Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells
Abstract
1. Introduction
- (1)
- It can be used as a soil conditioner in horticulture. It improves the soil itself (water retention, heat retention, aeration, etc.) and the microbiological properties of the soil in rice paddies and fields, creating an environment suitable for plant growth [8,9]. The [lactic acid bacteria + porosity] of bamboo flour acts on soil microorganisms [10].
- (2)
- (3)
- It can be used for deodorizing animal excrement and promoting compost fermentation. Ordinary bamboo powder can be spread on the floor of livestock sheds to reduce the odor of the sheds. The porous structure of bamboo powder absorbs bad odors [14].
- (4)
2. Materials and Methods
2.1. Bamboo Powder and Oyster Shells
2.2. Analysis of Nitrate and Phosphate Ions
2.3. Magnesium Chloride Treatment of Bamboo Powder
2.4. Nano-Size Oyster Shell Powder Hydrogel
2.5. Adsorption Experiments
2.6. Experimental Procedure Plan
- (i)
- The effect of mixing time was studied. The adsorbent and solution placed on each perforated plate, and each sample was quickly collected and filtered through a 0.4 μm membrane filter for analysis. The initial nitrate ion concentration was set at approximately 800 mg/L, and the nitrate nitrogen value was 200 mg/L. The pH was measured using a pH electrode (LAQUAtwin Horiba, Kyoto, Japan).
- (ii)
- The effect of temperature was studied. In Section 2.5, the temperature of the multi-shaker incubator was set at to 25 °C, but in the experiments, it was also changed to 35 °C and 45 °C, respectively.
- (iii)
- Adsorption isotherms were kept at a constant temperature and solid/liquid ratio and at different initial concentrations. The initial concentration was 0.05 M nitric acid standard solution. To study the effect of pH, a few drops of 1M NaOH or 1M H2SO4 were added.
3. Results and Discussion
3.1. Nitrate Removal Using Mg-Treated Bamboo Powder and Bamboo Charcoal
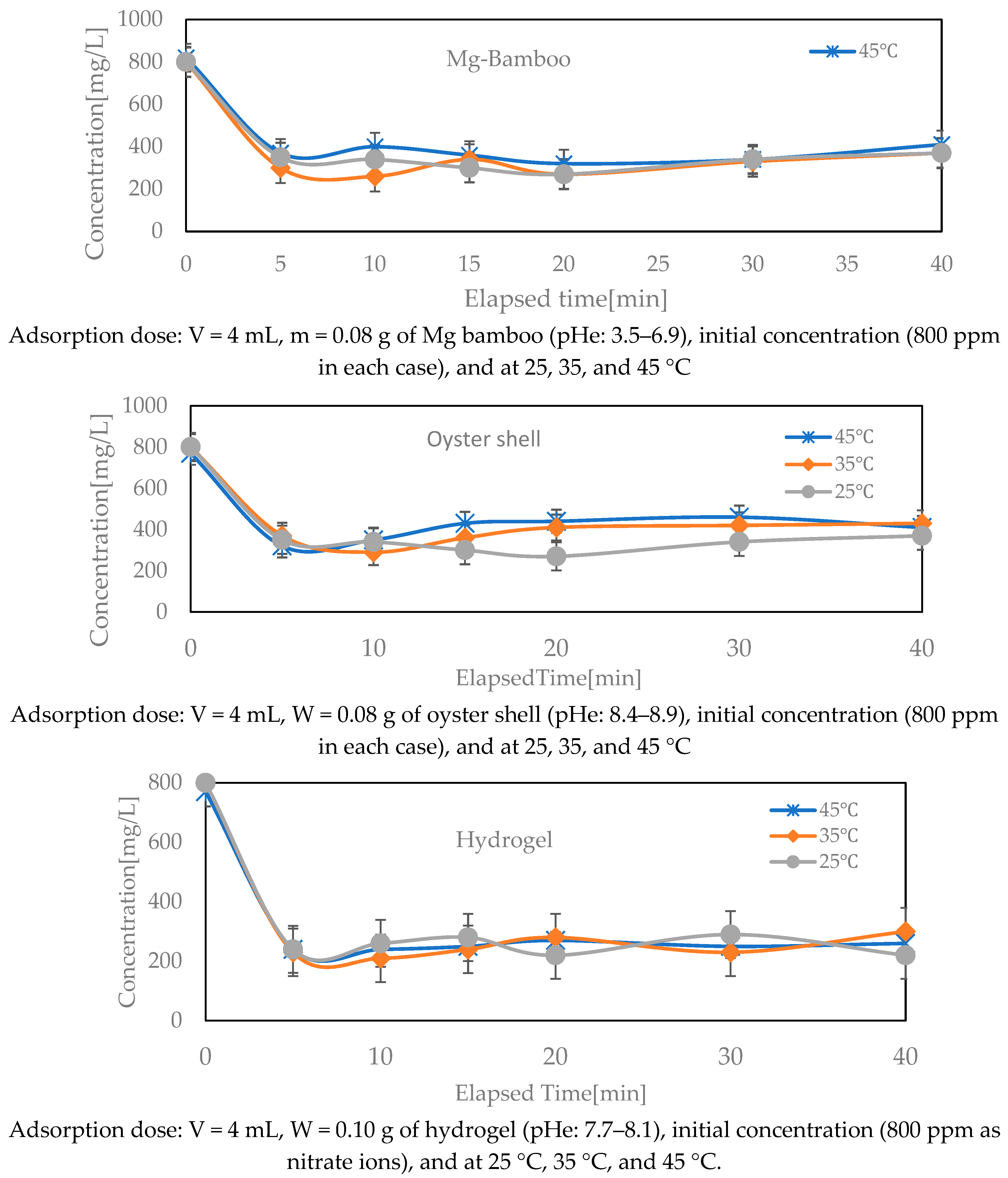
3.2. Effect of Temperature
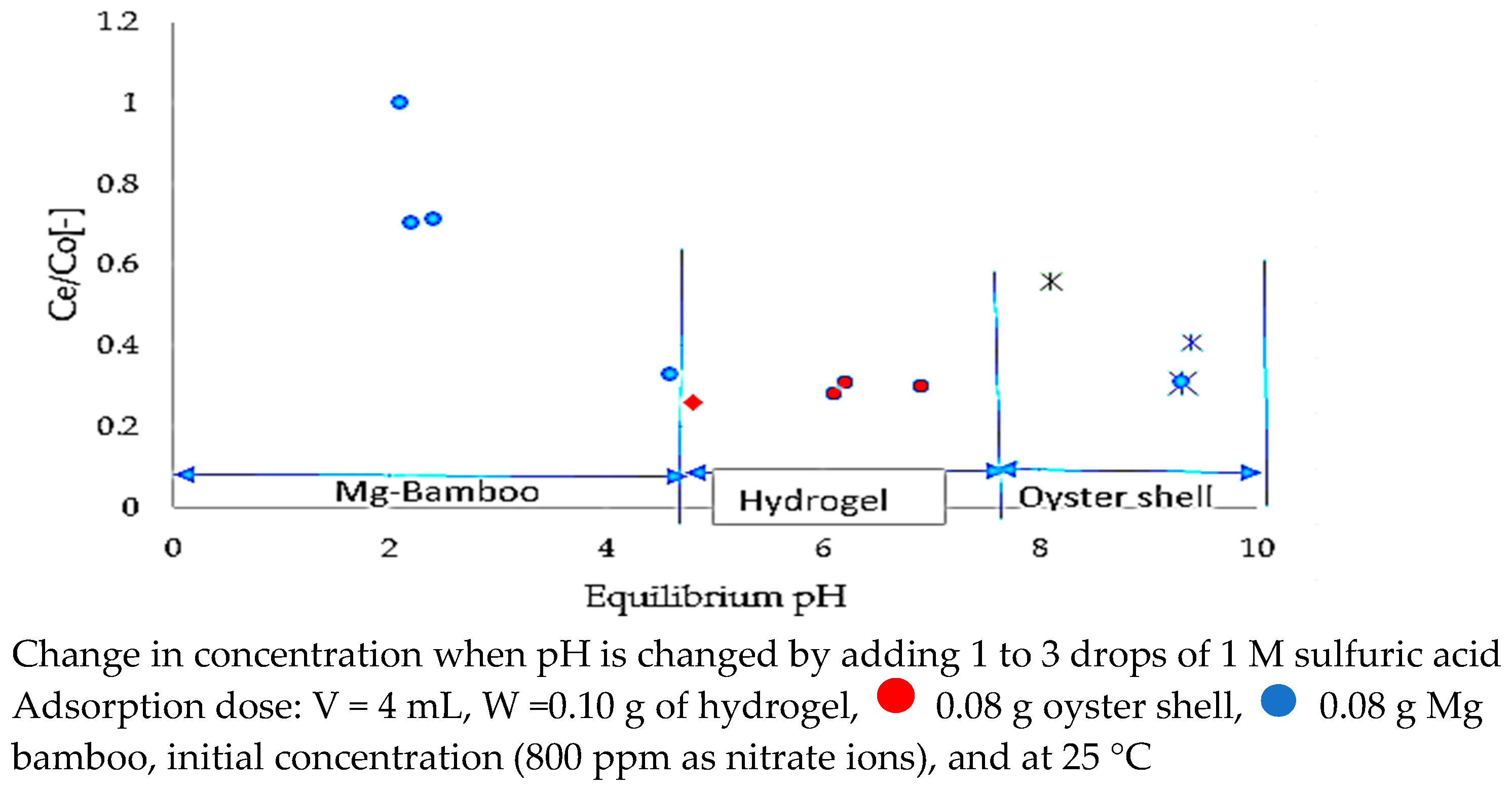
3.3. Nitrate Adsorption Mechanism
3.4. Nitrate Removal in the Presence of Phosphoric Acid
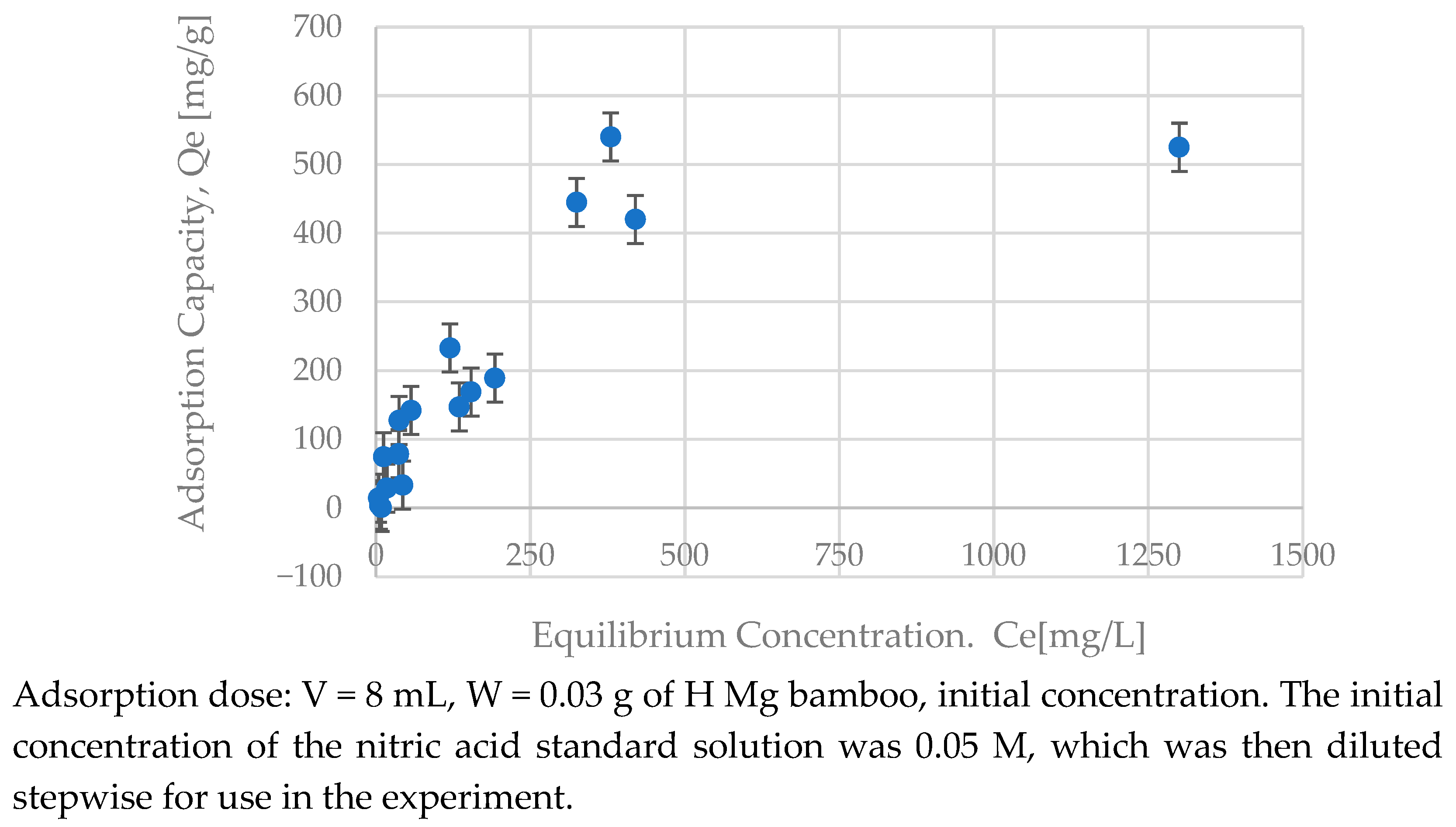
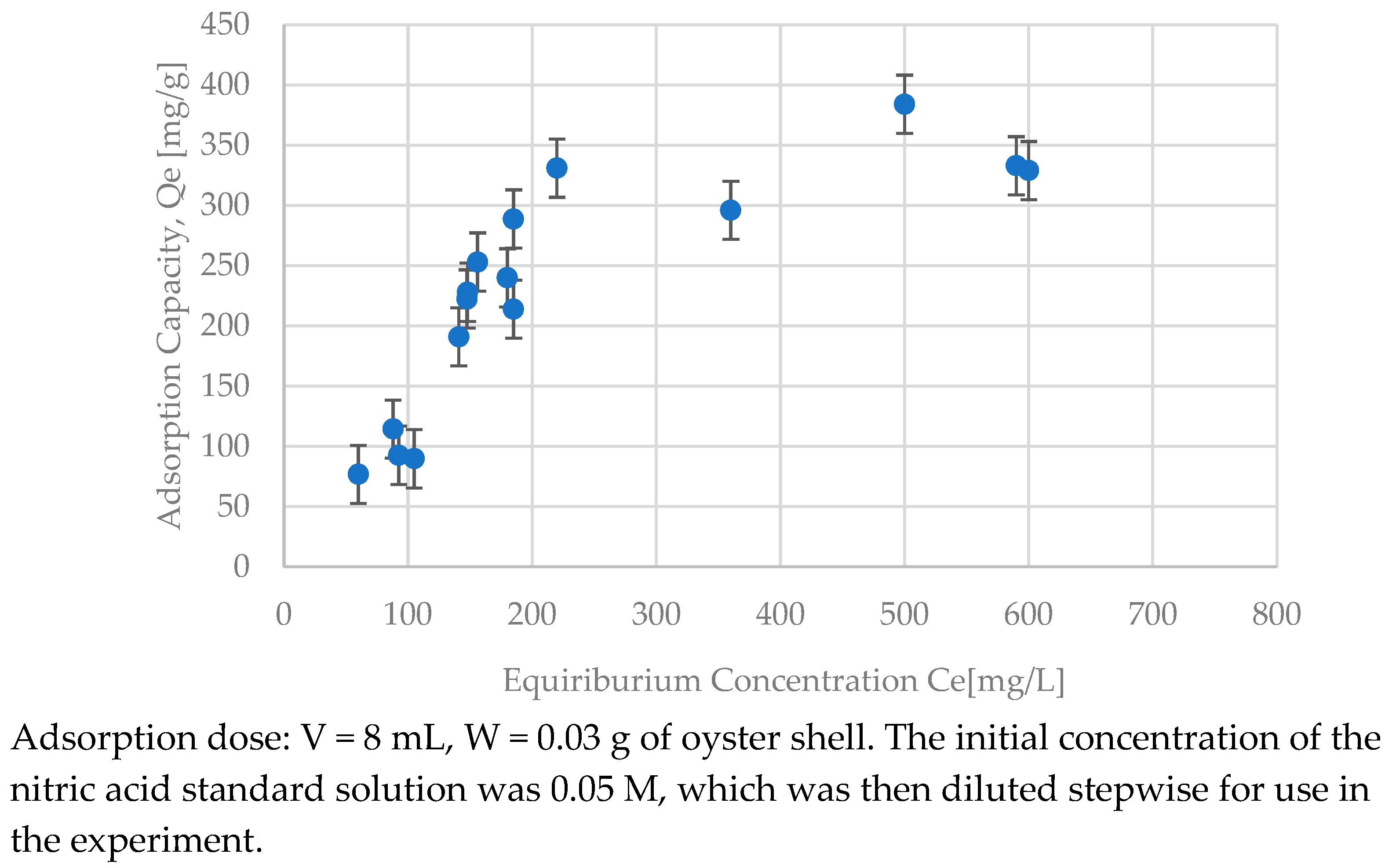
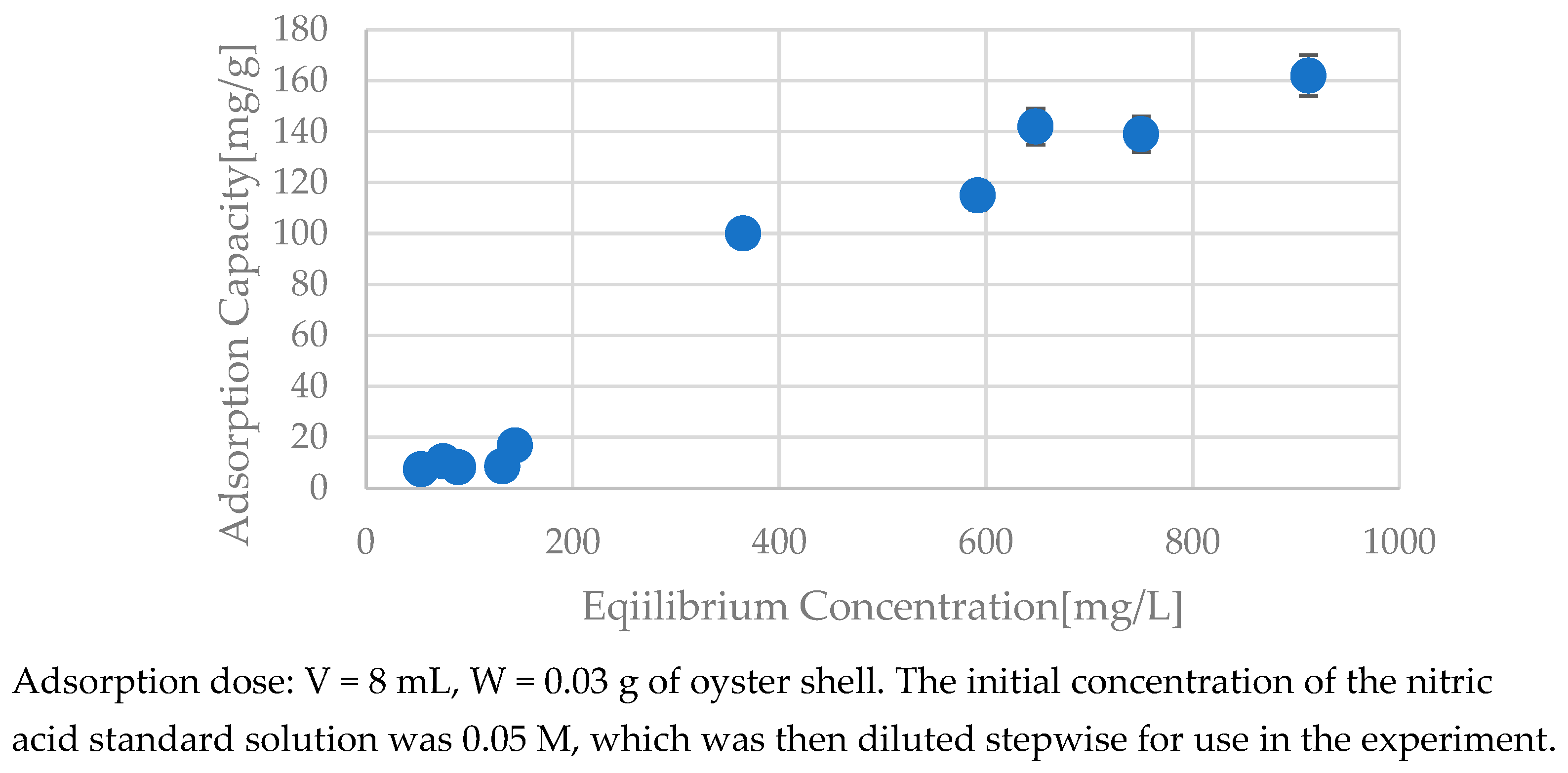
3.5. Analysis of the Adsorption Curve
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bryan, N.S.; van Grinsven, H. Chapter Three—The role of nitrate in human health. Adv. Agron. 2013, 119, 153–182. [Google Scholar] [CrossRef]
- Lamig, L.; Moreno, S.; Álvarez, J.M.; Gutiérrez, R.A. Molecular mechanisms underlying nitrate responses in plants. Curr. Biol. 2022, 32, R433–R439. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors, and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Takano, H. Effect of bamboo powder on the reduction of nitrate ion content in spinach. J. Educ. Res. Reg. Alliances 2020, 5, 27–39. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Van Labeke, M.C. Advanced strategies to reduce the nitrate content in vegetables. Front. Plant Sci. 2021, 12, 765636. [Google Scholar] [CrossRef]
- Fudano, T.; Kikukawa, H.; Kishida, F. Bamboo powder as an ingredient of potting substrates for horticultural plants. Landsc. Plan. Hortic. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Thakur, K.; Rajani, C.S.; Tomar, S.K.; Panmei, A. Fermented bamboo shoots: A riche niche for beneficial microbes. J. Bacteriol. Mycol. 2016, 2, 00030. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhou, X.; Mu, S. Effects of fermented bamboo powder supplementation on serum biochemical parameters, immune indices, and fecal microbial composition in growing–finishing pigs. Animals 2022, 12, 3127. [Google Scholar] [CrossRef]
- Balan, P.; Kim, S.H.; Moughan, P.J. Impact of oral immunoglobulins on animal health—A review. Anim. Sci. J. 2019, 90, 1099–1110. [Google Scholar] [CrossRef]
- Negi, M.; Thankachan, V.; Rajeev, A.; Vairamuthu, M.; Arundhathi, S.; Nidheesh, P.V. Bamboo powder is porous and therefore has the ability to adsorb heavy metals, phosphorus, and other constituents. Water 2025, 17, 454. [Google Scholar] [CrossRef]
- Lamaming, J.; Saalah, S.; Rajin MIsmail, N.M.; Yaser, A.Z. A review on bamboo as an adsorbent for removal of pollutants for wastewater treatment. Int. J. Chem. Eng. 2022, 2022, 7218759. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential application and regeneration of bamboo biochar for wastewater treatment: A review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Danková, Z.; Viglašová, E.; Galamboš, M.; Briančin, J. Production, characterization, and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef]
- de Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental using Life Cycle Assessment Analysis. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Liao, Y.; Fan, J.; Li, R.; Da, B.; Chen, D.; Zhang, Y. Influence of the usage of waste oyster shell powder on mechanical properties and durability of morta. Adv. Powder Technol. 2022, 33, 103503. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Sun, S.; Wang, Y.; Xu, L. Preparation of PVA/waste oyster shell powder composite as an efficient adsorbent of heavy metals from wastewater. Heliyon 2022, 8, e11938. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Singh, R.; Smith, J.A. Modified Crushed Oyster Shells for Fluoride Removal from Water. Sci. Rep. 2020, 10, 5759. [Google Scholar] [CrossRef]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef]
- Gao, B.; Yao, Y.; Xue, Y.W.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Banno, M.; Kuba, T.; Sano, K.; Kawamura, N.; Ichikawa, S.; Sakai, Y. Capacity and mechanism of nitrate anion adsorption onto bamboo charcoal. J. Jpn. Soc. Water Environ. 2009, 32, 369–374. [Google Scholar] [CrossRef][Green Version]
- Kuok, K.K.; Chiu, P.C.; Rahman, M.R.; Chin, M.Y.; Bakri, M.K. Sustainable bamboo and coconut shell activated carbon for purifying river water on Borneo Island. Waste Manag. Bull. 2024, 2, 39–48. [Google Scholar] [CrossRef]
- Jia, W.; Sun, X.; Gao, Y.; Yang, Y.; Yang, L. Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci. Total Environ. 2020, 740, 139534. [Google Scholar] [CrossRef]
- Dai, J.; Zheng, X.; Wang, H.; Zhang, H.; Zhang, L.; Lin, T.; Qin, R.; Qi, M. The removal of phosphorus in solution by the magnesium modified bio char bamboo. Mater. Sci. 2020, 19, 389–393. [Google Scholar]
- Okamoto, M.; Yokoyama, R.; Kubo, T.; Hosoya, K. Preparation of hybrid polymers with absorptivity for nitrate ion. Kobunshi Ronbunshu 2014, 71, 630–636. [Google Scholar] [CrossRef]
- Akizawa, H.; Hoshino, Y.; Suzuki, R.; Tsutsumi, K.; Toyoshima, K. Reduction of nitrogen from livestock wastewater using bamboo powder. Res. Bull. Aichi Agric. Res. Cent. 2019, 51, 123. [Google Scholar]
- Satake, S.; Tang, H. Experiments for nitrate nitrogen attenuation in ground wood chips. J. Ground Water Hydrol. 2016, 58, 183–194. [Google Scholar] [CrossRef][Green Version]
- Al-Agil, Z.H. Removal of nitrate from aqueous solution by bio-calcium from Iraqi eggshells. J. Ecol. Eng. 2023, 24, 10–18. [Google Scholar] [CrossRef]
- Salam, M.A.; Fageeh, O.; Al-Thabaiti, S.A.; Obaid, A.Y. Removal of nitrate ions from aqueous solution using zero-valent iron nanoparticles supported on high surface area Nano graphene’s. J. Mol. Liq. 2015, 212, 708–715. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Lingling, L.; Xu-Biao, L.; Lin, D.; Luo, S.-L. 4—Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal and Pollutants and Resource and Reutillization, Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]

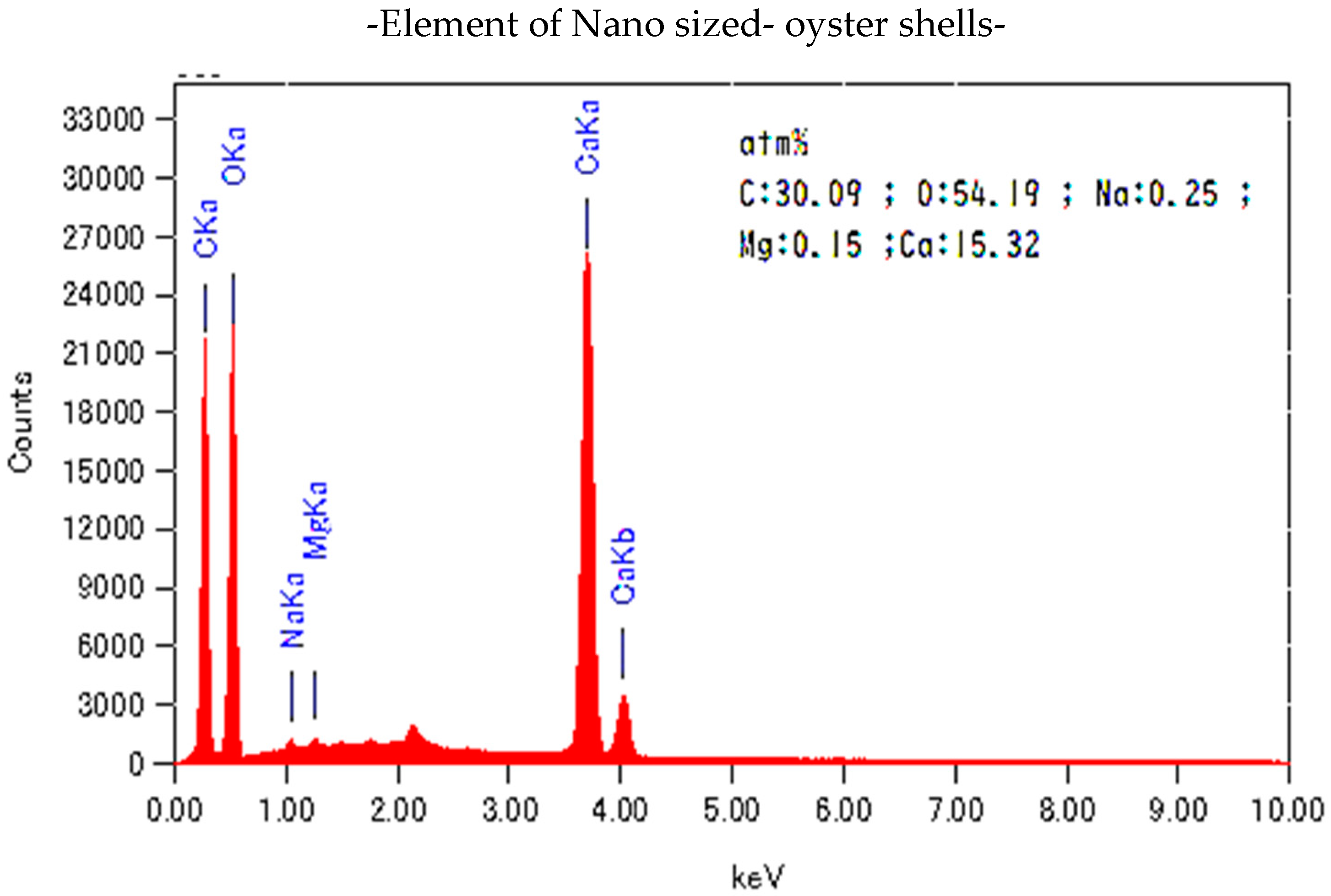
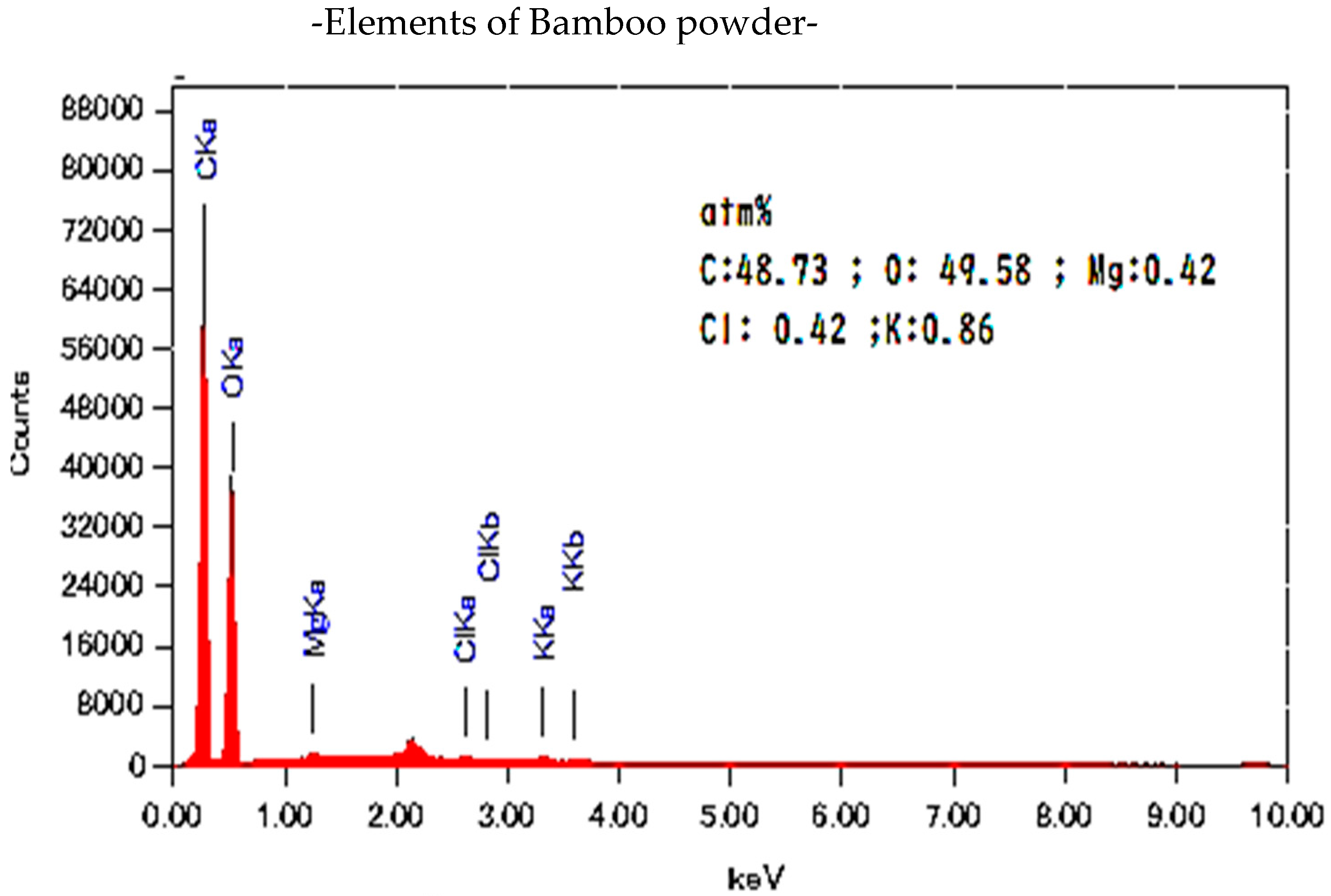


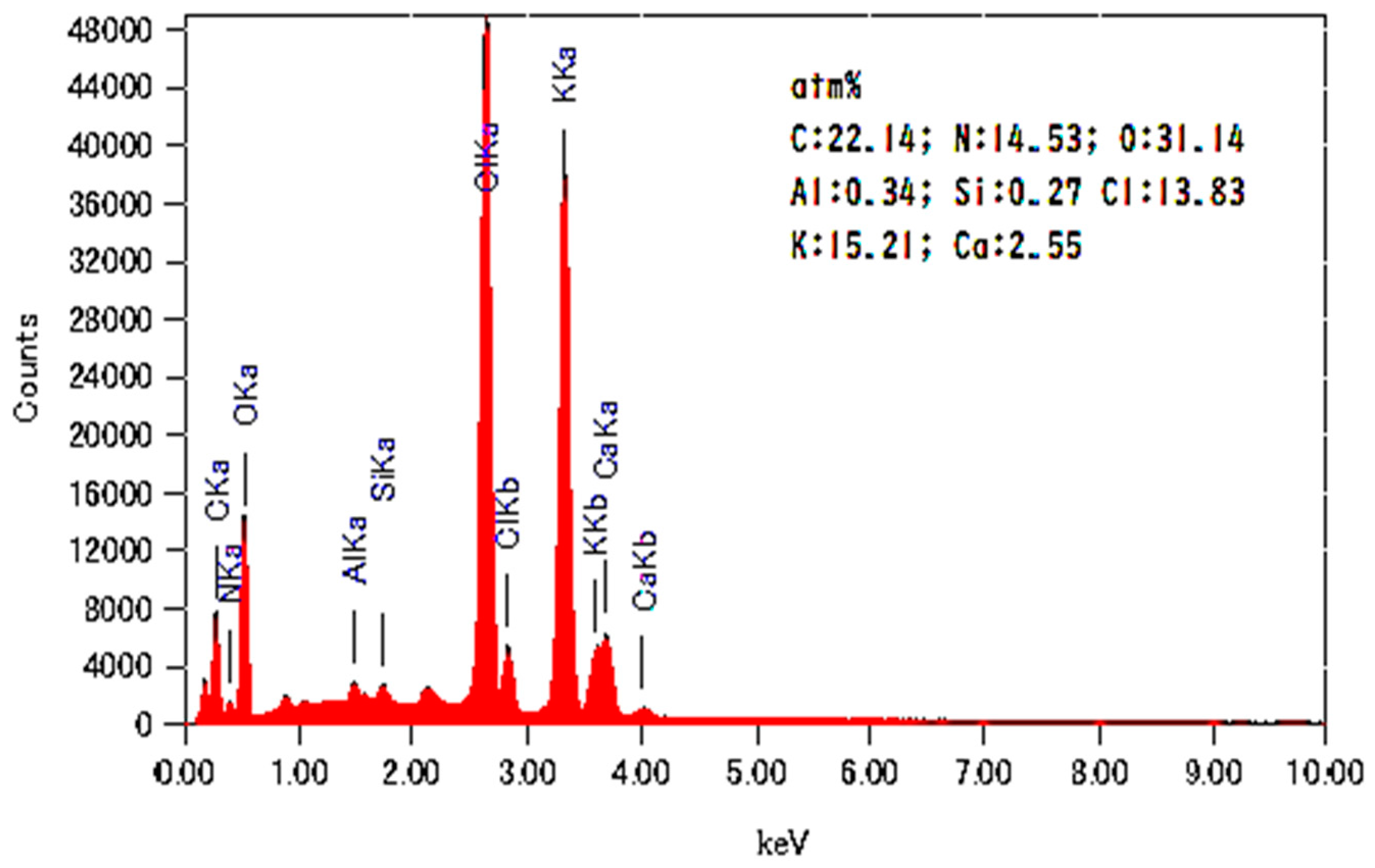


| Nano-Powder Oyster Shell | |
|---|---|
| Median diameter [μm] | 8.42 |
| Mode diameter [μm] | 9.55 |
| Arithmetic mean [μm] | 8.89 |
| Geometric mean [μm] | 4.03 |
| 50% cumulative [μm] | 0.42 μm (420 nm) |
| Level | |||||
| Mg-Bamboo | Temeprature, pH | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mg/L] |
| 1 | 25 °C | 4 | 0~40 | 0.08 | 800 |
| 2 | 35 °C | 4 | 0~40 | 0.08 | 800 |
| 3 | 45 °C | 4 | 0~40 | 0.08 | 800 |
| Oyster shell | Temeprature, pH | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mg/L] |
| 1 | 25 °C | 4 | 0~40 | 0.08 | 800 |
| 2 | 35 °C | 4 | 0~40 | 0.08 | 800 |
| 3 | 45 °C | 4 | 0~40 | 0.08 | 800 |
| Hydro gel | Temeprature, pH | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mg/L] |
| 1 | 25 °C | 4 | 0~40 | 0.1 | 800 |
| 2 | 35 °C | 4 | 0~40 | 0.1 | 800 |
| 3 | 45 °C | 4 | 0~40 | 0.1 | 800 |
| Level | |||||
| Mg-Bamboo | Adsorption Capacity [mg/g] | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mM] |
| 1 | 25 °C | 8 | 0~120 | 0.03 | 50 mM, 40 mM, 30 mM, 25 mM, 20 mM, 10 mM |
| 2 | 26 °C | 9 | 0~120 | 0.03 | 5 mM, 4 mM, 3 mM, 2.5 mM, 2 mM, 1 mM |
| Oyster shell | Adsorption Capacity [mg/g] | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mM] |
| 1 | 25 °C | 8 | 0~120 | 0.03 | 50 mM, 40 mM, 30 mM, 25 mM, 20 mM, 10 mM |
| 2 | 25 °C | 8 | 0~120 | 0.03 | 5 mM, 4 mM, 3 mM, 2.5 mM, 2 mM, 1 mM |
| Hydro gel | Co-Exsiting (N-P mixture) | Volume [mL] | Tim [min] | adsorbent [g] | Initial Concentration [mM] |
| 1 | 25 °C | 8 | 0~120 | 0.11 | Phosphate 1000 mg/L and Nitrate 1000 mg/L |
| 2 | 25 °C | 8 | 0~120 | 0.11 | P/(N + P) 0, 0.1, 0.2, 0.3, 0.4, 0,5 |
| 3 | 25 °C | 8 | 0~120 | 0.11 | P/(N + P) 0.6, 0.7, 0.8, 0.9, 1.0 |
| Langmuir Equation | Freundlich Equation | D-R Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KL | Qm | R2 | n | k | R2 | K | Qm | R2 | |
| Mg bamboo | 0.0047 | 625 | 0.916 | 1.42 | 5.71 | 0.911 | 0.0005 | 399 | 0.842 |
| Oyster shell | 0.0050 | 476 | 0.904 | 0.85 | 3.78 | 0.943 | 0.0016 | 354 | 0.938 |
| Hydrogel | - | - | 0.338 | 0.70 | 6.05 | 0.947 | 0.114 | 156 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiroyuki, H.; Mohamad Sarbani, N.M.; Misturu, A.; Nishimoto, J. Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells. Separations 2025, 12, 76. https://doi.org/10.3390/separations12040076
Hiroyuki H, Mohamad Sarbani NM, Misturu A, Nishimoto J. Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells. Separations. 2025; 12(4):76. https://doi.org/10.3390/separations12040076
Chicago/Turabian StyleHiroyuki, Harada, Nur Maisarah Mohamad Sarbani, Aoyagi Misturu, and Jun Nishimoto. 2025. "Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells" Separations 12, no. 4: 76. https://doi.org/10.3390/separations12040076
APA StyleHiroyuki, H., Mohamad Sarbani, N. M., Misturu, A., & Nishimoto, J. (2025). Adsorption of Nitrate Ions Using Magnesium-Loaded Bamboo Powder and Nano-Sized Crushed Oyster Shells. Separations, 12(4), 76. https://doi.org/10.3390/separations12040076







