Experimental Planning for Production of β-D-Glucan: Purification and Fluorescence Properties from Basidiomycete Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mushroom Samples
2.3. Methods
2.3.1. Growth and Maintenance Conditions of Mushroom Strains
2.3.2. Production of β-D-Glucan from Basidiomycete Mushroom Strains in Culture Media Containing Agro-Industrial Wastes
2.3.3. Isolation of of β-D-Glucan from Basidiomycete Mushroom Strains
2.3.4. Total Polysaccharides and Protein Assays
2.3.5. Congo Red Assay for Specific Determination of of β-D-Glucan with Triple Helical Structure
2.3.6. Alcian Blue Dye Colorimetric Assay for of β-D-Glucan
2.3.7. Experimental Design to Optimize Extracellular β-D-Glucan Production
2.3.8. Preparation of Stationary Phases for IMAC
2.3.9. Chromatographic Behavior of β-D-Glucans from Mushroom Strains on Immobilized Metal Chelates (IMAC)
2.3.10. Purification of β-D-Glucan from Basidiomycete Mushroom Strains by IMAC
2.3.11. Intrinsic Fluorescence Measurements of β-D-Glucans
2.3.12. FTIR Analysis of β-D-Glucans
2.3.13. Statistical Analysis
3. Results and Discussion
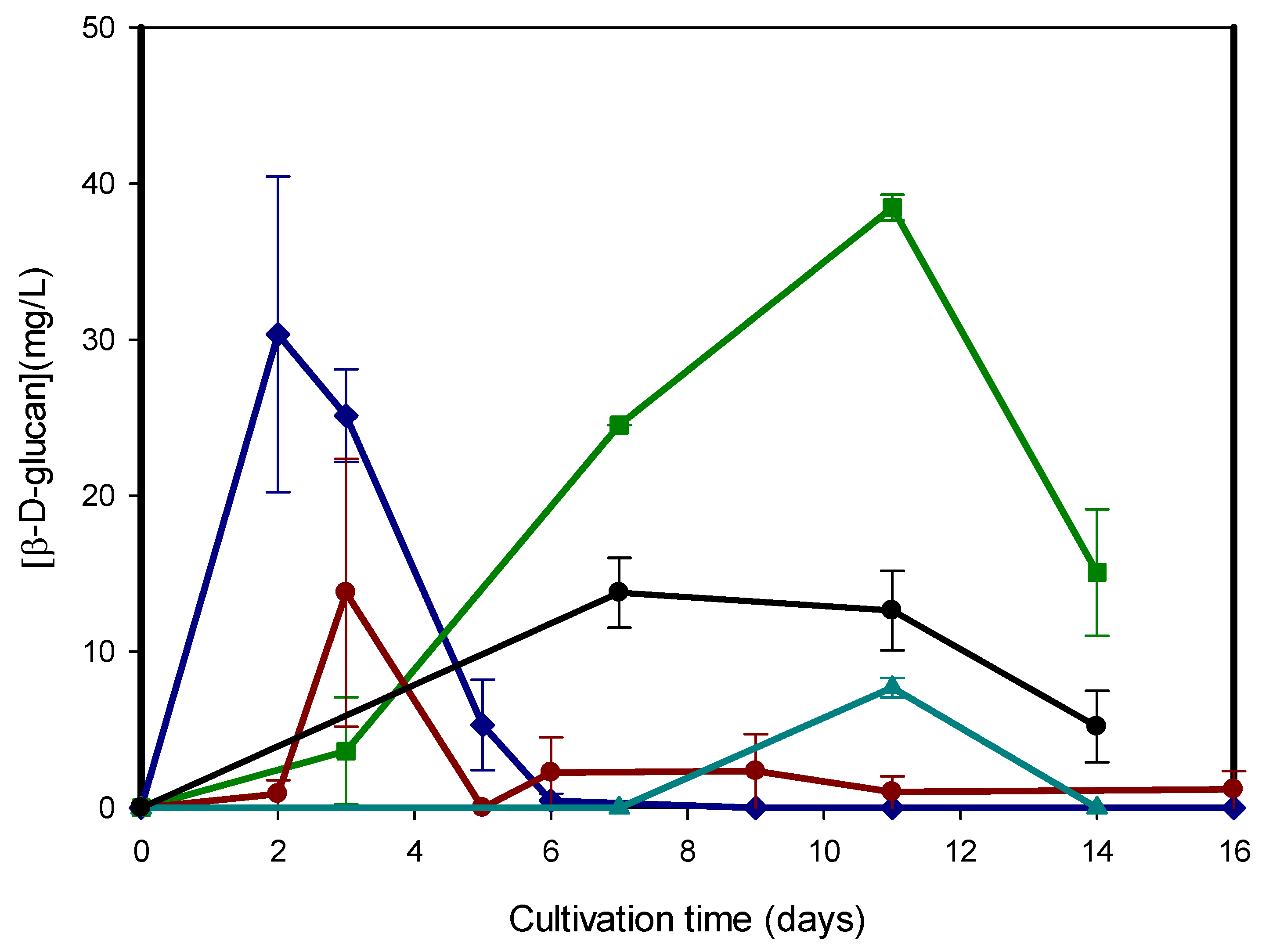
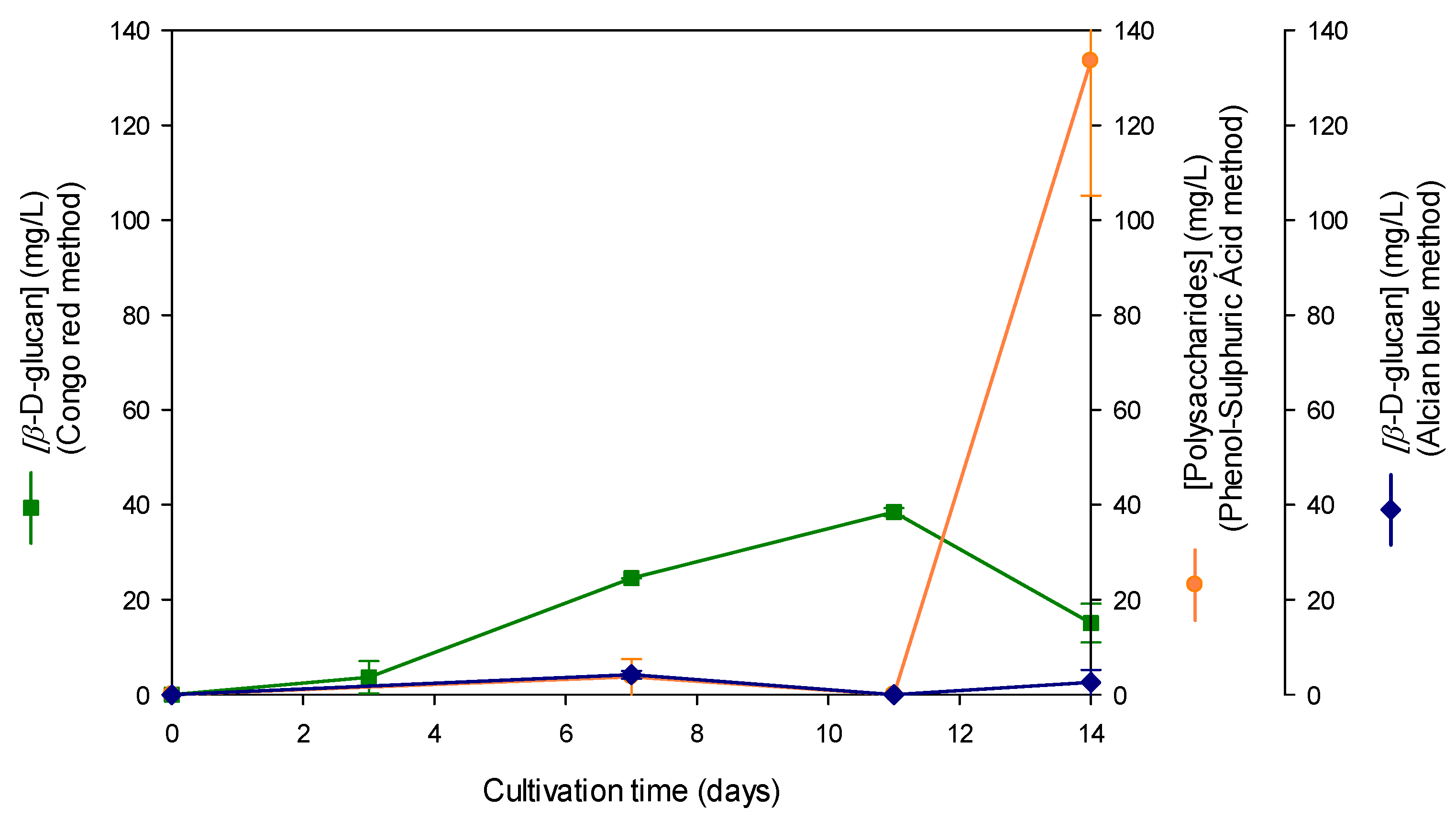
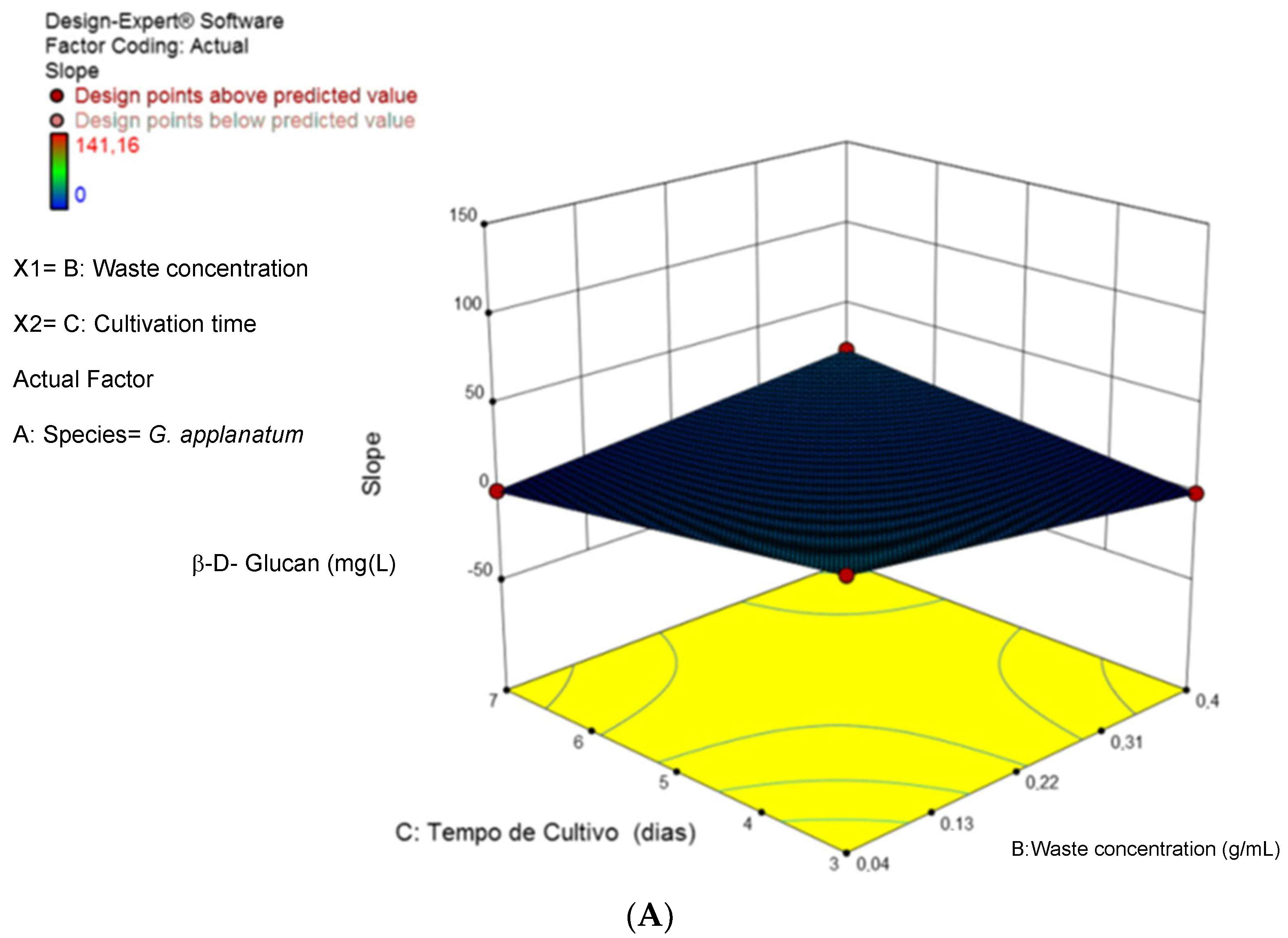
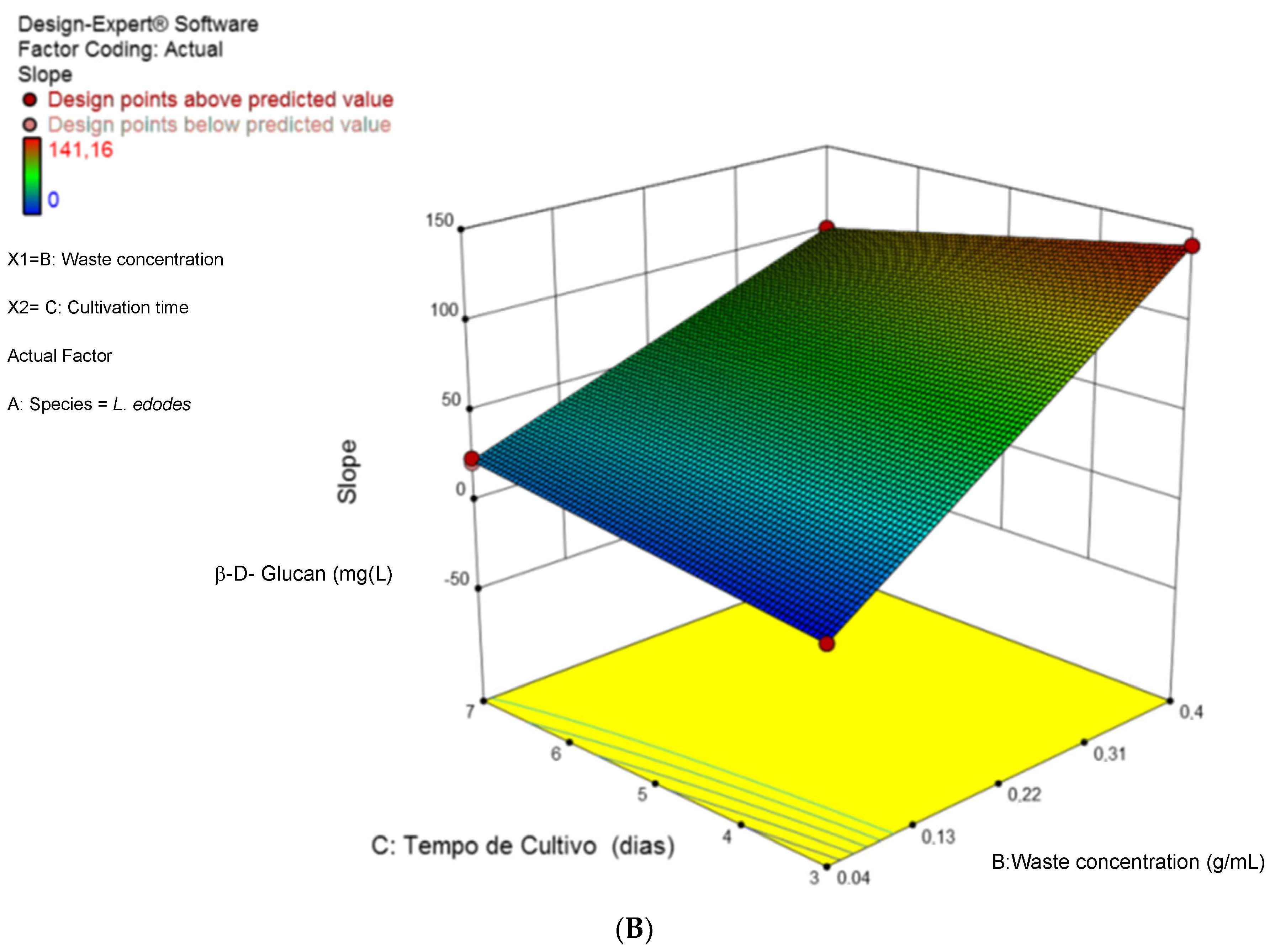
3.1. Optimization of Cultivation Conditions for β-D-Glucan Overproduction
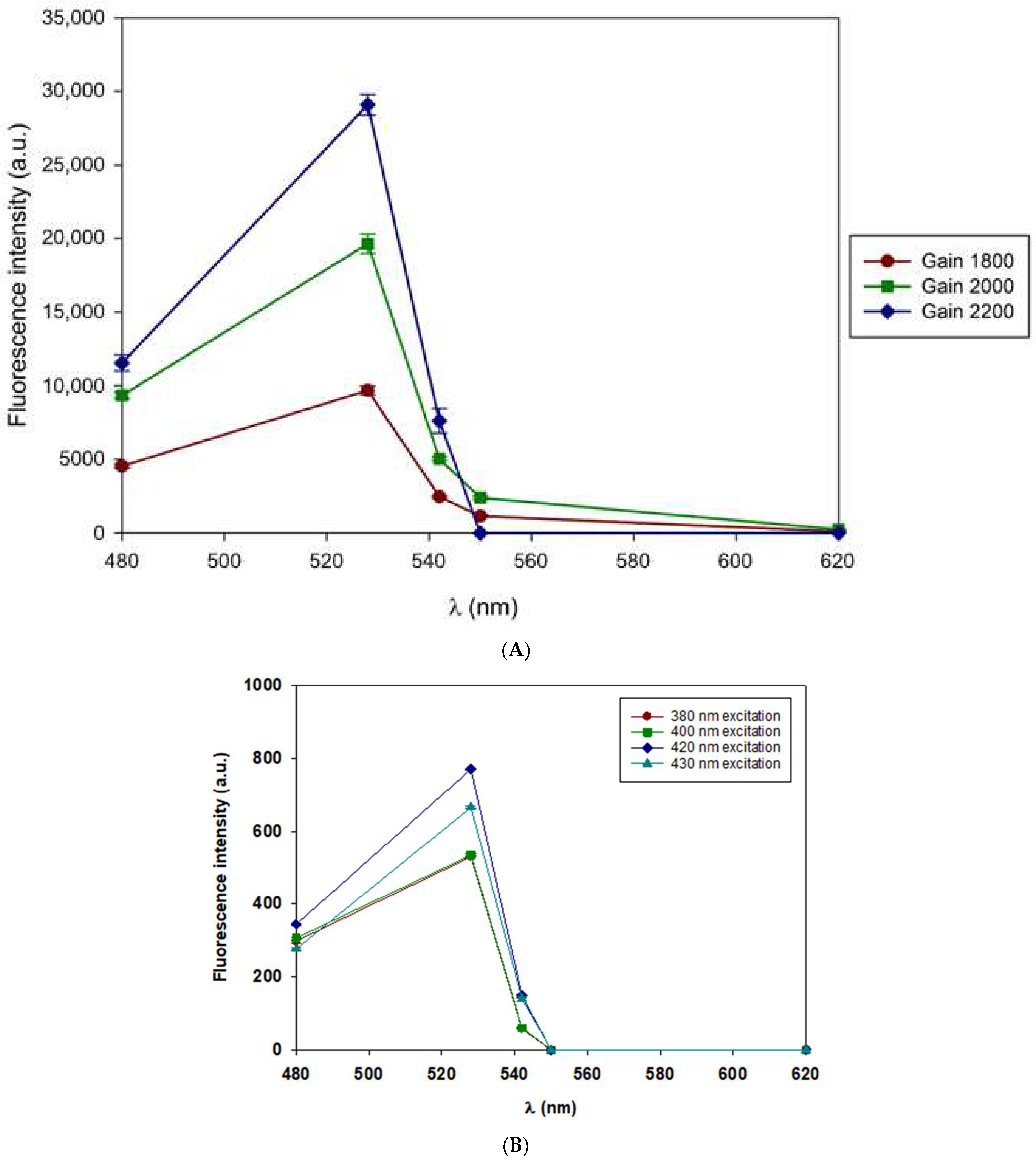
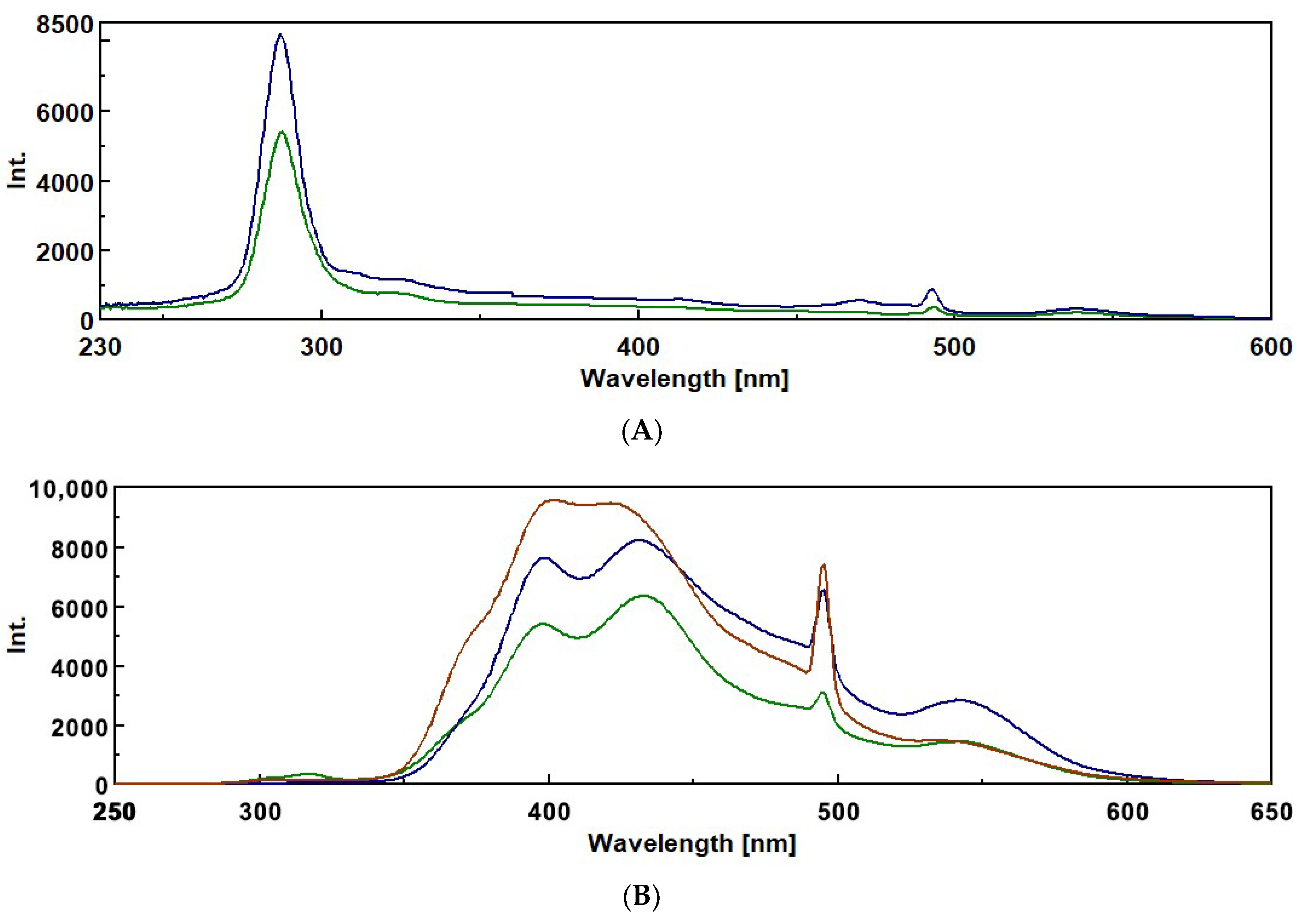
3.2. Intrinsic Fluorescence Spectroscopy
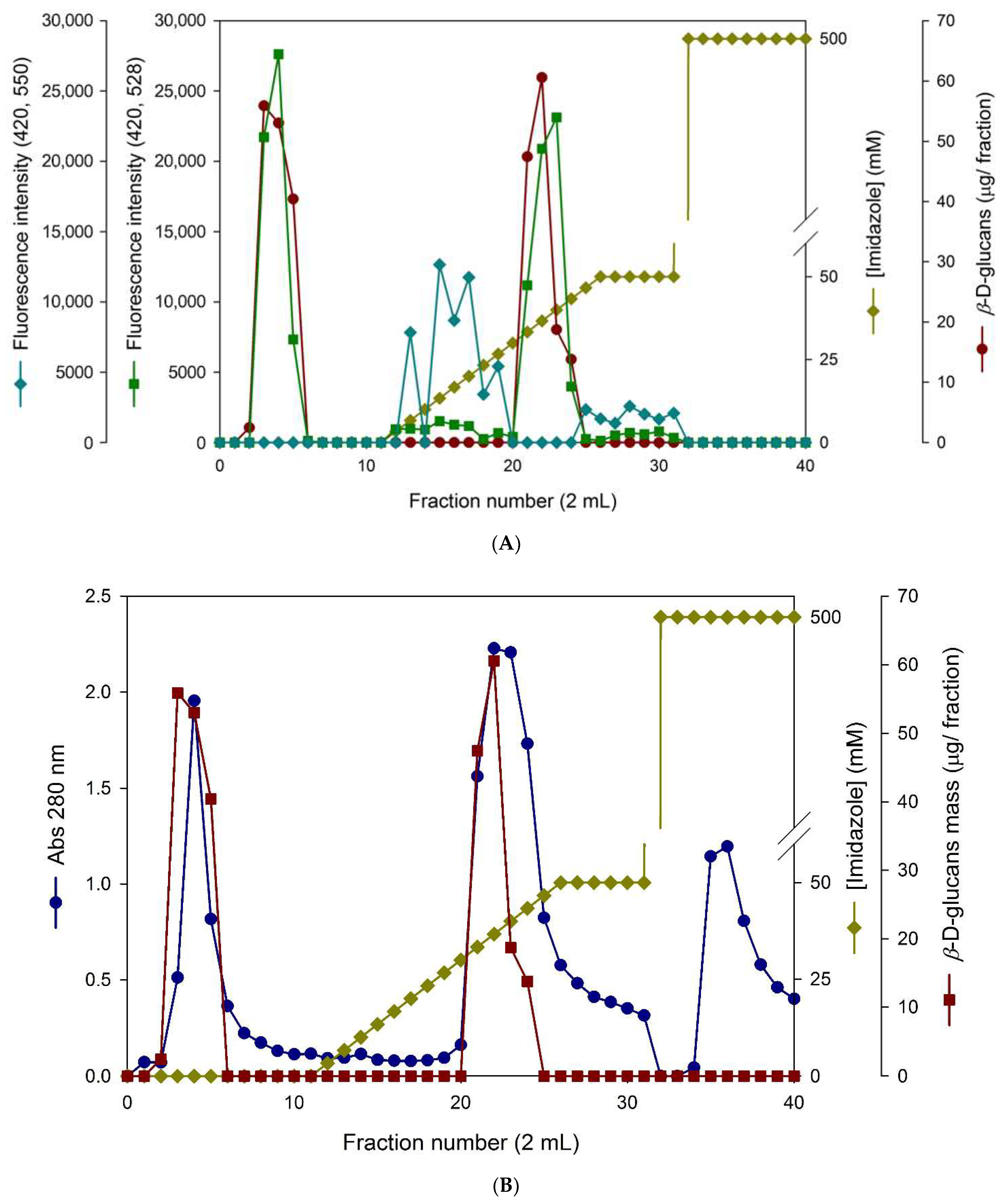
3.3. Immobilized Metal Affinity Chromatography
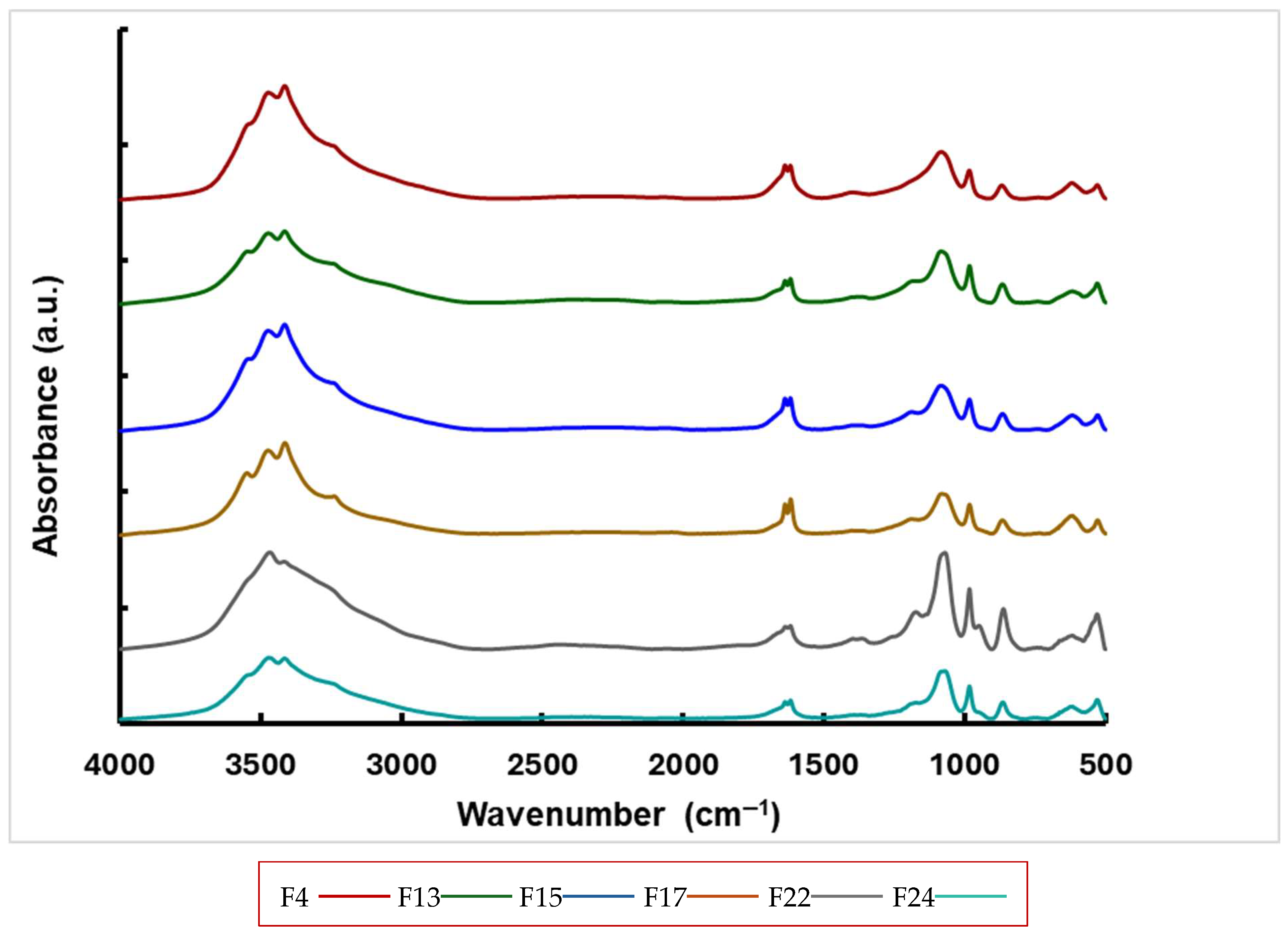
3.4. FTIR Spectroscopy
4. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDGE | 1,4-Butanediol diglycidyl ether |
| EPI | Epiclorohydrin |
| FW1 | Fraction extracted with cold H2O |
| FW2 | Fraction extracted with hot H2O |
| FKOH | Fraction extracted with KOH |
| FHCl | Fraction extracted with HCl |
| FNaOH | Fraction extracted with NaOH |
| IDA | Iminodiacetic acid |
| IMAC | Immobilized metal affinity chromatography |
| NTA | Nitrilotriacetic acid |
| PBS | Phosphate Buffered Saline |
| PDA | Potato Dextrose Agar |
References
- Luna, K.A.; Aguilar, C.N.; Ramírez-Guzmán, N.; Ruiz, H.A.; Martínez, J.L.; Chávez-González, M.L. Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds. Fermentation 2025, 11, 366. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of fruit waste substrates in mushroom production and manipulation of chemical composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Kowalski, S.; Gumul, D. The Use of Waste Products from the Food Industry to Obtain High Value-Added Products. Foods 2024, 13, 847. [Google Scholar] [CrossRef]
- Khatam, K.; Qazanfarzadeh, Z.; Jim’enez-Quero, A. Fungal fermentation: The blueprint for transforming industrial side streams and residues. Bioresour. Technol. 2026, 440, 133426. [Google Scholar] [CrossRef]
- Lysakova, V.; Streletskiy, A.; Sineva, O.; Isakova, E.; Krasnopolskaya, L. Screening of Basidiomycete Strains Capable of Synthesizing Antibacterial and Antifungal Metabolites. Int. J. Mol. Sci. 2025, 26, 9802. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Lehoczki, A.; Kryczyk-Poprawa, A.; Zábó, V.; Varga, J.T.; Bálint, M.; Fazekas-Pongor, V.; Csípő, T.; Rząsa-Duran, E.; Varga, P. Functional Foods in Modern Nutrition Science: Mechanisms, Evidence, and Public Health Implications. Nutrients 2025, 17, 2153. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Joshi, P.B.; Ahmad, Z.; Hemeg, H.A.; Olatunde, A.; Naz, S.; Hafeez, N.; Simal-Gandara, J. Edible mushrooms as potential functional foods in amelioration of hypertension. Phytother. Res. 2023, 37, 2644–2660. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Chinou, I.; Gortzi, O. A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases. Agriculture 2025, 15, 1329. [Google Scholar] [CrossRef]
- Case, S.; O’Brien, T.; Ledwith, A.E.; Chen, S.; Horneck Johnston, C.J.H.; Hackett, E.E.; O’Sullivan, M.; Charles-Messance, H.; Dempsey, E.; Yadav, S.; et al. b-glucans from Agaricus bisporus mushroom products drive Trained Immunity. Front. Nutr. 2024, 11, 1346706. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.-J.; Lim, K.-T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Li, W.Q.; Wu, X.-Q.; Cheng, J.-W.; Ma, S.-Y. Statistical optimization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem. Eng. J. 2010, 49, 104–112. [Google Scholar] [CrossRef]
- Singla, A.; Gupta, O.P.; Sagwal, V.; Kumar, A.; Patwa, N.; Mohan, N.; Ankush; Kumar, D.; Vir, O.; Singh, J.; et al. Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients 2024, 16, 900. [Google Scholar] [CrossRef]
- Pérez-Bassart, Z.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A. Compositional differences of β-glucan-rich extracts from three relevant mushrooms obtained through a sequential extraction protocol. Food Chem. 2023, 402, 134207. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of b-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef]
- Flores, G.A.; Cusumano, G.; Venanzoni, R.; Angelini, P. The Glucans Mushrooms: Molecules of Significant Biological and Medicinal Value. Polysaccharides 2024, 5, 212–224. [Google Scholar] [CrossRef]
- Martins, S.; Karmali, A.; Serralheiro, M.L. Chromatographic behaviour of monoclonal antibodies against wild-type amidase from Pseudomonas aeruginosa on immobilized metal chelates. Biomed. Chromatogr. 2011, 25, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Honda, Y.; Tsujimoto, T.; Uyama, H.; Azuma, J.I. Selective isolation of β-glucan from corn pericarp hemicelluloses by affinity chro matography on cellulose column. Carbohydr. Polym. 2014, 111, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Theint, P.P.; Sakkayawong, N.; Buajarern, S.; Singkhonrat, J. Development of an analytical fluorescence method for quantifying β-glucan content from mushroom extracts; utilizing curcumin as a green chemical fluorophore. J. Food Compos. Anal. 2025, 137 Pt A, 106896. [Google Scholar] [CrossRef]
- Koenig, S.; Rühmann, B.; Sieber, V.; Schmid, J. Quantitative assay of β-(1,3)–β-(1,6)–glucans from fermentation broth using aniline blue. Carbohydr. Polym. 2017, 174, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A high throughput colorimetric assay of β-1,3-D-glucans by Congo red dye. J. Microbiol. Meth. 2015, 109, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Semedo, M.C.; Karmali, A.; Fonseca, L. A novel colorimetric assay of β-D-glucans in basidiomycete strains by alcian blue dye in a 96-well microtiter plate. Biotechnol. Prog. 2015, 31, 1526–1535. [Google Scholar] [CrossRef]
- Freixo, M.R.; Karmali, A.; Frazão, C.; Arteiro, J.M. Production of laccase and xylanase from Coriolus versicolor grown on tomato pomace and their chromatographic behaviour on immobilized metal chelates. Process Biochem. 2008, 43, 1265–1274. [Google Scholar] [CrossRef]
- Martins, S.; Karmali, A.; Andrade, J.; Serralheiro, M. Immobilized metal affinity chromatography of monoclonal immunoglobulin M against mutant amidase from Pseudomonas aeruginosa. Mol. Biotechnol. 2006, 33, 103–113. [Google Scholar] [CrossRef]
- Gaberc-Porekar, V.; Menart, V. Perspectives of immobilized-metal affinity chromatography. J. Biochem. Biophys. Methods 2001, 49, 335–360. [Google Scholar] [CrossRef]
- Block, H.; Maertens, B.; Spriestersbach, A.; Brinker, N.; Kubicek, J.; Fabis, R.; Labahn, J.; Schäfer, F. Chapter 27 Immobilized-Metal Affinity Chromatography (IMAC): A Review. Methods Enzymol. 2009, 463, 439–473. [Google Scholar] [PubMed]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.; Todorović, N.; Jakovljević, D.; Griensven, L. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
| Cultivation Time (Days) | |||||||
|---|---|---|---|---|---|---|---|
| Agro-Industrial Wastes | Oatmeal | Yellow Lupine | Waste Coffee Ground | Banana Peel | Pear Peel | Pineapple Peel | Mango Peel |
| Basidiomycete Strains | |||||||
| F. fomentarius | 11 | 11 | 11 | +60 | 11 | 28 | 28 |
| G. applanatum | +60 | 56 | 9 | 12 | 12 | 12 | 12 |
| G. carnosum | 37 | 11 | 11 | 11 | 11 | 14 | 14 |
| G. lucidum violeta | 37 | 16 | 9 | 13 | 14 | 14 | 14 |
| I. lacteus | 5 | 5 | 13 | 13 | 13 | 9 | 13 |
| L. edodes | 18 | 16 | 18 | 18 | 11 | 14 | 11 |
| P. rufa | 37 | 30 | 15 | 30 | 15 | 14 | 30 |
| P. betulinus | 50 | 18 | 18 | 18 | +60 | 45 | 45 |
| P. ostreatus | 37 | 31 | 23 | 18 | 18 | 28 | 28 |
| Basidiomycete Strains | Agro-Industrial Residue | β-Glucan Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|
| Culture Media Filtrate | Cold Water Fraction | Hot Water Fraction | KOH Fraction | HCl Fraction | ||
| G. applanatum | Banana Peel | 0 | 0 | 0 | 0 | 0 |
| Waste Coffee Ground | 2.96 × 101 ± 5.79 × 10−1 | 0 | 8.04 × 10−1 ± 2.57 × 10−1 | 1.38 ± 2.82 × 10−1 | 3.16 ± 5.19 × 10−1 | |
| G. carnosum | Banana Peel | 0 | 0 | 1.83 × 10−1 ± 1.24 × 10−2 | 4.16 ± 1.69 × 10−1 | 9.76 ± 1.88 |
| Waste Coffee Ground | 4.26 ± 1.91 | 0 | 1.57 ± 1.31 × 10−2 | 1.03 × 101 ± 8.64 | 1.83 × 101 ± 1.27 | |
| L. edodes | Banana Peel | 0 | 0 | 0 | 3.46 ± 4.02 × 10−2 | 7.56 ± 5.19 × 10−1 |
| Waste Coffee Ground | 3.85 × 101 ± 4.41 | 0 | 9.14 × 10−1 ± 2.99 × 10−3 | 1.48 × 101 ± 2.97 | 1.56 ± 1.96 × 10−1 | |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value, Prob > F |
|---|---|---|---|---|---|
| Model | 3.87 × 104 | 7 | 5.53 × 103 | 1.34 × 104 | <0.0001 |
| A-Species | 1.13 × 104 | 1 | 1.13 × 104 | 2.74 × 104 | <0.0001 |
| B-Residue Concentration | 1.10 × 104 | 1 | 1.10 × 104 | 2.66 × 104 | <0.0001 |
| C-Time of Cultivation | 1.41 × 102 | 1 | 1.41 × 102 | 3.41 × 102 | <0.0001 |
| AB | 1.30 × 104 | 1 | 1.30 × 104 | 3.16 × 104 | <0.0001 |
| AC | 2.56 × 101 | 1 | 2.56 × 101 | 6.21 × 101 | <0.0001 |
| BC | 4.40 × 101 | 1 | 4.40 × 101 | 1.07 × 102 | <0.0001 |
| ABC | 3.23 × 103 | 1 | 3.23 × 103 | 7.83 × 103 | <0.0001 |
| Residual | 3.30 | 8 | 4.10 × 10−1 | ||
| Corrected Total | 3.87 × 104 | 15 |
| Stationary Phase | pH | Cu(II)-IDA | Ni(II)-IDA | Co(II)-IDA | Zn(II)-IDA |
| Sepharose 6B-BDGE (S1) | 8 | ± | − | − | ± |
| Sepharose 6B-EPI (S2) | 8 | − | − | − | ± |
| Sepharose 4B-BDGE (S3) | 8 | ±/− | − | − | ± |
| Sepharose 4B-EPI (S4) | 8 | + | ± | − | ± |
| Stationary phase | pH | Cu(II)-NTA | Ni(II)-NTA | Zn(II)-NTA | Co(II)-NTA |
| Sepharose 6B-BDGE (S5) | 8 | − | − | − | − |
| Sepharose 6B-EPI (S6) | 8 | ± | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, L.; Karmali, A. Experimental Planning for Production of β-D-Glucan: Purification and Fluorescence Properties from Basidiomycete Strains. Separations 2025, 12, 336. https://doi.org/10.3390/separations12120336
Marques L, Karmali A. Experimental Planning for Production of β-D-Glucan: Purification and Fluorescence Properties from Basidiomycete Strains. Separations. 2025; 12(12):336. https://doi.org/10.3390/separations12120336
Chicago/Turabian StyleMarques, Luís, and Amin Karmali. 2025. "Experimental Planning for Production of β-D-Glucan: Purification and Fluorescence Properties from Basidiomycete Strains" Separations 12, no. 12: 336. https://doi.org/10.3390/separations12120336
APA StyleMarques, L., & Karmali, A. (2025). Experimental Planning for Production of β-D-Glucan: Purification and Fluorescence Properties from Basidiomycete Strains. Separations, 12(12), 336. https://doi.org/10.3390/separations12120336





