Titanium Meets Carbon: Enhanced Reusable Filters for Oil–Water Separation and Environmental Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Substrate Material
2.1.2. TiO2 Nanopowder Material
2.1.3. Carbon Black Material
2.2. Coated Filter Preparation
2.3. Performance Testing
2.3.1. Oil Filtration Test
2.3.2. Contact Angle Test
3. Results and Discussion
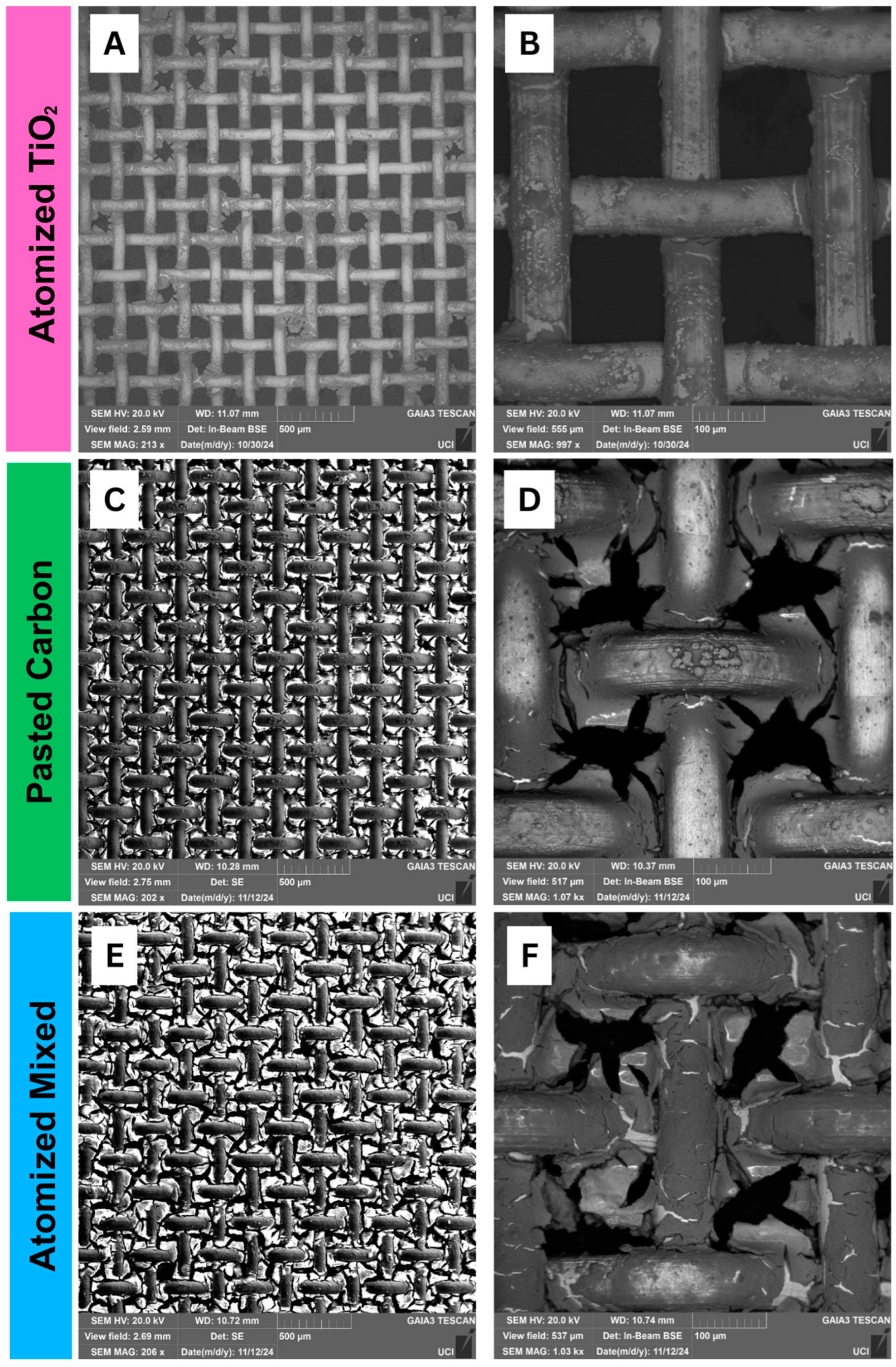
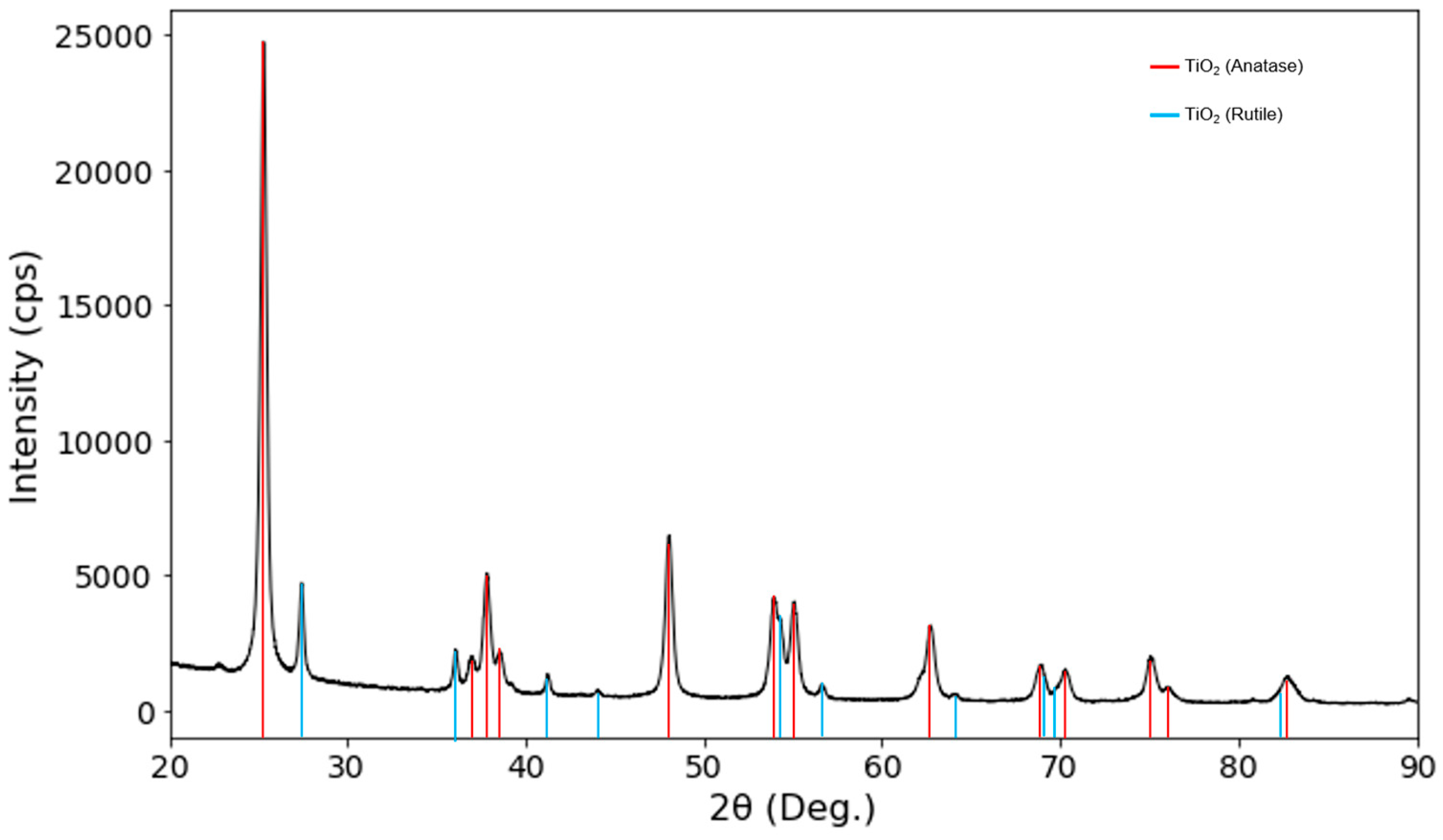
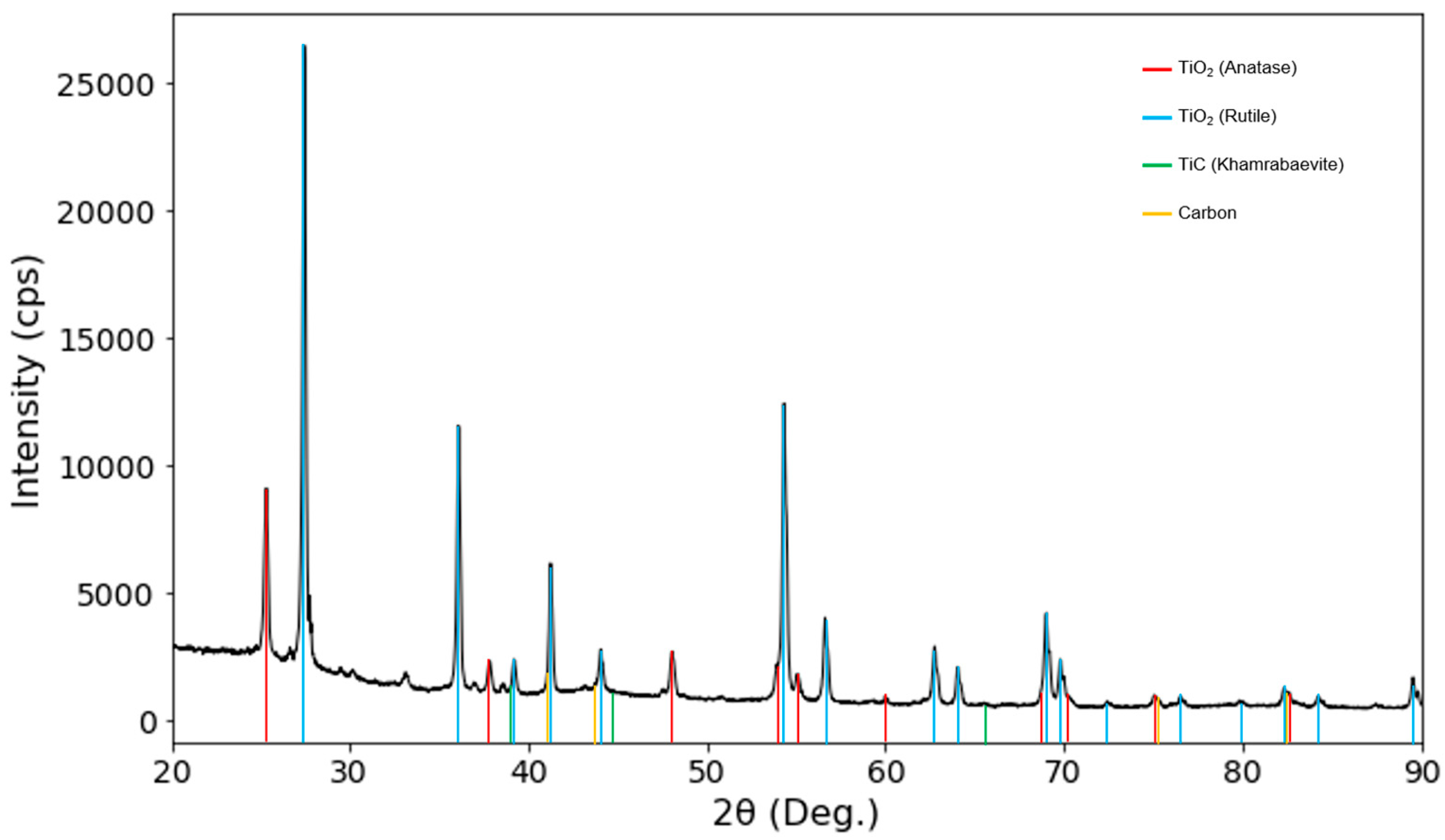
3.1. Structural Analysis
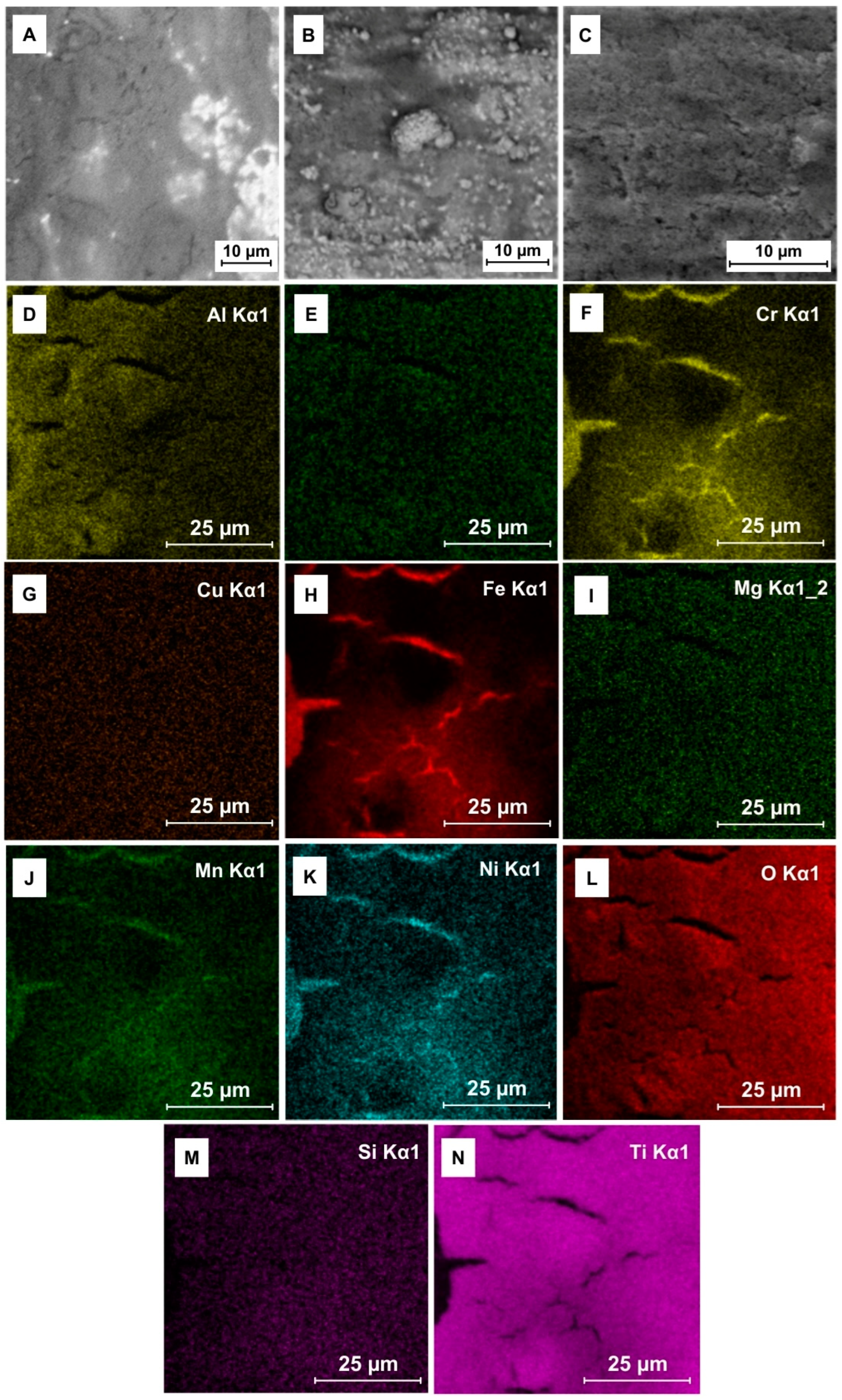
3.2. Elemental Analysis
3.3. Trace Element EDS
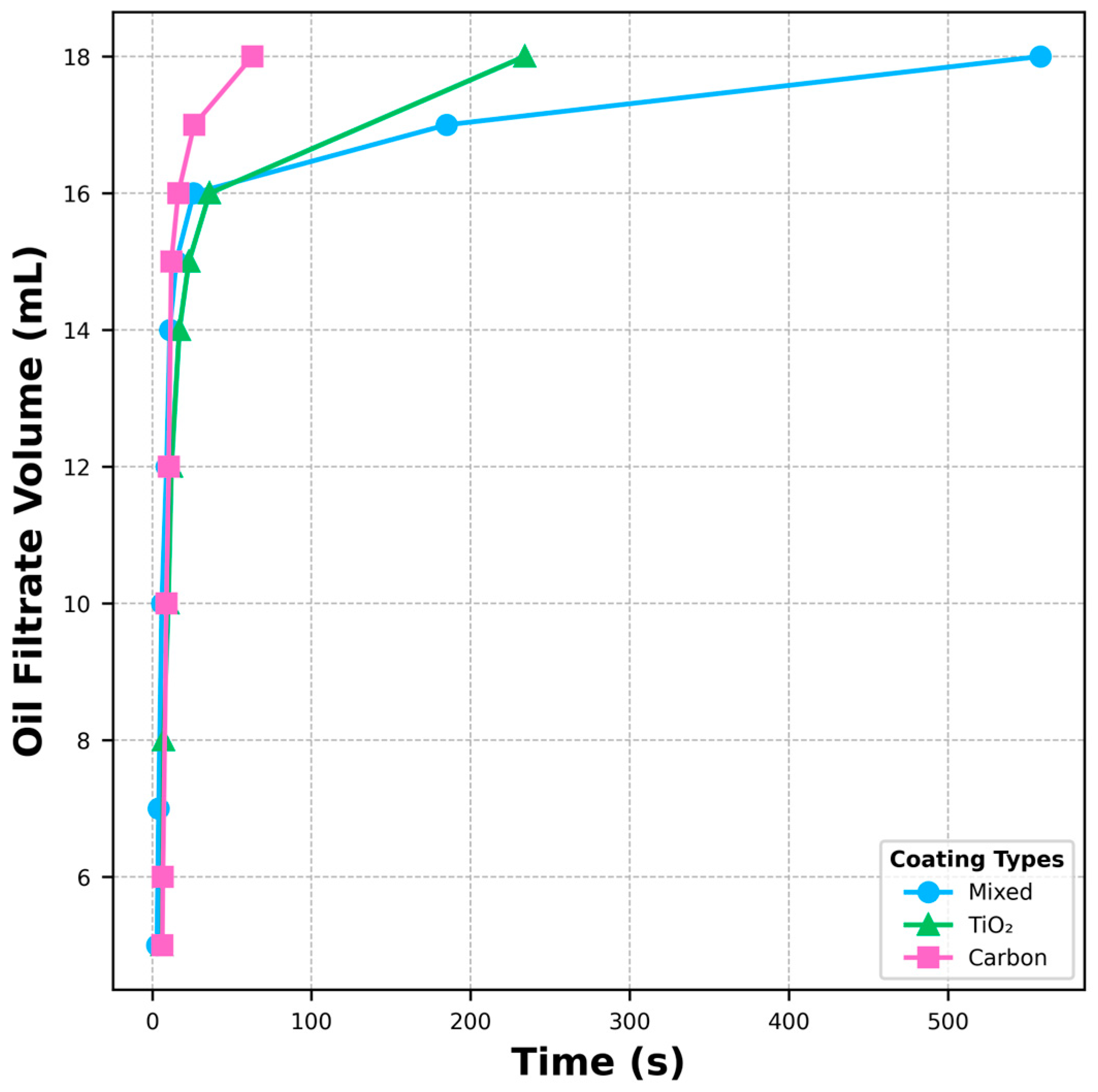
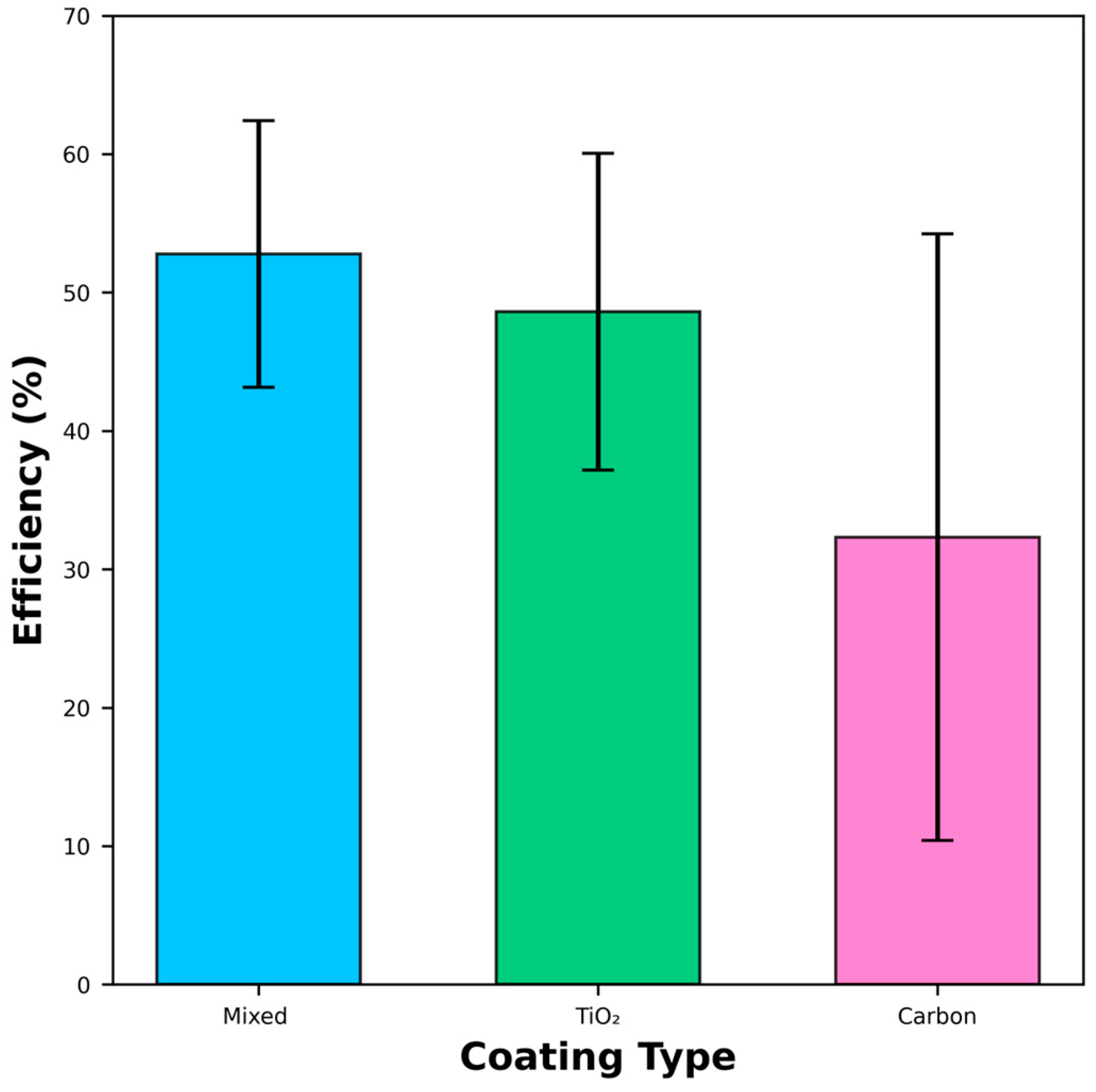
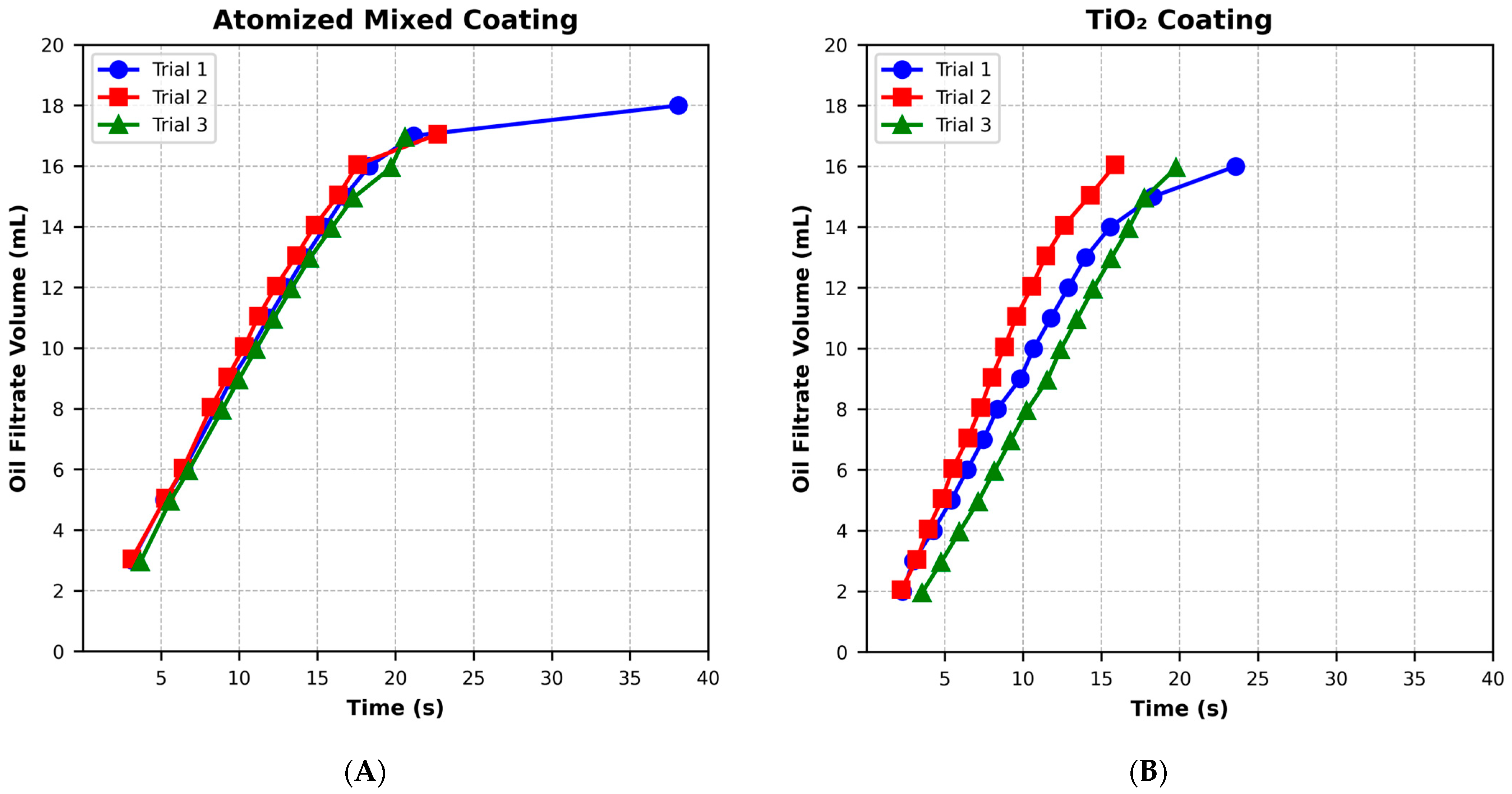
3.4. Oil Filtration and Oil–Water Separation Efficiency
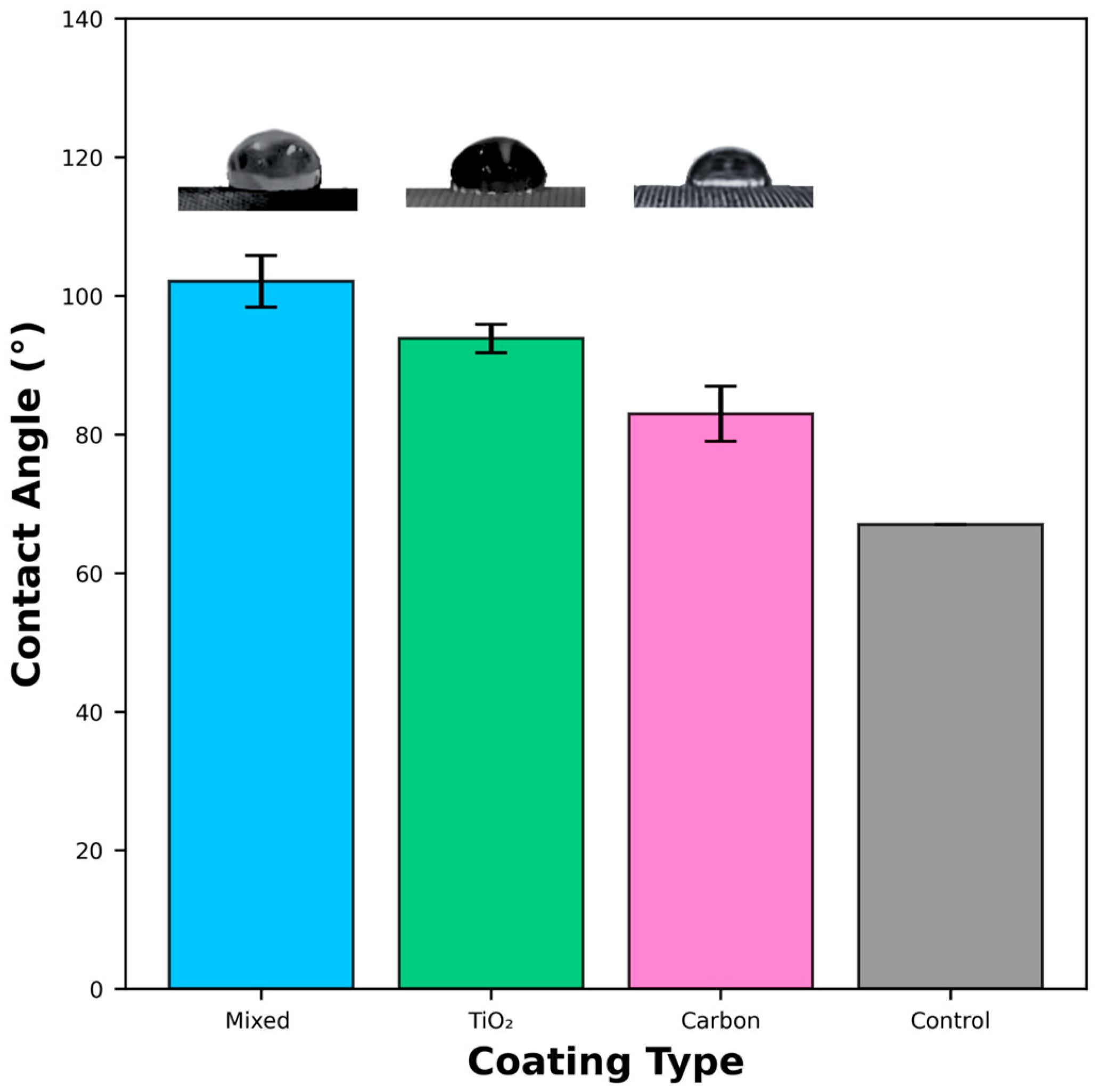
3.5. Wettability of Different Coating Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTE | Coefficient of thermal expansion |
| EDS | Energy-dispersive spectroscopy |
| IMRI | Irvine Materials Research Institute |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
References
- Our World Data. Oil Spills. Available online: https://ourworldindata.org/oil-spills (accessed on 1 January 2025).
- Friedland, A.; Relyea, R. Environmental Science for the AP® Course, 4th ed.; W.H. Freeman & Company: New York, NY, USA, 2023; ISBN 978-1-319-46948-1. [Google Scholar]
- Asif, Z.; Chen, Z.; An, C.; Dong, J. Environmental Impacts and Challenges Associated with Oil Spills on Shorelines. J. Mar. Sci. Eng. 2022, 10, 762. [Google Scholar] [CrossRef]
- 2023 DARRP Highlights: Over $92 Million to Restore 7 Polluted Waterways and Communities; National Oceanic and Atmospheric Administration (NOAA), Damage Assessment, Remediation, and Restoration Program (DARRP): Baton Rouge, LA, USA, 2023.
- US Environmental Protection Agency. Deepwater Horizon—BP Gulf of Mexico Oil Spill. Available online: https://www.epa.gov/enforcement/deepwater-horizon-bp-gulf-mexico-oil-spill (accessed on 25 January 2025).
- Our World Data. Energy Mix. Available online: https://ourworldindata.org/energy-mix (accessed on 1 January 2025).
- Oliveira, L.M.T.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.D.S.; McKay, G.; Meili, L. Sorption as a Rapidly Response for Oil Spill Accidents: A Material and Mechanistic Approach. J. Hazard. Mater. 2021, 407, 124842. [Google Scholar] [CrossRef] [PubMed]
- Egyetem, Ó. (Ed.) Proceedings of the International Conference “Global Environmental Changes and Environmental Health: Environmental and Economic Impact on Sustainable Development”, 7th ICEEE—2016, Budapest, Hungary, 17–19 November 2016; Óbuda University: Budapest, Hungary, 2017. ISBN 978-963-449-063-0.
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Ngaini, Z.; Nourouzi, M.M. Oil Removal from Aqueous State by Natural Fibrous Sorbent: An Overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Prince, R.C. Oil Spill Dispersants: Boon or Bane? Environ. Sci. Technol. 2015, 49, 6376–6384. [Google Scholar] [CrossRef]
- How Do Oil Spills out at Sea Typically Get Cleaned up?|Response.Restoration.Noaa.Gov. Available online: https://response.restoration.noaa.gov/about/media/how-do-oil-spills-out-sea-typically-get-cleaned.html (accessed on 16 March 2025).
- Ventikos, N. A High-Level Synthesis of Oil Spill Response Equipment and Countermeasures. J. Hazard. Mater. 2004, 107, 51–58. [Google Scholar] [CrossRef]
- Understanding Oil Spills And Oil Spill Response. Available online: https://www.epa.gov/sites/default/files/2018-01/documents/ospguide99.pdf (accessed on 16 March 2025).
- Fritt-Rasmussen, J.; Wegeberg, S.; Gustavson, K. Review on Burn Residues from In Situ Burning of Oil Spills in Relation to Arctic Waters. Water. Air. Soil Pollut. 2015, 226, 329. [Google Scholar] [CrossRef]
- Baniasadi, M.; Mousavi, S.M. A Comprehensive Review on the Bioremediation of Oil Spills. In Microbial Action on Hydrocarbons; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 223–254. ISBN 978-981-13-1839-9. [Google Scholar]
- Nakajima, A.; Koizumi, S.; Watanabe, T.; Hashimoto, K. Photoinduced Amphiphilic Surface on Polycrystalline Anatase TiO2 Thin Films. Langmuir 2000, 16, 7048–7050. [Google Scholar] [CrossRef]
- Ross, H.; Nguyen, H.; Nguyen, B.; Foster, A.; Salud, J.; Patino, M.; Gan, Y.X.; Li, M. Filter Modified with Hydrophilic and Oleophobic Coating for Efficient and Affordable Oil/Water Separation. Separations 2022, 9, 269. [Google Scholar] [CrossRef]
- Sico, J.; Tang, B.; Flores, D.; Mouawad, R.; Punsalan, R.; Gan, Y.X.; Li, M. Fabrication and Characterization of TiO2 Coatings on 304 Stainless-Steel Substrate for Efficient Oil/Water Separation. Coatings 2023, 13, 1920. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Zhang, Z.; Zhang, P.; Jiang, Z. The Photocatalytic Activity and Stability of a Nanosized TiO2 Film Prepared by Carbon Black Modified Method. Catal. Today 2004, 90, 305–312. [Google Scholar] [CrossRef]
- Mao, C.-C.; Weng, H.-S. Promoting Effect of Adding Carbon Black to TiO2 for Aqueous Photocatalytic Degradation of Methyl Orange. Chem. Eng. J. 2009, 155, 744–749. [Google Scholar] [CrossRef]
- Li, L.; Zhu, W.; Zhang, P.; Chen, Z.; Han, W. Photocatalytic Oxidation and Ozonation of Catechol over Carbon-Black-Modified Nano-TiO2 Thin Films Supported on Al Sheet. Water Res. 2003, 37, 3646–3651. [Google Scholar] [CrossRef]
- Ma, J.; Tang, Y.; Lu, G.; Wang, Y.; Niu, W.; Fu, D.; Zhang, K.; Bahnemann, D.W.; Pan, J.H. Incorporating Mesoporous Anatase TiO2 Spheres to Conductive Carbon Black Filled PVDF Membrane for Self-Cleaning Photo(Electro)Catalytic Filtration. J. Phys. Chem. C 2023, 127, 7998–8005. [Google Scholar] [CrossRef]
- Tan, K.H.; Rahman, H.A.; Taib, H. Coating Layer and Influence of Transition Metal for Ferritic Stainless Steel Interconnector Solid Oxide Fuel Cell: A Review. Int. J. Hydrogen Energy 2019, 44, 30591–30605. [Google Scholar] [CrossRef]
- Mashreghi, A. Determining the Volume Thermal Expansion Coefficient of TiO2 Nanoparticle by Molecular Dynamics Simulation. Comput. Mater. Sci. 2012, 62, 60–64. [Google Scholar] [CrossRef]
- Fang, D.; Qie, J.; Deng, H. Study on Thermal Physical Properties of 304 Stainless Steel. In Proceedings of the 6th International Symposium on High-Temperature Metallurgical Processing, Orlando, FL, USA, March 15–19 2015; Jiang, T., Hwang, J.-Y., Alvear, F.G.R.F., Yücel, O., Mao, X., Sohn, H.Y., Ma, N., Mackey, P.J., Battle, T.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 99–104, ISBN 978-3-319-48603-1. [Google Scholar]
- Desai, P.D.; Ho, C.Y. Thermal Linear Expansion of Nine Selected AISI Stainless Steels; Thermophysical and Electronic Properties Information Analysis Center; CINDAS: Lafayette, IN, USA, 1978; p. 12. [Google Scholar]
- Qin, P.; Ma, H.; Cui, Y.; Liu, R.; Ju, P.; Wang, F.; Liu, L. The Corrosion Behavior of 316 Stainless Steel under the Cooperative Effect of Plastic Stress and UV Illumination in 3.5 Wt% NaCl Solution. Corros. Sci. 2023, 223, 111466. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Corrosion Behavior of 316L and 304 Stainless Steels Exposed to Industrial-Marine-Urban Environment: Field Study. RSC Adv. 2016, 6, 47314–47324. [Google Scholar] [CrossRef]
- Halmešová, K.; Trojanová, Z.; Koukolíková, M.; Brázda, M.; Džugan, J.; Huang, W. Effect of Laser Power on Thermal Properties of Multimaterial Structure Inconel 718 and Stainless Steel 316L Processed by Directed Energy Deposition. J. Alloys Compd. 2022, 927, 167082. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Li, J.; Bian, X.; Guo, Z. Inhibitory Effect of Ni-P Coating on Thermal Expansion of Carbon Steel. Surf. Coat. Technol. 2017, 315, 484–489. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Photocatalysis—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/photocatalysis (accessed on 23 February 2025).
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium Dioxide Nanoparticles: Recent Progress in Antimicrobial Applications. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1860. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the Photocatalytic Antimicrobial Effects of a TiO2 Nanocomposite Food Packaging Film by in vitro and in vivo Tests. LWT Food Sci. Technol. 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon Black Reborn: Structure and Chemistry for Renewable Energy Harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Hsu, S.-W.; Yang, T.-S.; Chen, T.-K.; Wong, M.-S. Ion-Assisted Electron-Beam Evaporation of Carbon-Doped Titanium Oxide Films as Visible-Light Photocatalyst. Thin Solid Film. 2007, 515, 3521–3526. [Google Scholar] [CrossRef]
- Sadler, E.; Crick, C.R. Suction or Gravity-Fed Oil-Water Separation Using PDMS-Coated Glass Filters. Sustain. Mater. Technol. 2021, 29, e00321. [Google Scholar] [CrossRef]
- Kroeger, R.M.; DeKay, H.G. Measurement of Gravity Filtration. J. Am. Pharm. Assoc. 1951, 40, 213–215. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N. Characterization of Self-Cleaning RO Membranes Coated with TiO2 Particles under UV Irradiation. J. Membr. Sci. 2007, 303, 221–233. [Google Scholar] [CrossRef]
- Nishimoto, S.; Tomoishi, S.; Kameshima, Y.; Fujii, E.; Miyake, M. Self-Cleaning Efficiency of Titanium Dioxide Surface under Simultaneous UV Irradiation of Various Intensities and Water Flow. J. Ceram. Soc. Jpn. 2014, 122, 513–516. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of Self-Cleaning TiO2 Coatings on Fired Clay Brick Façades: Effects of UV Exposure and Wet & Dry Cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Li, W.; Liang, R.; Hu, A.; Huang, Z.; Zhou, Y.N. Generation of Oxygen Vacancies in Visible Light Activated One-Dimensional Iodine TiO2 Photocatalysts. RSC Adv. 2014, 4, 36959–36966. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic Degradation of Dyes and Organic Contaminants in Water Using Nanocrystalline Anatase and Rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef]
- Naniwa, S.; Kato, K.; Yamakata, A.; Yamamoto, A.; Yoshida, H. A Quantitative Study on the Relationship of Specific Surface Area and Carrier Lifetime to Photocatalytic Activity of Anatase TiO2 Nanoparticles. ACS Catal. 2023, 13, 15212–15218. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A Review on the Synthesis of the Various Types of Anatase TiO2 Facets and Their Applications for Photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Yao, S.; Ma, Y.; Xu, T.; Wang, Z.; Lv, P.; Zheng, J.; Ma, C.; Yu, K.; Wei, W.; Ostrikov, K. (Ken) Ti–C Bonds Reinforced TiO2@C Nanocomposite Na-Ion Battery Electrodes by Fluidized-Bed Plasma-Enhanced Chemical Vapor Deposition. Carbon 2021, 171, 524–531. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Anatase TiO2: Better Anode Material Than Amorphous and Rutile Phases of TiO2 for Na-Ion Batteries. Chem. Mater. 2015, 27, 6022–6029. [Google Scholar] [CrossRef]
- Gorthy, R.; Wasa, A.; Land, J.G.; Yang, Z.; Heinemann, J.A.; Bishop, C.M.; Krumdieck, S.P. Effects of Post-Deposition Heat Treatment on Nanostructured TiO2-C Composite Structure and Antimicrobial Properties. Surf. Coat. Technol. 2021, 409, 126857. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Dubey, R.S. Temperature-Dependent Phase Transformation of TiO2 Nanoparticles Synthesized by Sol-Gel Method. Mater. Lett. 2018, 215, 312–317. [Google Scholar] [CrossRef]
- Ghosh, T.B.; Dhabal, S.; Datta, A.K. On Crystallite Size Dependence of Phase Stability of Nanocrystalline TiO2. J. Appl. Phys. 2003, 94, 4577–4582. [Google Scholar] [CrossRef]
- Hirano, M.; Nakahara, C.; Ota, K.; Tanaike, O.; Inagaki, M. Photoactivity and Phase Stability of ZrO2-Doped Anatase-Type TiO2 Directly Formed as Nanometer-Sized Particles by Hydrolysis under Hydrothermal Conditions. J. Solid State Chem. 2003, 170, 39–47. [Google Scholar] [CrossRef]
- Rozman, N.; Sever Škapin, A.; Tobaldi, D.M.; Dražić, G.; Nadrah, P. Tailoring the Crystalline and Amorphous Phase Ratios of TiO2 through the Use of Organic Additives during Hydrothermal Synthesis. Ceram. Int. 2024, 50, 37033–37040. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New Approach of Modifying the Anatase to Rutile Transition Temperature in TiO2 Photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Vásquez, G.C.; Peche-Herrero, M.A.; Maestre, D.; Gianoncelli, A.; Ramírez-Castellanos, J.; Cremades, A.; González-Calbet, J.M.; Piqueras, J. Laser-Induced Anatase-to-Rutile Transition in TiO2 Nanoparticles: Promotion and Inhibition Effects by Fe and Al Doping and Achievement of Micropatterning. J. Phys. Chem. C 2015, 119, 11965–11974. [Google Scholar] [CrossRef]
- Yu, C.; Fu, L.; Xiao, H.; Lv, Q.; Gao, B. Effect of Carbon Content on the Microstructure and Bonding Properties of Hot-Rolling Pure Titanium Clad Carbon Steel Plates. Mater. Sci. Eng. A 2021, 820, 141572. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Theory of Carbon Doping of Titanium Dioxide. Chem. Mater. 2005, 17, 6656–6665. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. Selection of the HPLC Method in Chemical Analysis; Elsevier: Chantilly, VA, USA, 2016; ISBN 978-0-12-803711-9. [Google Scholar]
- Ranowsky, A. Contact Angle and Surface Tension—A Fascinating Liaison. Available online: https://www.cscscientific.com/csc-scientific-blog/how-does-contact-angle-relate-to-surface-tension (accessed on 19 March 2025).
- Wang, Y.; Gong, X. Special Oleophobic and Hydrophilic Surfaces: Approaches, Mechanisms, and Applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- Wan Ikhsan, S.N.; Yusof, N.; Aziz, F.; Ismail, A.F.; Jaafar, J.; Wan Salleh, W.N.; Misdan, N. Superwetting Materials for Hydrophilic-Oleophobic Membrane in Oily Wastewater Treatment. J. Environ. Manag. 2021, 290, 112565. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroumand, A.-H.; Laguana, K.; Dudley, E.; Cuadros-Arias, P.; Rubio, A.; Shin, Z.; Webster, J.; Li, M. Titanium Meets Carbon: Enhanced Reusable Filters for Oil–Water Separation and Environmental Remediation. Separations 2025, 12, 83. https://doi.org/10.3390/separations12040083
Boroumand A-H, Laguana K, Dudley E, Cuadros-Arias P, Rubio A, Shin Z, Webster J, Li M. Titanium Meets Carbon: Enhanced Reusable Filters for Oil–Water Separation and Environmental Remediation. Separations. 2025; 12(4):83. https://doi.org/10.3390/separations12040083
Chicago/Turabian StyleBoroumand, Amir-Hadi, Kayla Laguana, Eric Dudley, Pilar Cuadros-Arias, Adrian Rubio, Zachary Shin, Jack Webster, and Mingheng Li. 2025. "Titanium Meets Carbon: Enhanced Reusable Filters for Oil–Water Separation and Environmental Remediation" Separations 12, no. 4: 83. https://doi.org/10.3390/separations12040083
APA StyleBoroumand, A.-H., Laguana, K., Dudley, E., Cuadros-Arias, P., Rubio, A., Shin, Z., Webster, J., & Li, M. (2025). Titanium Meets Carbon: Enhanced Reusable Filters for Oil–Water Separation and Environmental Remediation. Separations, 12(4), 83. https://doi.org/10.3390/separations12040083






