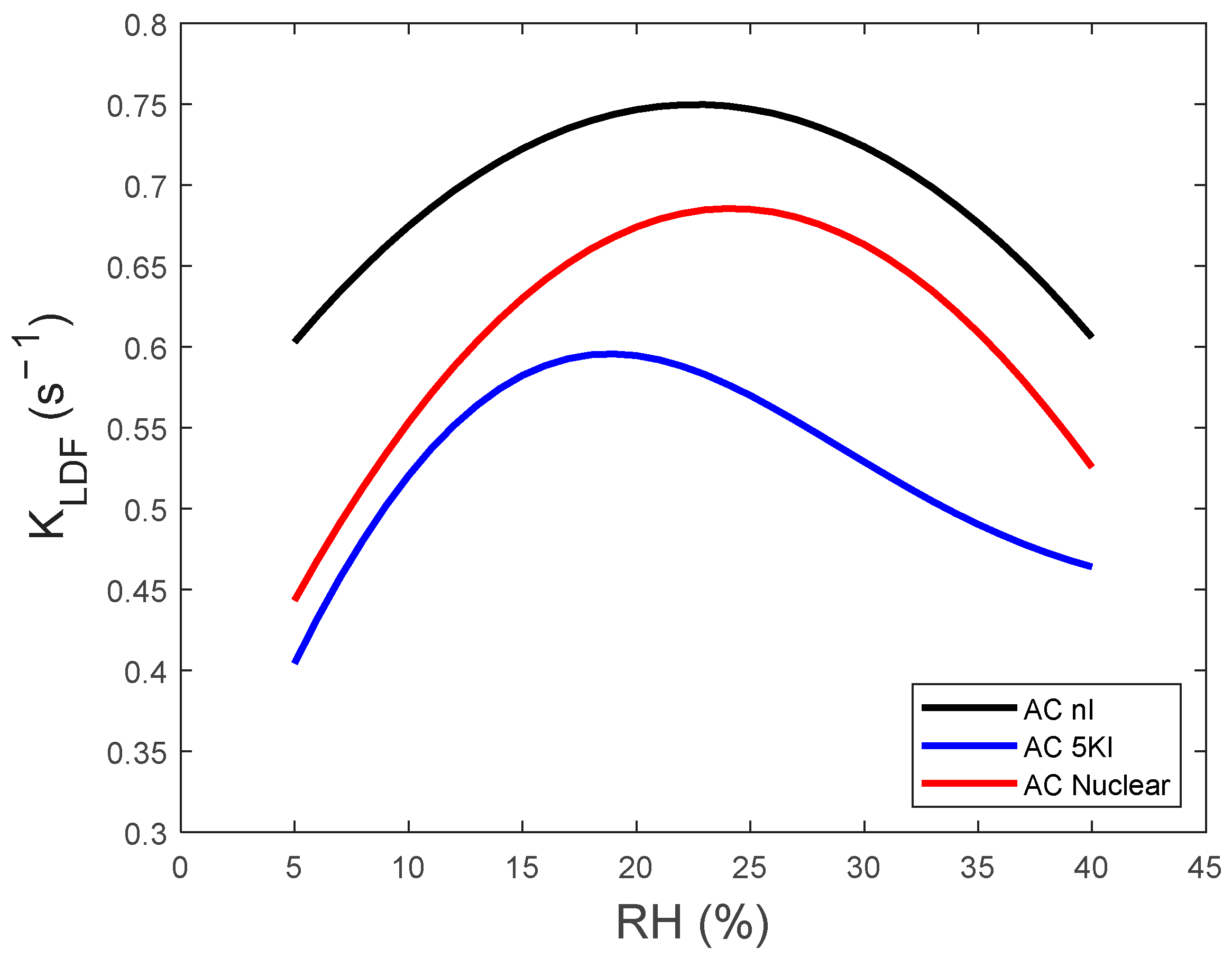
4.2. Adsorption Kinetics (LDF Model)
- (i)
- An initial increase in adsorption kinetics occurs as a function of the water vapor concentration. The RH threshold for this augmentation is about 24% for AC NI and AC Nuclear. This value is shifted to a lower RH of about 18% for AC 5KI in agreement with its more pronounced hydrophilicity, as commented before.
- (ii)
- A decrease in KLDF is, however, noticed for the further RH increase.
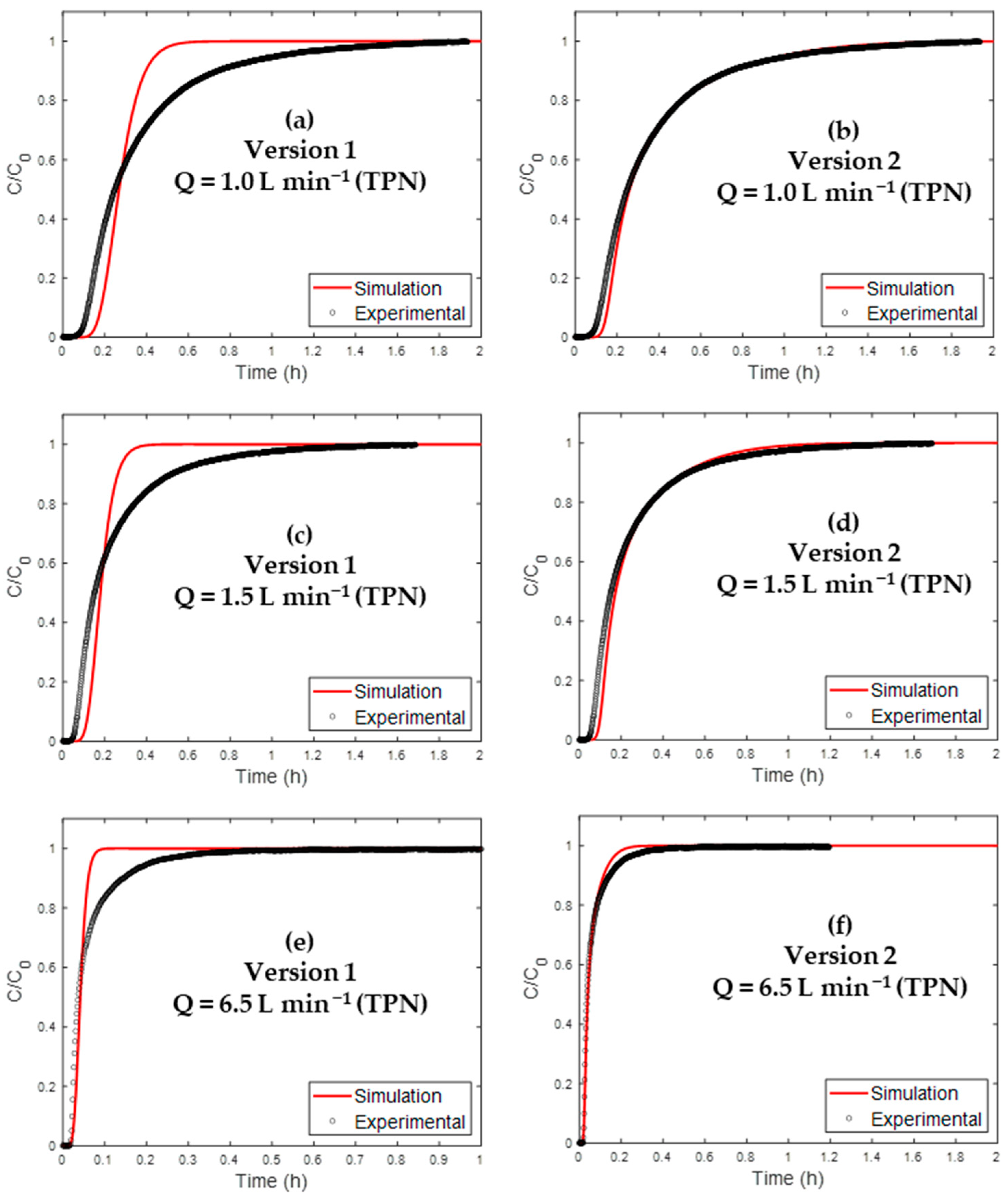
4.3. Simulation of Breakthrough Curves
- The ones linked to adsorbent column conditioning: bed density, bed porosity, and the initial RH, which determines the initial water quantity adsorbed on the AC;
- The second category related to the air flow parameters: water vapor concentration, face velocity, and axial dispersion;
- The last one, regarding the adsorption reaction: constants from kinetics and equilibrium models.
4.3.1. Model Established for Non-Impregnated AC
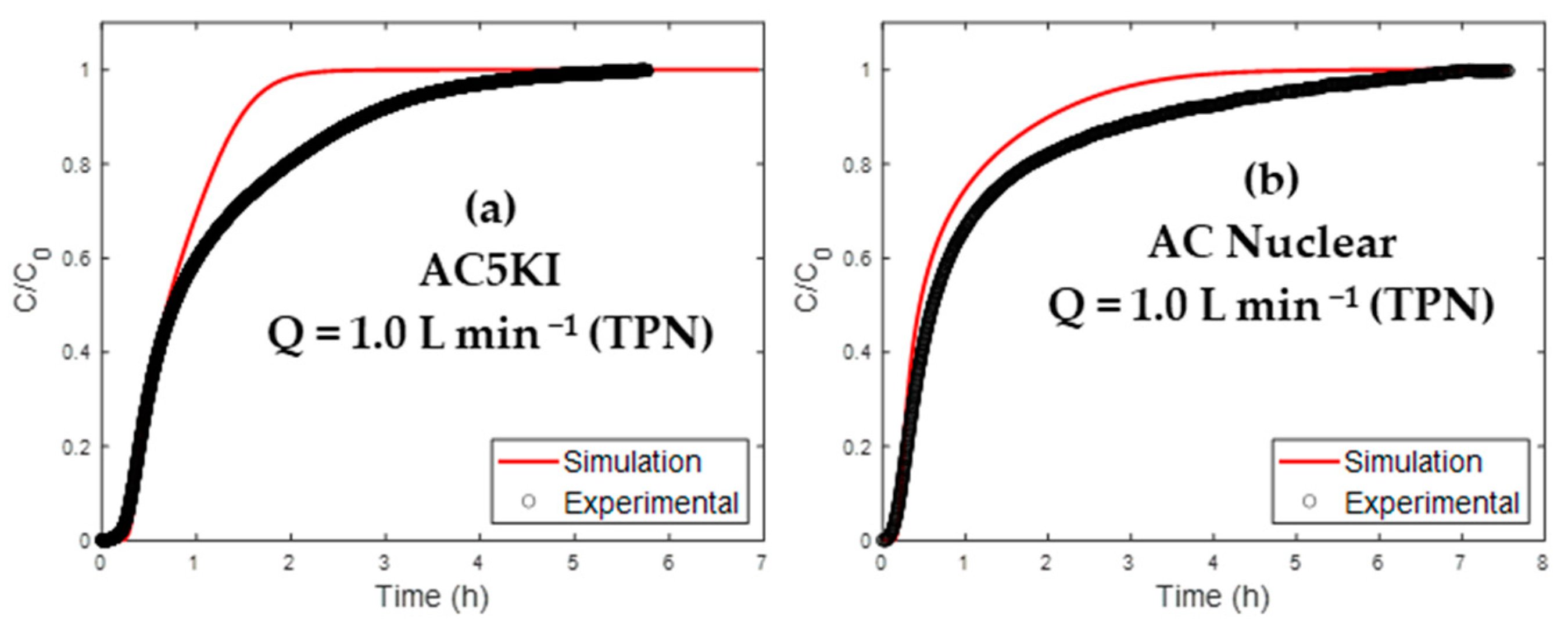
4.3.2. Extrapolation of AC Used in Nuclear Context
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| C | water concentration in the gas phase (kg m−3) |
| Z | coordinate in the axis following the column length (m) |
| D | internal diameter of the adsorption column (m) |
| Dax | axial dispersion (m2 s−1) |
| bulk flow speed (m s−1) | |
| bed porosity (-) | |
| (m s−1) | |
| time (s) | |
| fixed bed density (kg m−3) | |
| average adsorbed quantity (g of adsorbed H2O per g of adsorbent) | |
| Dm | m2 s−1 [34] |
| particle diameter (m) | |
| m2 s−1 [63] | |
| Re | |
| Sc | |
| m | sample mass used in the sorption microbalance (mg) |
| RH | relative humidity (%) |
| adsorbed quantity at equilibrium for a given RH (g g−1) | |
| KLDF | constant associated with the LDF model (s−1) |
| Dp | pore diffusivity (m2 s−1) |
| DK | Knudsen diffusivity (m2 s−1) |
| surface diffusivity (m2 s−1) | |
| tortuosity (-) | |
| feed concentration (kg m−3) | |
| adsorbed quantity in equilibrium with feed concentration (g g−1) | |
| pore diameter (m) | |
| adsorption enthalpy (J mol−1) | |
| model parameter based on the interaction (s = 2) [35] | |
| Psat | water vapor saturation pressure for a given temperature (bar) |
| R | ideal gas constant (8.314 J mol−1 K−1) |
| T | gas temperature (K) |
| molecular mass of water (18 g mol−1) | |
| molar fraction of the species in the gas (-) | |
| H2O adsorbed quantity in a monolayer (g g−1) | |
| KH | Henry constant (-) |
| K | constant of the adsorption equilibrium in the Klotz equation (-) |
| Ck | constant linked to the first association between the surface and the adsorbent in the Klotz equation (-) |
| maximum molecules association number in the clusters in the Klotz equation (-) | |
| t5% | |
| t95% |
References
- Chebbi, M.; Monsanglant-Louvet, C.; Parent, P.; Gerente, C.; Coq, L.L.; Mokili, B.M. Sorption properties of activated carbons for the capture of methyl iodide in the context of nuclear industry. Carbon Trends 2022, 7, 100164. [Google Scholar] [CrossRef]
- Kircher, J.F.; Barnes, R.H. Methyl Iodide Formation Under Postulated Nuclear Reactor Accident Conditions; International Atomic Energy Agency (IAEA): Vienna, Austria, 1968; ISBN 0074-1884. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:44057071 (accessed on 23 February 2024).
- Clément, B.; Cantrel, L.; Ducros, G.; Funke, F.; Herranz, L.; Rydl, A.; Weber, G.; Wren, C. State of the Art Report on Iodine Chemistry; Organisation for Economic Co-Operation and Development: Paris, France, 2007. [Google Scholar]
- Masson, O.; Baeza, A.; Bieringer, J.; Brudecki, K.; Bucci, S.; Cappai, M.; Carvalho, F.P.; Connan, O.; Cosma, C.; Dalheimer, A. Tracking of airborne radionuclides from the damaged Fukushima Dai-ichi nuclear reactors by European networks. Environ. Sci. Technol. 2011, 45, 7670–7677. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chebbi, M.; Monsanglant-Louvet, C.; Marcillaud, B.; Roynette, A.; Doizi, D.; Parent, P.; Laffon, C.; Grauby, O.; Ferry, D. KI and TEDA influences towards the retention of radiotoxic CH3I by activated carbons. J. Hazard. Mater. 2022, 431, 128548. [Google Scholar] [CrossRef] [PubMed]
- Scheele, R.D.; Burger, L.L.; Matsuzaki, C.L. Methyl Iodide Sorption by Reduced Silver Mordenite; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 1983. [Google Scholar]
- Chebbi, M.; Lin, H.; Azambre, B.; Monsanglant-Louvet, C.; Marcillaud, B.; Roynette, A.; Doizi, D. A novel methodology to estimate the importance of isotopic exchange in the CH3I adsorption by impregnated activated carbons. Sep. Purif. Technol. 2024, 330, 125427. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Monsanglant-Louvet, C.; Marcillaud, B.; Roynette, A.; Cantrel, L. Effects of water vapour and temperature on the retention of radiotoxic CH3I by silver faujasite zeolites. J. Hazard. Mater. 2021, 409, 124947. [Google Scholar] [CrossRef]
- Nakamura, M.; Ohba, T.; Branton, P.; Kanoh, H.; Kaneko, K. Equilibrium-time and pore width dependent hysteresis of water adsorption isotherm on hydrophobic microporous carbons. Carbon 2010, 48, 305–308. [Google Scholar] [CrossRef]
- Ho, K.; Chun, H.; Lee, H.C.; Lee, Y.; Lee, S.; Jung, H.; Han, B.; Lee, C.-H. Design of highly efficient adsorbents for removal of gaseous methyl iodide using tertiary amine-impregnated activated carbon: Integrated experimental and first-principles approach. Chem. Eng. J. 2019, 373, 1003–1011. [Google Scholar] [CrossRef]
- Ho, K.; Moon, S.; Lee, H.C.; Hwang, Y.K.; Lee, C.-H. Adsorptive removal of gaseous methyl iodide by triethylenediamine (TEDA)-metal impregnated activated carbons under humid conditions. J. Hazard. Mater. 2019, 368, 550–559. [Google Scholar] [CrossRef]
- Hassan, A.A.; Hassan, H.; Rupam, T.H.; Islam, M.A.; Saha, B.B. Development of novel ionic liquid-based silica gel composite adsorbents for designing high-efficiency adsorption heat pumps. Int. Commun. Heat Mass Transf. 2023, 146, 106862. [Google Scholar] [CrossRef]
- Kim, K.-M.; Oh, H.-T.; Lim, S.-J.; Ho, K.; Park, Y.; Lee, C.-H. Adsorption Equilibria of Water Vapor on Zeolite 3A, Zeolite 13X, and Dealuminated Y Zeolite. J. Chem. Eng. Data 2016, 61, 1547–1554. [Google Scholar] [CrossRef]
- Shi, L.; Kirlikovali, K.O.; Chen, Z.; Farha, O.K. Metal-organic frameworks for water vapor adsorption. Chem 2024, 10, 484–503. [Google Scholar] [CrossRef]
- Ecob, C.M.; Clements, A.J.; Flaherty, P.; Griffiths, J.G.; Nacapricha, D.; Taylor, C.G. Effect of humidity on the trapping of radioiodine by impregnated carbons. Sci. Total Environ. 1993, 130–131, 419–427. [Google Scholar] [CrossRef]
- Cardenas, C. Analyse et modélisation du comportement des caissons d’épuration de l’air équipant les engins de chantier pour la protection des opérateurs contre les gaz et vapeurs. Hal 2021, 240. Available online: https://www.theses.fr/s283000 (accessed on 25 January 2025).
- Paz, L.; Gentil, S.; Fierro, V.; Celzard, A. Assessing the performance of adsorbents for CO2/CH4 separation in pressure swing adsorption units: A review. J. Environ. Chem. Eng. 2024, 12, 114870. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Aguilera, P.G.; Ollero, P. Modeling and simulation of the adsorption of biogas hydrogen sulfide on treated sewage-sludge. Chem. Eng. J. 2014, 253, 305–315. [Google Scholar] [CrossRef]
- Cardenas, C.; Latifi, A.M.; Vallières, C.; Marsteau, S.; Sigot, L. Analysis of an Industrial Adsorption Process Based on Ammonia Chemisorption: Modeling and simulation. Comput. Chem. Eng. 2021, 154, 107474. [Google Scholar] [CrossRef]
- Helfferich, F.G. Principles of Adsorption & Adsorption Processes; Ruthven, D.M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Shamiri, A. A review of mathematical modeling of fixed-bed columns for carbon dioxide adsorption. Chem. Eng. Res. Des. 2014, 92, 961–988. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Knox, J.C.; Ebner, A.D.; LeVan, M.D.; Coker, R.F.; Ritter, J.A. Limitations of Breakthrough Curve Analysis in Fixed-Bed Adsorption. Ind. Eng. Chem. Res. 2016, 55, 4734–4748. [Google Scholar] [CrossRef]
- Khalighi, M.; Farooq, S.; Karimi, I.A. Nonisothermal pore diffusion model for a kinetically controlled pressure swing adsorption process. Ind. Eng. Chem. Res. 2012, 51, 10659–10670. [Google Scholar] [CrossRef]
- Rastegar, S.O.; Gu, T. Empirical correlations for axial dispersion coefficient and Peclet number in fixed-bed columns. J. Chromatogr. A 2017, 1490, 133–137. [Google Scholar] [CrossRef]
- Delgado, J.M.P.Q.P.Q. A Critical Review of Dispersion in Packed Beds. Heat Mass Transfer 2006, 42, 279–310. [Google Scholar] [CrossRef]
- Schlaich, A.; Barrat, J.; Coasne, B. Theory and Modeling of Transport in Nanoporous Materials: From Microscopic to Coarse-Grained Descriptions. arXiv 2024, arXiv:2406.03039. [Google Scholar] [CrossRef] [PubMed]
- Sircar, S.; Hufton, J.R. Why does the linear driving force model for adsorption kinetics work? Adsorption 2000, 6, 137–147. [Google Scholar] [CrossRef]
- Kärger, J.; Valiullin, R.; Brandani, S.; Caro, J.; Chmelik, C.; Chmelka, B.F.; Coppens, M.; Farooq, S.; Freude, D.; Jobic, H.; et al. Diffusion in nanoporous materials with special consideration of the measurement of determining parameters (IUPAC Technical Report). Pure Appl. Chem. 2025, 97, 1–89. [Google Scholar] [CrossRef]
- Díaz-blancas, V.; Aguilar-madera, C.G.; Flores-cano, J.V.; Leyva-ramos, R.; Padilla-ortega, E. Evaluation of mass transfer mechanisms involved during the adsorption of metronidazole on granular activated carbon in fixed bed column. J. Water Process Eng. 2020, 36, 101303. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, Y.; Kang, K.; Xie, Y.; Han, H.; Song, H.; Hu, J.; Bai, S. Water Vapor Adsorption/Desorption Behaviors on Activated Carbon Particles and Fixed Beds: Isotherms, Kinetics, and Axial Temperature. Ind. Eng. Chem. Res. 2023, 62, 18032–18046. [Google Scholar] [CrossRef]
- Hassan, M.M.; Raghavan, N.S.; Ruthven, D.M.; Boniface, H.A. Pressure Swing Adsorption; John Wiley & Sons: Hoboken, NJ, USA, 1985; Volume 31, pp. 2008–2016. [Google Scholar]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; Mc Graw-Hill Book Company: New York, NY, USA, 1997. [Google Scholar]
- Massman, W.J. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmos. Environ. 1998, 32, 1111–1127. [Google Scholar] [CrossRef]
- Sladek, K.J.; Gilliland, E.R.; Baddour, R.F. Diffusion on Surfaces II. Correlation of Diffusivities of Physically and Chemically Adsorbed Species. Ind. Eng. Chem. Fundam. 1974, 13, 100–105. [Google Scholar] [CrossRef]
- Furmaniak, S.; Gauden, P.A.; Terzyk, A.P.; Rychlicki, G. Water adsorption on carbons—Critical review of the most popular analytical approaches. Adv. Colloid Interface Sci. 2008, 137, 82–143. [Google Scholar] [CrossRef]
- Kim, M.B.; Ryu, Y.K.; Lee, C.H. Adsorption equilibria of water vapor on activated carbon and DAY zeolite. J. Chem. Eng. Data 2005, 50, 951–955. [Google Scholar] [CrossRef]
- Foley, N.J.; Thomas, K.M.; Forshaw, P.L.; Stanton, D.; Norman, P.R. Kinetics of Water Vapor Adsorption on Activated Carbon. Langmuir 1997, 13, 2083–2089. [Google Scholar] [CrossRef]
- Lee, H.S.; Matthews, C.J.; Braddock, R.D.; Sander, G.C.; Gandola, F. A MATLAB method of lines template for transport equations. Environ. Model. Softw. 2004, 19, 603–614. [Google Scholar] [CrossRef]
- Lin, H. Evaluation de la Contribution du Mécanisme D’échange Isotopique à L’épuration de L’iode Radioactive. 2022. Available online: http://www.theses.fr/2022SORUS073/document (accessed on 6 February 2025).
- Abbas, K.; Chebbi, M.; Azambre, B.; Monsanglant-Louvet, C.; Marcillaud, B.; Roynette, A. Unravelling the Potential of Iodine Isotopic Exchange in Ch3131i Capture by K127i-Impregnated Activated Carbons. Nucl. Mater. 2025, 608, 155719. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, W.K.; Moon, H. Adsorption and desorption of gaseous methyl iodide in a triethylenediamine-impregnated activated carbon bed. Sep. Technol. 1993, 3, 133–142. [Google Scholar] [CrossRef]
- Ho, K.; Park, D.; Park, M.K.; Lee, C.H. Adsorption mechanism of methyl iodide by triethylenediamine and quinuclidine-impregnated activated carbons at extremely low pressures. Chem. Eng. J. 2020, 396, 125215. [Google Scholar] [CrossRef]
- Velasco, L.F.; Devos, A.; Lodewyckx, P. The importance of outgassing conditions when using water vapour to characterize activated carbons. Carbon 2019, 152, 409–415. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and v sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G. Adsorption, Ion Exchange and Catalysis: Design of Operations and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–602. [Google Scholar] [CrossRef]
- Chebbi, M. Piégeage d’Espèces Iodées Volatiles sur des Adsorbants Poreux de Type Zéolithique dans le Contexte d ’ un Accident Nucléaire Grave. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2016. [Google Scholar]
- Buttersack, C. General Cluster Sorption Isotherm. Microporous Mesoporous Mater. 2021, 316, 110909. [Google Scholar] [CrossRef]
- Horikawa, T.; Sekida, T.; Hayashi, J.; Katoh, M.; Do, D.D. A new adsorption–desorption model for water adsorption in porous carbons. Carbon 2011, 49, 416–424. [Google Scholar] [CrossRef]
- Velasco, L.F.; Snoeck, D.; Mignon, A.; Misseeuw, L.; Ania, C.O.; Van Vlierberghe, S.; Dubruel, P.; de Belie, N.; Lodewyckx, P. Role of the surface chemistry of the adsorbent on the initialization step of the water sorption process. Carbon 2016, 106, 284–288. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Linares-Solano, A.; Rand, B. Water adsorption on activated carbons: Study of water adsorption in micro- and mesopores. J. Phys. Chem. B 2001, 105, 7998–8006. [Google Scholar] [CrossRef]
- Chauveau, R. Modélisation multiparamètre du phénomène d ’ adsorption: Détermination du temps de percée des cartouches de masques à gaz. 2018. Available online: https://hal.univ-lorraine.fr/tel-01751295v1 (accessed on 20 January 2025).
- Grismer, M.E. Kinetics of water vapor adsorption on soils. Soil Sci. 1987, 143, 367–371. [Google Scholar] [CrossRef]
- Qi, N.; Appel, W.S.; LeVan, M.D.; Finn, J.E. Adsorption dynamics of organic compounds and water vapor in activated carbon beds. Ind. Eng. Chem. Res. 2006, 45, 2303–2314. [Google Scholar] [CrossRef]
- Harding, A.W.; Foley, N.J.; Norman, P.R.; Francis, D.C.; Thomas, K.M. Diffusion barriers in the kinetics of water vapor adsorption/desorption on activated carbons. Langmuir 1998, 14, 3858–3864. [Google Scholar] [CrossRef]
- Rani, S.; Prusty, B.K.; Pal, S.K. Adsorption kinetics and diffusion modeling of CH4 and CO2 in Indian shales. Fuel 2018, 216, 61–70. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zuo, S.; Chen, S.; Xiao, S.; Liu, Z. Investigation of adsorption kinetics of CH4 and CO2 on shale exposure to supercritical CO2. Energy 2021, 236, 121410. [Google Scholar] [CrossRef]
- Xie, W.; Fu, X.; Wang, H.; Sun, Y.; Vandeginste, V. Adsorption kinetics of water vapor in shale and their dependence on in-situ conditions and the composition of reservoirs. Geoenergy Sci. Eng. 2025, 249, 213779. [Google Scholar] [CrossRef]
- Mittal, H.; Al, A.; Alhassan, S.M. Microporous and Mesoporous Materials Adsorption isotherm and kinetics of water vapors on novel superporous hydrogel composites. Microporous Mesoporous Mater. 2020, 299, 110106. [Google Scholar] [CrossRef]
- Yan, J.; Yu, Y.; Ma, C.; Xiao, J.; Xia, Q.; Li, Y.; Li, Z. Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101(Cr)@GO with super-high capacity. Appl. Therm. Eng. 2015, 84, 118–125. [Google Scholar] [CrossRef]
- AbdulKareem, F.A.; Mohd. Shariff, A.; Ullah, S.; See, T.L.; Keong, L.K.; Mellon, N. Adsorption performance of 5A molecular sieve zeolite in water vapor–binary gas environment: Experimental and modeling evaluation. J. Ind. Eng. Chem. 2018, 64, 173–187. [Google Scholar] [CrossRef]
- Born, M.H.B.; De Witte, N.; Denayer, J.F.M.; Van Assche, T.R.C. An insulated column for lab-scale non-isothermal breakthrough: Impact of thermal effects during CO2 adsorption. Chem. Eng. Sci. 2023, 276, 118810. [Google Scholar] [CrossRef]
- Houston, W.V. The viscosity of air. Phys. Rev. 1937, 52, 751. [Google Scholar] [CrossRef]
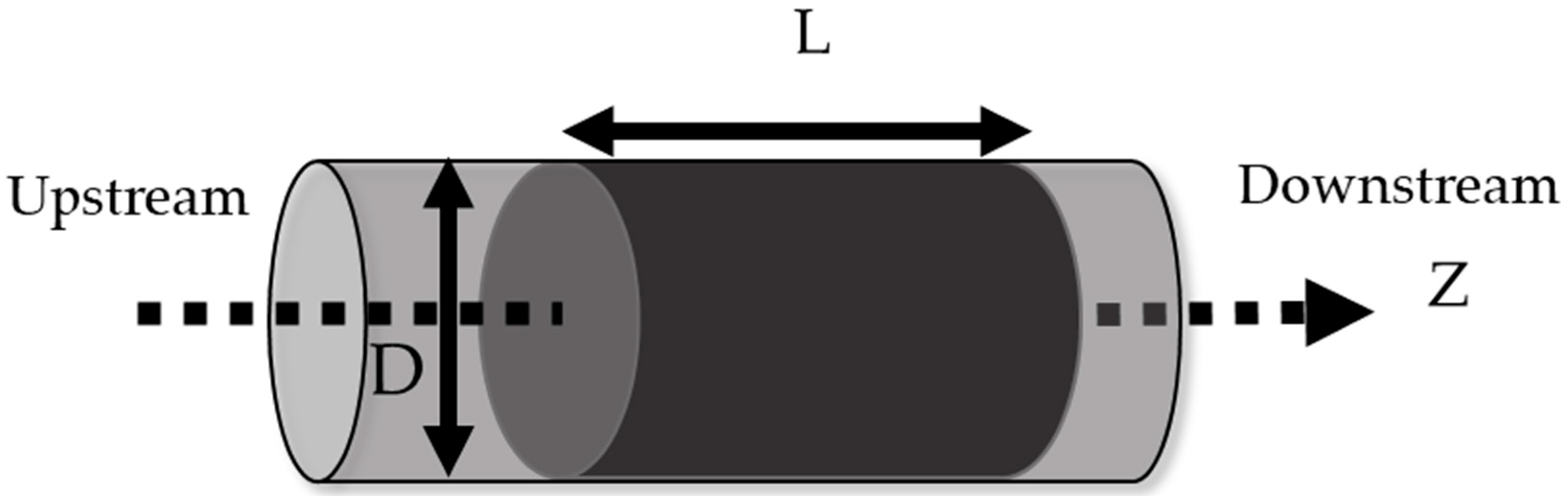
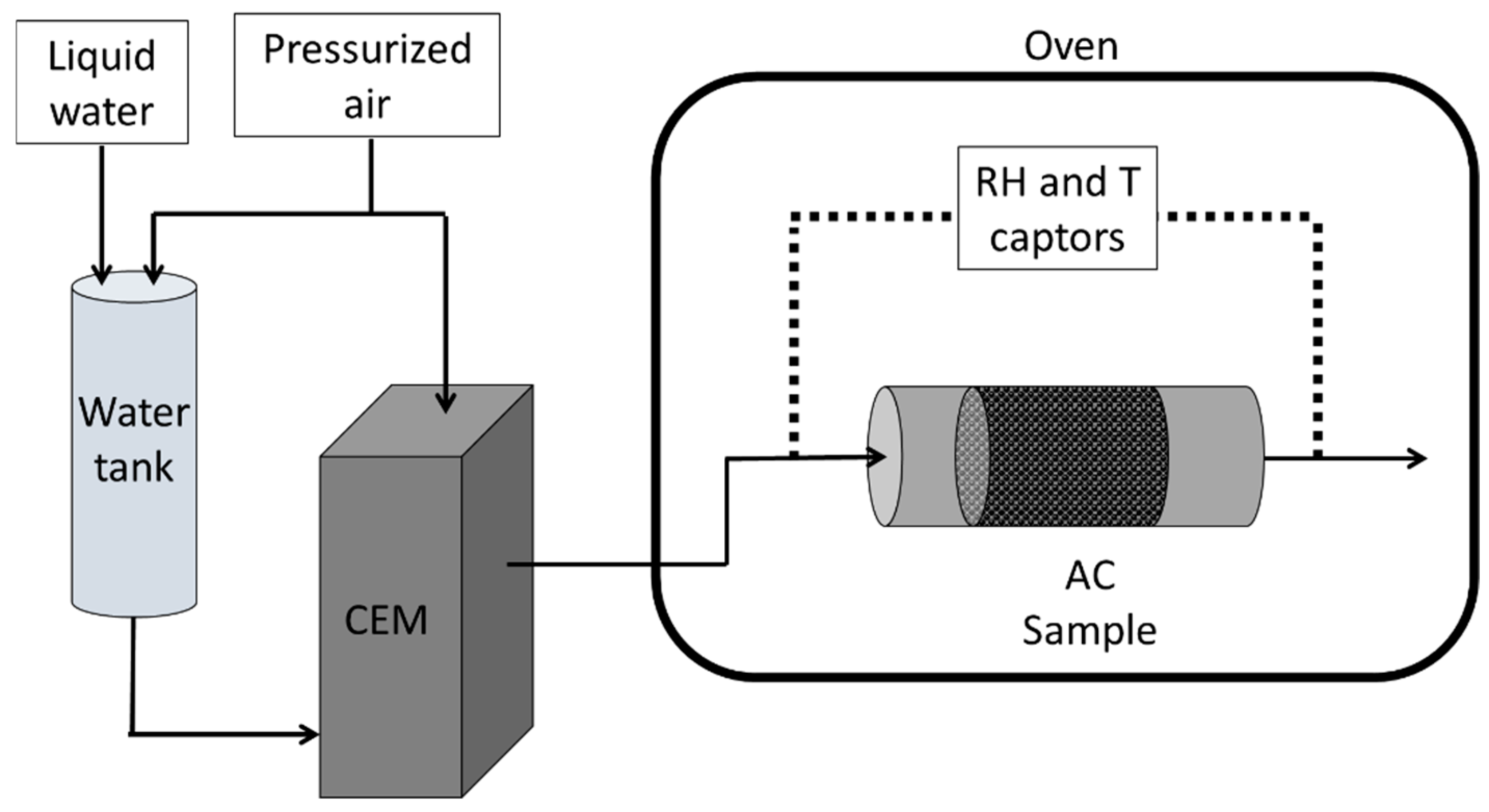

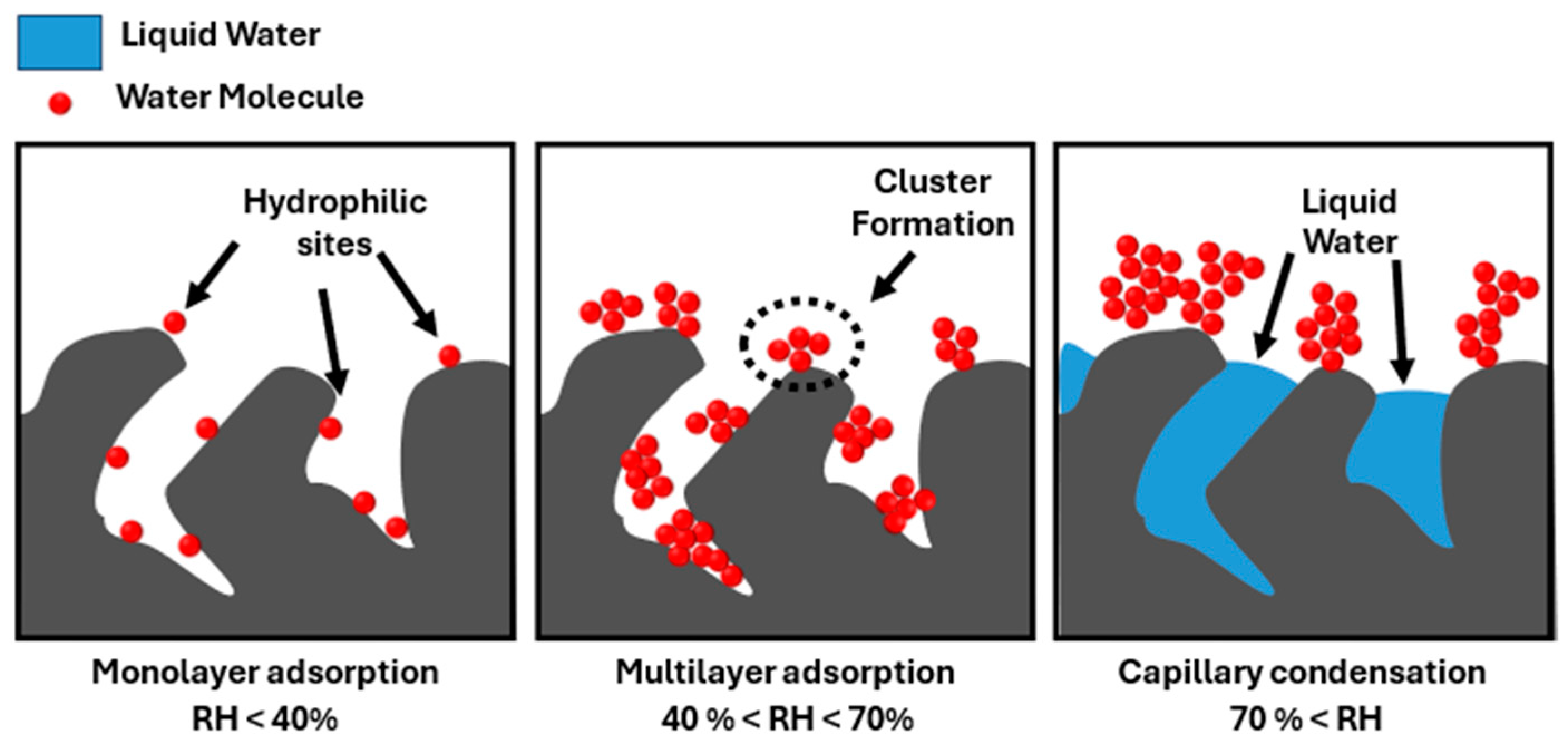
| Impregnation (%wt) | SBET (m2 g−1) | Vpore (cm3 g−1) | % Microporosity (%) | |
|---|---|---|---|---|
| AC nI | - | 1142 33 | 0.472 0.016 | 96.0 3.3 |
| AC 5KI | 5% KI | 1132 34 | 0.483 0.017 | 94.4 3.1 |
| AC Nuclear | 1% KI + 5% TEDA | 976 10 | 0.415 0.005 | 93.5 0.9 |
| Materials | Gas Flow Rate (L min−1 NTP) | Temperature (°C) | RH (%) | [H2O] (g m−3) |
|---|---|---|---|---|
| AC nI | 1.0 | 30 | 40 | 12 |
| 1.5 | ||||
| 6.5 | ||||
| AC 5KI | 1.0 | |||
| AC Nuclear | 1.0 |
| Adsorbent | qeq (g g−1) [RH = 20%] | qeq (g g−1) [RH = 40%] | qeq (g g−1) [RH = 70%] | qsat(g g−1) [RH =95%] |
|---|---|---|---|---|
| AC nI | 0.011 | 0.026 | 0.204 | 0.256 |
| AC 5KI | 0.029 | 0.076 | 0.204 | 0.236 |
| AC Nuclear | 0.017 | 0.045 | 0.191 | 0.223 |
| AC nI | AC 5KI | AC Nuclear | |
|---|---|---|---|
| Klotz’s equation parameters (Equation (15)) | |||
| qm (g g−1) | 0.0104 ± 0.0002 | 0.025 ± 0.002 | 0.0130 ± 0.0004 |
| K | 1.604 ± 0.003 | 1.75 ± 0.03 | 1.772 ± 0.007 |
| mK | 26.3 ± 0.5 | 10.7 ± 0.6 | 18.3 ± 0.5 |
| CK | 4.6 ± 0.9 | 8 ± 3 | 8 ± 2 |
| R2 | 0.99986 | 0.99774 | 0.99963 |
| Henry’s model parameters (Supplementary Materials (S5)) | |||
| KH (-) | 0.059 ± 0.002 | 0.179 ± 0.006 | 0.097 ± 0.004 |
| R2 | 0.99371 | 0.97564 | 0.98582 |
| Adsorbent | DK (m2 s−1) | Dm (m2 s−1) | DS (m2 s−1) | Dp (m2 s−1) |
|---|---|---|---|---|
| AC nI | 3.03 × 10−8 | 2.63 × 10−5 | 4.50 × 10−8 | 7.24 × 10−8 |
| AC 5KI | 2.02 × 10−7 | |||
| AC Nuclear | 1.24 ×10−7 |
| AC nI | AC 5KI | AC Nuclear | |||
|---|---|---|---|---|---|
| T (°C) | 30 | 30 | 30 | 30 | 30 |
| (kg m−3) | 605 | 605 | 605 | 607 | 640 |
| RH0 (%) | 5 | 5 | 5 | 5 | 5 |
| RHfeed air (%) | 40 | 40 | 40 | 40 | 40 |
| Porosity—ε (%) | 35 | 35 | 35 | 35 | 35 |
| Q (L min−1) NTP | 1.0 | 1.5 | 6.5 | 1.0 | 1.0 |
| Dax (m2 s−1) | 2.5 | 3.6 | 13.0 | 2.5 | 2.5 |
| Version | Isotherm Equation |
|---|---|
| 1 | Henry |
| 2 | Klotz |
| Version | Q = 1.0 L min−1 (NTP) | ||||
|---|---|---|---|---|---|
| R2 | t5% model (min) | Error (%) * | t95% model (min) | Error (%) * | |
| 1 | 0.8798 | 9.8 | 58 | 26.4 | 57 |
| 2 | 0.9909 | 8.5 | 35 | 58.8 | 4 |
| Material | R2 | t5% model (min) | Error (%) * | t95% model (min) | Error (%) * |
|---|---|---|---|---|---|
| AC 5KI | 0.8736 | 19.2 | 15 | 100.6 | 51 |
| AC Nuclear | 0.9156 | 11.9 | 23 | 160.4 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, F.C.B.; Chebbi, M.; Monsanglant-Louvet, C.; Marcillaud, B.; Roynette, A. Simulation of Water Vapor Sorption Profiles on Activated Carbons in the Context of the Nuclear Industry. Separations 2025, 12, 126. https://doi.org/10.3390/separations12050126
Martins FCB, Chebbi M, Monsanglant-Louvet C, Marcillaud B, Roynette A. Simulation of Water Vapor Sorption Profiles on Activated Carbons in the Context of the Nuclear Industry. Separations. 2025; 12(5):126. https://doi.org/10.3390/separations12050126
Chicago/Turabian StyleMartins, Felipe Cabral Borges, Mouheb Chebbi, Céline Monsanglant-Louvet, Bénoit Marcillaud, and Audrey Roynette. 2025. "Simulation of Water Vapor Sorption Profiles on Activated Carbons in the Context of the Nuclear Industry" Separations 12, no. 5: 126. https://doi.org/10.3390/separations12050126
APA StyleMartins, F. C. B., Chebbi, M., Monsanglant-Louvet, C., Marcillaud, B., & Roynette, A. (2025). Simulation of Water Vapor Sorption Profiles on Activated Carbons in the Context of the Nuclear Industry. Separations, 12(5), 126. https://doi.org/10.3390/separations12050126








