Extraction and Characterization of TiO2 Pigments from Commercial Paints for Environmental Studies
Abstract
1. Introduction
2. Materials and Methods
3. Results
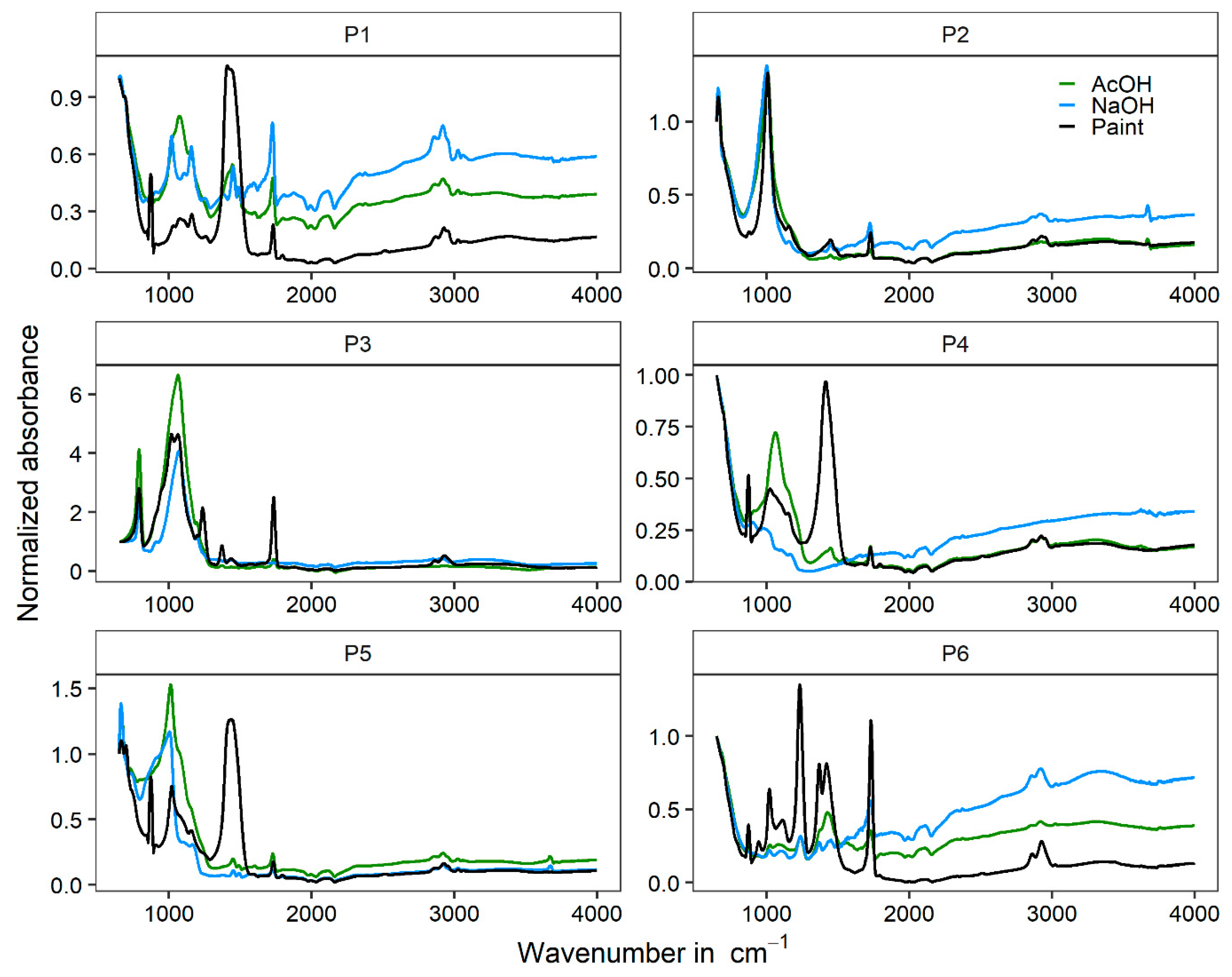
3.1. Paints’ Composition
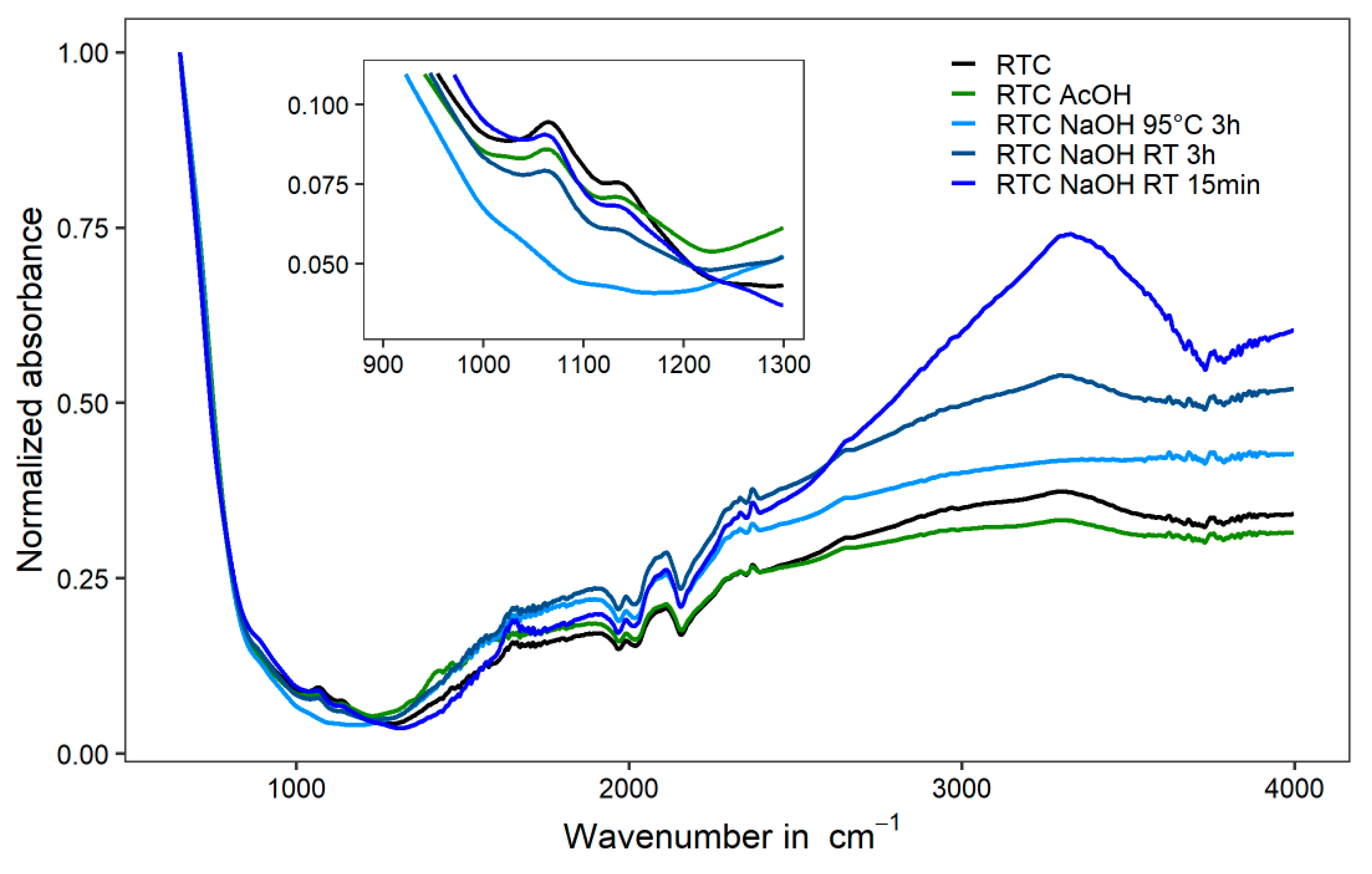
3.2. Extraction Method
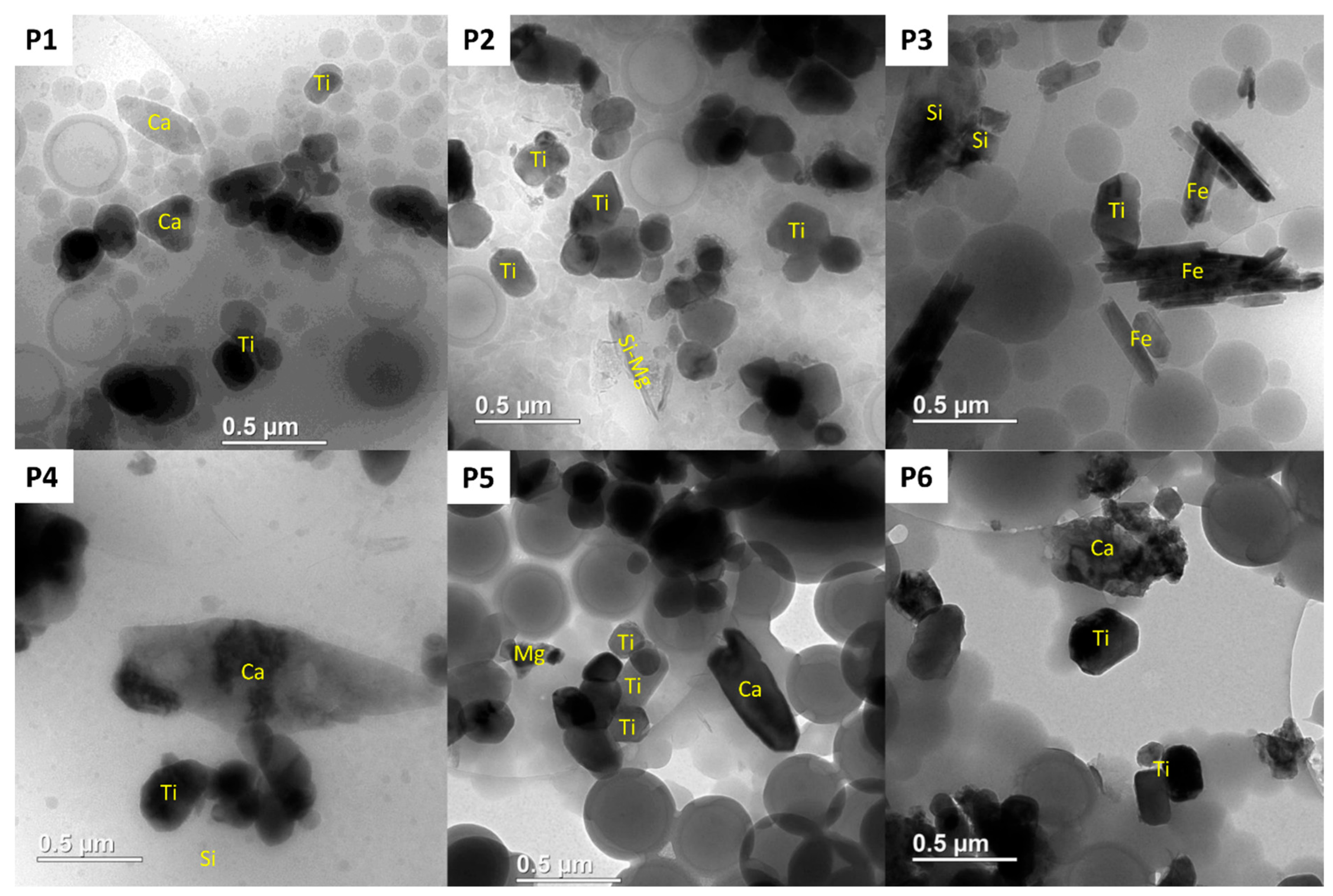
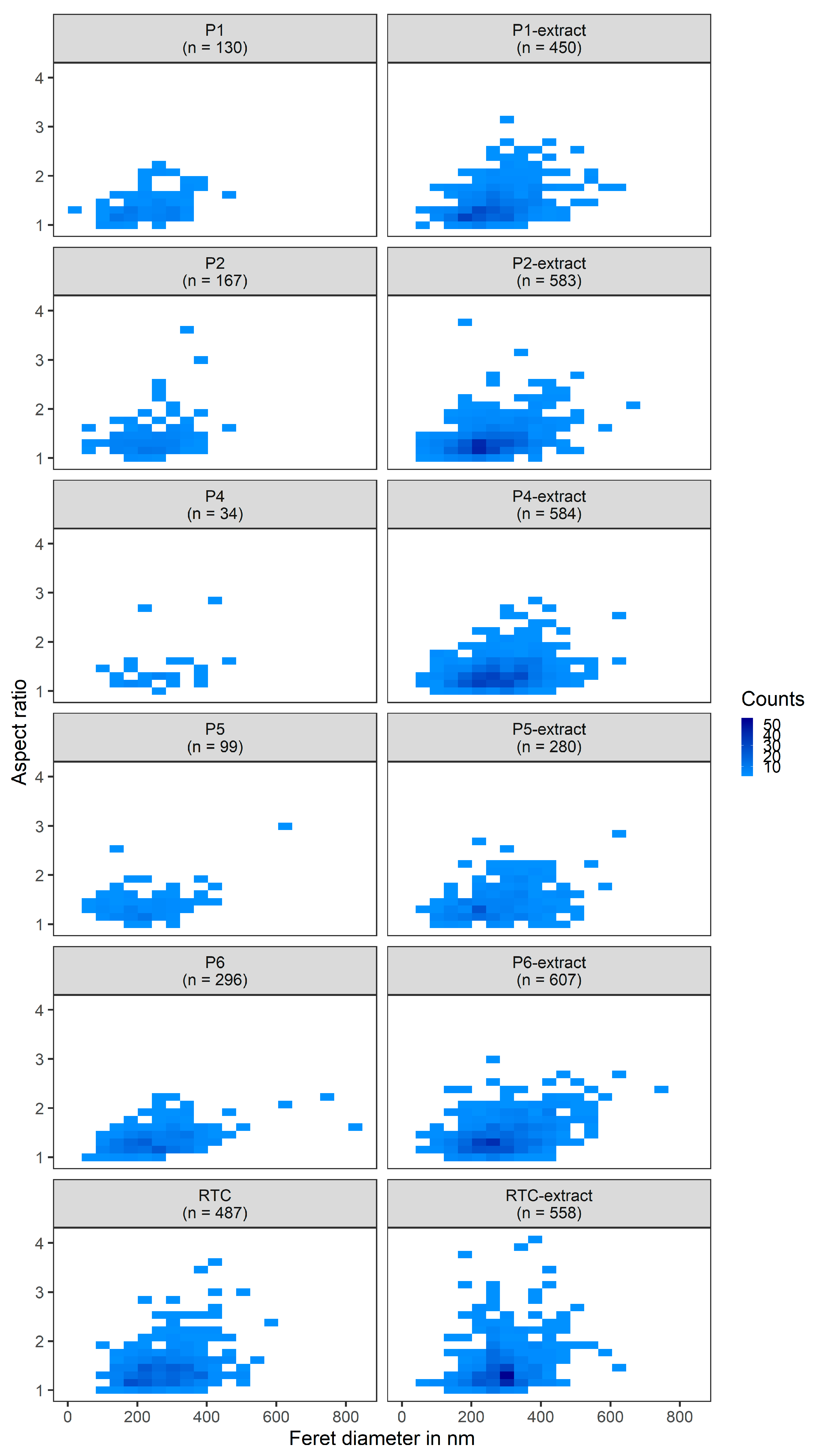
3.3. TiO2 Pigments Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cryo-TEM | Cryogenic-transmission electron microscopy |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| TGA-QMS | Thermogravimetry coupled with quadrupole mass spectrometry |
| IR | Infrared spectroscopy |
| EDTA | Ethylenediaminetetraacetic acid |
| AcOH | Acetic acid |
| UPW | Ultrapure water |
| XEDS | X-ray energy dispersive spectroscopy |
| SEM | Scanning electron microscopy |
| XRD | X-ray powder diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding Nano: Challenges Involved in Monitoring the Presence and Fate of Engineered Titanium Dioxide Nanoparticles in Aquatic Environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Karlsson, M.C.F.; Álvarez-Asencio, R.; Bordes, R.; Larsson, A.; Taylor, P.; Steenari, B.M. Characterization of paint formulated using secondary TiO2 pigments recovered from waste paint. J. Coat. Technol. Res. 2019, 16, 607–614. [Google Scholar] [CrossRef]
- Kirsch, S.; Pfaua, A.; Frechen, T.; Schrof, W.; Pföhler, P.; Francke, D. Scrub resistance of highly pigmented paints: A study on abrasion mechanisms of different scrub techniques. Prog. Org. Coat. 2001, 43, 99–110. [Google Scholar]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F., Jr. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557, 740–753. [Google Scholar]
- Azimzada, A.; Farner, J.M.; Jreije, I.; Hadioui, M.; Liu-Kang, C.; Tufenkji, N.; Shaw, P.; Wilkinson, K.J. Single-and multi-element quantification and characterization of TiO2 nanoparticles released from outdoor stains and paints. Front. Environ. Sci. 2020, 8, 91. [Google Scholar]
- Azimzada, A.; Farner, J.M.; Hadioui, M.; Liu-Kang, C.; Jreije, I.; Tufenkji, N.; Wilkinson, K.J. Release of TiO2 nanoparticles from painted surfaces in cold climates: Characterization using a high sensitivity single-particle ICP-MS. Environ. Sci. Nano 2020, 7, 139–148. [Google Scholar]
- Baalousha, M.; Wang, J.; Nabi, M.; Loosli, F.; Valenca, R.; Mohanty, S.K.; Afrooz, N.; Cantando, E.; Aich, N. Stormwater green infrastructures retain high concentrations of TiO2 engineered (nano)-particles. J. Hazard. Mater. 2020, 392, 122335. [Google Scholar] [PubMed]
- Wang, J.; Nabi, M.; Mohanty, S.K.; Afrooz, A.N.; Cantando, E.; Aich, N.; Baalousha, M. Detection and quantification of engineered particles in urban runoff. Chemosphere 2020, 248, 126070. [Google Scholar] [PubMed]
- Nabi, M.M.; Wang, J.; Goharian, E.; Baalousha, M. Temporal variation in TiO2 engineered particle concentrations in the Broad River during dry and wet weathers. Sci. Total Environ. 2021, 807, 151081. [Google Scholar]
- Wang, J.; Nabi, M.M.; Erfani, M.; Goharian, E.; Baalousha, M. Identification and Quantification of Anthropogenic Nanomaterials in Urban Rain and Runoff Using Single Particle-Inductively Coupled Plasma-Time of Flight-Mass Spectrometry. Environmental Science Nano 2022, 9, 714–729. [Google Scholar]
- Nabi, M.M.; Wang, J.; Baalousha, M. Detection and quantification of anthropogenic titanium, cerium, and lanthanum-bearing particles home dust. Environ. Sci. Nano 2023, 10, 1372–1384. [Google Scholar]
- Sharma, V.K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A Review. J. Environ. Sci. Health Part A 2009, 44, 1485–1495. [Google Scholar]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs-a critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar]
- Liu, H.H.; Cohen, Y. Multimedia environmental distribution of engineered nanomaterials. Environ. Sci. Technol. 2014, 48, 3281–3292. [Google Scholar]
- Phalyvong, K.; Sivry, Y.; Pauwels, H.; Gélabert, A.; Tharaud, M.; Wille, G.; Bourrat, X.; Ranville, J.F.; Benedetti, M.F. Assessing CeO2 and TiO2 nanoparticle concentrations in the Seine River and its tributaries near Paris. Front. Environ. Sci. 2021, 8, 271. [Google Scholar]
- Phalyvong, K.; Sivry, Y.; Pauwels, H.; Gélabert, A.; Tharaud, M.; Wille, G.; Bourrat, X.; Benedetti, M.F. Occurrence and origins of cerium dioxide and titanium dioxide nanoparticles in the Loire river (France) by single particle ICP-MS and FEG-SEM imaging. Front. Environ. Sci. 2020, 8, 141. [Google Scholar]
- Liu, Z.; Rui, M.; Yu, S. Occurrence of titanium dioxide nanoparticle in Taihu Lake (China) and its removal at a full-scale drinking water treatment plant. Environ. Sci. Pollut. Res. 2021, 29, 23352–23360. [Google Scholar]
- Campos, D.A.; Schaumann, G.E.; Philippe, A. Natural TiO2-Nanoparticles in Soils: A Review on Current and Potential Extraction Methods. Crit. Rev. Anal. Chem. 2020, 52, 735–755. [Google Scholar]
- Khomami, N.T.S.; Patel, P.M.; Jusi, C.P.; Trouillet, V.; David, J.; Schaumann, G.E.; Philippe, A. Influential parameters of surface waters on the formation of coating onto TiO2 nanoparticles under natural conditions. Environ. Sci. Nano 2021, 8, 3153–3166. [Google Scholar] [CrossRef]
- Xu, F. Review of analytical studies on TiO2 nanoparticles and particle aggregation, coagulation, flocculation, sedimentation, stabilization. Chemosphere 2018, 212, 662–677. [Google Scholar]
- Sani-Kast, N.; Labille, J.; Ollivier, P.; Slomberg, D.; Hungerbühler, K.; Scheringer, M. A network perspective reveals decreasing material diversity in studies on nanoparticle interactions with dissolved organic matter. Proc. Natl. Acad. Sci. USA 2017, 114, E1756–E1765. [Google Scholar] [PubMed]
- Tayyebi Sabet Khomami, N.; Welle, A.; Kunz, S.; Philippe, A. Sorption of Fulvic Acids onto Titanium Dioxide Nanoparticles Extracted from Commercial Sunscreens: ToF-SIMS and High-Dimensional Data Analysis. Coatings 2022, 12, 335. [Google Scholar]
- Karlsson, M.C.; Abbas, Z.; Bordes, R.; Cao, Y.; Larsson, A.; Taylor, P.; Steenari, B.-M. Characterisation of silicon, zirconium and aluminium coated titanium dioxide pigments recovered from paint waste. Dye. Pigment. 2019, 162, 145–152. [Google Scholar]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar]
- Fitzpatrick, R.W.; Chittleborough, D. Titanium and zirconium minerals. Soil Mineral. Environ. Appl. 2002, 7, 667–690. [Google Scholar]
- Khomami, N.T.S.; Philippe, A.; Abu Quba, A.A.; Lechtenfeld, O.J.; Guigner, J.-M.; Heissler, S.; Schaumann, G.E. Validation of a field deployable reactor for in situ formation of NOM-engineered nanoparticle corona. Environ. Sci. Nano 2020, 7, 486–500. [Google Scholar] [CrossRef]
- SDBS Database. (National Institute of Advanced Industrial Science and Technology). Available online: https://sdbs.db.aist.go.jp (accessed on 1 October 2023).
- Philippe, A.; Košík, J.; Welle, A.; Guigner, J.-M.; Clemens, O.; Schaumann, G.E. Extraction and characterization methods for titanium dioxide nanoparticles from commercialized sunscreens. Environ. Sci. Nano 2018, 5, 191–202. [Google Scholar]
- O’Brien, L.C.; Root, H.B.; Wei, C.C.; Jensen, D.; Shabestary, N.; De Meo, C.; Eder, D.J. M2+• EDTA binding affinities: A modern experiment in thermodynamics for the physical chemistry laboratory. J. Chem. Educ. 2015, 92, 1547–1551. [Google Scholar]
- Fertani-Gmati, M.; Jemal, M. Thermochemistry and kinetics of silica dissolution in NaOH aqueous solution. Thermochim. Acta 2011, 513, 43–48. [Google Scholar]
- Niibori, Y.; Kunita, M.; Tochiyama, O.; Chida, T. Dissolution rates of amorphous silica in highly alkaline solution. J. Nucl. Sci. Technol. 2000, 37, 349–357. [Google Scholar]

| ID | Water in % | TiO2 mg/g | Al mg/g | Ca mg/g | Fe mg/g | Na mg/g | Organic Molecules in % | Carbonate in % |
|---|---|---|---|---|---|---|---|---|
| RTC | - | 922 ± 12 | 22 ± 2 | 71 ± 7 | <LOD | 8 ± 6 | 2.1 ± 0.13 | <LOD |
| P1 | 42 ± 4 | 109 ± 16 | 11 ± 3 | 81 ± 9 | <LOD | 3.4 ± 0.1 | 20.8 ± 0.6 | 22.5 ± 0.3 |
| P2 | 50 ± 5 | 122 ± 9 | 17 ± 2 | 8.3 ± 0.2 | <LOD | 4.8 ± 0.4 | 18.4 ± 0.4 | 3.0 ± 0.4 |
| P3 | 38 ± 2 | 11.7 ± 0.3 | <LOD | <LOD | 12.4 ± 0.3 | 4.00 ± 0.7 | 34.9 ± 0.5 | <LOD |
| P4 | 45 ± 6 | 142 ± 33 | 9 ± 2 | 109 ± 22 | <LOD | <LOD | 11.4 ± 0.2 | 25.2 ± 0.2 |
| P5 | 44 ± 3 | 49 ± 2 | 15 ± 8 | 69 ± 3 | <LOD | 3.20 ± 0.3 | 17.1 ± 0.3 | 19.1 ± 0.3 |
| P6 | 44 ± 3.3 | 144 ± 21 | <LOD | 56 ± 8 | <LOD | 5 ± 2 | 43.5 ± 0.5 | 15.0 ± 0.5 |
| LOD | - | 1.7 | 5 | 2 | 3 | 2 | 0.025 | 0.025 |
| ID | Method | TiO2 mg/g | Al mg/g | Ca mg/g | Fe mg/g | Na mg/g | Recovery TiO2 in % | Organic Molecules in % | CO32− in % |
|---|---|---|---|---|---|---|---|---|---|
| RTC | AcOH | 922 ± 31 | 21.7 ± 0.8 | 2.4 ± 0.2 | <LOD | 11.3 ± 0.2 | 98 ± 3 | 1.62 | 0.08 |
| RTC | NaOH | 973 ± 33 | 4.9 ± 0.2 | 1.2 ± 0.1 | <LOD | 9 ± 1 | 106 ± 2 | <LOD | <LOD |
| P1 | NaOH | 506 ± 22 | 18 ± 5 | <LOD | <LOD | 10 ± 2 | 109 ± 27 | 39.2 | <LOD |
| P1 | AcOH | 922 ± 5 | 38.4 ± 0.7 | 22.9 ± 4.3 | <LOD | 11.3 ± 1.1 | 118 ± 2 | 26.5 | <LOD |
| P2 | NaOH | 307 ± 16 | 21 ± 4 | <LOD | 2.6 ± 0.5 | 10 ± 1 | 90 ± 10 | 19.4 | 1.0 |
| P2 | AcOH | 285 ± 17 | 35 ± 5 | 3.8 ± 0.2 | <LOD | 13.0 ± 0.3 | 91 ± 4 | 9.6 | <LOD |
| P3 | NaOH | 56 ± 3 | <LOD | <LOD | 60 ± 1 | 8 ± 1 | 78 ± 5 | 41.5 | <LOD |
| P3 | AcOH | 25.0 ± 0.3 | <LOD | 2.8 ± 0.4 | 28 ± 2 | 12.5 ± 1.1 | 91 ± 4 | 19.7 | 1.0 |
| P4 | NaOH | 725 ± 31 | 46 ± 4 | <LOD | 2.8 ± 0.8 | 7.2 ± 0.8 | 95 ± 18 | 2.2 | <LOD |
| P4 | AcOH | 317 ± 28 | 20.2 ± 1.1 | 19.4 ± 1.0 | <LOD | 10.0 ± 0.9 | 67 ± 5 | 14.1 | 3.6 |
| P5 | NaOH | 278 ± 9 | 74 ± 2 | <LOD | 2.2 ± 0.2 | 9.8 ± 0.2 | 107 ± 14 | 19.4 | <LOD |
| P5 | AcOH | 166 ± 8 | 44 ± 12 | 7.3 ± 0.6 | <LOD | 14 ± 3 | 122 ± 6 | 21.6 | 1.7 |
| P6 | NaOH | 358 ± 101 | <LOD | <LOD | <LOD | 8.7 ± 0.6 | 73 ± 30 | 34.8 | <LOD |
| P6 | AcOH | 618 ± 59 | 6.3 ± 1.7 | 20 ± 18 | <LOD | 18 ± 13 | 92 ± 6 | 11.0 | 5.9 |
| LOD | 2 | 5 | 5 | 2 | 2 | 0.025 | 0.025 |
| Element | Binding Energy in eV | Corresponding Species | RTC | RTC AcOH |
|---|---|---|---|---|
| Al2p3 | 74.3 | Al-oxides/hydroxides | 13.6 | 12.8 |
| C1s | 285.0 | C-C and C-H | 27.0 | 20.1 |
| C1s | 286.5 | C-O | 3.3 | 5.1 |
| C1s | 288.9 | O-C=O | 1.2 | 3.6 |
| Ti2p3 | 458.7 | TiO2 | 6.1 | 6.2 |
| O1s | 529–533 | Metal oxide, C-O and C=O | 47.5 | 51.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippe, A.; Ndoli-Kessie, S.; Fricke, C.; Guigner, J.-M.; Heider, B.; Di Lodovico, E. Extraction and Characterization of TiO2 Pigments from Commercial Paints for Environmental Studies. Separations 2025, 12, 91. https://doi.org/10.3390/separations12040091
Philippe A, Ndoli-Kessie S, Fricke C, Guigner J-M, Heider B, Di Lodovico E. Extraction and Characterization of TiO2 Pigments from Commercial Paints for Environmental Studies. Separations. 2025; 12(4):91. https://doi.org/10.3390/separations12040091
Chicago/Turabian StylePhilippe, Allan, Sylvester Ndoli-Kessie, Christian Fricke, Jean-Michel Guigner, Benjamin Heider, and Eliana Di Lodovico. 2025. "Extraction and Characterization of TiO2 Pigments from Commercial Paints for Environmental Studies" Separations 12, no. 4: 91. https://doi.org/10.3390/separations12040091
APA StylePhilippe, A., Ndoli-Kessie, S., Fricke, C., Guigner, J.-M., Heider, B., & Di Lodovico, E. (2025). Extraction and Characterization of TiO2 Pigments from Commercial Paints for Environmental Studies. Separations, 12(4), 91. https://doi.org/10.3390/separations12040091






