Exploration on the Extraction of Phenolic Acid from Abutilon theophrasti and Antioxidant and Antibacterial Activities
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Determination of Total Phenolic Acid Content
2.3. Optimization of Extraction Parameters
2.4. Comparison of UARE with Traditional Methods
2.5. Separation and Identification of Phenolic Acid
2.6. Determination of Phytochemicals by HPLC
2.7. Antioxidant Activity
2.8. Antibacterial Activity
2.9. Data Analysis
3. Results and Discussion
3.1. Optimization of Extraction Condition
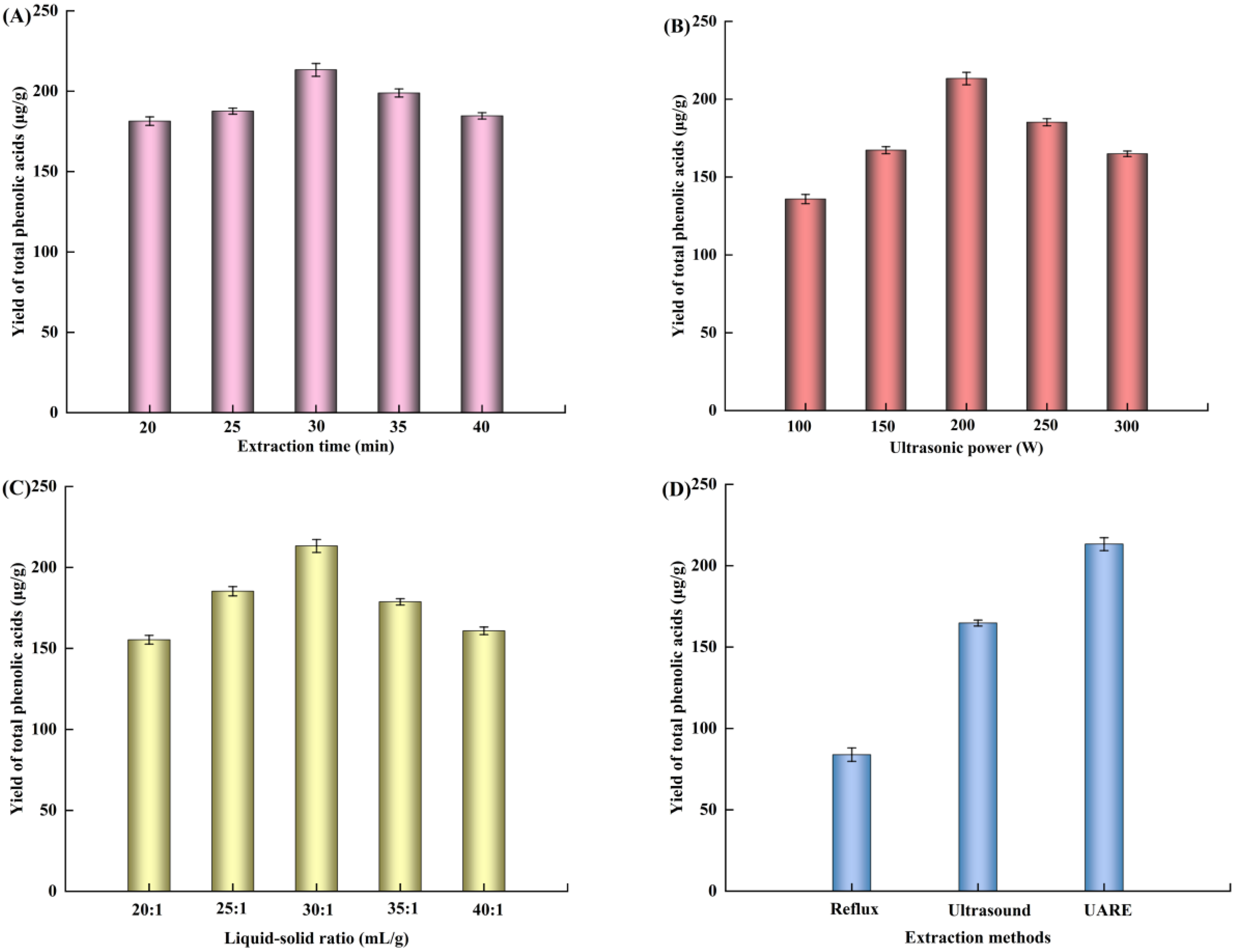
3.1.1. Effect of Extraction Time
3.1.2. Effect of Ultrasonic Power
3.1.3. Effect of Liquid–Solid Ratio
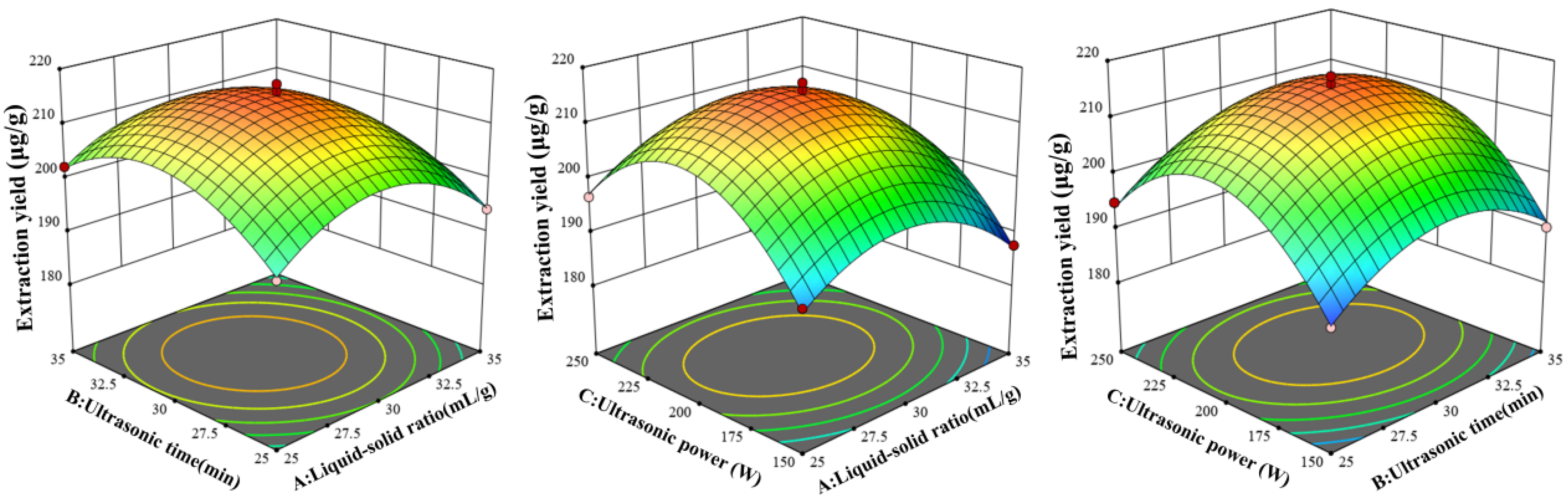
3.1.4. Results of BBD and RSM Analysis
− 0.005X23 − 0.51X32
3.2. Comparison of Different Extraction Processes
3.3. SEM
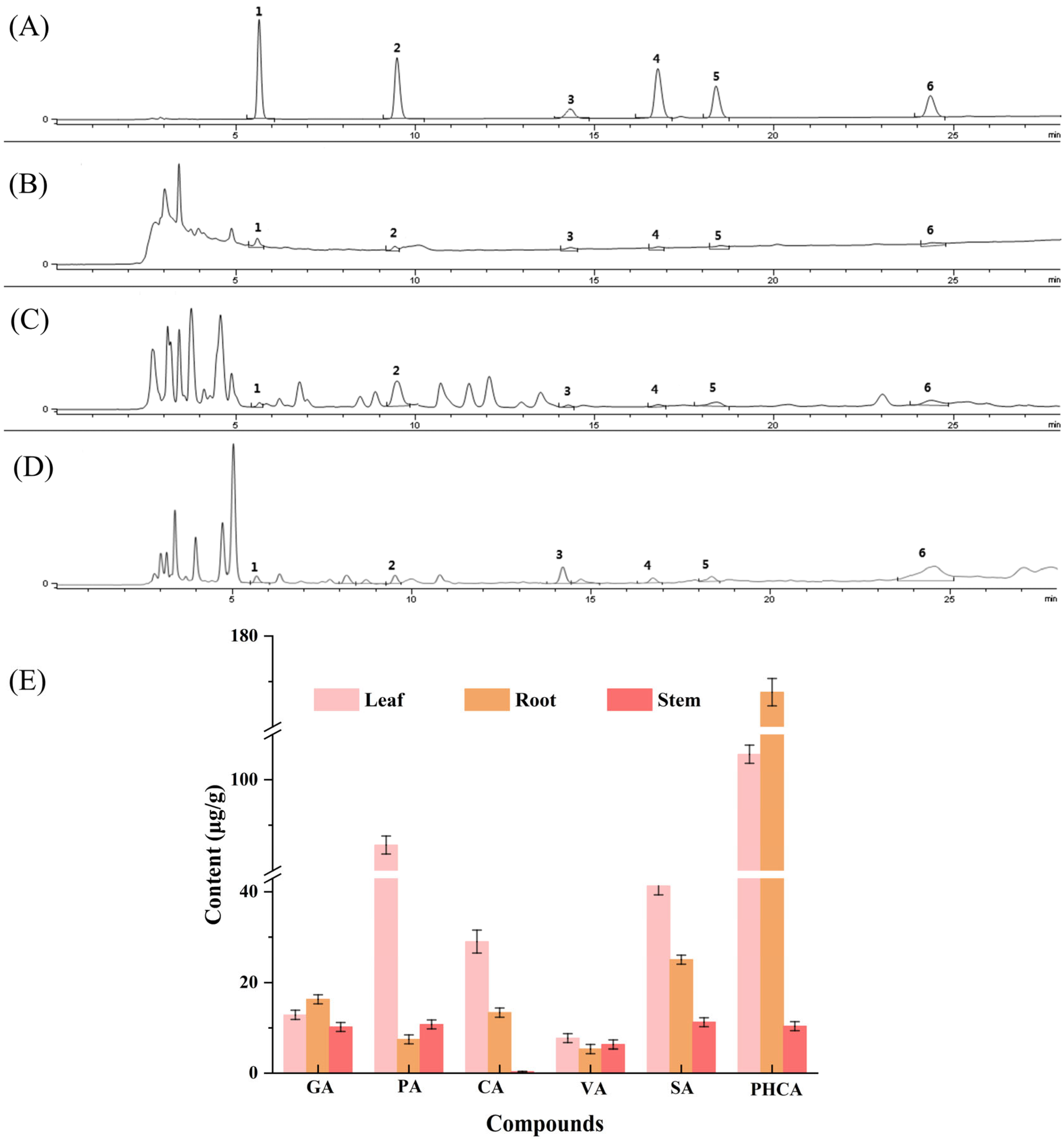
3.4. Separation and Identification of Phenolic Acids
3.5. Determination of the Contents of Six Phenolic Acid
3.6. Results of Antioxidant Activity
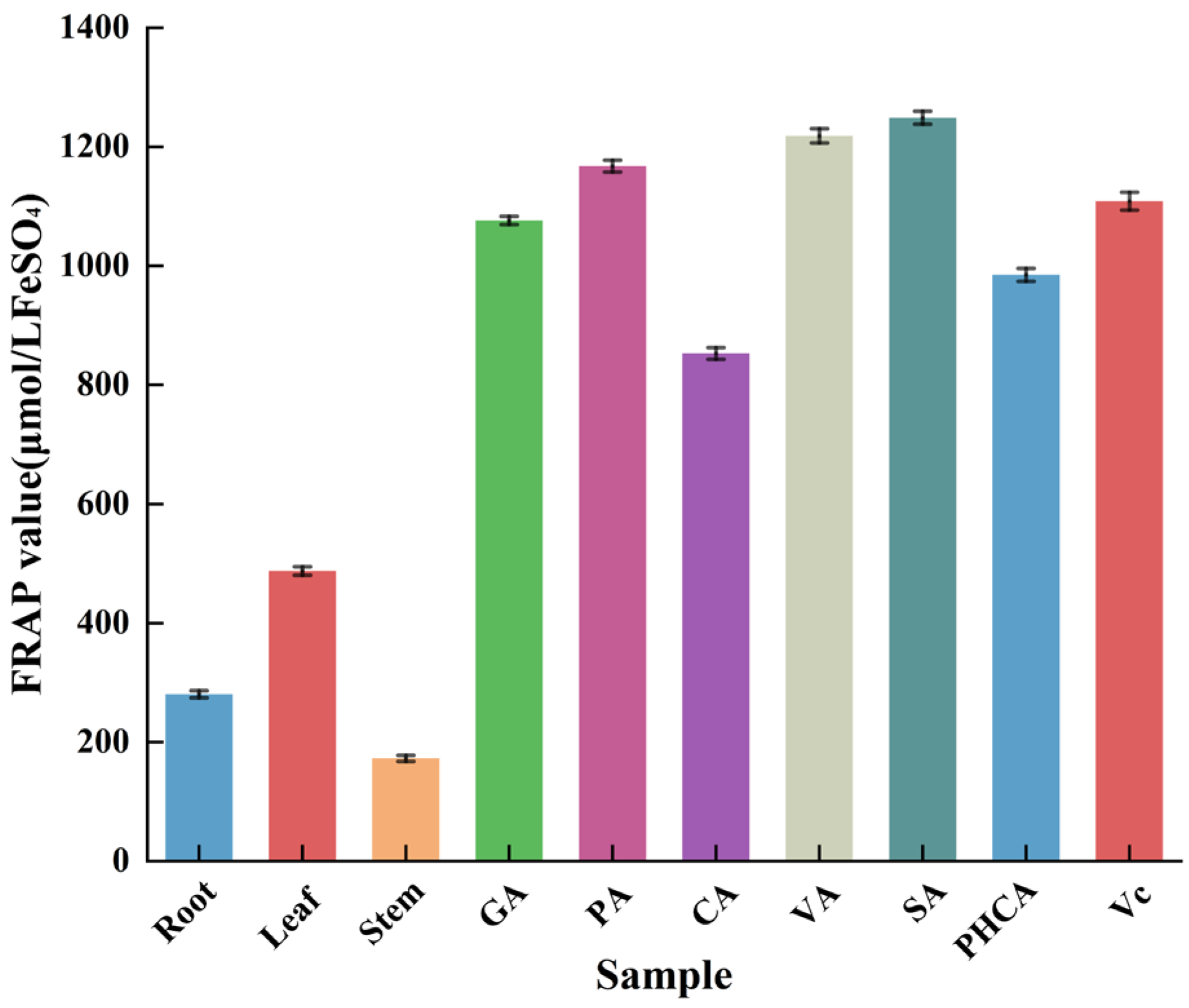
3.6.1. The FRAP of Extracts and Six Phenolic Acids
3.6.2. The DPPH of Extracts and Six Phenolic Acids
3.6.3. The ABTS of Extracts and Six Phenolic Acids
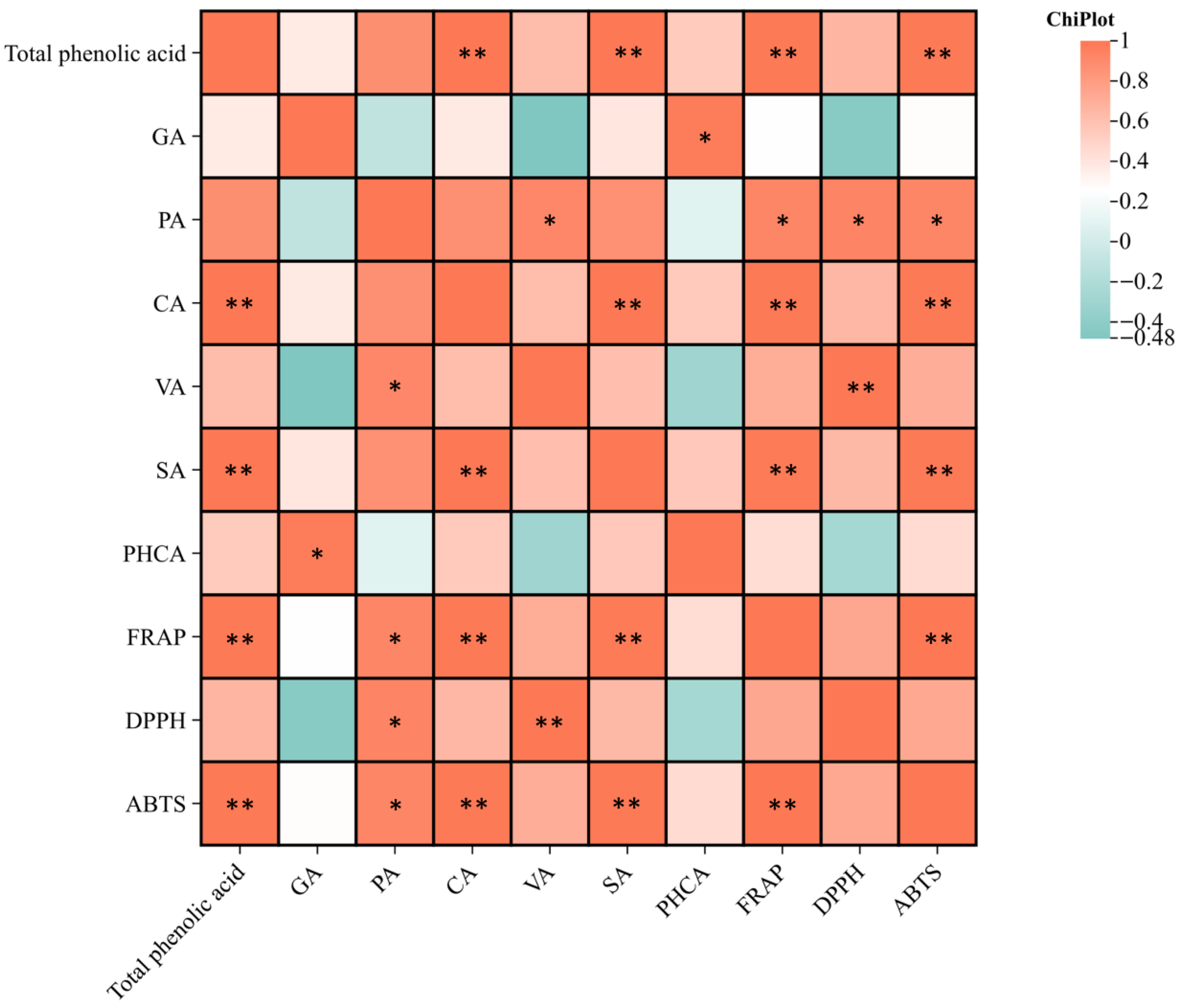
3.6.4. Correlation Analysis
3.7. Antibacterial Activity of Extract and Six Phenolic Acids
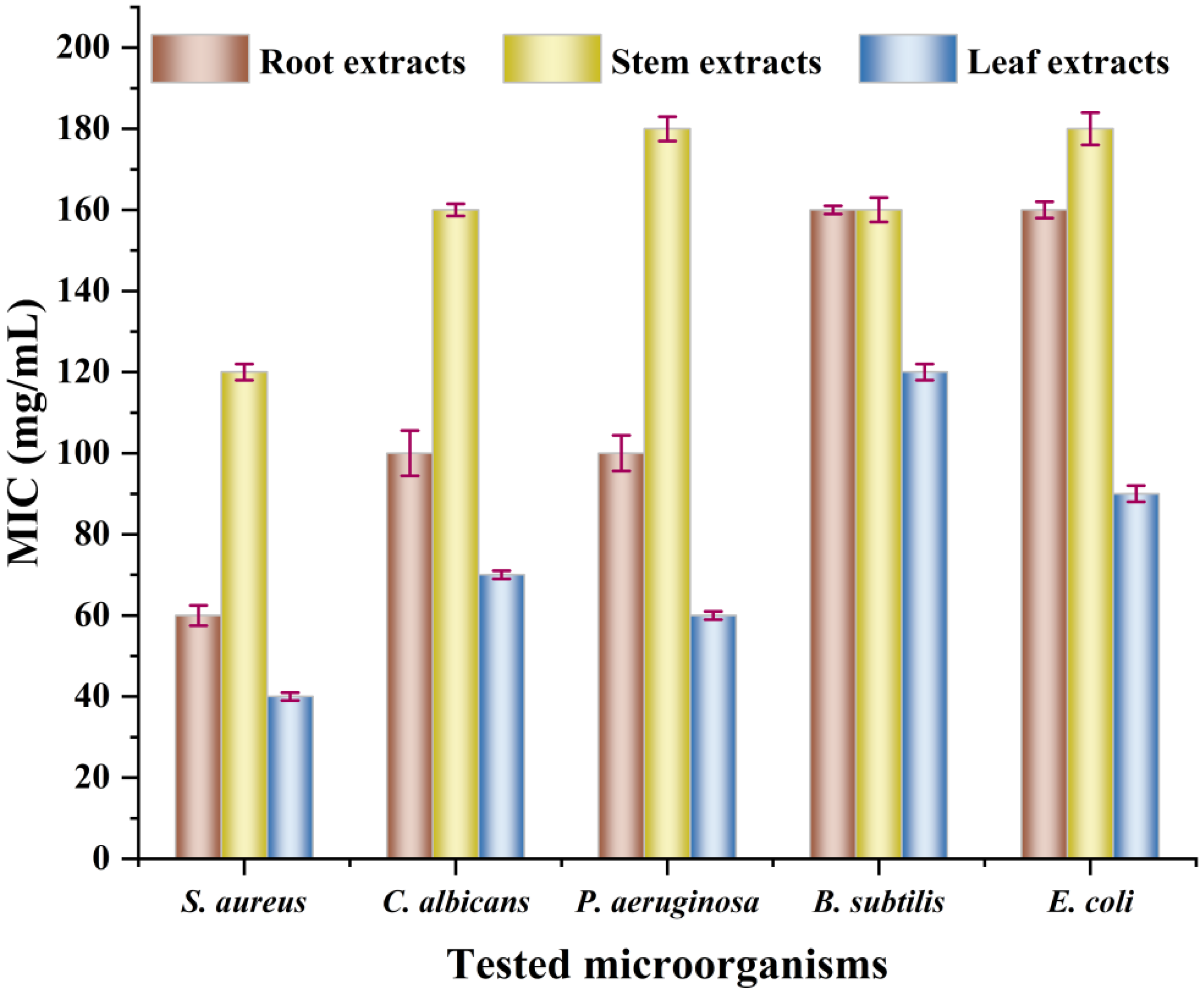
3.7.1. Antibacterial Activity of Extracts from Different Parts
3.7.2. Antibacterial Activity of Six Phenolic Acids
3.8. The Minimum Inhibitory Concentrations of Extract and Six Phenolic Acids
3.8.1. The Minimum Inhibitory Concentrations of Extracts from Different Parts
3.8.2. The Minimum Inhibitory Concentrations of Six Phenolic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rico, C.M.; Wagner, D.C.; Ofoegbu, P.C.; Kirwa, N.J.; Clubb, P.; Coates, K.; Zenobio, J.E.; Adeleye, A.S. Toxicity assessment of perfluorooctanesulfonic acid (PFOS) on a spontaneous plant, velvetleaf (Abutilon theophrasti), via metabolomics. Sci. Total Environ. 2024, 907, 167894. [Google Scholar] [PubMed]
- Sandhya, K.; Bhagavanth Reddy, G.; Ayodhya, D.; Venkatesh, B.; Noorjahan, M.; Girija Mangatayaru, K. Biogenic and hydrothermal synthesis, characterization, antioxidant and anticancer activities of PdNPs using Abutilon indicum leaf extract. Inorg. Chem. Commun. 2024, 166, 112629. [Google Scholar] [CrossRef]
- Tian, C.; Wang, H.; Guo, Y.; Qiu, P.; Cui, C.; Liu, M. Abutilon theophrasti medic. Episperms as a total flavonoids fraction for pharmaceutical applications: In vitro antioxidant, antibacterial, anti-inflammatory activities, extraction technology and HPLC-MS profiles. Ind. Crops Prod. 2019, 134, 100–106. [Google Scholar]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Biogenic silver nanoparticles from Abutilon indicum: Their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids Surf. B Biointerfaces 2015, 128, 276–286. [Google Scholar] [PubMed]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.-H.; Rajesh Banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications- A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Luo, F.; Zeng, F.; Zhao, Z.; Ren, X.; He, Y.; Yang, X.; Zhang, F.; Pang, H.; Shi, W. A novel green and efficient strategy for natural products by extraction with acidic electrolytic oxidation water-assisted deep eutectic solvent. ACS Sustain. Chem. Eng. 2025, 13, 9316–9330. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants-a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar]
- Hashempour, H.; Mohammadnejad, M.; Moharrami, S. Development of cold plasma pretreatment ultrasound-assisted extraction for enhancing phenolic acids extractability from Salvia sclarea. Microchem. J. 2024, 207, 112052. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, Y.; Chen, P.; Peng, P.; Liu, S.; Li, D.; Ruan, R. Effect of alkaline and high-pressure homogenization on the extraction of phenolic acids from potato peels. Innov. Food Sci. Emerg. Technol. 2016, 37, 91–97. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Z.; Jiang, H.; Wei, F.; Wu, Y.; Song, L. Recent advances in the health benefits of phenolic acids in whole grains and the impact of processing techniques on phenolic acids: A Comprehensive Review. J. Agric. Food Chem. 2024, 72, 24131–24157. [Google Scholar] [CrossRef]
- Sahu, S.; Kumari, D.; Kusam; Kuila, A.; Gurjar, R.S.; Sharma, K.; Verma, R. Deep eutectic solvent extraction of polyphenol from plant materials: Current status and future prospects in food applications. Food Chem. 2025, 482, 144125. [Google Scholar]
- Duan, S.; Kwon, S.J.; Jeong, D.Y.; Kim, J.H.; Park, Y.R.; Kim, C.K.; Kim, J.-H.; Eom, S.H. Antioxidant activities in Kenaf (Hibiscus cannabinus) shoots during growth stages and destination of chlorogenic acid and kaempferol glycosides. Antioxidants 2024, 13, 532. [Google Scholar] [CrossRef]
- Cao, S.; Liang, J.; Chen, M.; Xu, C.; Wang, X.; Qiu, L.; Zhao, X.; Hu, W. Comparative analysis of extraction technologies for plant extracts and absolutes. Front. Chem. 2025, 13, 1536590. [Google Scholar] [CrossRef]
- Liang, Y.F.; Wen, Q.; Yang, Z.F.; Li, C.Q.; Li, F.R. Optimization of ultrasound-assisted extraction of glycyrrhizic acid and glabridin from Glycyrrhiza glabra L. using response surface methodology and development of a green analytical method. Sustain. Chem. Pharm. 2024, 37, 101385. [Google Scholar] [CrossRef]
- Peng, W.; Wang, Y.; Wang, L.; Wang, W.; Huang, J.; Zhou, R.; Bo, R.; Liu, C.; Liu, M.; Li, J. Eco-friendly extraction of polysaccharides from Moringa oleifera Lam. leaves using COSMO-RS screened natural deep eutectic solvents. Food Res. Int. 2025, 217, 116859. [Google Scholar]
- Wijesekara, T.; Xu, B. A critical review on the stability of natural food pigments and stabilization techniques. Food Res. Int. 2024, 179, 114011. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Fang, J.; Song, Z.; Geng, J.; Zhao, J.; Fang, Y.; Wang, C.; Li, M. Green and efficient extraction of flavonoids from Perilla frutescens (L.) Britt. leaves based on natural deep eutectic solvents: Process optimization, component identification, and biological activity. Food Chem. 2024, 452, 139508. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, T.; Liu, L.; Tan, Z. CO2-responsive deep eutectic solvents for the enhanced extraction of hesperidin from Fertile orange peel. Food Chem. 2024, 432, 137255. [Google Scholar] [PubMed]
- Niu, L.; Zhang, S.; Si, X.; Fang, Y.; Wang, S.; Li, L.; Sheng, Z. Ultrasonic-assisted extraction of luteolin from peanut shells using ionic liquid and its molecular mechanism. Ultrason. Sonochemistry 2025, 113, 107228. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and modern techniques for bioactive compounds recovery from plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, H.; Li, Z.; Zhang, W.; Guo, L.; Zhang, Y.; Jiang, Y. Intelligent transformation of ultrasound-assisted novel solvent extraction plant active ingredients: Tools for machine learning and deep learning. Food Chem. 2025, 486, 144649. [Google Scholar] [CrossRef]
- Belokurov, S.S.; Narkevich, I.A.; Flisyuk, E.V.; Kaukhova, I.E.; Aroyan, M.V. Modern extraction methods for medicinal plant raw material (review). Pharm. Chem. J. 2019, 53, 559–563. [Google Scholar] [CrossRef]
- Nisoa, M.; Kaewpradit, S.; Nahar, L.; Sarker, S.D.; Charoensup, R.; Puttarak, P.; Yusakul, G. Extraction of curcumin and curcuminoids: From conventional methods to innovative extraction using deep eutectic solvents. Microchem. J. 2025, 215, 114269. [Google Scholar] [CrossRef]
- Musilová, J.; Franková, H.; Fedorková, S.; Lidiková, J.; Vollmannová, A.; Sulírová, K.; Árvay, J.; Kasal, P. Comparison of polyphenols, phenolic acids, and antioxidant activity in sweet potato (Ipomoea batatas L.) tubers after heat treatments. J. Agric. Food Res. 2024, 18, 101271. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Huang, B.; Zhuang, Y.; Hu, Y.; Fei, P. Pectin modified with phenolic acids: Evaluation of their emulsification properties, antioxidation activities, and antibacterial activities. Int. J. Biol. Macromol. 2021, 174, 485–493. [Google Scholar] [CrossRef]
- Yin, R.; Jiang, J.; Ma, X.; Xie, Y.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Cheng, W.; Gao, F. Dynamic changes of physicochemical parameters, antioxidant activity, organic acids, polyphenols, and volatile components in prune vinegar during fermentation. Food Biosci. 2024, 59, 104042. [Google Scholar] [CrossRef]
- Babaei-Rad, S.; Mumivand, H.; Mollaei, S.; Khadivi, A. Postharvest UV-B and UV-C treatments combined with fermentation enhance the quality characteristics of Capparis spinosa L. fruit, improving total phenols, flavonoids, anthocyanins, phenolic acids, and antioxidant activity. Food Chem. 2025, 483, 144306. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the Folin-Ciocalteu method for the estimation of (Poly) phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [PubMed]
- Donoso-Bustamante, V.; Osorio, E.; Arias-Santé, M.F.; De Camargo, A.C.; Rincón-Cervera, M.Á.; Amalraj, J.; Carrasco, B.; Palomo, I.; Araya-Maturana, R. Antioxidant activity of sinapic acid anilides: DPPH, ABTS, FRAP, electrochemical and theoretical analysis. LWT 2025, 222, 117656. [Google Scholar] [CrossRef]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Wang, L.; Mu, W.; Liu, Y.; Wang, X.; Zheng, X. Antibacterial properties of reduced graphene oxide fibers fabricated by hydrothermal method. J. Ind. Eng. Chem. 2025, 141, 297–304. [Google Scholar]
- Li, Y.; Mu, Y.; Cao, Y.; Xu, D.; Liu, X.; Xu, G. Synthesis and evaluation of novel 1-methyl-1h-pyrazol-5-amine derivatives with disulfide moieties as potential antimicrobial agents. J. Agric. Food Chem. 2024, 72, 20658–20669. [Google Scholar]
- Xu, R.; Chen, K.; Han, X.; Lou, Y.; Gu, S.; Gao, Y.; Shang, S.; Song, Z.; Song, J.; Li, J. Design and synthesis of antifungal candidates containing triazole scaffold from natural rosin against valsa mali for crop protection. J. Agric. Food Chem. 2023, 71, 9718–9727. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Fu, M.; Sun, X.; Fei, C.; Li, D.; Zhang, D.; Tuo, X.; Gao, S.; Han, X.; Xiu, J.; Wang, J.; et al. Optimization and characterization of pectin extracted from hawthorn by deep eutectic solvent. Int. J. Biol. Macromol. 2024, 256, 128688. [Google Scholar]
- Gong, J.; Tian, M.; Liu, Y.; Zhang, Y.; Xing, J.; Liang, Q.; Xie, X.; Li, C.; Zhao, C. Smart dual-responsive ionic liquid as transformable and recyclable media for extracting and separating phytochemicals from Wild Rumex acetosa L. ACS Sustain. Chem. Eng. 2025, 13, 251–266. [Google Scholar] [CrossRef]
- Cheng, J.; Tian, M.; Gul, Z.; Liang, C.; Qiao, B.; Gao, Y.; Zhao, C.; Li, C. pH-responsive functionalized surface active ionic liquid as an enhanced medium for efficient extraction and in-situ separation of flavonoids in Vitex negundo L. Leaves. Microchem. J. 2023, 193, 109080. [Google Scholar] [CrossRef]
- Nie, F.; Feng, C.; Ahmad, N.; Tian, M.; Liu, Q.; Wang, W.; Lin, Z.; Li, C.; Zhao, C. A new green alternative solvent for extracting echinacoside and acteoside from Cistanche deserticola based on ternary natural deep eutectic solvent. J. Ind. Eng. Chem. 2023, 118, 499–510. [Google Scholar] [CrossRef]
- Qin, L.; Yang, L.; Yang, J.; Weber, R.; Ranguelova, K.; Liu, X.; Lin, B.; Li, C.; Zheng, M.; Liu, G. Photoinduced formation of persistent free radicals, hydrogen radicals, and hydroxyl radicals from catechol on atmospheric particulate matter. iScience 2021, 24, 102193. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. Fe3+ reducing power as the most common assay for understanding the biological functions of Antioxidants. Processes 2025, 13, 1296. [Google Scholar] [CrossRef]
- Hao, J.W.; Wang, W.T.; Chen, N.D.; Shen, Y. Identification of 13 natural antioxidants in green calyx plum using AAPH, ABTS, and FRAP-coupled HPLC-DAD via QTOF-MS/MS. Food Chem. 2025, 477, 143568. [Google Scholar]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT 2021, 146, 111411. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Faúndez, X.; Báez, M.E.; Martínez, J.; Zúñiga-López, M.C.; Espinoza, J.; Fuentes, E. Evaluation of the generation of reactive oxygen species and antibacterial activity of honey as a function of its phenolic and mineral composition. Food Chem. 2023, 426, 136561. [Google Scholar] [CrossRef]





| NO. | Factor | Y (μg/g) | ||
|---|---|---|---|---|
| X1 (mL/g) | X2 (min) | X3 (W) | ||
| 1 | 30 | 25 | 250 | 193.71 |
| 2 | 35 | 30 | 150 | 196.08 |
| 3 | 25 | 35 | 200 | 191.54 |
| 4 | 30 | 30 | 200 | 211.96 |
| 5 | 30 | 30 | 200 | 213.29 |
| 6 | 30 | 30 | 200 | 215.96 |
| 7 | 30 | 30 | 200 | 217.29 |
| 8 | 30 | 25 | 150 | 186.66 |
| 9 | 30 | 35 | 150 | 187.62 |
| 10 | 35 | 30 | 250 | 199.97 |
| 11 | 25 | 30 | 150 | 189.47 |
| 12 | 30 | 35 | 250 | 191.74 |
| 13 | 35 | 25 | 200 | 198.29 |
| 14 | 35 | 35 | 200 | 196.59 |
| 15 | 25 | 30 | 250 | 196.82 |
| 16 | 25 | 25 | 200 | 193.84 |
| 17 | 30 | 30 | 200 | 215.96 |
| Source | Sum of Squares | F-Value | p-Value |
|---|---|---|---|
| Model | 1767.44 | 100.19 | <0.0001 |
| X1 | 32.68 | 16.67 | 0.0047 |
| X2 | 23.29 | 11.88 | 0.0107 |
| X3 | 70.45 | 35.94 | 0.0005 |
| X1X2 | 3.74 | 1.91 | 0.2094 |
| X1X3 | 0.0529 | 0.027 | 0.8742 |
| X2X3 | 0.25 | 0.1275 | 0.7315 |
| X12 | 364.11 | 185.75 | <0.0001 |
| X22 | 305.95 | 156.08 | <0.0001 |
| X32 | 804.96 | 410.65 | <0.0001 |
| Residual | 13.72 | ||
| Lack of fit | 4.46 | 0.643 | 0.6266 |
| R2 | 0.9923 | ||
| Adjusted-R2 | 0.9824 |
| Diameter (mm) | Concentration (mg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 50 | 25 | 10 | 5 | 2.5 | 1 | 0.5 | 0.25 | 0 | ||
| Root | St | 15.0 | 12.4 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Es | 10.8 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Bs | 12.2 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Ca | 11.8 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Pa | 12.3 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Stem | St | 13.6 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Es | 10.9 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Bs | 11.1 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Ca | 11.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Pa | 10.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Leaf | St | 16.8 | 14.6 | 11.2 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Es | 12.3 | 10.8 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Bs | 15.8 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Ca | 14.1 | 11.2 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Pa | 14.7 | 11.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Diameter (mm) | Concentration (mg/mL) | |||||
|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 10 | 5 | ||
| GA | St | 25.0 | 14.5 | 9.2 | 9.0 | 9.0 |
| Es | 13.3 | 11.4 | 9.0 | 9.0 | 9.0 | |
| Bs | 20.5 | 13.5 | 11.2 | 9.4 | 9.0 | |
| Ca | 22.8 | 19.6 | 15.2 | 10.1 | 9.0 | |
| Pa | 19.9 | 12.9 | 9.0 | 9.0 | 9.0 | |
| PA | St | 23.2 | 13.3 | 9.0 | 9.0 | 9.0 |
| Es | 16.9 | 13.5 | 10.5 | 9.0 | 9.0 | |
| Bs | 21.0 | 12.7 | 9.9 | 9.0 | 9.0 | |
| Ca | 22.1 | 20.2 | 9.0 | 9.0 | 9.0 | |
| Pa | 17.5 | 15.6 | 9.0 | 9.0 | 9.0 | |
| CA | St | 12.5 | 10.2 | 9.0 | 9.0 | 9.0 |
| Es | 10.2 | 9.9 | 9.0 | 9.0 | 9.0 | |
| Bs | 18.8 | 13.3 | 9.0 | 9.0 | 9.0 | |
| Ca | 17.7 | 12.0 | 9.0 | 9.0 | 9.0 | |
| Pa | 12.7 | 10.2 | 9.0 | 9.0 | 9.0 | |
| VA | St | 17.6 | 16.0 | 15.4 | 14.5 | 11.6 |
| Es | 12.0 | 11.2 | 10.7 | 9.6 | 9.0 | |
| Bs | 13.3 | 12.1 | 11.4 | 10.5 | 9.0 | |
| Ca | 16.6 | 15.9 | 14.8 | 9.0 | 9.0 | |
| Pa | 15.6 | 14.2 | 13.9 | 12.1 | 9.0 | |
| SA | St | 16.8 | 15.1 | 13.5 | 11.2 | 9.0 |
| Es | 10.8 | 9.7 | 9.0 | 9.0 | 9.0 | |
| Bs | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Ca | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Pa | 11.2 | 9.7 | 9.0 | 9.0 | 9.0 | |
| PHCA | St | 14.6 | 12.3 | 9.0 | 9.0 | 9.0 |
| Es | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | |
| Bs | 15.3 | 14.0 | 11.4 | 9.0 | 9.0 | |
| Ca | 11.1 | 10.5 | 10.3 | 9.0 | 9.0 | |
| Pa | 15.9 | 13.2 | 11.2 | 9.0 | 9.0 | |
| Sample | Dsample (mm) | Dcontrol (mm) | Ddisk (mm) | RIA (%) | |
|---|---|---|---|---|---|
| GA | St | 14.5 | 20.4 | 9.0 | 48.2 |
| Es | 11.4 | 24.8 | 9.0 | 15.2 | |
| Bs | 13.5 | 30.6 | 9.0 | 20.8 | |
| Ca | 19.6 | 26.5 | 9.0 | 60.6 | |
| Pa | 12.9 | 26.4 | 9.0 | 22.4 | |
| PA | St | 13.3 | 20.4 | 9.0 | 37.7 |
| Es | 13.5 | 24.8 | 9.0 | 28.5 | |
| Bs | 12.7 | 30.6 | 9.0 | 17.4 | |
| Ca | 20.2 | 26.5 | 9.0 | 64.0 | |
| Pa | 15.6 | 26.4 | 9.0 | 38.0 | |
| CA | St | 10.2 | 20.4 | 9.0 | 10.5 |
| Es | 9.9 | 24.8 | 9.0 | 5.7 | |
| Bs | 13.3 | 30.6 | 9.0 | 19.9 | |
| Ca | 12.0 | 26.5 | 9.0 | 17.1 | |
| Pa | 10.2 | 26.4 | 9.0 | 6.9 | |
| VA | St | 16.0 | 20.4 | 9.0 | 61.4 |
| Es | 11.2 | 24.8 | 9.0 | 13.9 | |
| Bs | 12.1 | 30.6 | 9.0 | 14.9 | |
| Ca | 15.9 | 26.5 | 9.0 | 39.4 | |
| Pa | 14.2 | 26.4 | 9.0 | 29.9 | |
| SA | St | 15.1 | 20.4 | 9.0 | 53.3 |
| Es | 9.7 | 24.8 | 9.0 | 4.4 | |
| Bs | 9.0 | 30.6 | 9.0 | 0.0 | |
| Ca | 9.0 | 26.5 | 9.0 | 0.0 | |
| Pa | 9.7 | 26.4 | 9.0 | 4.0 | |
| PHCA | St | 12.3 | 20.4 | 9.0 | 29.5 |
| Es | 9.0 | 24.8 | 9.0 | 0.0 | |
| Bs | 14.0 | 30.6 | 9.0 | 23.2 | |
| Ca | 10.5 | 26.5 | 9.0 | 8.6 | |
| Pa | 13.2 | 26.4 | 9.0 | 24.1 | |
| MIC (mg/mL) | |||||
|---|---|---|---|---|---|
| S. aureus | C. albicans | P. aeruginosa | B. subtilis | E. coli | |
| GA | 15 | 12.5 | 30 | 7.5 | 35 |
| PA | 30 | 30 | 35 | 20 | 15 |
| CA | 45 | 45 | 35 | 45 | 45 |
| VA | 2.5 | 12.5 | 7.5 | 7.5 | 7.5 |
| SA | 7.5 | - | 45 | - | 45 |
| PHCA | 40 | 35 | 15 | 20 | - |
| PC | 0.125 | 0.125 | 0.5 | 0.25 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Zhao, W.; Liu, J.; Liang, Q.; Chen, K.; Lin, Q.; Yang, Y.; Zhao, C.; Li, C. Exploration on the Extraction of Phenolic Acid from Abutilon theophrasti and Antioxidant and Antibacterial Activities. Separations 2025, 12, 288. https://doi.org/10.3390/separations12110288
Xie X, Zhao W, Liu J, Liang Q, Chen K, Lin Q, Yang Y, Zhao C, Li C. Exploration on the Extraction of Phenolic Acid from Abutilon theophrasti and Antioxidant and Antibacterial Activities. Separations. 2025; 12(11):288. https://doi.org/10.3390/separations12110288
Chicago/Turabian StyleXie, Xiaofei, Wenyan Zhao, Jiaying Liu, Qi Liang, Kuiwang Chen, Quanyu Lin, Ying Yang, Chunjian Zhao, and Chunying Li. 2025. "Exploration on the Extraction of Phenolic Acid from Abutilon theophrasti and Antioxidant and Antibacterial Activities" Separations 12, no. 11: 288. https://doi.org/10.3390/separations12110288
APA StyleXie, X., Zhao, W., Liu, J., Liang, Q., Chen, K., Lin, Q., Yang, Y., Zhao, C., & Li, C. (2025). Exploration on the Extraction of Phenolic Acid from Abutilon theophrasti and Antioxidant and Antibacterial Activities. Separations, 12(11), 288. https://doi.org/10.3390/separations12110288








