Metabolic Profiling and Pharmacokinetics Characterization of Yinhua Pinggan Granules with High-Performance Liquid Chromatography Combined with High-Resolution Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal Experiments and Serum Sample Collection
2.3. Serum Pharmacochemical Analysis
2.3.1. Biological Sample Pretreatment
2.3.2. Qualitive UHPLC-MS Analysis
2.3.3. Data Analysis
2.4. Serum Pharmacokinetic Analysis
2.4.1. Pretreatment of Blood Samples
2.4.2. Calibration Standard and QC Samples
2.4.3. Quantitative UHPLC-MS Analysis
2.4.4. Method Validation
2.4.5. Pharmacokinetic Parameter Calculation
2.4.6. Statistical Analysis
3. Results and Discussion
3.1. Identification of In Vivo Prototype Compounds of YPG
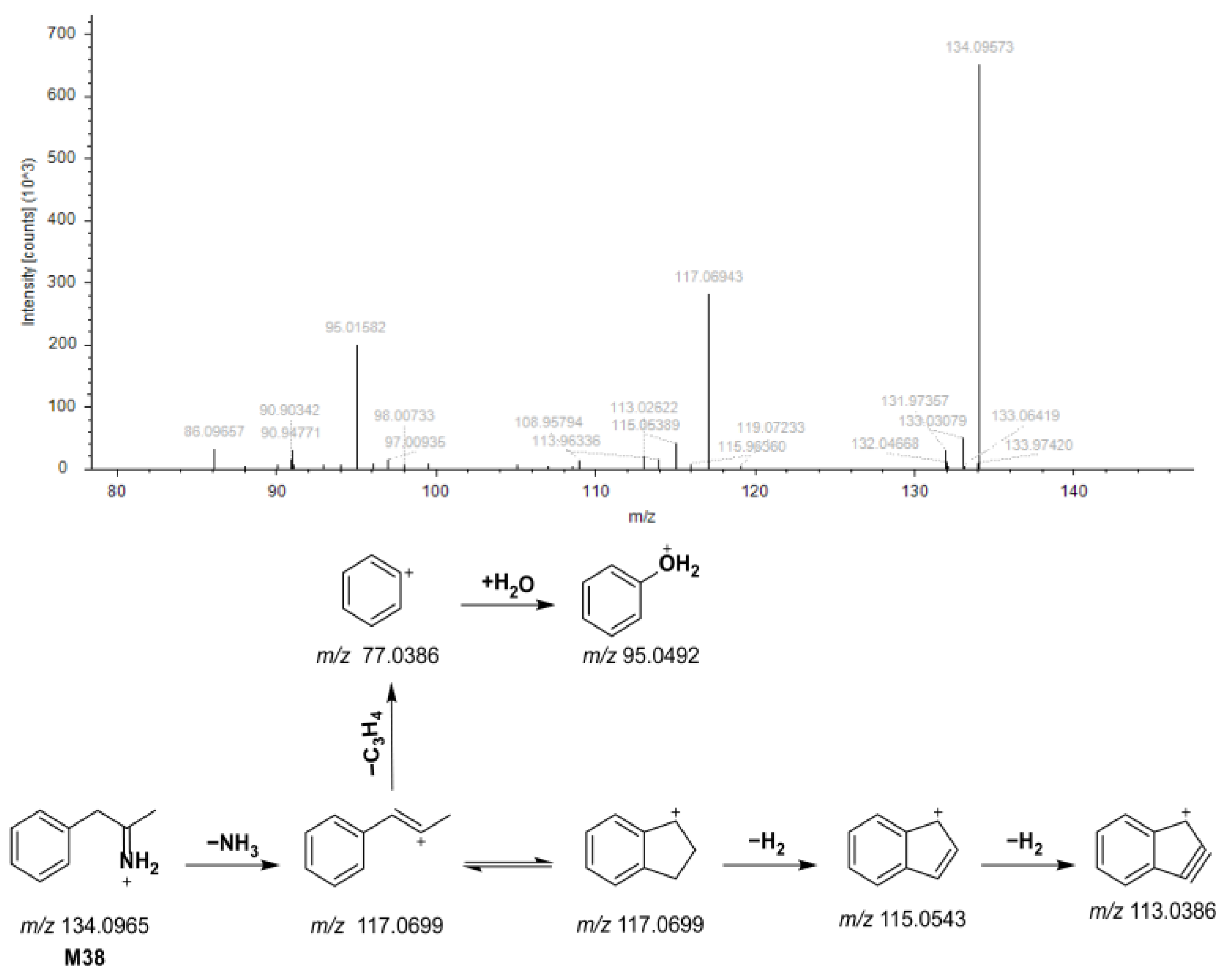
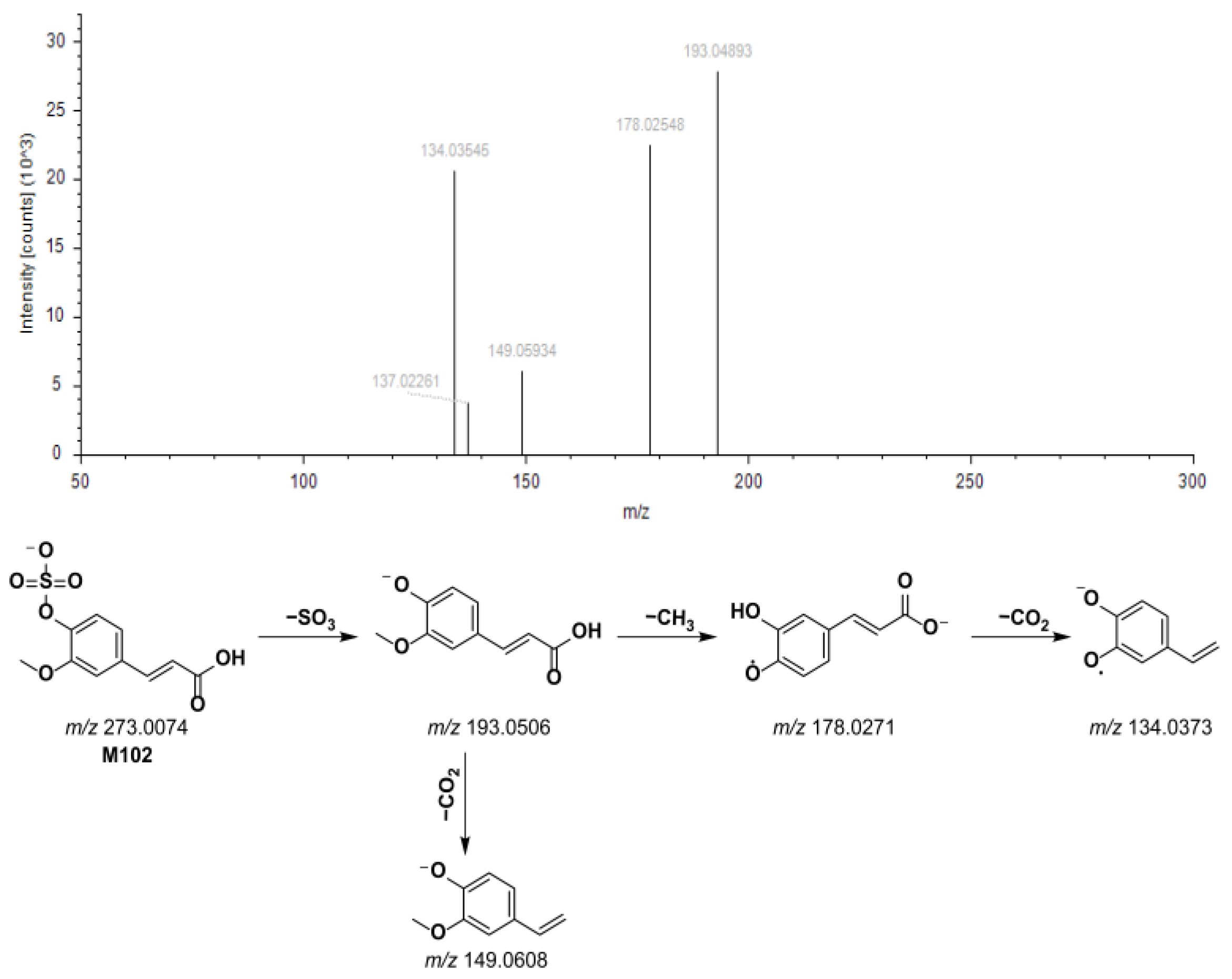
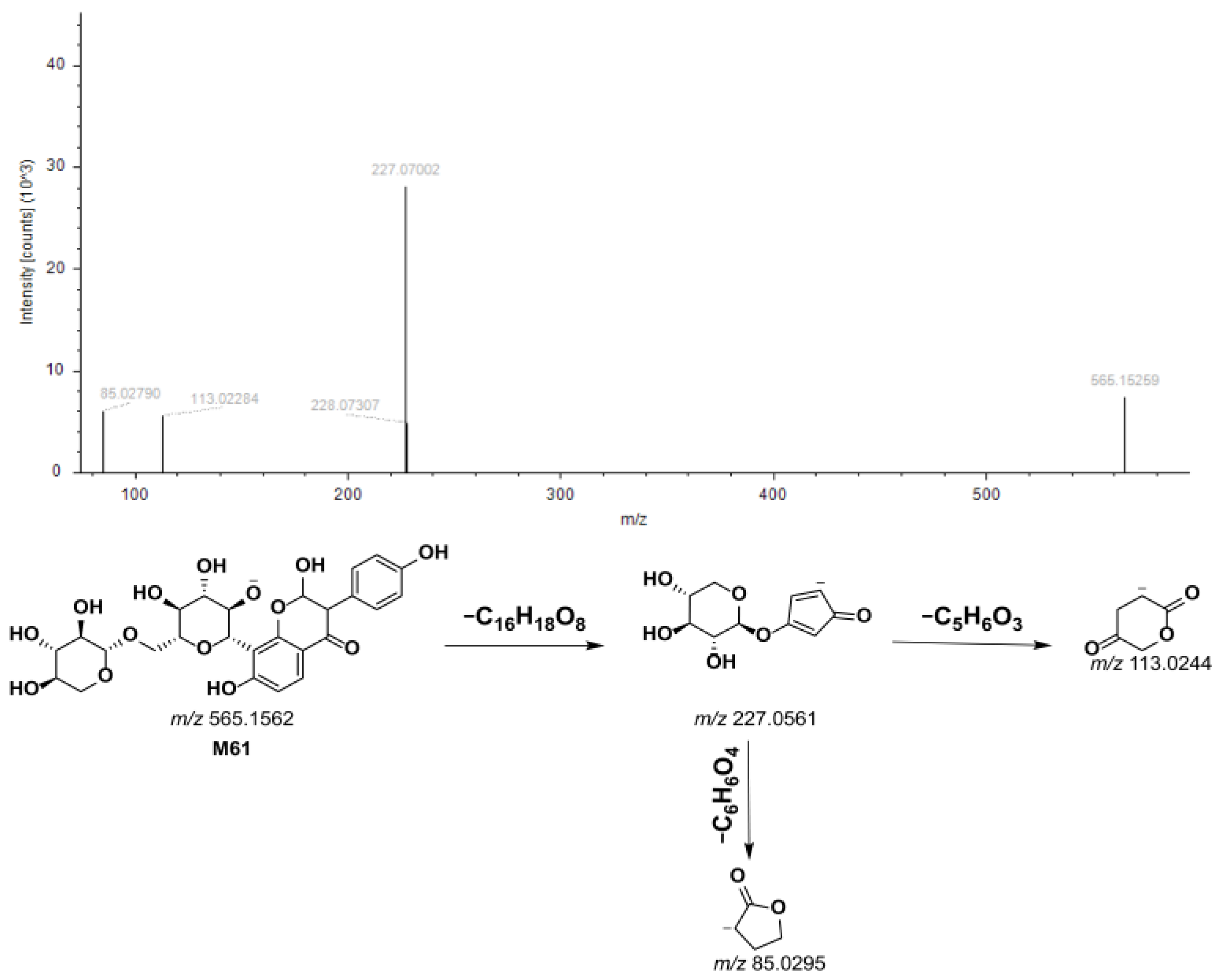
3.2. Annotation of YPG-Related Metabolites in Rat Biosamples
3.2.1. P2-Related Metabolites
3.2.2. N4-Related Metabolites
3.2.3. N45-Related Metabolites
3.2.4. P15-Related Metabolites
3.3. Pharmacokinetics of Seven Representative Components
3.3.1. Establishment of Quantitative UHPLC-MS Method
3.3.2. Analysis of Pharmacokinetic Parameters
3.3.3. Method Validation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| YPG | Yinhua Pingguan Granules |
| QC | quality control |
| UHPLC-MS | ultra-high-performance liquid chromatography coupled with high-resolution orbitrap mass spectrometry |
| TCM | traditional Chinese medicine |
| NMPA | National Medical Products Administration |
| Q-14 | Huashi Baidu decoction |
| HESI | electrospray ionization source |
| RT | retention time |
| DAS | Drug and Statistics |
| BPCs | base peak chromatograms |
| Tmax | time to peak concentration |
| Cmax | peak plasma concentration |
| AUC0–∞ | area under the drug-time curve |
| t1/2 | terminal elimination half-life |
| MRT0–∞ | mean residence time |
| CLz/F | clearance |
| Vz/F | apparent volume of distribution |
References
- Wang, J.; Hu, H.; Du, H.; Luo, M.; Cao, Y.; Xu, J.; Chen, T.; Guo, Y.; Li, Q.; Chen, W.; et al. Clinical Efficacy Protocol of Yinhuapinggan Granules: A Randomized, Double-Blind, Parallel, and Controlled Clinical Trial Program for the Intervention of Community-Acquired Drug-Resistant Bacterial Pneumonia as a Complementary Therapy. Front. Pharmacol. 2022, 13, 852604. [Google Scholar]
- Kubo, T.; Nishimura, H. Antipyretic effect of Mao-to, a Japanese herbal medicine, for treatment of type A influenza infection in children. Phytomedicine 2007, 14, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, S.; Kashiwagi, K.; Ajisaka, K.; Masui, S.; Takeoka, H.; Ikematsu, H.; Kashiwagi, S. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J. Infect. Chemother. 2012, 18, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Kataoka, E.; Aoki, Y.; Hokari, R.; Kiyohara, H.; Yamada, H. Alleviative Effects of a Kampo (a Japanese Herbal) Medicine “Maoto (Ma-Huang-Tang)” on the Early Phase of Influenza Virus Infection and Its Possible Mode of Action. Evid. Based Complement. Alternat. Med. 2014, 2014, 187036. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhou, H.; Yang, J.; Lu, Y.; He, Y.; Wan, H. Preliminary study of Yinhuapinggan granule against H1N1 influenza virus infection in mice through inhibition of apoptosis. Pharm. Biol. 2020, 58, 979–991. [Google Scholar] [CrossRef]
- Du, H.; Zhou, H.; Wan, H.; Yang, J.; Lu, Y.; He, Y.; Wan, H. Antiviral effects and mechanisms of Yinhuapinggan granule against H1N1 influenza virus infection in RAW264.7 cells. Inflammopharmacology 2018, 26, 1455–1467. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.; Huang, J.; Pan, W.; Ma, Q.; Shi, Y.; Li, C.; Jin, Z.; Jia, Z.; Jiang, H.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar]
- Lee, D.; Li, Q.; Liu, J.; Efferth, T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 2021, 80, 153337. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Chen, C.; Gu, Y.; Zhu, C.; Wang, S.; Chen, J.; Zhang, L.; Lv, L.; Zhang, G.; et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B 2021, 11, 222–236. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Li, J.; Fu, J.; Zhao, M.; Zhang, W.; Weng, W.; Li, Q. Network Pharmacology and LC-MS Approachs to Explore the Active Compounds and Mechanisms of Yuanjiang Decoction for Treating Bradyarrhythmia. Comput. Biol. Med. 2023, 152, 106435. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Zeng, J.; Ji, E.; Xu, J.; Tang, C.; Huo, H.; Zhang, Y.; Li, H.; Yang, H. Precise Investigation of the Efficacy of Multicomponent Drugs Against Pneumonia Infected with Influenza Virus. Front. Pharmacol. 2021, 12, 604009. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Guan, S.; Jing, H.; Ji, W.; Kong, X.; Du, S.; Jia, M.; Wang, H. Efficacy and Safety of Traditional Chinese Medicine Adjuvant Therapy for Severe Pneumonia: Evidence Mapping of the Randomized Controlled Trials, Systematic Reviews, and Meta-Analyses. Front. Pharmacol. 2023, 14, 1227436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, X.; Wang, L.; Zhang, J.; Lv, R.; Tan, L.; Chen, Y.; Tao, R.; Li, X.; Chen, Y.; et al. Improvement Influenza Vaccine Immune Responses with Traditional Chinese Medicine and Its Active Ingredients. Front. Microbiol. 2023, 14, 1111886. [Google Scholar] [CrossRef] [PubMed]
- Yalkun, I.; Wan, H.; Ye, L.; Yu, L.; He, Y.; Li, C.; Wan, H. Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry. Molecules 2024, 29, 2300. [Google Scholar] [CrossRef]
- Hu, L.; Yao, Z.; Qin, Z.; Liu, L.; Song, X.; Dai, Y.; Kiyohara, H.; Yamada, H.; Yao, X. In Vivo Metabolic Profiles of Bu-Zhong-Yi-Qi-Tang, a Famous Traditional Chinese Medicine Prescription, in Rats by Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 171, 81–98. [Google Scholar] [CrossRef]
- Mahana, A.; Hammoda, H.M.; Khalifa, A.A.; Elblehi, S.S.; Harraz, F.M.; Shawky, E. Integrated Serum Pharmacochemistry and Network Pharmacology Analyses Reveal the Bioactive Metabolites and Potential Functional Mechanism of Ground Cherry (Physalis pruinosa L.) in Treatment of Type 2 Diabetes Mellitus in Rats. J. Ethnopharmacol. 2023, 300, 115750. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji Formulation Inhibits Hepatocellular Carcinoma Progression in Patients by Targeting the AKT/CyclinD1/P21/P27 Pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef]
- Guo, Z.; Duan, Y.; Zhao, Z.; Yang, D.; Xu, X. HPLC Fingerprint Analysis of Cibotii Rhizoma from Different Regions and Identification of Common Peaks by LC-MS. Pharmaceuticals 2024, 17, 313. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Qian, J.; Wang, Y.; Wang, S. Characterization of the Chemical Constituents of Jie-Geng-Tang and the Metabolites in the Serums and Lungs of Mice after Oral Administration by LC-Q-TOF-MS. Chin. J. Nat. Med. 2021, 19, 284–294. [Google Scholar] [CrossRef]
- Han, F.; Li, Y.; Ma, L.; Liu, T.; Wu, Y.; Xu, R.; Song, A.; Yin, R. A Rapid and Sensitive UHPLC-FT-ICR MS/MS Method for Identification of Chemical Constituents in Rhodiola Crenulata Extract, Rat Plasma and Rat Brain after Oral Administration. Talanta 2016, 160, 183–193. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Song, J.; Fan, B.; Nie, Y.; Bai, D.; Lei, H. The Profiling and Identification of the Absorbed Constituents and Metabolites of Guizhi Decoction in Rat Plasma and Urine by Rapid Resolution Liquid Chromatography Combined with Quadrupole-Time-of-Flight Mass Spectrometry. Int. J. Mol. Sci. 2016, 17, 1409. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Fang, Y.; Shen, Z.; Shao, Y.; Ma, Q.; Yang, Z.; Zhao, J.; Li, H.; Li, R.; Dong, S.; et al. Broad Antiviral and Anti-Inflammatory Activity of Qingwenjiere Mixture against SARS-CoV-2 and Other Human Coronavirus Infections. Phytomedicine 2021, 93, 153808. [Google Scholar] [CrossRef]
- Li, Z.; Hou, J.; Deng, Y.; Zhi, H.; Wu, W.; Yan, B.; Chen, T.; Tu, J.; Zhu, Z.; Wu, W.; et al. Exploring the Protective Effects of Danqi Tongmai Tablet on Acute Myocardial Ischemia Rats by Comprehensive Metabolomics Profiling. Phytomedicine 2020, 74, 152918. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, J.; Cheng, J.; Kang, L.; Li, W.; Zhong, Y.; Wei, C.; Fu, L.; Qi, J.; et al. Bioactive Compounds from Huashi Baidu Decoction Possess Both Antiviral and Anti-Inflammatory Effects against COVID-19. Proc. Natl. Acad. Sci. USA 2023, 120, e2301775120. [Google Scholar] [CrossRef]
- Gong, P.; Cui, N.; Wu, L.; Liang, Y.; Hao, K.; Xu, X.; Tang, W.; Wang, G.; Hao, H. Chemicalome and Metabolome Matching Approach to Elucidating Biological Metabolic Networks of Complex Mixtures. Anal. Chem. 2012, 84, 2995–3002. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Xu, F.; Wang, H.; Zhang, Y.; Liu, G.; Shang, M.; Wang, X.; Cai, S. Exploring the In Vivo Existence Forms (23 Original Constituents and 147 Metabolites) of Astragali Radix Total Flavonoids and Their Distributions in Rats Using HPLC-DAD-ESI-IT-TOF-MSn. Molecules 2020, 25, 5560. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, M.; Li, J.; Liu, J.; Wang, M.; Zhao, C. Exploration of the Anti-Liver Injury Active Components of Shaoyao Gancao Decoction by Network Pharmacology and Experiments in Vivo. Phytomedicine 2023, 112, 154717. [Google Scholar] [CrossRef]
- Kong, S.; Ou, S.; Liu, Y.; Xie, M.; Mei, T.; Zhang, Y.; Zhang, J.; Wang, Q.; Yang, B. Surface-Enhanced Raman Spectroscopy Analysis of Astragalus Saponins and Identification of Metabolites After Oral Administration in Rats by Ultrahigh-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry Analysis. Front. Pharmacol. 2022, 13, 828449. [Google Scholar] [CrossRef]
- Chen, Q.; Wan, J.; Zhang, Y.; He, Y.; Bao, Y.; Yu, L.; Yang, J. Pharmacokinetic-Pharmacodynamic Modeling Analysis for Hydroxysafflor Yellow A-Calycosin in Compatibility in Normal and Cerebral Ischemic Rats: A Comparative Study. Biomed. Pharmacother. 2022, 150, 112950. [Google Scholar] [CrossRef]
- Truver, M.T.; Smith, C.R.; Garibay, N.; Kopajtic, T.A.; Swortwood, M.J.; Baumann, M.H. Pharmacodynamics and Pharmacokinetics of the Novel Synthetic Opioid, U-47700, in Male Rats. Neuropharmacology 2020, 177, 108195. [Google Scholar] [CrossRef]
- Arora, P.; Gudelsky, G.; Desai, P.B. Gender-Based Differences in Brain and Plasma Pharmacokinetics of Letrozole in Sprague-Dawley Rats: Application of Physiologically-Based Pharmacokinetic Modeling to Gain Quantitative Insights. PLoS ONE 2021, 16, e0248579. [Google Scholar] [CrossRef] [PubMed]
- Pinterova-Leca, N.; Horsley, R.R.; Danda, H.; Žídková, M.; Lhotková, E.; Šíchová, K.; Štefková, K.; Balíková, M.; Kuchař, M.; Páleníček, T. Naphyrone (Naphthylpyrovalerone): Pharmacokinetics, Behavioural Effects and Thermoregulation in Wistar Rats. Addict. Biol. 2021, 26, e12906. [Google Scholar] [CrossRef] [PubMed]
- Krotulski, A.J.; Garibay, N.; Walther, D.; Walton, S.E.; Mohr, A.L.A.; Logan, B.K.; Baumann, M.H. Pharmacokinetics and Pharmacodynamics of the Synthetic Cannabinoid, 5F-MDMB-PICA, in Male Rats. Neuropharmacology 2021, 199, 108800. [Google Scholar] [CrossRef] [PubMed]
- Syrová, K.; Šíchová, K.; Danda, H.; Lhotková, E.; Jorratt, P.; Pinterová-Leca, N.; Vejmola, Č.; Olejníková-Ladislavová, L.; Hájková, K.; Kuchař, M.; et al. Acute Pharmacological Profile of 2C-B-Fly-NBOMe in Male Wistar Rats-Pharmacokinetics, Effects on Behaviour and Thermoregulation. Front. Pharmacol. 2023, 14, 1120419. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China: 2020 Edition; China Medical Science Press: Beijing, China, 2020; Volume IV, pp. 515–517. ISBN 978-7-5214-1599-5. [Google Scholar]
- Gao, J.; Xu, E.; Wang, H.; Wang, L.; Chen, S.; Wang, C.; Meng, F. Integrated Serum Pharmacochemistry, Network Pharmacology, and Pharmacokinetics to Clarify the Effective Components and Pharmacological Mechanisms of the Proprietary Chinese Medicine Jinkui Shenqi Pill in Treating Kidney Yang Deficiency Syndrome. J. Pharm. Biomed. Anal. 2024, 247, 116251. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Qiao, X.; Qian, Y.; Ye, M. Chemistry of the Chinese herbal medicine Puerariae Radix (Ge-Gen): A review. J. Chin. Pharm. Sci. 2014, 23, 347–360. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Liang, T.; Zhang, K.; Gao, Y.; Xu, L. Puerarin improves metabolic function leading to hepatoprotective effects in chronic alcohol-induced liver injury in rats. Phytomedicine 2013, 20, 849–852. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, S.; Liang, P.; Wang, Y.; Zhang, X.; Jia, Q.; Fu, J.; Han, S.; He, L. Screening and Evaluation of Anti-SARS-CoV-2 Components from Ephedra Sinica by ACE2/CMC-HPLC-IT-TOF-MS Approach. Anal. Bioanal. Chem. 2021, 413, 2995–3004. [Google Scholar] [CrossRef]
- Krizevski, R.; Bar, E.; Shalit, O.; Sitrit, Y.; Ben-Shabat, S.; Lewinsohn, E. Composition and Stereochemistry of Ephedrine Alkaloids Accumulation in Ephedra Sinica Stapf. Phytochemistry 2010, 71, 895–903. [Google Scholar] [CrossRef]
- Yun, N.; Kim, H.J.; Park, S.C.; Park, G.; Kim, M.K.; Choi, Y.H.; Jang, Y.P. Localization of Major Ephedra Alkaloids in Whole Aerial Parts of Ephedrae Herba Using Direct Analysis in Real Time-Time of Flight-Mass Spectrometry. Molecules 2021, 26, 580. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Q.; Hu, J.; Zhang, Y.; Li, J. Research Progress on Chemical Constituents of Lonicerae Japonicae Flos. BioMed Res. Int. 2016, 2016, 8968940. [Google Scholar]
- Shah, M.-A.; Kang, J.-B.; Park, D.-J.; Kim, M.-O.; Koh, P.-O. Chlorogenic Acid Alleviates Cerebral Ischemia-Induced Neuroinflammation via Attenuating Nuclear Factor Kappa B Activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-Inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Luo, M.; Jiang, Q.; Liu, S.; Jia, Q.; Bai, Z.; Wu, F.; Xie, J. Advancements and challenges in pharmacokinetic and pharmacodynamic research on the traditional Chinese medicine saponins: A comprehensive review. Front. Pharmacol. 2024, 15, 1393409. [Google Scholar] [CrossRef]
- Ma, S.; Wei, T.; Zhang, B.; Zhang, Y.; Lai, J.; Qu, J.; Liu, J.; Yin, P.; Shang, D. Integrated pharmacokinetic properties and tissue distribution of multiple active constituents in Qing-Yi Recipe: A comparison between granules and decoction. Phytomedicine 2024, 129, 155645. [Google Scholar] [CrossRef]
- Choi, M.K.; Jin, S.; Jeon, J.H.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Han, Y.H.; Song, I.S. Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J. Ginseng Res. 2020, 44, 229–237. [Google Scholar] [CrossRef]
- Rao Gajula, S.; Talari, S.; Chilvery, S.; Godugu, C.; Sonti, R. A Unique in Vivo Pharmacokinetic Profile, in Vitro Metabolic Stability and Hepatic First-Pass Metabolism of Garcinol, a Promising Novel Anticancer Phytoconstituent, by Liquid Chromatography–Mass Spectrometry. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad017. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Sayi, J.; Gustafsson, L.; Bertilsson, L.; Ingelman-Sundberg, M. Phenotype–Genotype Variability in the Human CYP3A Locus as Assessed by the Probe Drug Quinine and Analyses of Variant CYP3A4 Alleles. Biochem. Biophys. Res. Commun. 2005, 338, 299–305. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.; Wang, Y.; Zhang, L.; Qi, S.; Fang, Q.; Xu, Y.; Chen, J.; Cheng, X.; Liu, P.; et al. Pharmacokinetics and Anti-Liver Fibrosis Characteristics of Amygdalin: Key Role of the Deglycosylated Metabolite Prunasin. Phytomedicine 2022, 99, 154018. [Google Scholar] [CrossRef] [PubMed]





| Components | RT/min | Formula | Ion | Observed Mass (m/z) |
|---|---|---|---|---|
| quinic acid | 3.35 | C7H12O6 | [M − H]− | 191.0561 |
| amygdalin | 7.16 | C16H18O9 | [M − H]− | 353.0878 |
| chlorogenic acid | 7.27 | C21H20O9 | [M − H]− | 415.1035 |
| puerarin | 7.46 | C20H27NO11 | [M + HCOO]− | 502.1566 |
| 3′-methoxypuerarin | 7.66 | C22H22O10 | [M − H]− | 445.1140 |
| polydatin | 11.78 | C20H22O8 | [M + HCOO]− | 435.1297 |
| glycyrrhizic acid | 20.06 | C42H62O16 | [M − H]− | 821.3965 |
| Icariin (IS) | 19.19 | C33H40O15 | [M + HCOO]− | 721.2349 |
| Parameter | Quinic Acid | Chlorogenic Acid | Amygdalin | Puerarin | 3′-Methoxypuerarin | Polydatin | Glycyrrhizic Acid |
|---|---|---|---|---|---|---|---|
| Tmax/h | 1.71 ± 0.81 | 0.50 ± 0.16 | 0.43 ± 0.53 | 0.50 ± 0.16 | 0.50 ± 0.16 | 0.42 ± 0.13 | 0.50 ± 0.32 |
| Cmax/μg·L−1 | 3068.45 ± 448.78 | 460.090 ± 88.4920 | 22.07 ± 9.97 | 986.44 ± 210.85 | 248.34 ± 58.36 | 300.67 ± 60.55 | 41.78 ± 14.72 |
| AUC0–∞/ug·h·L−1 | 12,041.89 ± 1760.23 | 784.15 ± 163.27 | 94.63 ± 42.87 | 2203.81 ± 467.73 | 689.33 ± 124.96 | 574.39 ± 109.85 | 184.60 ± 59.78 |
| t1/2/h | 2.30 ± 0.96 | 3.18 ± 0.86 | 3.48 ± 2.45 | 3.97 ± 1.20 | 4.11 ± 0.95 | 4.60 ± 3.06 | 4.84 ± 0.99 |
| MRT0–∞/h | 3.94 ± 0.99 | 3.44 ± 0.56 | 6.21 ± 3.93 | 4.50 ± 1.23 | 4.94 ± 1.42 | 4.89 ± 2.93 | 7.46 ± 1.20 |
| Vz/F/L·kg−1 | 3761.66 ± 1455.95 | 82,627.60 ± 24,792.24 | 698,253.42 ± 235,745.13 | 36,676.36 ± 10,564.02 | 123,671.03 ± 40,580.80 | 158,774.23 ± 103,757.89 | 549,761.86 ± 126,372.03 |
| CLz/F/L·kg−1 | 1163.34 ± 163.20 | 18,151.94 ± 3434.65 | 168,323.84 ± 62,470.31 | 6485.99 ± 1357.43 | 20,505.31 ± 3528.00 | 24,720.59 ± 4734.52 | 82,393.60 ± 29,787.64 |
| Components | Calibration Curve | Linearity (ng/mL) | Correlation Coefficient (R2) |
|---|---|---|---|
| quinic acid | y = 0.0816x + 1.7136 | 50.00–7000 | 0.9999 |
| amygdalin | y = 0.0225x − 0.0022 | 0.25–100 | 0.9998 |
| chlorogenic acid | y = 0.0284x − 0.2839 | 1.00–1500 | 0.9993 |
| puerarin | y = 0.0398x − 0.2120 | 10.00–3000 | 0.9999 |
| 3′-methoxypuerarin | y = 0.0351x − 0.2379 | 5.00–1000 | 0.9994 |
| polydatin | y = 0.0173x − 0.1199 | 1.00–1000 | 0.9989 |
| glycyrrhizic acid | y = 0.0304x − 0.0041 | 0.10–150 | 0.9992 |
| Components | Concentration (ng/mL) | Precision RSD (%) | Accuracy RE (%) | Stability (%) | |||
|---|---|---|---|---|---|---|---|
| Intraday | Interday | Intraday | Interday | Freeze–Thaw | Long-Term | ||
| quinic acid | 300 | 2.6 | 7.2 | −2.1 | 5.7 | 4.7 | 3.4 |
| 3000 | 5.2 | 6.8 | −0.6 | 1.2 | 6.1 | 1.3 | |
| 6000 | 6.7 | 5.6 | −1.6 | 1.1 | 2.3 | 4.3 | |
| amygdalin | 5 | 10.6 | 11.3 | −4.6 | 4.2 | 2.6 | 14.8 |
| 45 | 3.7 | 6.9 | 6.2 | 1.3 | 6.7 | 5.7 | |
| 90 | 4.1 | 6.9 | −5.0 | 6.6 | 5.4 | 2.4 | |
| chlorogenic acid | 50 | 4.0 | 7.3 | 4.0 | −0.7 | 1.7 | 3.6 |
| 600 | 3.0 | 6.4 | 4.2 | −6.0 | 5.8 | 7.7 | |
| 1200 | 3.7 | 5.7 | −4.0 | 0.6 | 4.0 | 9.6 | |
| puerarin | 150 | 4.3 | 8.3 | 3.3 | 0.6 | 2.9 | 0.7 |
| 1400 | 4.3 | 6.8 | 5.1 | −0.9 | 8.0 | 3.2 | |
| 2800 | 4.2 | 3.5 | −1.3 | −4.1 | 2.0 | 3.3 | |
| 3′-methoxypuerarin | 50 | 2.8 | 5.7 | −2.7 | 1.4 | 4.5 | 1.3 |
| 450 | 3.9 | 5.6 | 4.5 | 3.1 | 6.0 | 3.8 | |
| 900 | 4.1 | 5.1 | −2.3 | 3.5 | 4.9 | 1.3 | |
| polydatin | 50 | 4.3 | 6.6 | 4.1 | −0.1 | 3.3 | 2.5 |
| 450 | 3.5 | 5.9 | 4.1 | −4.6 | 3.8 | 3.9 | |
| 900 | 2.8 | 4.5 | −0.7 | 1.4 | 2.9 | 5.9 | |
| glycyrrhizic acid | 5 | 10.1 | 9.5 | 14.1 | −7.3 | 13.1 | 6.8 |
| 70 | 5.4 | 8.4 | 4.5 | 2.8 | 6.8 | 3.2 | |
| 140 | 3.8 | 4.6 | 1.45 | 0.5 | 5.8 | 3.7 | |
| Components | Concentration (ng/mL) | Recovery (%) | Matrix Effects (%) | ||
|---|---|---|---|---|---|
| Mean ± SD | RSD | Mean ± SD | RSD | ||
| quinic acid | 300 | 92.3 ± 5.5 | 5.9 | 100.4 ± 5.2 | 5.2 |
| 3000 | 94.6 ± 1.7 | 1.8 | 95.7 ± 3.2 | 2.3 | |
| 6000 | 91.8 ± 3.3 | 3.6 | 95.6 ± 5.9 | 6.2 | |
| amygdalin | 5 | 98.5 ± 3.3 | 3.3 | 103.0 ± 2.8 | 2.7 |
| 45 | 93.0 ± 4.6 | 4.9 | 95.7 ± 4.5 | 4.7 | |
| 90 | 97.1 ± 3.4 | 3.5 | 96.2 ± 3.1 | 3.2 | |
| chlorogenic acid | 50 | 96.6 ± 3.8 | 3.9 | 102.2 ± 2.4 | 2.3 |
| 600 | 95.8 ± 5.1 | 5.3 | 97.2 ± 6.1 | 6.3 | |
| 1200 | 93.9 ± 4.2 | 4.5 | 95.1 ± 4.3 | 4.5 | |
| puerarin | 150 | 98.8 ± 1.9 | 1.9 | 103.6 ± 4.4 | 4.3 |
| 1400 | 98.3 ± 1.5 | 1.6 | 100.2 ± 4.2 | 4.2 | |
| 2800 | 98.4 ± 2.2 | 2.2 | 101.1 ± 2.8 | 2.8 | |
| 3′-methoxypuerarin | 50 | 97.4 ± 2.7 | 2.8 | 103.1 ± 3.5 | 3.4 |
| 450 | 98.2 ± 3.0 | 3.1 | 103.0 ± 4.2 | 4.2 | |
| 900 | 97.2 ± 2.8 | 2.8 | 99.9 ± 2.4 | 2.4 | |
| polydatin | 50 | 96.3 ± 2.5 | 2.5 | 94.1 ± 3.4 | 3.6 |
| 450 | 97.2 ± 4.8 | 4.9 | 94.8 ± 3.3 | 3.5 | |
| 900 | 92.1 ± 3.2 | 3.4 | 94.8 ± 3.9 | 4.1 | |
| glycyrrhizic acid | 5 | 95.2 ± 2.7 | 2.8 | 101.1 ± 6.9 | 6.8 |
| 70 | 96.0 ± 4.7 | 4.9 | 96.6 ± 3.9 | 4.1 | |
| 140 | 95.2 ± 3.0 | 3.2 | 93.6 ± 4.8 | 5.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, N.; Wan, H.; Yalkun, I.; He, Y.; Lu, Y.; Li, C.; Wan, H. Metabolic Profiling and Pharmacokinetics Characterization of Yinhua Pinggan Granules with High-Performance Liquid Chromatography Combined with High-Resolution Mass Spectrometry. Separations 2025, 12, 113. https://doi.org/10.3390/separations12050113
Gu N, Wan H, Yalkun I, He Y, Lu Y, Li C, Wan H. Metabolic Profiling and Pharmacokinetics Characterization of Yinhua Pinggan Granules with High-Performance Liquid Chromatography Combined with High-Resolution Mass Spectrometry. Separations. 2025; 12(5):113. https://doi.org/10.3390/separations12050113
Chicago/Turabian StyleGu, Ningning, Haofang Wan, Imranjan Yalkun, Yu He, Yihang Lu, Chang Li, and Haitong Wan. 2025. "Metabolic Profiling and Pharmacokinetics Characterization of Yinhua Pinggan Granules with High-Performance Liquid Chromatography Combined with High-Resolution Mass Spectrometry" Separations 12, no. 5: 113. https://doi.org/10.3390/separations12050113
APA StyleGu, N., Wan, H., Yalkun, I., He, Y., Lu, Y., Li, C., & Wan, H. (2025). Metabolic Profiling and Pharmacokinetics Characterization of Yinhua Pinggan Granules with High-Performance Liquid Chromatography Combined with High-Resolution Mass Spectrometry. Separations, 12(5), 113. https://doi.org/10.3390/separations12050113









