Sustainable Soil Volatilome: Discrimination of Land Uses Through GC-MS-Identified Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
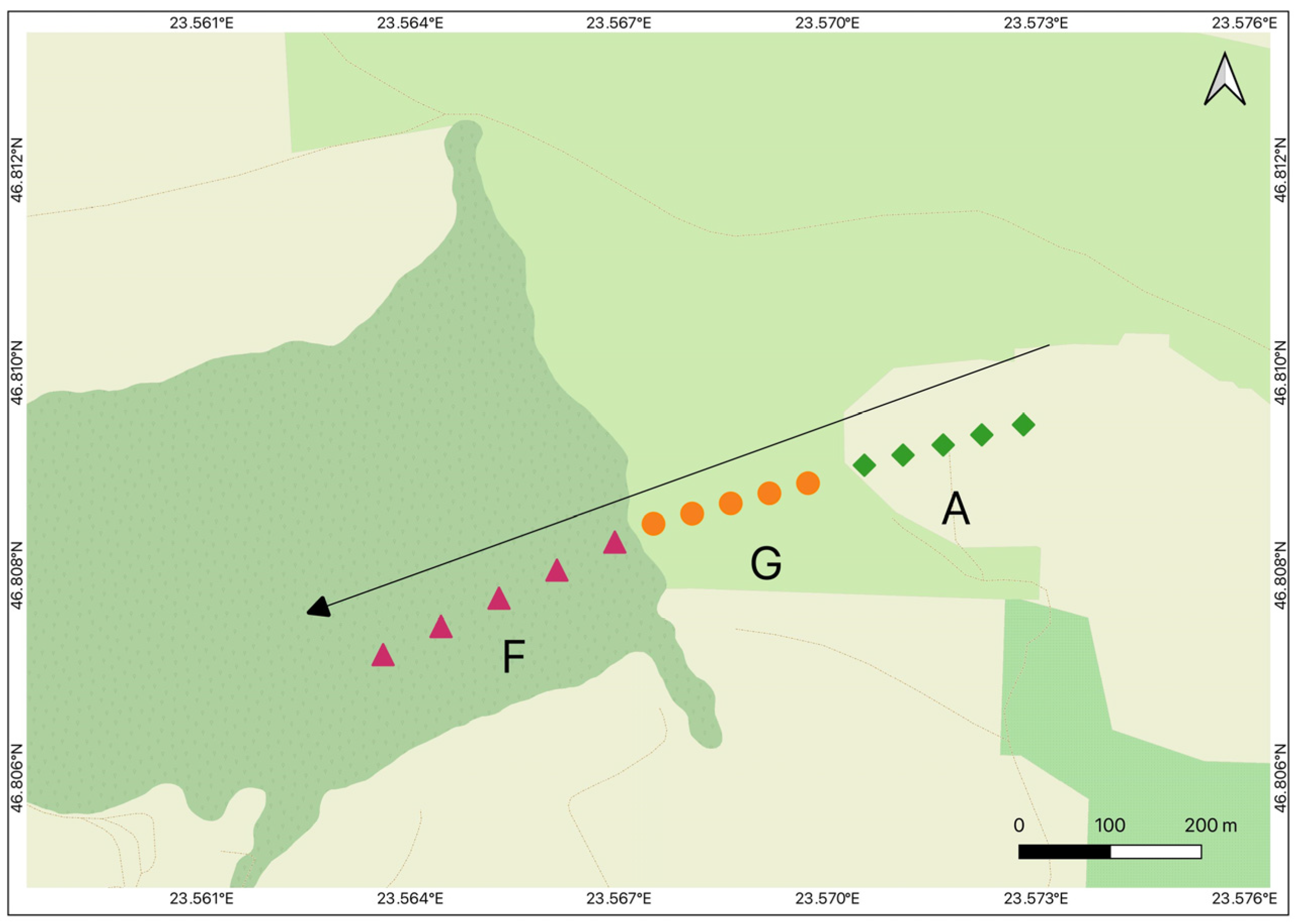
2.1. Sampling Design
2.2. Soil Characteristics
2.3. HS-SPME-GC-MS Analysis of Soil Volatilome Profile
2.4. Data Preprocessing and Data Pretreatment
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Volatilome Profile
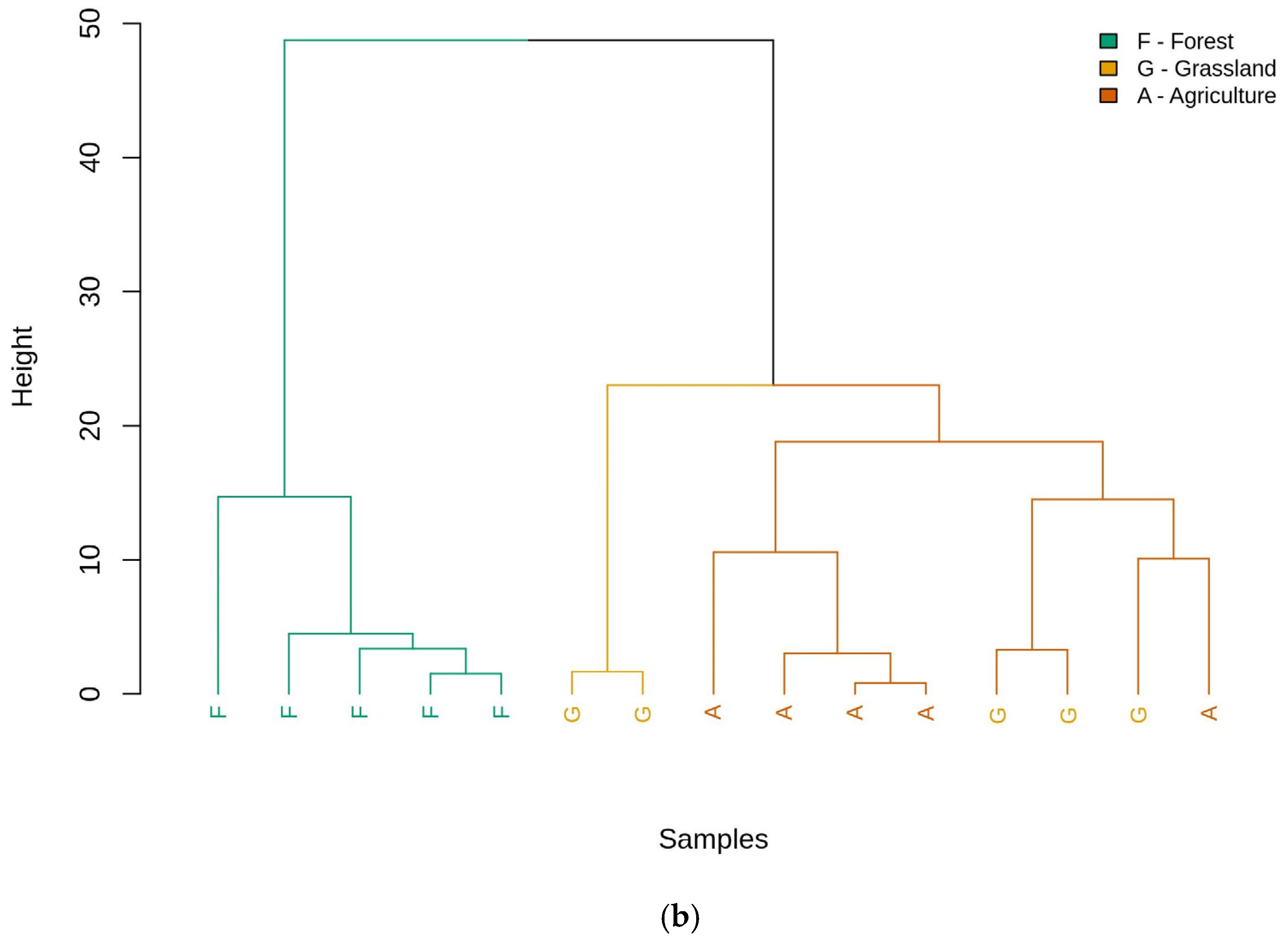
3.3. Exploratory Analysis
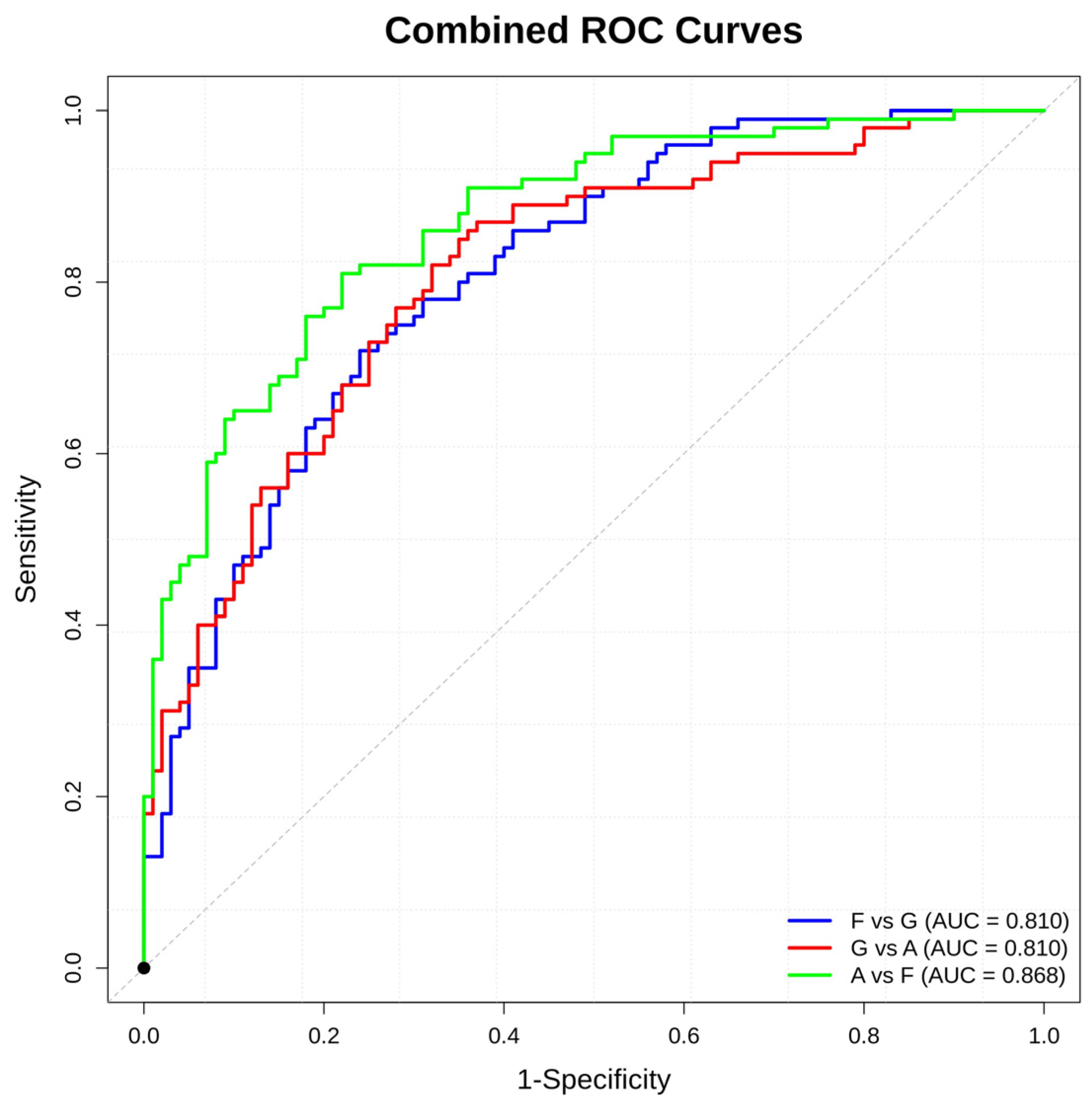
3.4. Supervised Analysis
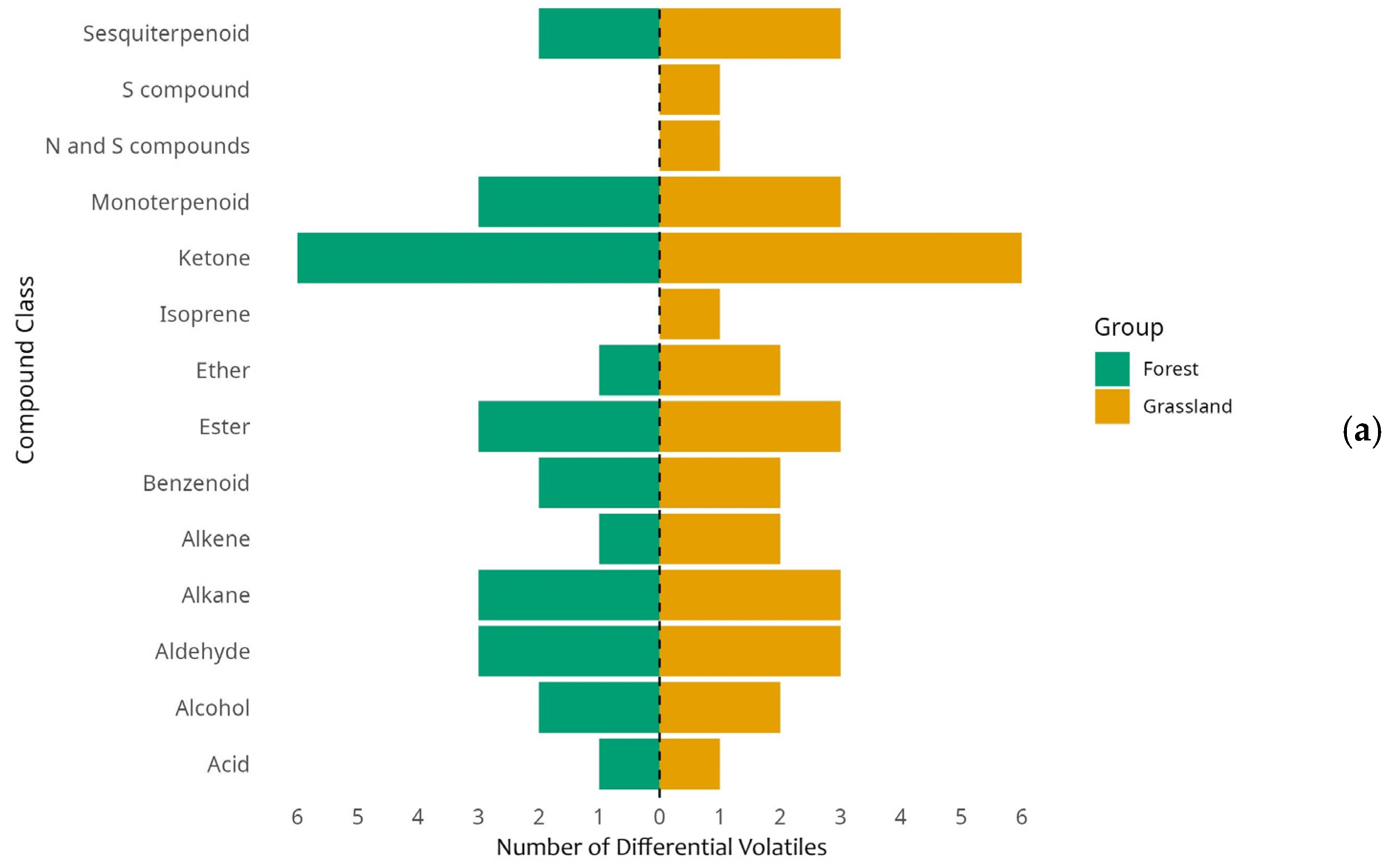
3.5. Differential Volatile Organic Compounds
4. Discussion
4.1. Soil Volatilomics in Decoding Complex Land Use-Specific Patterns
4.2. Ecological Implications
4.3. Further Research Directions and Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plassart, P.; Prevost-Boure, N.C.; Dequiedt, S.; Stone, D.; Creamer, R.; Griffiths, R.I.; Bailey, M.J.; Lemanceau, P. Soil parameters, land use, and geographical distance drive soil bacterial communities along a European transect. Sci. Rep. 2019, 9, 605. [Google Scholar]
- Burton, V.J.; Contu, S.; De Palma, A.; Hill, S.L.L.; Albrecht, H.; Bone, J.S.; Carpenter, D.; Corstanje, R.; De Smedt, P.; Farrell, M.; et al. Land use and soil characteristics affect soil organisms differently from above-ground assemblages. BMC Ecol. Evol. 2022, 22, 135. [Google Scholar]
- Igor, B.; Telak, L.J.; Pereira, P. Agriculture Management Impacts on Soil Properties and Hydrological Response in Istria (Croatia). Agronomy 2020, 10, 282. [Google Scholar] [CrossRef]
- Li, T.; Ciu, L.; Filipovic, V.; Tang, C.; Lai, Y.; Wehr, B.; Song, X.; Chapman, S.; Liu, H.; Dalal, R.C.; et al. From soil health to agricultural productivity: The critical role of soil constraint management. Catena 2025, 250, 108776. [Google Scholar]
- Gorska, E.B.; Stepien, W.; Hewelke, E.; Lata, J.C.; Gworek, B.; Gozdowski, D.; Sas-Paszt, L.; Bazot, S.; Lisek, A.; Gradowski, M.; et al. Response of soil microbiota to various soil management practices in 100-year-old agriculture field and identification of potential bacterial ecological indicator. Ecol. Indic. 2024, 158, 111545. [Google Scholar]
- Liu, Y.; Zhang, M.; Wang, X.; Wang, C. The impact of different grazing intensity and management measures on soil organic carbon density in Zhangye grassland. Sci. Rep. 2024, 14, 17556. [Google Scholar]
- Teague, R.; Kreuter, U. Managing grazing to restore soil health ecosystem function, and ecosystem services. Front. Sustain. Food Syst. 2020, 4, 534187. [Google Scholar]
- Yang, Z.; Miao, P.; Zheng, Y.; Guo, J. Impacts of grazing on vegetation and soil physicochemical properties in northern Yinshan Mountain grasslands. Sustainability 2023, 15, 16028. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.; You, Y.; Guo, X.; Wu, C.; Liu, S.; Sun, O.J. Differential effects of forest-floor litter and roots on soil organic carbon formation in a temperate oak forest. Soil. Biol. Biochem. 2023, 180, 109017. [Google Scholar]
- Zhao, X.; Tian, P.; Wang, Q. Roots have greater effects on the accumulation of soil microbial residue carbon in microaggregate fractions than leaf litter in a subtropical forest. Geoderma 2024, 442, 116803. [Google Scholar]
- Zhu, L.; Huang, R.; Guan, H.; Wang, J.; Huang, Z.; Fang, H.; Zou, X.; Li, J.; Liu, P. Litter, root, and mycorrhiza manipulations and seasonal effects on soil physicochemical properties and microbial communities in a subtropical coniferous and broad-leaved mixed forest. Appl. Soil. Ecol. 2024, 204, 105721. [Google Scholar] [CrossRef]
- Swiatek, B.; Pietrzykowski, M. Impact of Leaf Litter and Fine Roots in the Pool of Carbon, Nitrogen and Phosphorus Accumulated in Soil in Various Scenarios of Regeneration and Reconstruction of Forest Ecosystems. Forests 2022, 13, 1207. [Google Scholar] [CrossRef]
- Li, F.; Gao, W.; Lin, Y.; Liu, J.; Su, Y.; Zhang, Y.; Cai, K. Soil volatile organic compounds: Source-sink, function, mechanism, detection, and application analysis in environmental ecology. TrAC Trends Anal. Chem. 2025, 184, 118125. [Google Scholar] [CrossRef]
- Boone, R.W.C.; Meurs, J.; Rinnan, R.; de Caluwe, H.; Wakely, A.A.; Takke, J.W.C.; Cristescu, S.M.; Van der Putten, W.; de Kroon, H.; Robroek, B.J.M. Microbial scents: Soil microbial Volatile Organic Compounds (mVOCs) as biomarkers for grasslands across a land use gradient. Soil. Biol. Biochem. 2025, 204, 109749. [Google Scholar] [CrossRef]
- Honeker, L.K.; Graves, K.R.; Tfaily, M.M.; Krechmer, J.E.; Meredith, L.K. The Volatilome: A Vital Piece of the Complete Soil Metabolome. Front. Environ. Sci. 2021, 9, 649905. [Google Scholar] [CrossRef]
- Baloch, F.B.; Zeng, N.; Gong, H.; Zhang, Z.; Zhang, N.; Baloch, S.B.; Ali, S.; Li, B. Rhizobacterial volatile organic compounds: Implications for agricultural ecosystems’ nutrient cycling and soil health. Heliyon 2024, 10, e40522. [Google Scholar] [CrossRef]
- Wang, L.; Lun, X.; Wu, J.; Wang, Q.; Tao, J.; Dou, X.; Zhang, Z. Investigation of biogenic volatile organic compounds emissions in the Qinghai-Tibetan Plateau. Sci. Total Environ. 2023, 902, 165877. [Google Scholar] [CrossRef]
- Mu, Z.; Asensio, D.; Llusia, J.; Filella, I.; Ogaya, R.; Yi, Z.; Penuelas, J. Annual and seasonal variations in soil volatile organic compound concentrations in a Mediterranean shrubland and holm oak forest. Geoderma 2022, 405, 115401. [Google Scholar] [CrossRef]
- Yang, K.; Llusia, J.; Preece, C.; Ogaya, R.; Tur, L.M.; Mu, Z.; Chengming, Y.; Xu, Z.; Tan Yu Penuelas, J. Impacts of seasonality, drought, nitrogen fertilization, and litter on soil fluxes of biogenic volatile organic compounds in a Mediterranean forest. Sci. Total Environ. 2024, 906, 167354. [Google Scholar] [CrossRef]
- Leff, J.W.; Fierer, N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil. Biol. Biochem. 2008, 40, 1629–1636. [Google Scholar] [CrossRef]
- Legros, T.; Temime-Roussel, B.; Kammer, J.; Quivet, E.; Wortham, H.; Reiter, I.M.; Santonja, M.; Fernandez, C.; Ormeno, E. Decline of soil volatile organic compounds from a Mediterranean deciduous forest under a future drier climate. Atmos. Environ. 2025, 340, 120909. [Google Scholar]
- Brown, R.W.; Bull, I.D.; Journeaux, T.; Chadwick, D.R.; Jones, D.L. Volatile organic compounds (VOCs) allow sensitive differentiation of biological soil quality. Soil. Biol. Biochem. 2021, 156, 108187. [Google Scholar]
- Parastar, H.; Weller, P. Benchtop volatilomics supercharged: How machine learning based design of experiment helps optimizing untargeted GC-IMS gas phase metabolomics. Talanta 2024, 272, 125788. [Google Scholar] [CrossRef] [PubMed]
- Skawinski, M.; Van Schooten, F.J.; Smolinska, S. A comprehensive guide to volatolomics data analysis. J. Breath Res. 2025, 19, 015001. [Google Scholar]
- Yuan, Z.; Liu, Q.; Mo, L.; Pang, Z.; Hu, C. Integrating the Soil Microbiota and Metabolome Reveals the Mechanism through Which Controlled Release Fertilizer Affects Sugarcane Growth. Int. J. Mol. Sci. 2023, 24, 14086. [Google Scholar] [CrossRef]
- Song, Y.; Yao, S.; Li, X.; Wang, T.; Jiang, X.; Bolan, N.; Warren, C.R.; Northen, T.R.; Chang, S.X. Soil metabolomics: Deciphering underground metabolic webs in terrestrial ecosystems. Eco-Environ. Health 2024, 3, 227–237. [Google Scholar]
- Sitton, J.D.; Story, B.A. Estimating soil classification via quantitative and qualitative field testing for use in constructing compressed earth blocks. Procedia Eng. 2016, 145, 860–867. [Google Scholar]
- Chen, Y.C.; Vowles, P.D.; McTainsh, G.H.; Simpson, R.W.; Cohen, D.D.; Bailey, G.M. Use of a modified Walkley-Black method to determine the organic and elemental carbon content of urban aerosols collected on glass fibre filters. Chemosphere 1995, 31, 4403–4411. [Google Scholar]
- Lai, Z.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; Kind, T.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry chemoinformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Oliveira, L.F.C.; Pereira, T.F.D.; Matos, T.S.; Sayawa, A.C.H.F.; Sussulini, A. Sustainable food metabolome: Discrimination of agroecological, organic, and conventional pinto beans (Phaseolus vulgaris L.) through the volatile organic compounds profile. Microchem. J. 2024, 200, 110374. [Google Scholar]
- Pripdeevech, P.; Janta, R.; Sripahco, T.; Meesang, W.; Aiyathiti, C.; Prabamroong, T.; Mahatheeranont, S.; Poshyachind, S.; Pongpiachan, S.; Khruengsai, S. Seasonal volatile organic compound dynamics in urban and forest environments in Thailand: Implications for air quality and secondary pollutants. Environ. Pollut. 2025, 365, 125565. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ye, C.; Lin, W.; Chen, Y.; Zeng, L.; Yu, X.; Cui, J.; Zhang, C. Volatile organic compounds at a highland forest site in the southeast of the Tibetan Plateau: Source apportionment and reactivity contributions. Environ. Pollut. 2025, 366, 125410. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Ingrisch, J.; Meredith, L.K.; Pfannerstill, E.Y.; Klupfel, T.; Meeran, K.; Byron, J.; Purser, J.; Gil-Loaiza, J.; Haren, J.; et al. Effects of drought and recovery on soil volatile organic compound fluxes in an experimental rainforest. Nat. Commun. 2023, 14, 5064. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammary, A.A.G.; Al-Shihmani, L.S.; Fernandez-Galvez, J.; Caballero-Calvo, A. Optimizing sustainable agriculture: A comprehensive review of agronomic practices and their impacts on soil attributes. J. Environ. Manag. 2024, 364, 121487. [Google Scholar] [CrossRef]
- Li, F.; Zi, H.; Sonne, C.; Li, X. Microbiome sustains forest ecosystem functions across hierarchical scales. Eco-Environ. Health 2023, 2, 24–31. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, S.; Du, J.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Brown, R.E.; Chadwick, D.R.; Zang, H.; Jones, D.L. Use of metabolomics to quantify changes in soil microbial function in response to fertiliser nitrogen supply and extreme drought. Soil Biol. Biochem. 2021, 160, 108351. [Google Scholar] [CrossRef]
- Schramski, J.R.; Dell, A.I.; Grady, J.M.; Silby, R.M.; Brown, J.H. Metabolic theory predicts whole-ecosystem properties. Proc. Natl. Acad. Sci. USA 2015, 112, 2617–2622. [Google Scholar] [CrossRef]
- Pascual-Garcia, A.; Bell, T. Community-level signatures of ecological succession in natural bacterial communities. Nat. Commun. 2020, 11, 2386. [Google Scholar] [CrossRef]
- Gao, G.; Hannula, E.S.; Bodegom, P.M.; Bezemer, T.M.; Vries, T.F.; Hassink, J.; Zandt, M.H.; Moinet, G.Y.K. Land use intensity differently affects soil microbial functional communities in arable fields. Appl. Soil Ecol. 2024, 204, 105723. [Google Scholar]
- Bloor, J.M.G.; Si-Moussi, S.; Taberlet, P.; Carrere, P.; Hedde, M. Analysis of complex trophic networks reveals the signature of land-use intensification on soil communities in agroecosystems. Sci. Rep. 2021, 11, 18260. [Google Scholar]
- Zou, Y.; Zhang, Y.; Liu, X.; Song, H.; Cai, Q.; Wang, S.; Yi, C.; Chen, J. Research progress of benzothiazole and benzoxazole derivatives in the discovery of agricultural chemicals. Int. J. Mol. Sci. 2023, 24, 10807. [Google Scholar] [CrossRef] [PubMed]
- Dossin, E.; Elyette, M.; Pierrick, D.; Castellon, A.; Monge, A.; Pospisil, P. Prediction Models of Retention Indices for Increased Confidence in Structural Elucidation during Complex Matrix Analysis: Application to Gas Chromatography Coupled with High-Resolution Mass Spectrometry. Anal. Chem. 2016, 88, 7539–7547. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef]
- Battaloglu, R.; Yagiz, F. Determination of Essential Oils Components of Maclura pomifera (Osage Orange) Fruit from Turkey. Int. J. Sec. Metab. 2017, 4, 459–462. [Google Scholar]
- Belhassan, A.; Chtita, S.; Lakhlifi, T.; Bouachrine, M. QSRR study of linear retention indices for volatile compounds using statistical methods. Chem. Sci. Trans. 2018, 7, 558–575. [Google Scholar]
- Fidelis, C.; Sampaio, P.; Krainavic, P.; Augusto, F.; Barata, L. Correlation between maturity of tree and GC × GC–qMS chemical profiles of essential oil from leaves of Aniba rosaeodora Ducke. Microchem. J. 2013, 109, 73–77. [Google Scholar] [CrossRef]



| Soil Properties | KW Test | Post Hoc | |||||
|---|---|---|---|---|---|---|---|
| χ2 | p | Significance 1 | F-G | G-A | A-F | ||
| Bulk density (g cm−3) | 10.02 | 0.007 | ** | p = 0.035 * | ns | p = 0.003 ** | |
| Texture (%) | Silt | 8.74 | 0.013 | * | p = 0.024 * | p = 0.011 * | ns |
| Clay | 1.39 | 0.5 | ns | ns | ns | ns | |
| Sand | 3.37 | 0.185 | ns | ns | ns | ns | |
| pH | 3.4 | 0.183 | ns | ns | ns | ns | |
| OC (%) | 5.06 | 0.08 | ns | ns | ns | ns | |
| Water-soluble ions (mg kg−1) | Ca2+ | 4.75 | 0.093 | ns | ns | ns | ns |
| Mg2+ | 0.74 | 0.689 | ns | ns | ns | ns | |
| K+ | 9.5 | 0.009 | ** | ns | p = 0.007 ** | p = 0.02 * | |
| Na+ | 1.69 | 0.429 | ns | ns | ns | ns | |
| Cl− | 7.31 | 0.026 | * | p = 0.015 * | ns | ns | |
| 5.2 | 0.074 | ns | ns | ns | ns | ||
| 9.75 | 0.008 | ** | ns | p = 0.026 * | p = 0.005 ** | ||
| Figures of Merit | R | Cutt Point | Q | Accuracy | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| F vs. G | 0.74 | 0.714 | 0.822 | 0.74 | 0.81 | 0.72 | 0.76 |
| G vs. A | 0.747 | 0.697 | 0.839 | 0.75 | 0.81 | 0.82 | 0.68 |
| A vs. F | 0.795 | 0.751 | 0.838 | 0.795 | 0.868 | 0.81 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacs, E.D.; Rusu, T.; Kovacs, M.H. Sustainable Soil Volatilome: Discrimination of Land Uses Through GC-MS-Identified Volatile Organic Compounds. Separations 2025, 12, 92. https://doi.org/10.3390/separations12040092
Kovacs ED, Rusu T, Kovacs MH. Sustainable Soil Volatilome: Discrimination of Land Uses Through GC-MS-Identified Volatile Organic Compounds. Separations. 2025; 12(4):92. https://doi.org/10.3390/separations12040092
Chicago/Turabian StyleKovacs, Emoke Dalma, Teodor Rusu, and Melinda Haydee Kovacs. 2025. "Sustainable Soil Volatilome: Discrimination of Land Uses Through GC-MS-Identified Volatile Organic Compounds" Separations 12, no. 4: 92. https://doi.org/10.3390/separations12040092
APA StyleKovacs, E. D., Rusu, T., & Kovacs, M. H. (2025). Sustainable Soil Volatilome: Discrimination of Land Uses Through GC-MS-Identified Volatile Organic Compounds. Separations, 12(4), 92. https://doi.org/10.3390/separations12040092









