Systematic Isolation and Characterization of Regenerated Hemicellulose and Lignin from Soybean Feedstocks Using Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
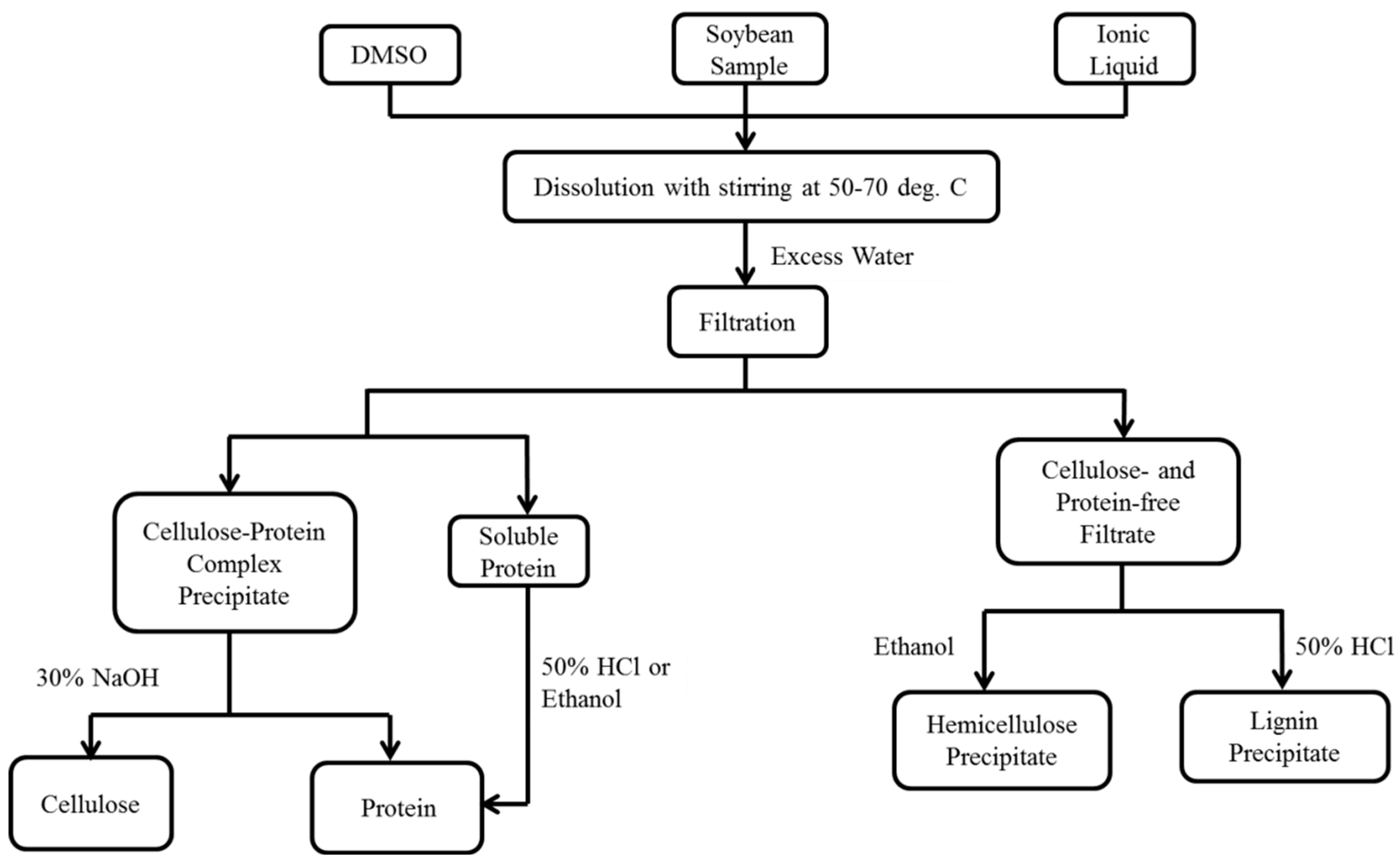
2.2. Dissolution of Soybean Meal, Flakes, and Hulls in ILs
2.3. Hemicellulose Regeneration
2.4. Lignin Regeneration
2.5. FTIR Analysis of Regenerated Hemicellulose and Lignin
2.6. Pyrolysis-Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
3. Results and Discussion
3.1. Hemicellulose and Lignin Regeneration
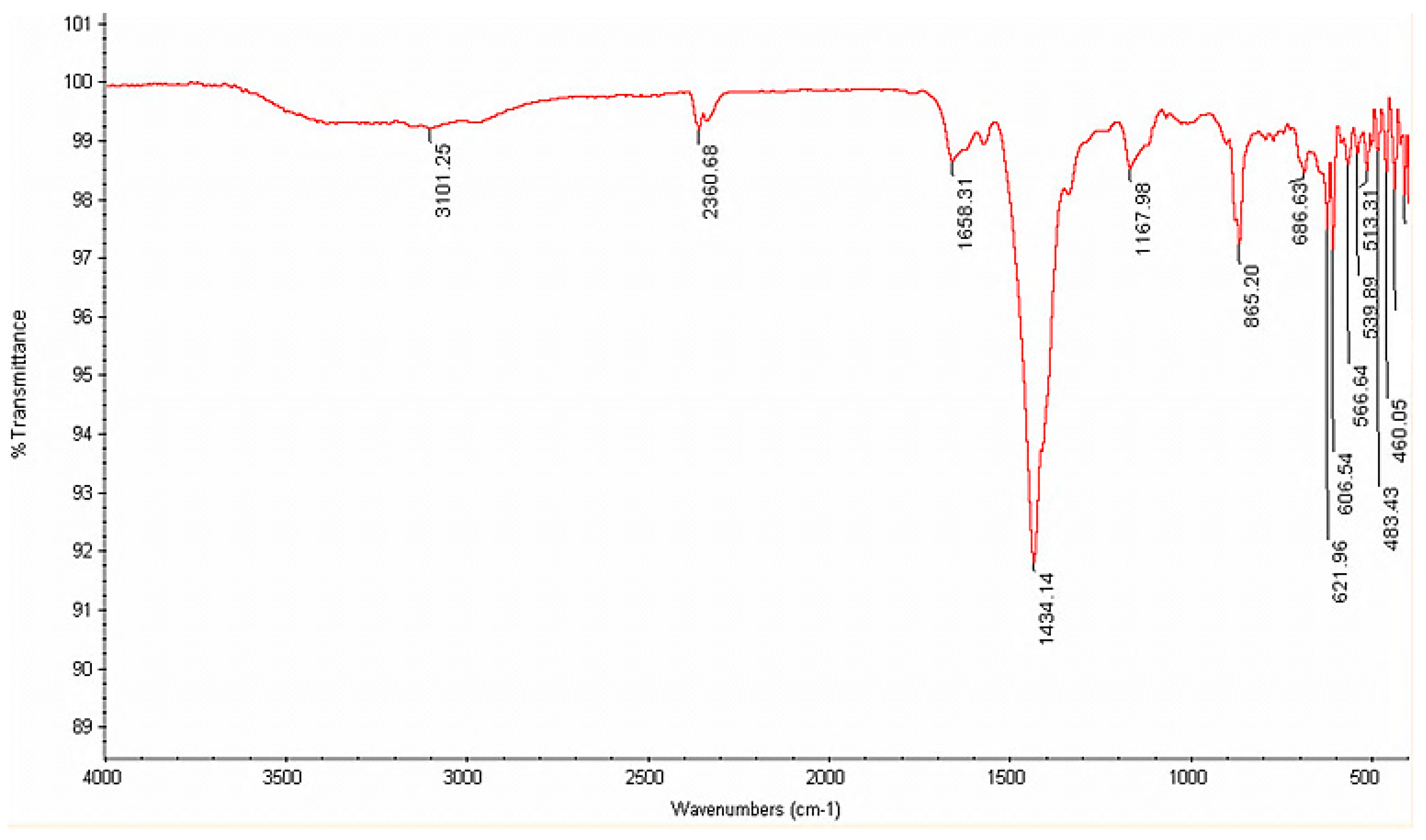
3.2. FTIR Analysis of Regenerated Hemicellulose
3.3. FTIR Analysis of Regenerated Lignin
3.4. Pyrolysis-GC/MS Analysis of Regenerated Lignin
| Ionic Liquid | Soybean Feedstock | Regenerated Hemicellulose (%) | Regenerated Lignin (%) |
|---|---|---|---|
| EmimAc | Meal | 6.20 ± 0.25 | 8.87 ± 0.14 |
| Flakes | 6.13 ± 0.29 | 8.850 ± 0.040 | |
| Hulls | 12.240 ± 0.010 | 18.03 ± 0.14 | |
| AmimCl | Meal | 6.80 ± 0.55 | 8.51 ± 0.18 |
| Flakes | 6.23 ± 0.71 | 8.950 ± 0.045 | |
| Hulls | 12.37 ± 0.55 | 18.41 ± 0.37 | |
| BmimCl | Meal | 6.50 ± 0.29 | 8.46 ± 0.27 |
| Flakes | 6.10 ± 0.61 | 8.61 ± 0.80 | |
| Hulls | 11.14 ± 0.10 | 18.93 ± 0.54 |
| Phenolic Moieties Identified | Identity on Pyrogram | Area (%) a | Lignin Moiety |
|---|---|---|---|
| Phenol | A | 2.01 | H |
| 2-methoxyphenol | B | 2.63 | G |
| 4-methylphenol | C | 0.94 | H |
| 2-methoxy-4-methylphenol | D | 2.90 | G |
| 3-ethylphenol | E | 0.84 | H |
| 2-methylbenzaldehyde | F | 5.33 | * |
| 2-methoxy-4-vinylphenol | G | 3.48 | G |
| 2,6-dimethoxyphenol | H | 0.74 | S |
| Vanillin | I | 0.26 | G |
| 3-tertbutyl-4-hydroxyanisole | J | 0.66 | G |
| 2,6-dimethoxy-4-propenylphenol | K | 1.25 | S |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier-transform infrared spectrometry |
| Py-GC/MS | Pyrolysis-Gas Chromatography/Mass Spectrometry |
| [Bmim]Cl | 1-butyl-3-methylimidazolium chloride |
| [Amim]Cl | 1-allyl-3-methylimidazolium chloride |
| [Emim]Ac | 1-ethyl-3-methylimidazolium acetate |
| IL | Ionic liquid |
| DMSO | Dimethyl sulfoxide |
| CP-F | Cellulose- and protein-free |
| CPH-F | Cellulose-, protein- and hemicellulose-free |
References
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding factors that limit enzymatic hydrolysis of biomass. Appl. Biochem. Biotechnol. 2005, 121–124, 1081–1099. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Molecules 2010, 15, 8641. [Google Scholar] [CrossRef] [PubMed]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655. [Google Scholar] [CrossRef] [PubMed]
- Rowell, R.M. Emerging technology for materials and chemicals from biomass. In Proceedings of ACS Symposium Series 476; American Chemical Society: Washington, DC, USA, 1992; Chapter 2. [Google Scholar]
- Hasegawa, I.; Inoue, Y.; Muranaka, Y.; Yasukawa, T.; Mae, K. Selective production of organic acids and depolymerization of lignin by hydrothermal oxidation with diluted hydrogen pPeroxide. Energy Fuels 2011, 25, 791–796. [Google Scholar] [CrossRef]
- Fu, C.X.; Mielenz, J.R.; Xiao, X.R.; Ge, Y.X.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R.J. Ionic liquid crystals: Hexafluorophosphate salts. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef]
- Dadi, A.P.; Schall, C.A.; Varanasi, S. Mitigation of cellulose recalcitrance to enzymatic hydrolysis by ionic liquid pretreatment. Appl. Biochem. Biotechnol. 2007, 136–140, 407–421. [Google Scholar]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vofgel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed]
- Samayam, I.P.; Schall, C.A. Saccharification of ionic liquid pretreated biomass with commercial enzyme mixtures. Bioresour. Technol. 2010, 101, 3561–3566. [Google Scholar] [CrossRef]
- MacGregor, A.W.; Fincher, G.B. Cereal grain carbohydrates. In Barley: Chemistry and Technology; MacGregor, A.W., Bhatty, R.S., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1993; pp. 73–130. [Google Scholar]
- Bjorkman, A. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Svensk Papperstidn. 1956, 59, 477–485. [Google Scholar]
- Bjorkman, A. Lignin and lignin-carbohydrate complexes. Ind. Eng. Chem. 1957, 49, 1395–1398. [Google Scholar] [CrossRef]
- Wu, S.; Argyropoulos, D.S. An improved method for isolating lignin in high yield and purity. J. Pulp Pap. Sci. 2003, 29, 235–240. [Google Scholar]
- Martin-Sampedro, R.; Capanema, E.A.; Hoeger, I.; Villar, J.C.; Orlando, J.; Rojas, O.R. Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J. Agric. Food Chem. 2011, 59, 8761–8769. [Google Scholar] [CrossRef]
- Feather, M.S.; Whistler, R.L. Isolation and characterization of the principal hemicellulose from corn germ. Archive Biochem. Biophys. 1962, 98, 111–115. [Google Scholar] [CrossRef]
- Igartuburu, J.M.; Pando, E.; Rodriguez-Luis, F.; Gil-Serrano, A. Structure of a hemicellulose B fraction in dietary fiber from the seed of grape variety palomino (Vitis vinifera cv. Palomino). J. Nat. Prod. 1998, 61, 881–886. [Google Scholar] [CrossRef]
- Sun, R.; Mott, L.; Bolton, J. Isolation and fractional characterization of ball-milled and enzyme lignins from oil palm trunk. J. Agric. Food Chem. 1998, 46, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv. Biochem. Eng. Biotechnol. 2007, 108, 41–65. [Google Scholar]
- NREL. Enzyme Sugar-Ethanol Platform Project; National Renewable Energy Laboratory: Golden, CO, USA, 2002. [Google Scholar]
- Fang, J.M.; Sun, R.C.; Tomkinson, J. Isolation and characterization of hemicelluloses and cellulose from rye straw by alkaline peroxide extraction. Cellulose 2000, 7, 87–107. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.-L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.-C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6314. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Liang, C.Y. The infrared spectra of crystalline polysaccharides. VIII. Xylans. J. Polym. Sci. 1962, 59, 357–378. [Google Scholar] [CrossRef]
- Robert, P.; Marquis, M.; Barron, C.; Guillon, F.; Saulnier, L. FT-IR Investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. J. Agric. Food Chem. 2005, 53, 7014–7018. [Google Scholar] [CrossRef] [PubMed]
- Stevanic, J.; Salmen, L. Orientation of the wood polymers in the cell wall of spruce wood fibres. Holzforschung 2009, 63, 497–503. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Song, X.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Fractional characterization of hemicellulosic polymers isolated from Caragana korshinskii Kom. Ind. Eng. Chem. Res. 2011, 50, 6877–6885. [Google Scholar] [CrossRef]
- Lasch, T.P.; Ullrich, O.; Backmann, J.; Naumann, D.; Grune, T. Hydrogen peroxide-induced structural alterations of RNase A. J. Biol. Chem. 2001, 276, 9492–9502. [Google Scholar] [CrossRef] [PubMed]
- Ravi, J.; Hills, A.E.; Cerasoli, E.; Rakowska, P.D.; Ryadnov, M.G. FTIR markers of methionine oxidation for early detection of oxidized protein therapeutics. Eur. Biophys. J. 2011, 40, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Pu, Y.; Raman, B.; Ragauskas, A.J. Structural Characterization and Comparison of Switchgrass Ball-milled Lignin Before and After Dilute Acid Pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cateto, C.; Pu, Y.; Samuel, R.; Ragauskas, A.J. Structural Characterization of Switchgrass Lignin after Ethanol Organosolv Pretreatment. Energy Fuels 2012, 26, 740–745. [Google Scholar] [CrossRef]
- Atalla, R.H.; Agarwal, U.P. Raman Microprobe Evidence for Lignin Orientation in the Cell Walls of Native Woody Tissue. Science 1985, 227, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure and Reactions; Walter de Gruyter: Berlin, Germany, 1989; pp. 217–220. [Google Scholar]
- Sarkanen, K.V.; Ludwig, C.H. Lignin: Occurrence, Formation, Structure and Reactions; Wiley-Interscience: New-York, NY, USA, 1971; p. 916. [Google Scholar]
- Sarkanen, K.V.; Chang, H.M.; Allan, G.G. Species variation in lignins. III. Hardwood lignins. TAPPI 1967, 50, 583–590. [Google Scholar]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Bain, C.D.; Nuzzo, R.G.; Whitesides, G.M. Structure and wetting properties of ω-alkoxy-n-alkanethiolate monolayers on gold and silver. J. Phys. Chem. 1995, 99, 7663–7676. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular conformation in oligo(ethylene glycol)-terminated self-assembled monolayers on gold and silver surfaces determines their ability to resist protein adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Zeller, H.; Novak, P.; Landgraf, R. Blood glucose measurements by infrared spectroscopy. Int. J. Artif. Intern. Organ. 1989, 12, 129–135. [Google Scholar] [CrossRef]
- Martin, W.B.; Mirov, S.; Venugopalan, R. Using two discrete frequencies within the middle infrared to quantitatively determine glucose in serum. J. Biomed. Opt. 2002, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.J.; Hatfield, R.D. Pyrolysis-GC-MS characterization of forage materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Rencoret, J.; Gutierrez, A.; Nieto, L.; Jimenez-Barbero, J.; Faulds, C.B.; Kim, H.; Ralph, J.; Martinez, A.T.; del Rio, J.C. Lignin Composition and Structure in Young versus Adult Eucalyptus globulus Plants. Plant Physiol. 2011, 155, 667–682. [Google Scholar] [CrossRef]
- Kuroda, k.; Nakagawa-izumi, A.; Mazumder, B.B.; Ohtani, Y.; Sameshima, K. Evaluation of chemical composition of the core and bast lignins of variety Chinpi-3 kenaf (Hibiscus cannabinus L.) by pyrolysis–gas chromatography/mass spectrometry and cupric oxide oxidation. Ind. Crop Prod. 2005, 22, 223–232. [Google Scholar] [CrossRef]
- Ross, K.; Mazza, G. Comparative Analysis of Pyrolysis Products from a Variety of Herbaceous Canadian Crop Residues. World J. Agric. Sci. 2011, 7, 763–776. [Google Scholar]
- Karstens, T. Method for Separating Xylose from Lignocelluloses Rich in Xylan, in Particular Wood. DE10158120A, 27 November 2001. [Google Scholar]
- Savannah River Nuclear Solutions LLC. Separation of Lignin from Lignocellulosic Materials. U.S. Patent US20110253326A1, 18 April 2011. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essel, V.; Raynie, D.E. Systematic Isolation and Characterization of Regenerated Hemicellulose and Lignin from Soybean Feedstocks Using Ionic Liquids. Separations 2025, 12, 37. https://doi.org/10.3390/separations12020037
Essel V, Raynie DE. Systematic Isolation and Characterization of Regenerated Hemicellulose and Lignin from Soybean Feedstocks Using Ionic Liquids. Separations. 2025; 12(2):37. https://doi.org/10.3390/separations12020037
Chicago/Turabian StyleEssel, Victor, and Douglas E. Raynie. 2025. "Systematic Isolation and Characterization of Regenerated Hemicellulose and Lignin from Soybean Feedstocks Using Ionic Liquids" Separations 12, no. 2: 37. https://doi.org/10.3390/separations12020037
APA StyleEssel, V., & Raynie, D. E. (2025). Systematic Isolation and Characterization of Regenerated Hemicellulose and Lignin from Soybean Feedstocks Using Ionic Liquids. Separations, 12(2), 37. https://doi.org/10.3390/separations12020037







