Migration and Enrichment of Rare Earth Elements in the Flotation Process of Rare Earth-Bearing Collophanite
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Samples and Reagents
2.2. Experimental Methods
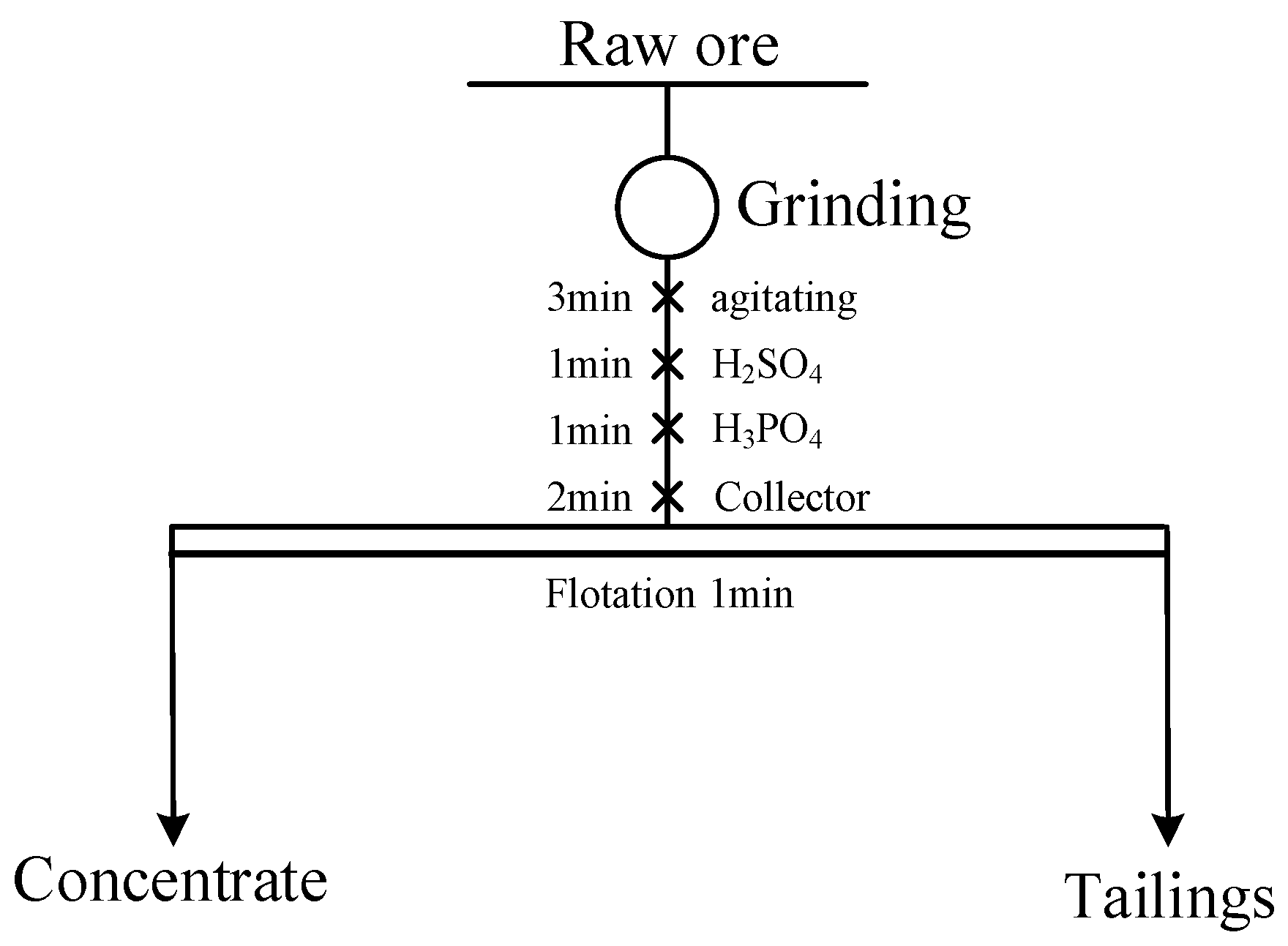
2.2.1. Reverse Flotation Experiment on Rare Earth-Bearing Collophanite
2.2.2. X-Ray Powder Diffraction Analysis of Rare Earth-Bearing Collophane
2.2.3. X-Ray Fluorescence Spectroscopy Analysis of Rare Earth-Bearing Collophane
2.2.4. Atomic Emission Spectroscopy Analysis
2.2.5. Mass Spectrometry Analysis
2.2.6. Mineral Embedding Characteristics Analysis
3. Results and Discussion
3.1. Process Mineralogical Study of Rare Earth-Bearing Collophane
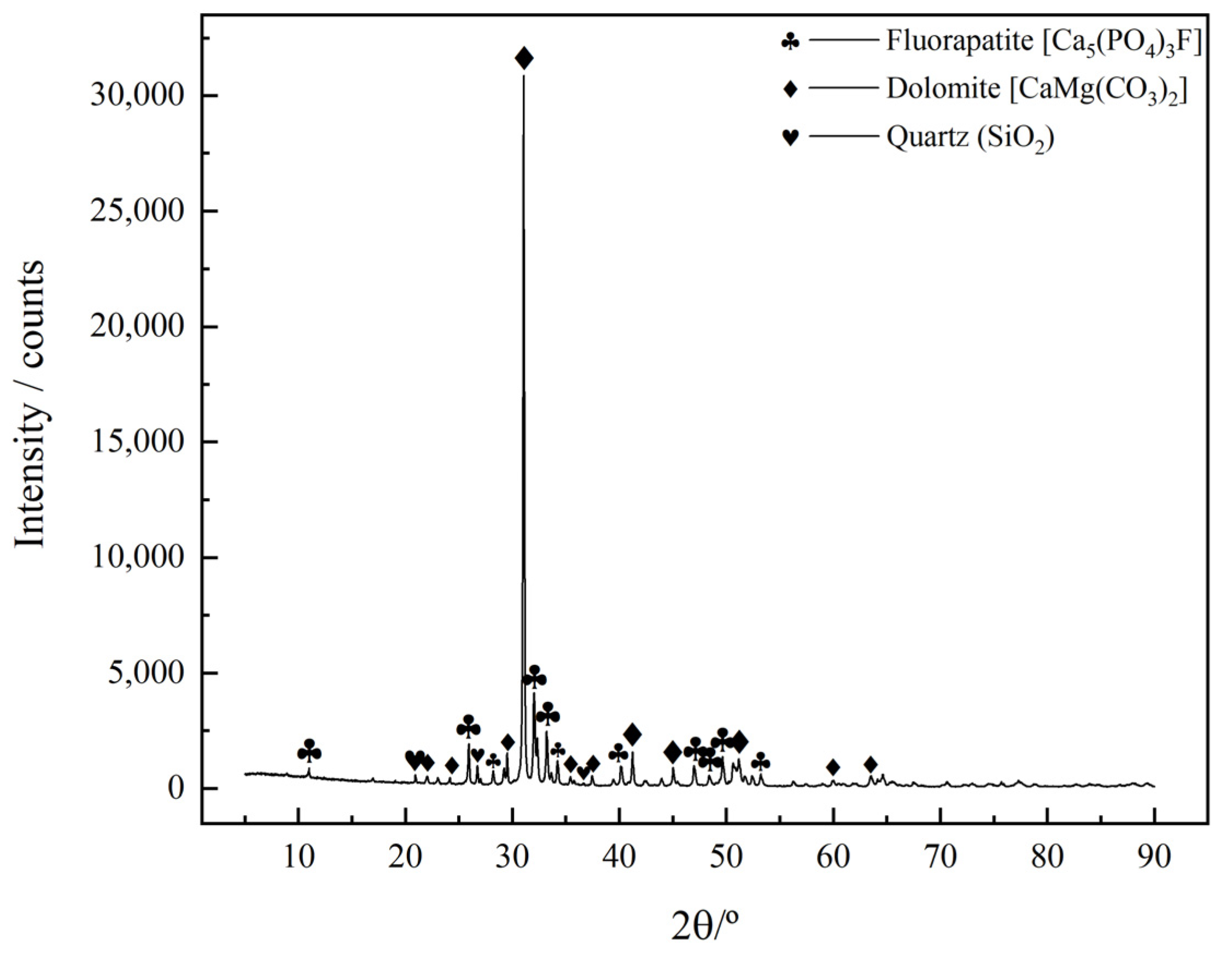
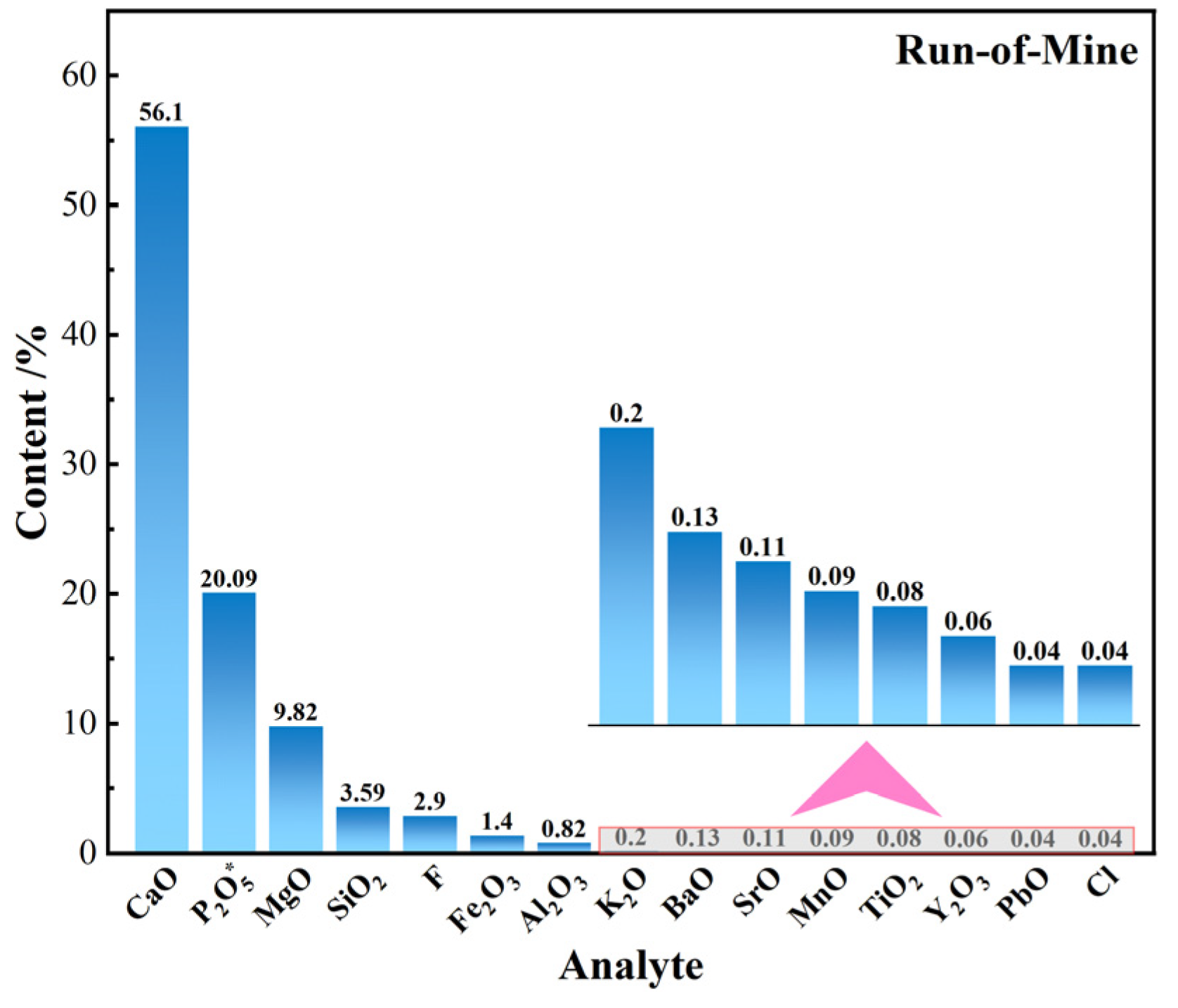
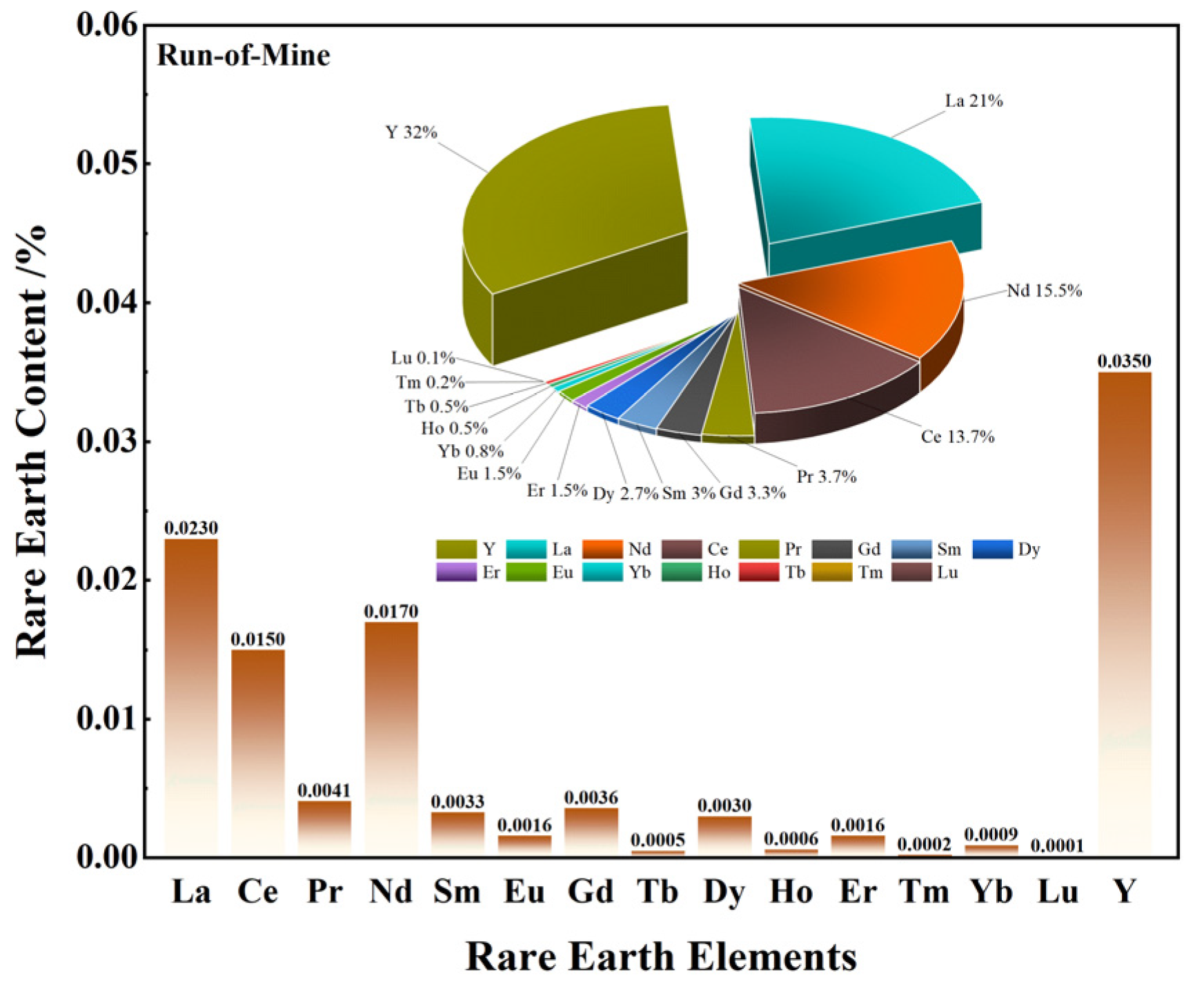
3.1.1. Phase and Multi-Element Analysis
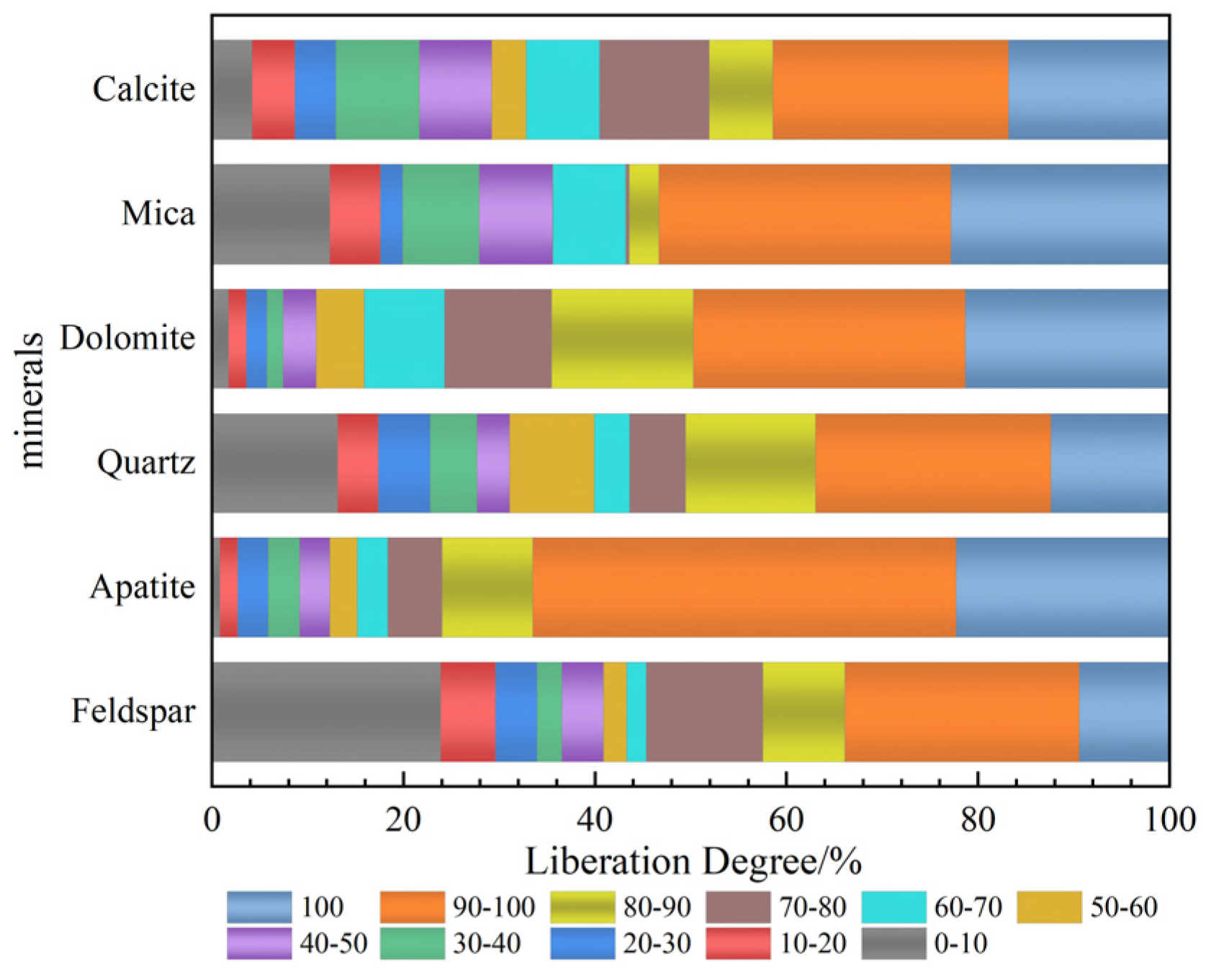
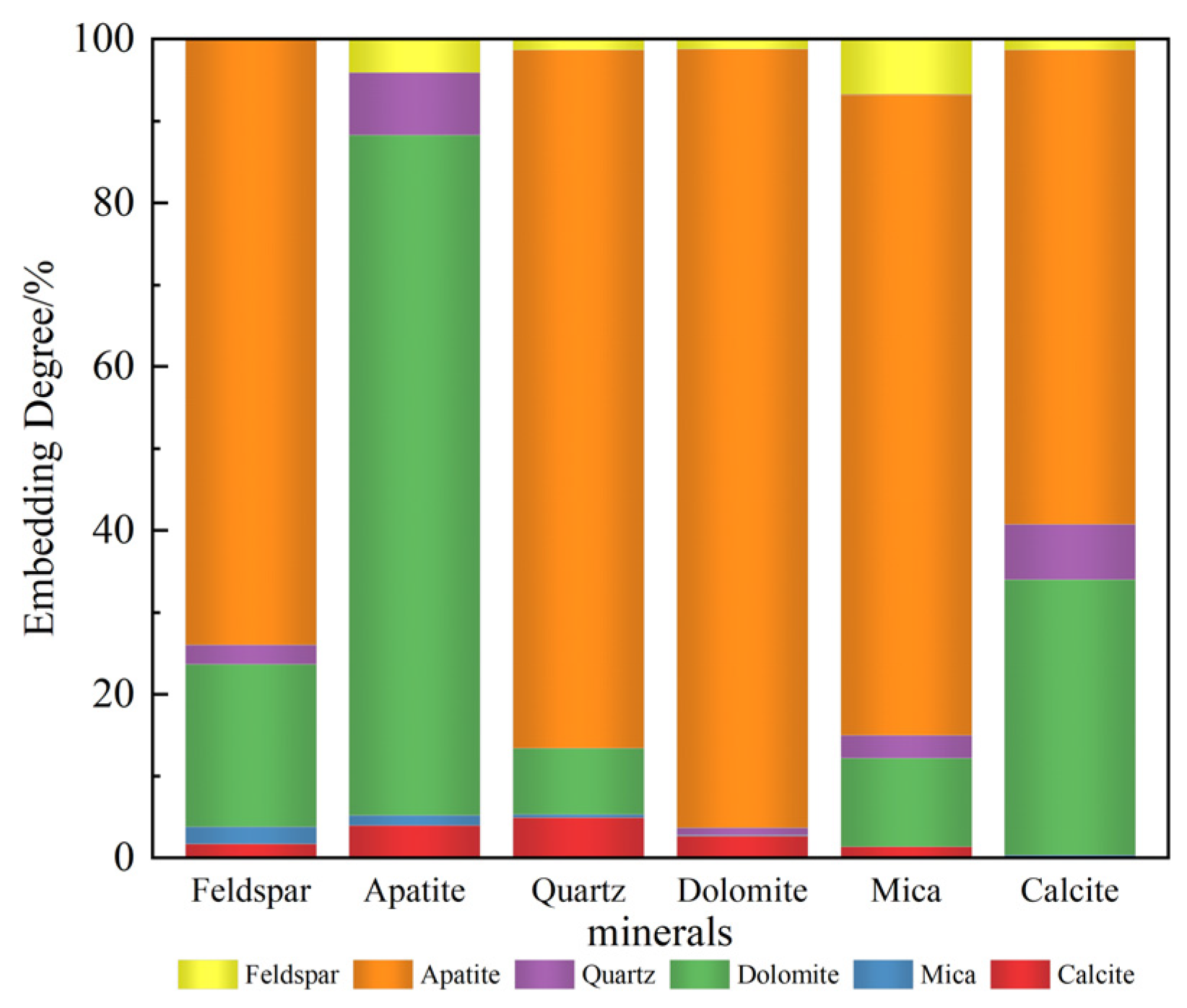
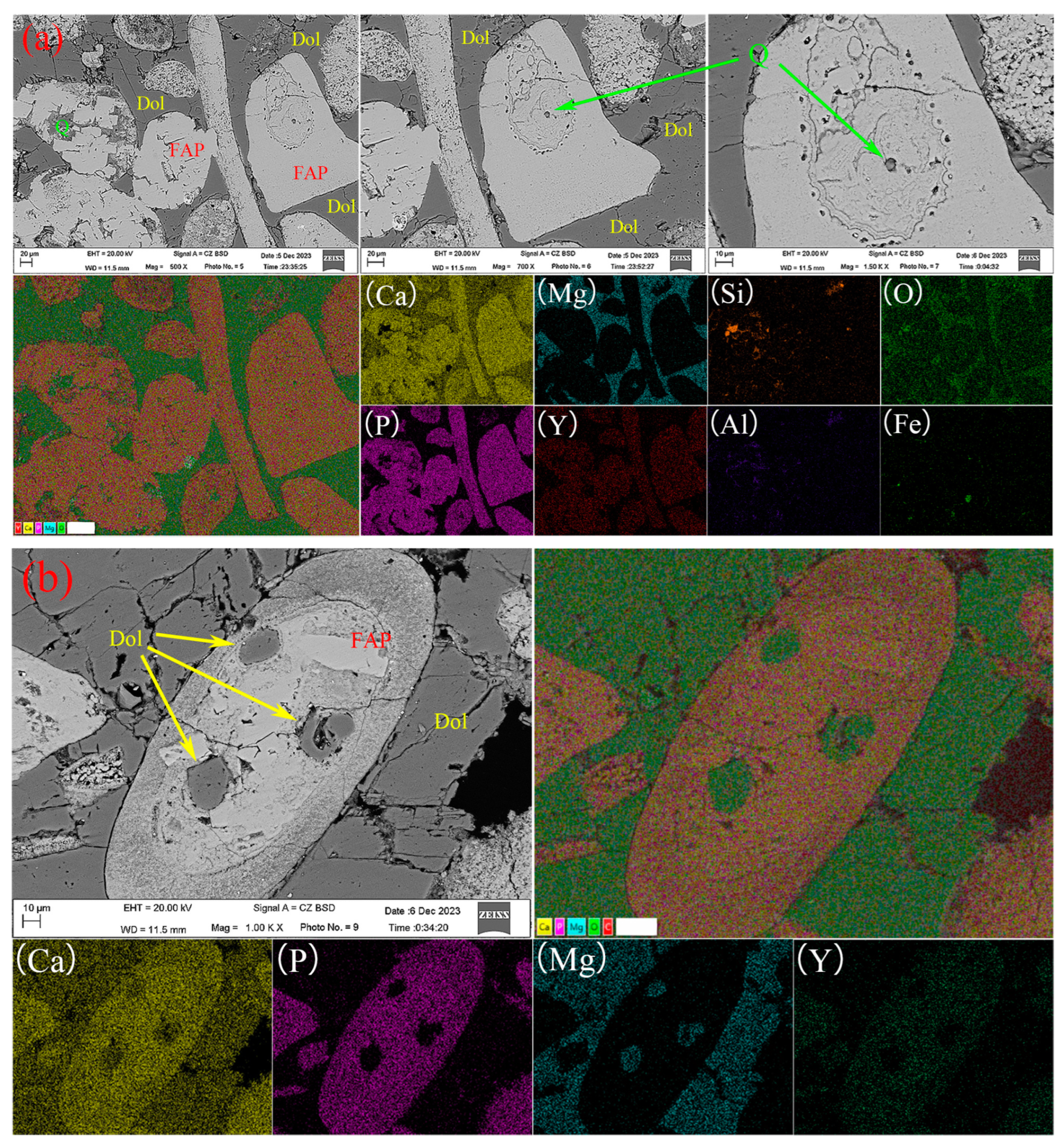
3.1.2. Mineral Embedding Characteristics and Composition Analysis
3.2. Flotation Study of Rare Earth-Bearing Collophane
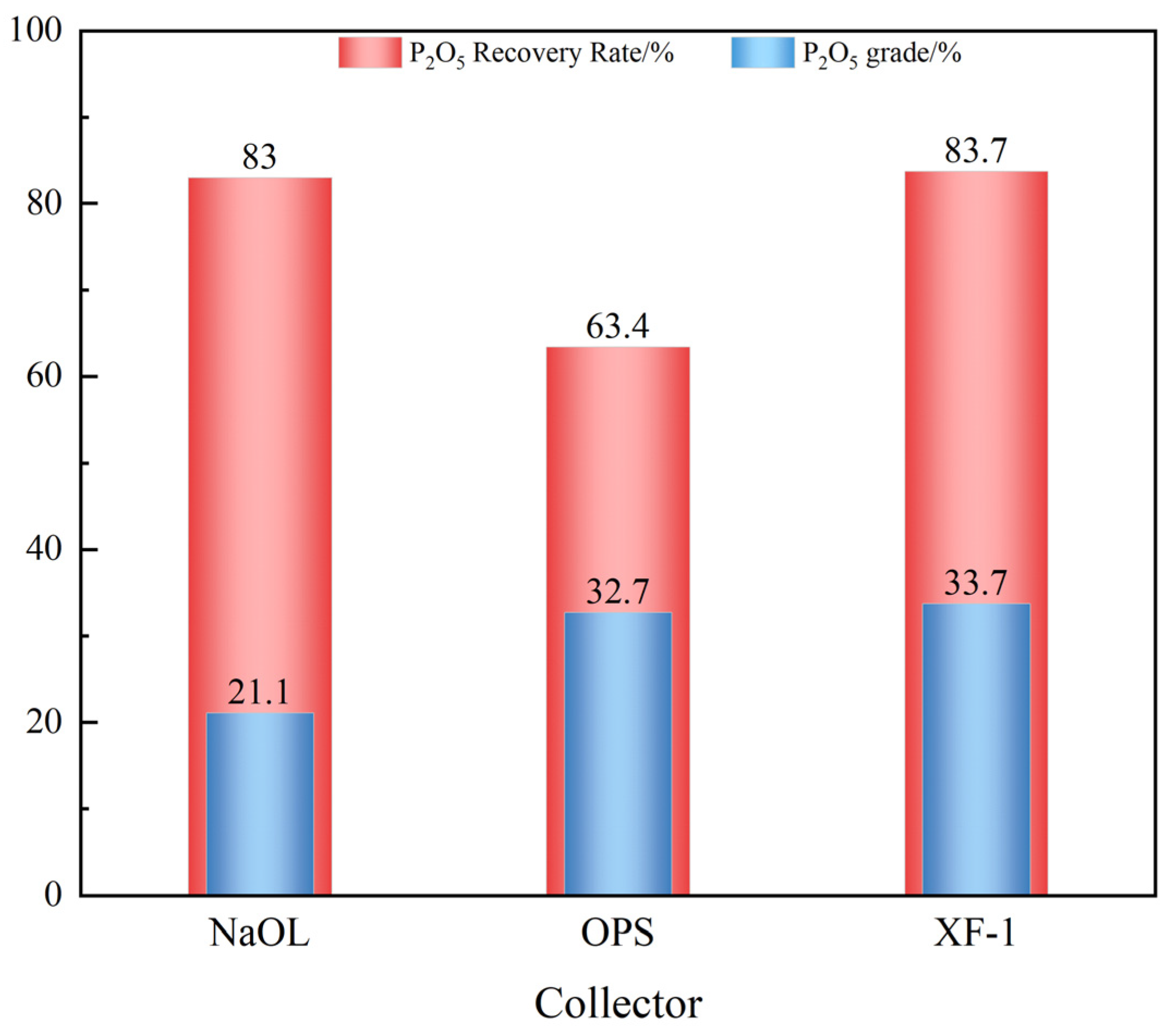
3.2.1. Collector Comparison Test
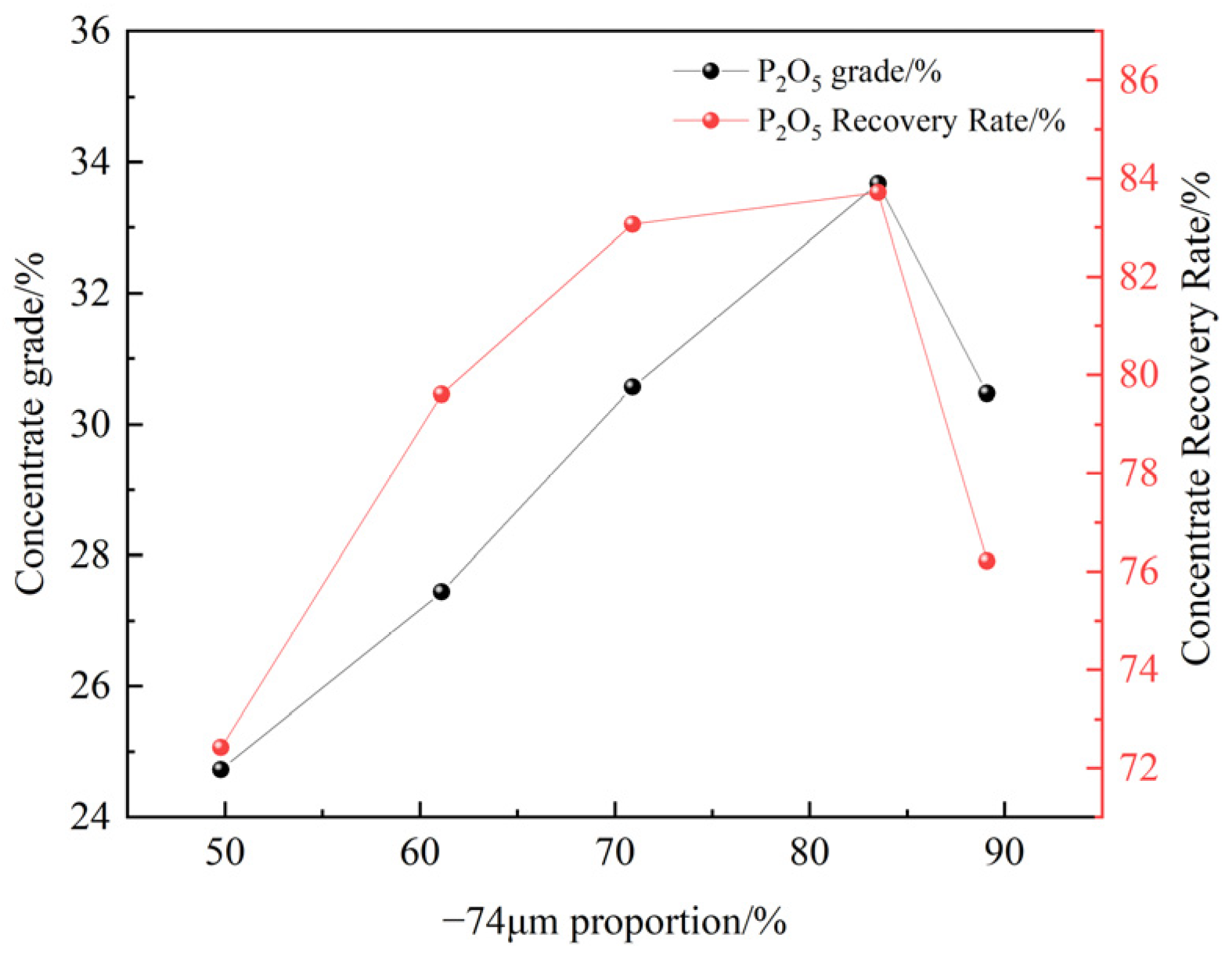
3.2.2. Grinding Fineness Test
3.2.3. Collector Dosage Test
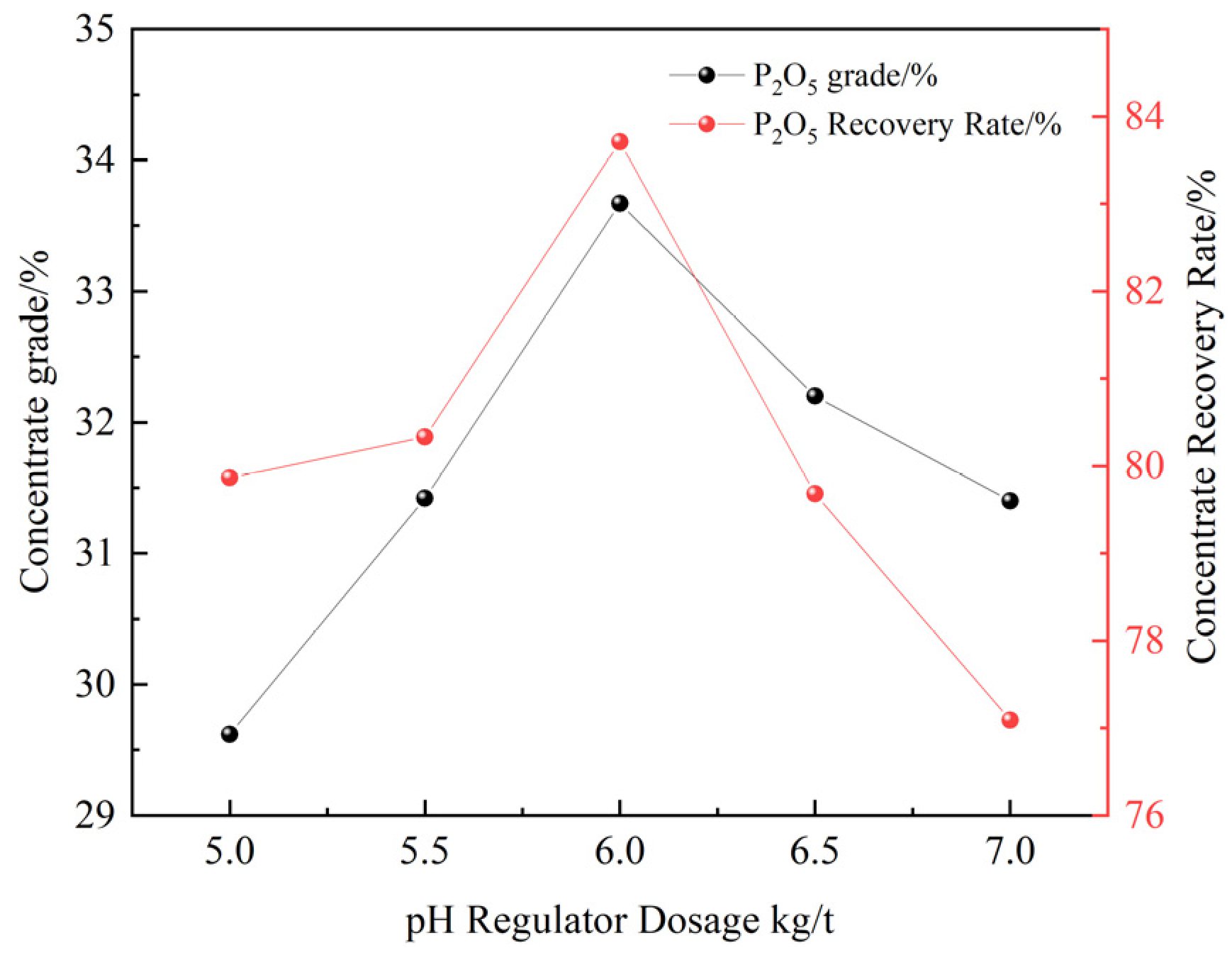
3.2.4. pH Regulator Dosage Test
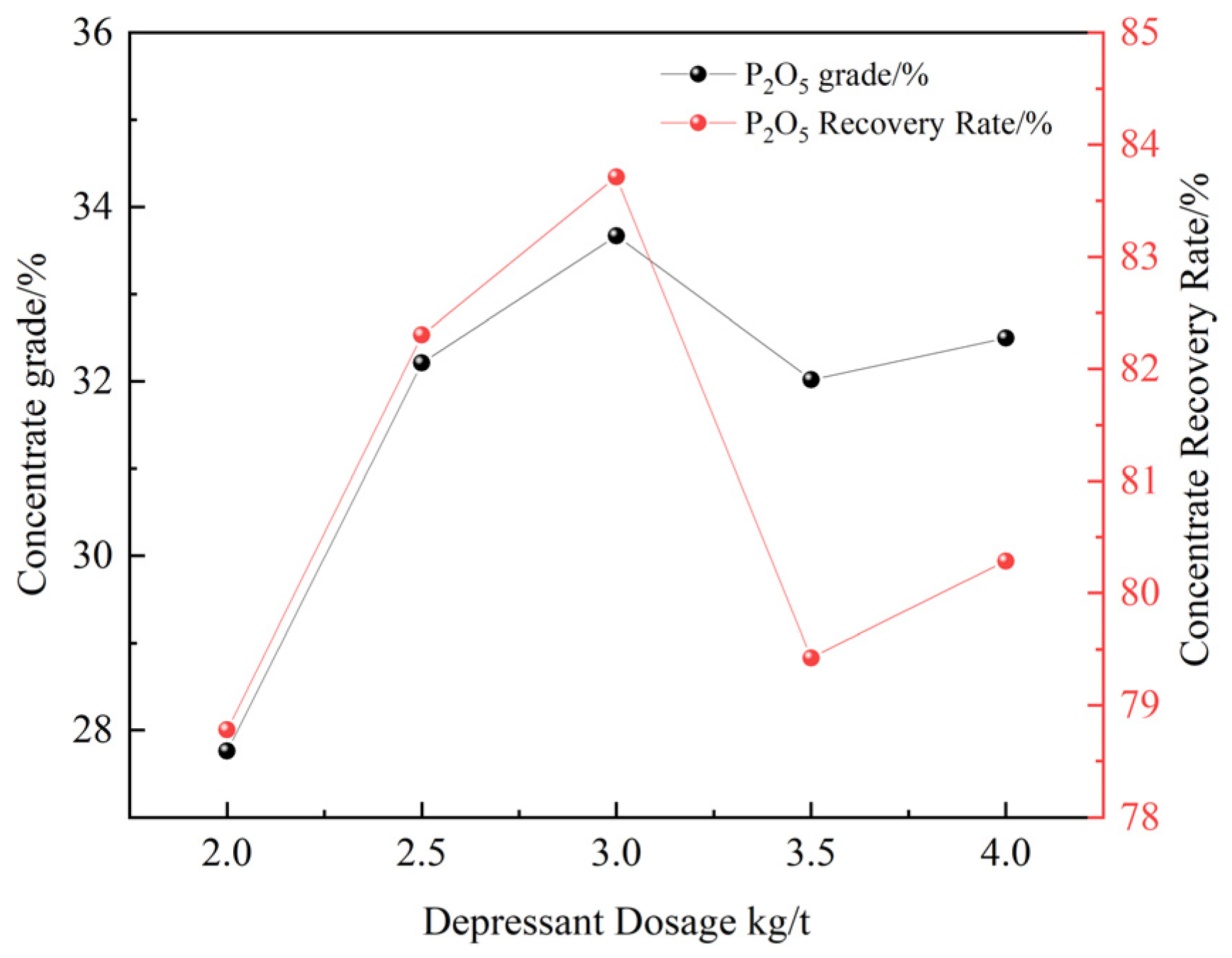
3.2.5. Depressant Dosage Test
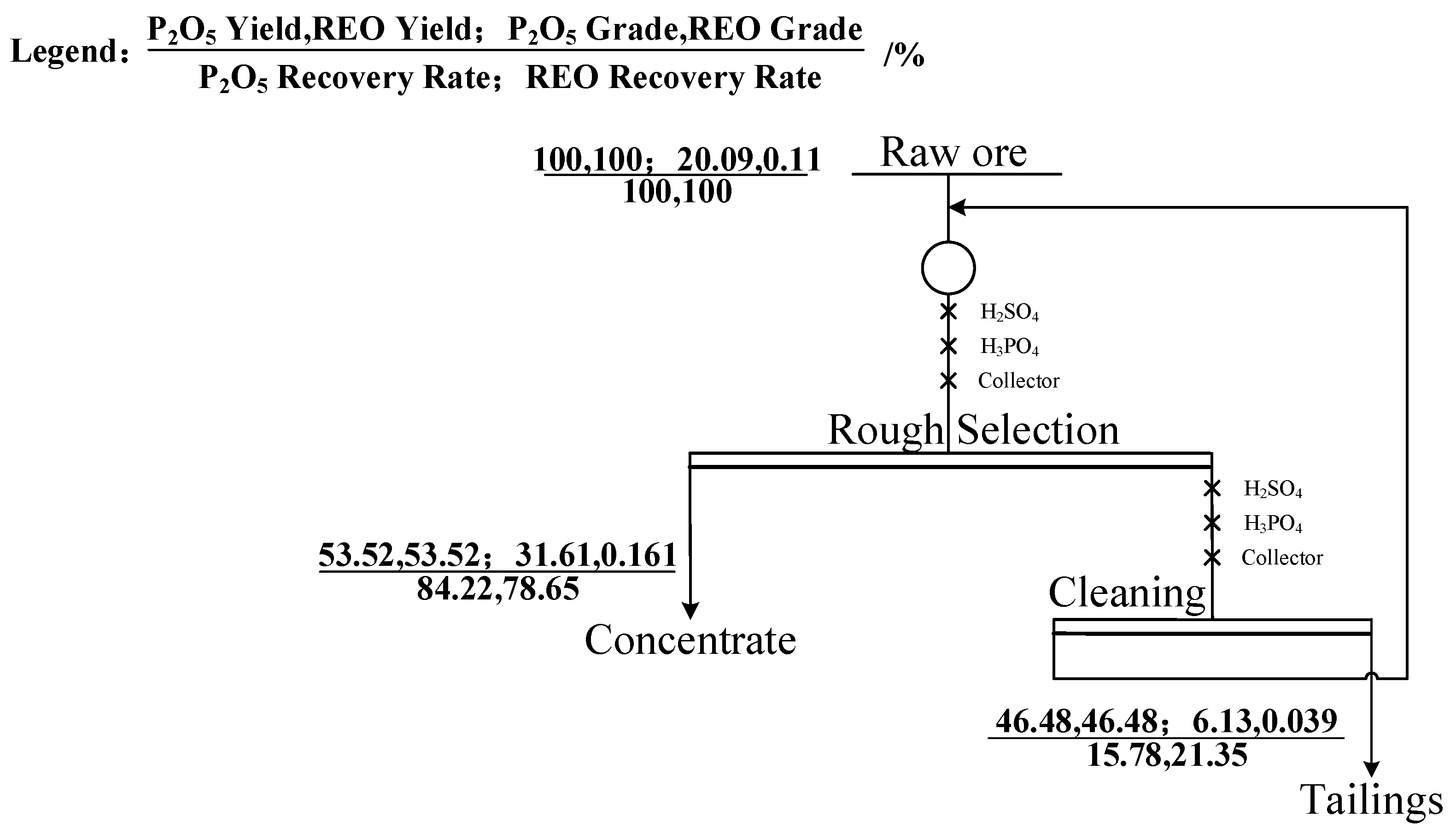
3.2.6. Closed-Circuit Experiment
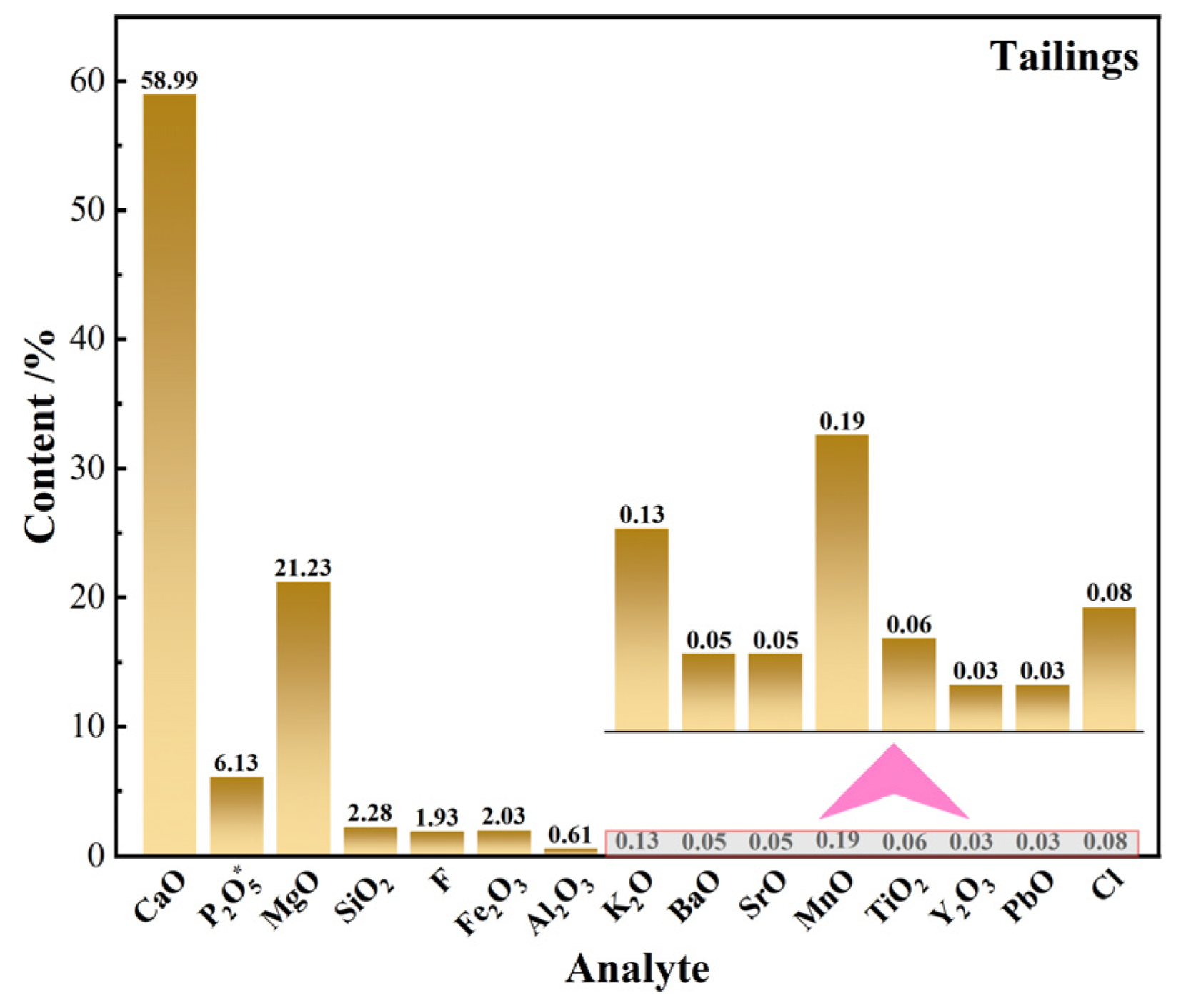
3.3. Mineral Composition and Chemical Analysis of Flotation Concentrate and Tailings
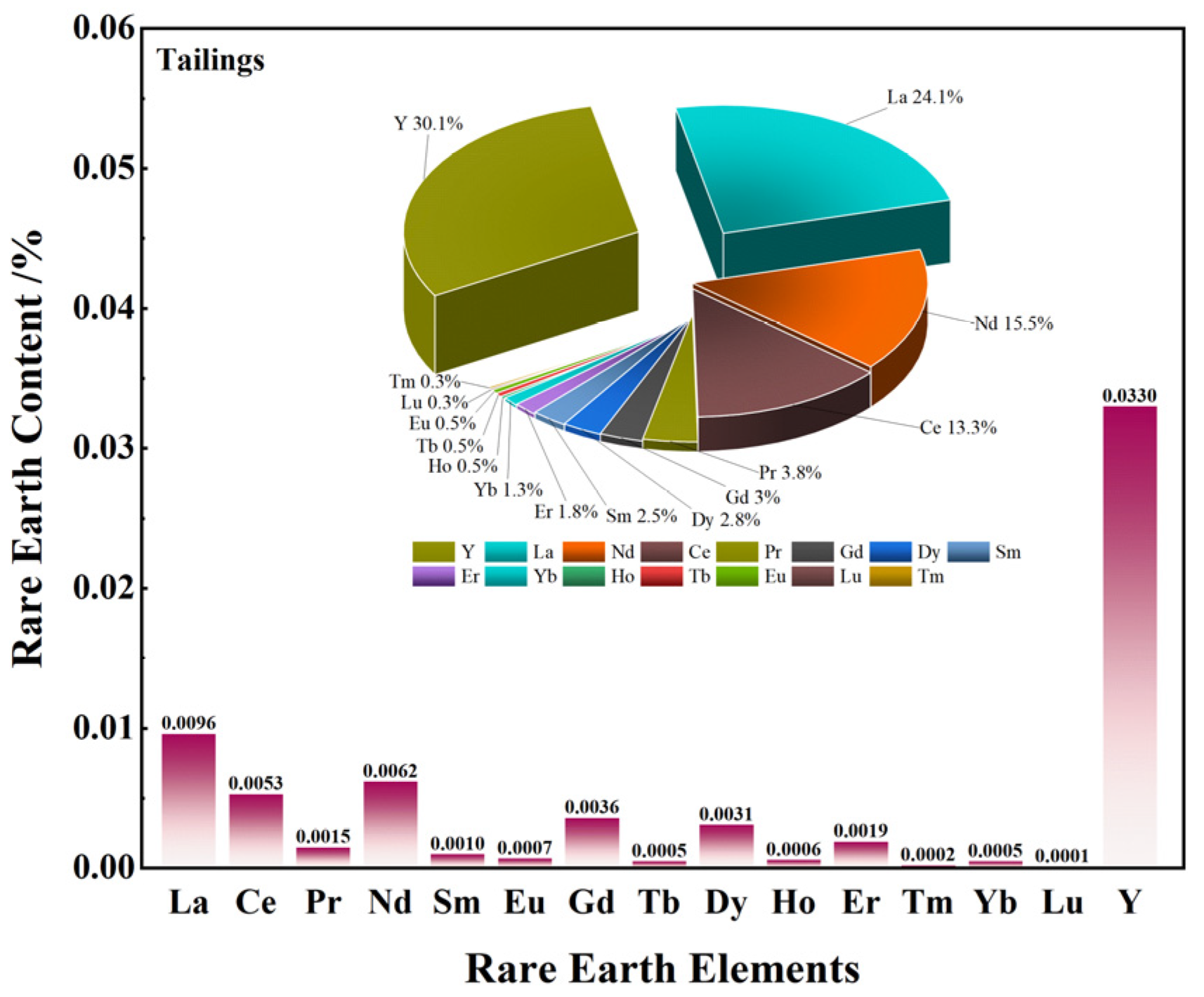
3.3.1. Phase and Multi-Element Analysis
3.3.2. Analysis of Mineral Intergrowth Relationships in Flotation Concentrate and Tailings
4. Conclusions
- The primary mineral phases of the rare earth-bearing collophane from Zhijin are fluorapatite and dolomite, with a P2O5 content of 20.09% and a total rare earth oxide (REO) content of 0.11%. Among the rare earth elements, yttrium (Y) has the highest proportion, accounting for 32.2%. SEM-EDS and BPMA analyses revealed that fluorapatite and dolomite primarily exist as intergrowths and inclusions, with rare earth elements hosted in fluorapatite, and no independent rare earth minerals were detected;
- A comparative test of three collectors (NaOL, OPS, and XF-1) showed that XF-1 had the best magnesium removal performance. Based on this, XF-1 was selected as the collector for subsequent flotation condition tests. The optimal flotation conditions were determined as a grinding fineness of -74 μm (83%), XF-1 collector dosage of 300 g/t, pH regulator (H2SO4) dosage of 6 kg/t, and depressant (H3PO4) dosage of 3 kg/t. Under these optimal conditions, a closed-circuit flotation test (one rougher–one scavenger) yielded a phosphate concentrate with 31.61% P2O5 and a recovery rate of 84.22%, as well as an REO content of 0.161% and a recovery rate of 78.65%;
- Mineralogical composition and multi-element analysis of the flotation concentrate and tailings showed that the P2O5 grade in the flotation concentrate increased by 11.52 percentage points, while the REO content increased by 0.051 percentage points, indicating that rare earth elements were successfully enriched in the phosphate concentrate. In contrast, the P2O5 content in the flotation tailings decreased from 20.09% in the raw ore to 6.13%, while the MgO content increased significantly, confirming the effective removal of dolomite as the primary gangue mineral;
- The study further examined the recovery and enrichment of rare earth elements. According to the ICP-MS analysis, the overall rare earth element recovery in the flotation concentrate reached 78.65%, with a significant enrichment of heavy rare earth element Y and light rare earth element La, increasing by 0.018 and 0.017 percentage points, respectively. Meanwhile, the REO content in the flotation tailings dropped to 0.039%, a decrease of 0.071 percentage points compared to the raw ore, while the distribution ratio of rare earth elements remained largely unchanged.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, M.; Tadesse, B.; Dick, C.; Logan, A.; Dyer, L.; Albijanic, B. Understanding the role of water quality in separation of rare earth minerals from iron oxide minerals in a flotation circuit. Miner. Eng. 2023, 205, 108461. [Google Scholar] [CrossRef]
- Shuai, Z.C.; Zhu, Y.M.; Gao, P.; Han, Y. Rare earth elements resources and beneficiation: A review. Miner. Eng. 2024, 218, 109011. [Google Scholar] [CrossRef]
- Liu, H.D.; He, M.Q.; Jiang, Z.X.; Zheng, M.Y. Geochemical Study of Rare Earth Elements in the Phosphorite Deposit of the Yingping Section of Wengfu Phosphate Mine. J. Kunming Univ. Sci. Technol. (Nat. Sci. Ed.) 2025, 1–13. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective. In Non-Renewable Resource Issues; International Year of Planet Earth; Springer: Dordrecht, The Netherlands, 2010; pp. 131–155. [Google Scholar]
- Huang, X.; Li, H.; Wang, C.; Wang, G.; Xue, X.; Zhang, G. Current Status and Progress of Rare Earth Industry in China. Rare Met. 2007, 3, 279–288. [Google Scholar]
- Xie, Y.L.; Qin, X.Y.; Dai, Z.W.; Geng, Z. Types of Associated Rare Earth Element Resources and Resource Potential in China. J. Geomech. 2024, 30, 723–746. [Google Scholar] [CrossRef]
- Min, W.; Xiaoming, S.; Mingyang, M. Rare earth elements compositions and genesis of Xinhua large-scale phosphorite deposit in Western Guizhou, China. J. Rare Earths 2005, 23, 323–330. [Google Scholar]
- Chen, J.Y.; Yang, R.D.; Zhang, J. Study on the Occurrence State of Rare Earth Elements in the Rare Earth-Bearing Phosphate Deposit in Zhijin, Guizhou. Acta Mineral. Sin. 2010, 30, 123–129. [Google Scholar]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Liu, J.; Zeng, G.; Xaing, P.; Hu, P.; Xiang, W. Distribution, Characteristics, and Development Status of Phosphate Ores. Geol. China 2021, 48, 82–101. [Google Scholar]
- Meng, Q.; Li, W.; Wang, S. Comprehensive Exploration Report on Phosphate (Rare Earth) Deposits in the Zhijin Area, Guizhou Province; Guizhou Geological and Mineral Exploration and Development Bureau, 104th Geological Brigade: Duyun, China, 2016. [Google Scholar]
- Liu, S.R. Microbeam Analysis Study on the Mineralization of Xinhua Phosphate Deposit in Zhijin. Ph.D. Thesis, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, China, 2008. [Google Scholar]
- Chen, J.Y.; Yang, R.D. Rare earth element geochemistry of Cambrian. phosphorites from the Yangtze Region. J. Rare Earths 2013, 31, 101–112. [Google Scholar] [CrossRef]
- Xiqiang, L.; Hui, Z.; Yong, T.; Yunlong, L. REE Geochemical Characteristic of Apatite: Implications for Ore Genesis of the Zhijin Phosphorite. Minerals 2020, 10, 1012. [Google Scholar] [CrossRef]
- Cheng, D.; Ao, X.; Yuan, X.; Liu, Q. Effect of dissolved metal ions from mineral surfaces on the surface wettability of phosphate ore by flotation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134995. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; El-Bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R. Selective flotation of calcite and dolomite from apatite using bio-based alternatives to conventional collectors: Castor and mustard oils. Miner. Eng. 2024, 208, 108597. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Liu, M.; Jin, Y.; Xiao, L.; Yu, H. Employing sulfur-phosphorus mixed acid as a depressant: A novel investigation in flotation of collophanite. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 47, 3015–3028. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Wang, X. New insights on depressive mechanism of citric acid in the selective flotation of dolomite from apatite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130075. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Q. Flotation separation of dolomite and apatite using polyaspartic acid as inhibitor. Physicochem. Probl. Miner. Process. 2021, 57, 117–130. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.A.; Xue, N.; Li, Z.; Zhu, Y.; Nie, Y. Flotation separation of apatite from dolomite with tara gum as depressant: An experimental and molecular dynamics simulation study. Colloids Surf. A Physicochem. Eng. Asp. 2024; 136044. [Google Scholar] [CrossRef]
- Sun, K.; Liu, T.; Zhang, Y.; Liu, X.; Wang, B.; Xu, C. Application and Mechanism of Anionic Collector Sodium Dodecyl Sulfate (SDS) in Phosphate Beneficiation. Minerals 2017, 7, 29. [Google Scholar] [CrossRef]
- Zafar, Z.I.; Anwar, M.M.; Pritchard, D.W. Optimization of thermal beneficiation of a low grade dolomitic phosphate rock. Int. J. Miner. Process. 1995, 43, 123–131. [Google Scholar] [CrossRef]
- Farid, Z.; Assimeddine, M.; Abdennouri, M.; Barka, N.; Sadiq, M. Flotation Enhancement of sedimentary phosphate ores by cornstarch as an environmental depressant: Modeling and analysis using full factorial design (FFD) and artificial neural network (ANN) approaches. Environ. Funct. Materials. 2023, 2, 243–254. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. The Occurrence States of Rare Earth Elements Bearing Phosphorite Ores and Rare Earth Enrichment Through the Selective Reverse Flotation. Minerals 2019, 9, 698. [Google Scholar] [CrossRef]
- El-Midany, A.A.; Arafat, Y. Enhancing Phosphate Grade Using Oleic Acid-Sodium Dodecyl Sulfate Mixtures. Chem. Eng. Commun. 2016, 203, 660–665. [Google Scholar] [CrossRef]
- Yao, Z.; Fang, J.; Zhang, L.; Zhao, M.; Dai, Z. Research Progress on Flotation Technology and Reagents for Collophane in China. Conserv. Util. Miner. Resour. 2017, 4, 107–112. [Google Scholar]
- Hernáinz, F.; Calero, M.; Blázquez, G. Flotation of Low-Grade Phosphate Ore. Adv. Powder Technol. 2004, 15, 421–433. [Google Scholar] [CrossRef]
- HG/T 2673–1995; Phosphate Rock for Wet-Process Phosphoric Acid Production. Ministry of Chemical Industry of the People’s Republic of China: Beijing, China, 1996.







| No. | Name | Purity | Usage | Purpose | Dosage (kg/t) |
|---|---|---|---|---|---|
| 1 | NaOL | Analytical | 3% aqueous solution | Collector | 0.1~1.5 kg/t |
| 2 | OPS | Analytical | Collector | 0.1~1.5 kg/t | |
| 3 | XF-1 | Industrial | Collector | 0.1~1.5 kg/t | |
| 4 | H2SO4 | Analytical | pH regulator | 5~7 kg/t | |
| 5 | H3PO4 | Analytical | Depressant | 2~4 kg/t |
| Element | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic mass | 89 | 139 | 140 | 141 | 146 | 148 | 153 | 157 | 159 | 163 | 165 | 166 | 169 | 172 | 175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Kou, J.; Wen, X.; Xu, H. Migration and Enrichment of Rare Earth Elements in the Flotation Process of Rare Earth-Bearing Collophanite. Separations 2025, 12, 90. https://doi.org/10.3390/separations12040090
Lin J, Kou J, Wen X, Xu H. Migration and Enrichment of Rare Earth Elements in the Flotation Process of Rare Earth-Bearing Collophanite. Separations. 2025; 12(4):90. https://doi.org/10.3390/separations12040090
Chicago/Turabian StyleLin, Jiawei, Jue Kou, Xiaojin Wen, and Hongda Xu. 2025. "Migration and Enrichment of Rare Earth Elements in the Flotation Process of Rare Earth-Bearing Collophanite" Separations 12, no. 4: 90. https://doi.org/10.3390/separations12040090
APA StyleLin, J., Kou, J., Wen, X., & Xu, H. (2025). Migration and Enrichment of Rare Earth Elements in the Flotation Process of Rare Earth-Bearing Collophanite. Separations, 12(4), 90. https://doi.org/10.3390/separations12040090






