1. Introduction
As a colourless, highly toxic, and flammable gas, phosphine (PH
3) has become one of the important directions of industrial gas research in recent years as its application in chemical, semiconductor, and environmental protection fields has gradually expanded. In the semiconductor field, the research and application of high-purity PH
3 are quite extensive. High-purity PH
3 is the main phosphorus source for N-type doping, and after high-temperature decomposition, phosphorus atoms can be doped and diffused into the silicon lattice [
1] to regulate the electrical conductivity of silicon. The high purity of PH
3 can also be used for the synthesis of phosphorus-based thin films via chemical vapor deposition (CVD) and atomic layer deposition (ALD) [
2,
3,
4], as an alternative to the traditional POCl
3 for reducing pollution. As semiconductor nodes micronise to below 7 nm, trace impurities in PH
3 are likely to lead to threshold voltage drift, resulting in junction leakage, and thus the demand for PH
3 purity (typically ≥99.9999%) is increasing [
5,
6]. High-purity PH
3 has gradually become an indispensable material in semiconductor fabrication, and its purity directly affects device performance and exergy.
Currently, PH
3 is mainly produced using chemical synthesis and hydrolysis of metal phosphides. In traditional processes, PH
3 is prepared by the reaction of calcium phosphate with water [
7], but the reaction process produces excessive impure gases, which lead to the difficulty of purification in the subsequent production of high-purity PH
3. Hydrolysis of metal phosphides mainly uses aluminium phosphide or zinc phosphide and water reaction to obtain pure PH
3, which can only be used in the production of small-scale high-purity PH
3 due to the high cost of raw materials and as they are difficult to obtain. Furthermore, it is difficult to put into practical application. Accordingly, our research focus is on the preparation of PH
3 by the simpler acidic and alkali methods. For the acidic method, phosphoric acid is used as a catalyst and a reusable reaction solution, while red phosphorus is used as the sole raw material to produce highly effective and high-purity phosphine, which is beneficial for subsequent high-purity processing. The alkaline method uses long-chain alcohols as dispersants to react P
4 with NaOH to obtain PH
3. Due to the simple reaction conditions and mild reaction process, this method has been applied in practical production for more than 50 years.
As a core foundational material in high-end manufacturing sectors (semiconductors, artificial intelligence, photovoltaics, etc.), the efficiency and energy consumption of specialty gas purification processes directly determine the technological upper limits of downstream industries [
8,
9]. The primary methods for purifying specialty gas include adsorption, distillation, and membrane separation [
10,
11]. In order to meet the demand of the semiconductor industry for electronic-grade, high-purity acetylene [
7], it is necessary to purify industrial acetylene through adsorption and desorption experiments, which are relatively simple to operate and low cost. But the adsorbent has a limited adsorption capacity and needs to be replaced or regenerated periodically. The current main research focus is thus concentrated on the design and study of adsorption catalysts. Xia et al. developed a Zr-PMA framework that achieved a purity of over 5N for C
3H
8 at room temperature through a “pore size–electrostatic potential matching” design [
12]. Cai et al. [
13] reported a flexible metal–organic framework (Ca-TCPb) with a temperature-controlled gating mechanism, which was able to effectively separate C
3F
8 and C
3F
6, and which could directly separate C
3F
8 with a purity of over 99.999%, which is of great significance for the electronics industry [
14,
15]. For phosphorus-containing specialty gases (e.g., PH
3), the challenge lies in separating impurities (such as AsH
3, H
2, N
2) that share similar physicochemical properties with PH
3, are highly toxic, and present greater separation difficulties. Existing technologies lack highly selective adsorbents specifically designed for the PH
3 molecular structure, and traditional purification processes (e.g., cryogenic adsorption, chemical absorption) struggle to simultaneously meet 6N-grade purity requirements and industrial energy consumption standards. Membrane separation technology is constrained by material selectivity and corrosion resistance [
16]. Prolonged use may lead to performance degradation of the separation membrane due to erosion by the separated substances. Distillation is a process that separates different components in a mixture by taking advantage of the differences in boiling points of various substances. It involves multiple evaporation and condensation steps to enhance the purity of the gas. Choosing an appropriate purification method can effectively improve the purity and exergy efficiency of ultra-high-purity special gases. When dealing with the separation of large amounts of gases, distillation, with its low cost and high efficiency, can better meet practical applications. Also, considering that PH
3 is a toxic flammable gas, when obtaining high-purity PH
3 with a purity greater than 6N, low-temperature distillation for separation and purification is recommended.
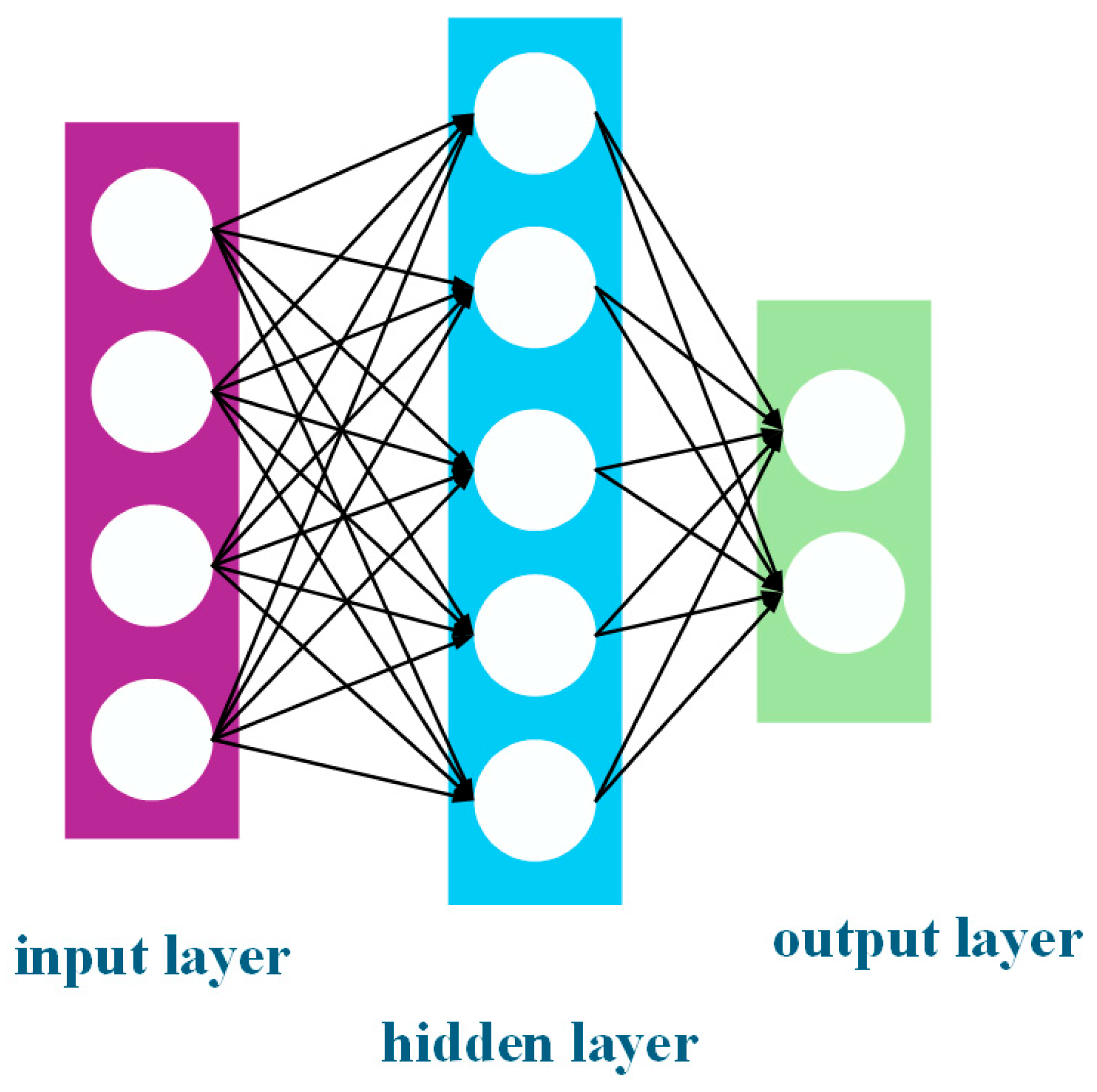
In the context of sustainable development and green manufacturing, the design of the separation process needs to take into account product quality, energy consumption control, and environmental impact. The traditional design methods that rely on experience or simplified models are no longer sufficient to meet the requirements of precise optimisation, and gradient algorithms are prone to becoming stuck in local optima [
17]. Therefore, an integrated simulation and intelligent optimisation hybrid platform has become a breakthrough direction. Song et al. [
18] built an ActiveX interface between Aspen HYSYS and MATLAB, and they used the genetic algorithm (GA) and NSGA-II to achieve single-objective and multi-objective optimisation, respectively, reducing the energy consumption of the nitrogen expansion liquefaction process by 15.2% and increasing the liquefaction rate to 0.81. Similarly, Nishiyama et al. [
19] used the MEIGO plugin of Aspen Plus and MATLAB to optimise the glycerol ketalisation process, reducing the reactor volume to 24.17 m
3 at a 80% conversion rate. In the study of the separation process, algorithms can also demonstrate unique advantages: the GA is good at global optimisation of single objectives, while NSGA-II achieves coordinated optimisation of energy consumption and yield through Pareto sorting [
20]; meanwhile, MEIGO can efficiently handle constrained optimisation problems of black-box models [
19]. Economic analysis shows that multi-objective optimisation results have more advantages in analysing energy and investment payback periods [
16,
21]. By optimising energy losses in the separation process through exergy analysis, the application value of simulation–optimisation integration technology in complex chemical systems can be clearly illustrated, providing methodological support for energy-efficient utilisation and a resource circular economy. However, in the field of gas purification, there is currently no research on the purification of high-purity phosphine by analysing algorithms. Therefore, the two processes of producing high-purity PH
3 using acidic and alkali methods will be simulated separately, and a comprehensive comparison will be made from the perspective of exergy efficiency. Aspen Plus is a widely used chemical process simulation software. For gas/liquid systems, Aspen Plus includes mixers, separators, flash modules, heat exchangers, reactors, pumps, and compressors for various types of multi-stage gas–liquid separation operations [
22]. It can directly simulate core separation units in purification processes and output key metrics such as energy consumption and component recovery rates through its results analysis module [
18,
23]. Therefore, in this paper, we used the Aspen Plus V14 software to analyse and optimise the costs and energy required in the process flow, and we utilised the MATLAB 2023 software to conduct an optimisation study on various equipment parameters that affect the exergy efficiency of the process emissions. We obtained the optimal solution for the entire process. Our research provide new ideas for the field of high-purity gas production and offer a reference for subsequent high-purity gas production work. The work in this paper has guiding significance for the exergy efficiency and process optimisation of high-purity gases.
2. Feed Parameters and Process Assumptions
In the acidic process, we used phosphoric acid as a catalyst to generate PH
3 by disproportionation reaction between yellow phosphorus and water at 280 °C, and we carried out subsequent distillation to obtain high-purity phosphine; the specific feed parameters are shown in
Table 1. The alkaline process uses sodium hydroxide to react with molten P
4, and the by-product method obtains PH
3, followed by distillation and purification operations. The feed parameters are shown in
Table 2. However, the alkaline process will produce the by-product H
2 to affect the purity of the overall PH
3, so it should be treated in the subsequent purification. The specific reactions are as follows:
Acidic process primary reaction:
Alkaline process primary reaction:
Since the alkaline process produces a large amount of NaH
2PO
2 and Na
2HPO
3 with low commercial value, it is difficult to subsequently recycle them. Due to the low-valence phosphorus forms of NaH
2PO
2 and Na
2HPO
3, we consider producing PH
3 through secondary utilisation. After separating NaH
2PO
2 and Na
2HPO
3 by adding phosphoric acid to the distillate, NaH
2PO
2 and Na
2HPO
3 can be added to the distillate. Then, PH
3 is produced by thermal decomposition of NaH
2PO
2 and Na
2HPO
3 in the temperature range of 190–310 °C and then distilled into a purification distillation tower to obtain high-purity PH
3. The secondary pyrolysis of by-products can greatly improve the overall exergy and utilisation of PH
3 in the alkaline process. The specific reactions are as follows:
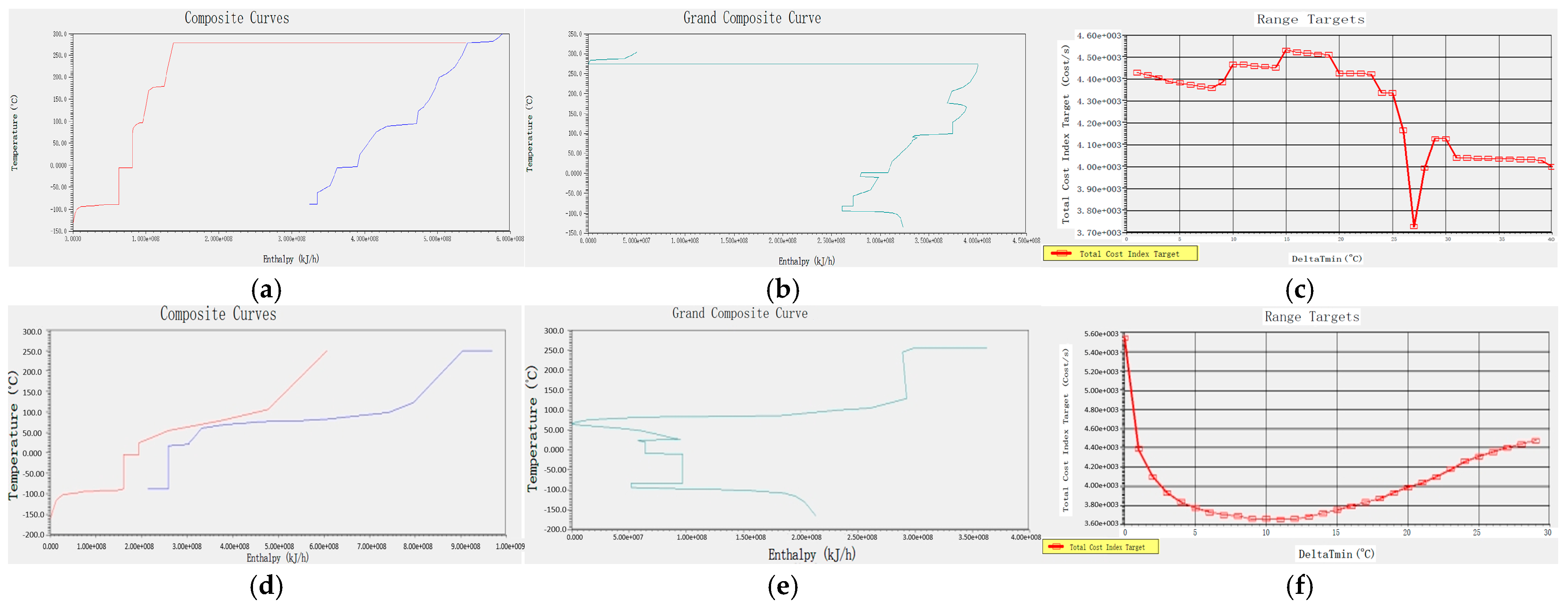
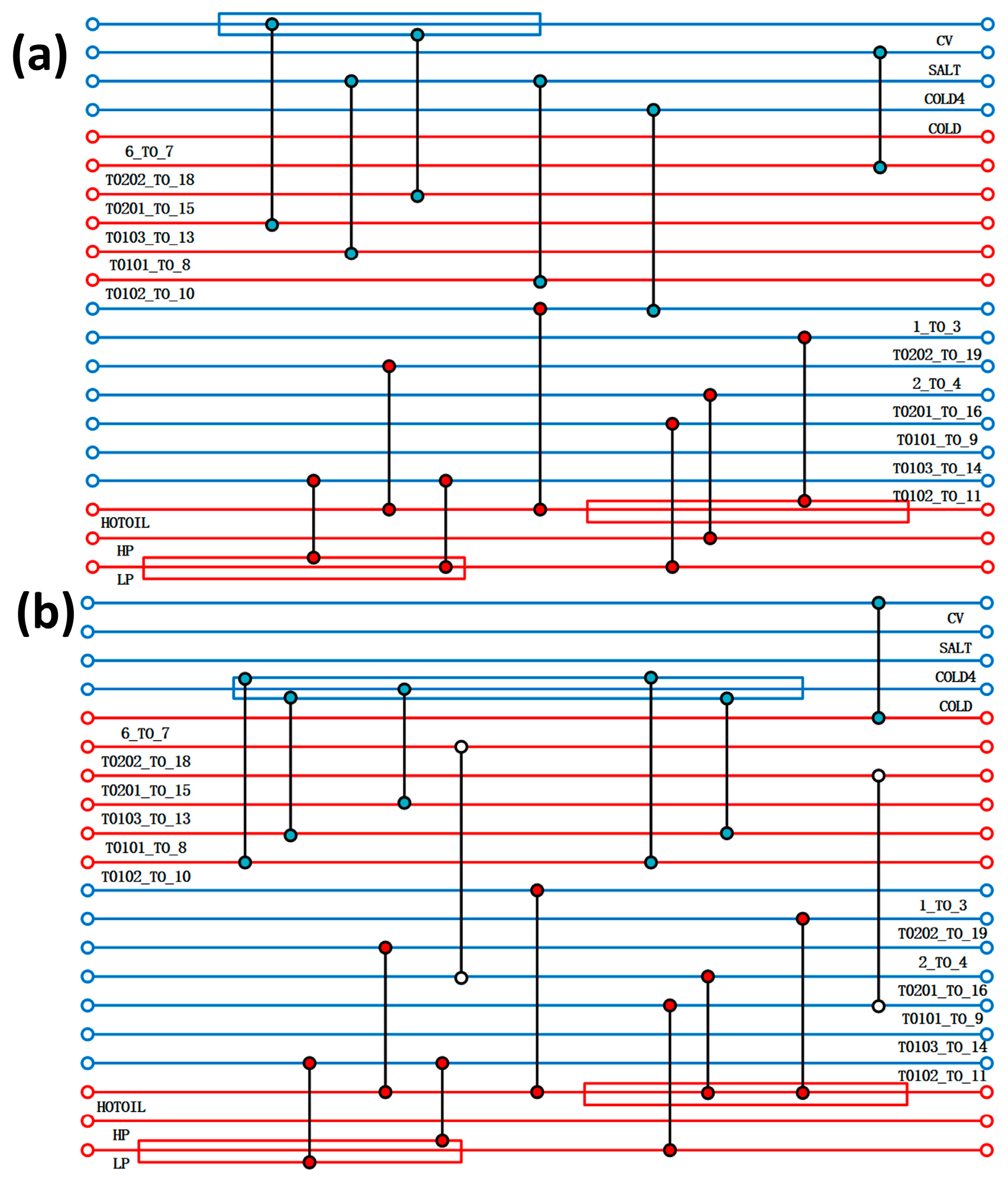
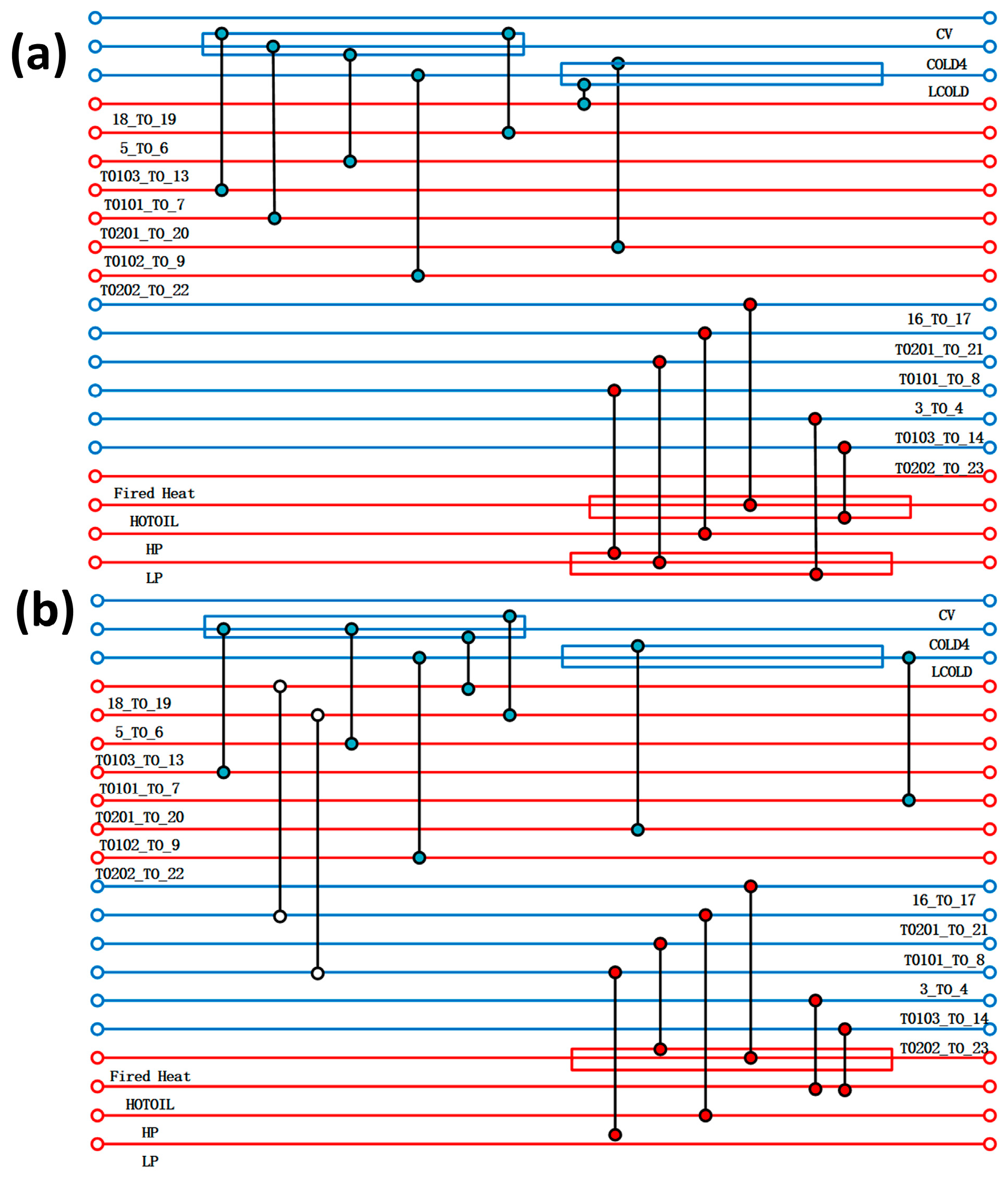
Based on a typical high-purity PH3 production device, the initial reaction pressure is set to 0.1 MPa, the temperature is set to 25 °C, and the impure gas content is within the normal production range. Before simulating the entire reaction process, the following assumptions should be made: (1) simplify the analysis process and ignore the product compression, drying, and transportation processes; and (2) the system operates in a steady state, ignoring the pressure drop in the tower and pipeline. The heat transfer process remains adiabatic, without considering the heat dissipation of the equipment. The pressure and heat losses of the pump, pressure-reducing valve, and heat exchanger are ignored. This paper employs the Electrolyte-NRTL (ELECNRTL) model to describe the aforementioned process system. The Aspen Plus V14 software is used to simulate the thermodynamics and kinetics of acidic and alkaline processes, as well as to conduct utility simulation and energy/cost analysis. Additionally, the Aspen Energy Analysis V14 software within Aspen Plus V14 is utilised to describe the complex chemical and thermodynamic characteristics of acidic and alkaline processes, and to conduct subsequent heat exchange network simulation and optimisation processes.
3. Exergy Consumption Estimation and Analysis Methods
According to the second law of thermodynamics, we consider the overall effective exergy efficiency of the reaction as an important criterion for evaluation. The total exergy can be divided into four parts, and since the changes in velocity and height in the model can be ignored, only the calculation of physical and chemical exergy is considered here [
6]. Before carrying out the calculation of the value of the exergy, the baseline environmental state should be determined firstly, and the more commonly applied models are the Japanese Kameyama–Yoshida model and the Polish Szargut model [
5], which is adopted for the baseline environmental model in this paper due to the wider application of the Kameyama–Yoshida environmental model in Japan [
23]. The ambient temperature was set to 298.15 K and the ambient pressure was set to 101.3 kPa. The standard molar chemical hydrazones are shown in
Tables S1 and S2. The equations for physical and chemical exergy are as follows:
where
EPHY is the physical exergy, kW;
h0 is the enthalpy of the stream in the reference state (
T0,
P0), kJ;
s0 is the entropy in the reference state, kJ/K;
h is the enthalpy of the stream strand, kJ;
s is the entropy of the stream strand, kJ/K; and
F is the flow rate of the stream strand, kg/s.
ECHEM is the chemical heat, kW, and
Ei,CH is the standard mole fraction of the stream strand for component i, kJ/kmol.
R is the gas constant,
R = 8.314 J/(mol·K).
xi is the molar fraction of component i in the stream strand.
T is the temperature, K, and
Fmol represents the molar flow rate, kmol/s. The calculation of the equilibrium equations of the exergy balance and the calculation of the exergy efficiencies involved in an acidic process and an alkaline process are shown below:
where
Ein denotes the exergy entering the system, kJ;
Eout denotes the exergy leaving the system, kJ;
Ir denotes the exergy loss and also the exergy degradation caused by process dissipation, kJ;
W is the net output work of the system or equipment, kJ; and
ηgen denotes the exergy efficiency. Therefore, based on the simulation results, two processes for the production of high-purity PH
3 are analysed in terms of exergy and are compared and discussed in detail.
4. Process Simulation
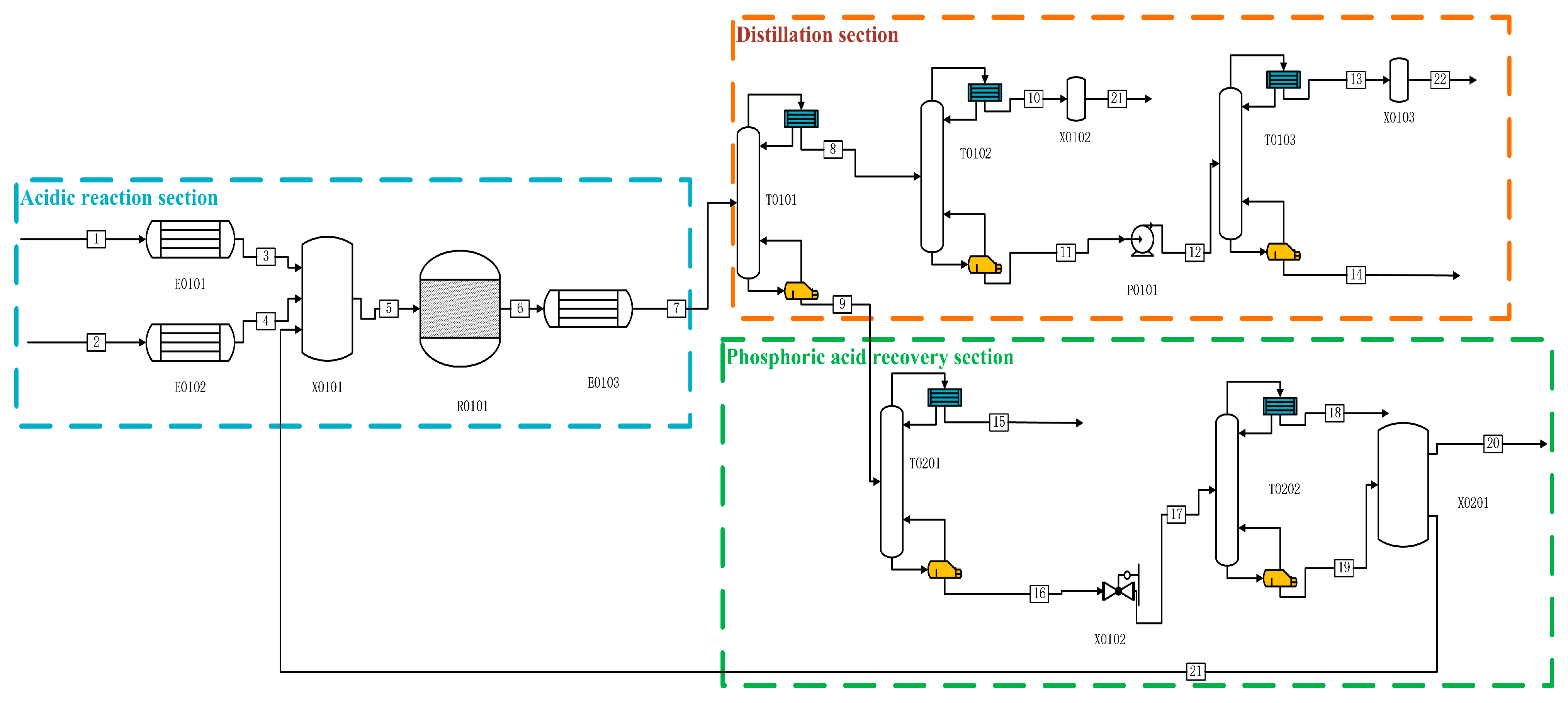
The Aspen simulations in both production processes are shown in
Figure 1 and
Figure 2. In the acidic process in
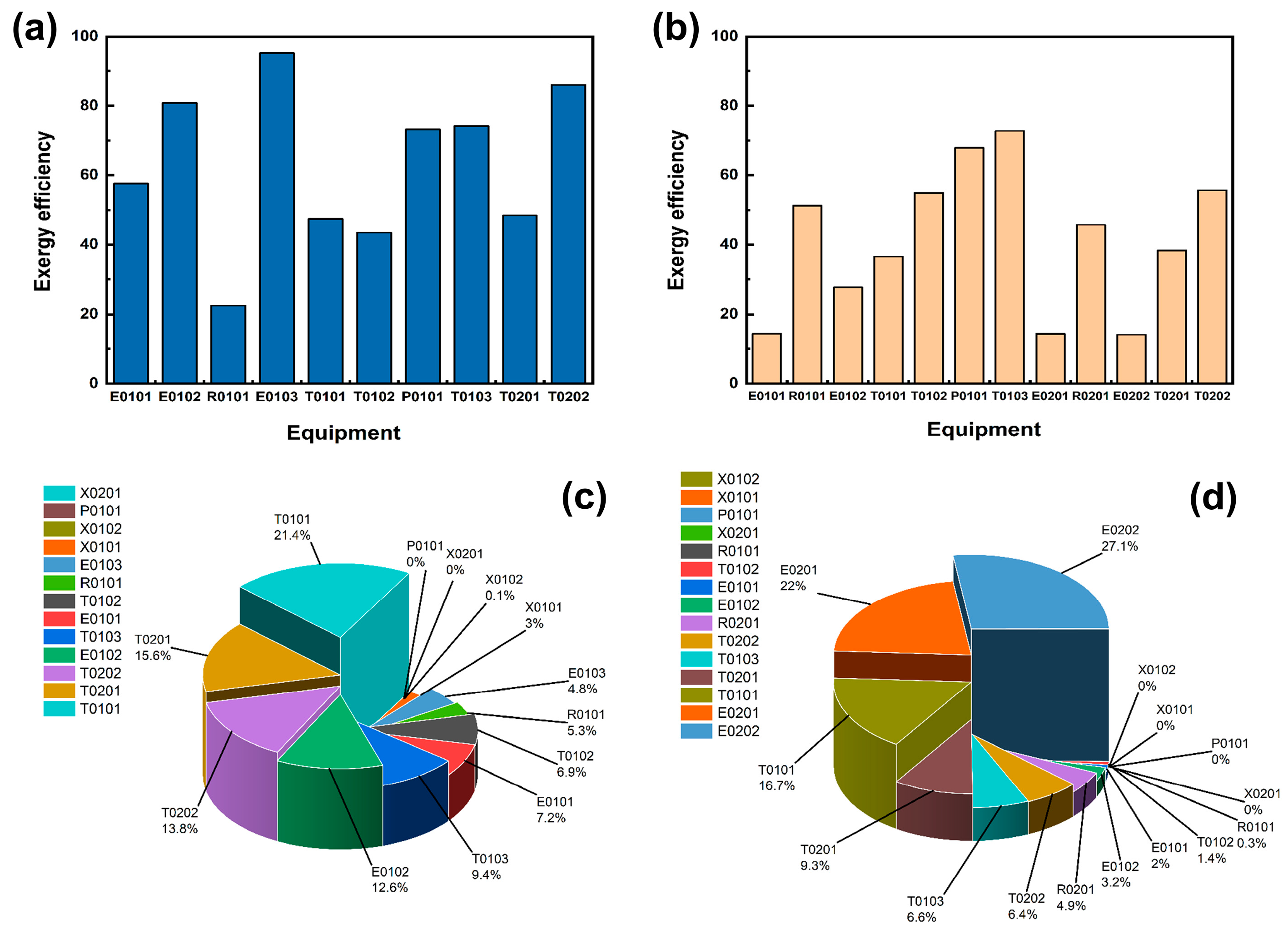
Figure 1, P is fed from the one-flow stream and preheated to 280 °C by E0101, and H
2O, as well as catalyst H
3PO
4 and other impure gases, are fed from the two-flow stream, which is heated to 280 °C by the heat exchanger E0102, and then mixed into the reactor R0101 for reaction. After the reaction is complete, the product first enters into E0103 for preheating to 70 °C. After that, it enters into the primary distillation column T0101 for heating. After entering the primary distillation tower T0101, the reaction production of PH
3 and other impure gases, entering the T0102 for the second distillation, distillation of the kettle liquid through P0101 into the final purification tower for the deep-cooled distillation, and distillation of the top of the tower (mass fraction ≥ 99.9999%) of high-purity phosphine, the exergy was 87.84%. After the reaction, the remaining P, H
2O, H
3PO
4, etc., are distilled from the tower kettle liquid of T0101 and enter T0201 for separation. Other impure gases are separated from the top of the tower. The tower kettle distillate flows into the final phosphoric acid separation tower T0202 through a pressure-reducing valve, where P and most of the water are distilled from the top of the tower. The ultra-high-purity H
3PO
4 distilled from the tower kettle is partially refluxed to the initial mixer X0101 for circulation, and most of the H
3PO
4 is distilled as a by-product.
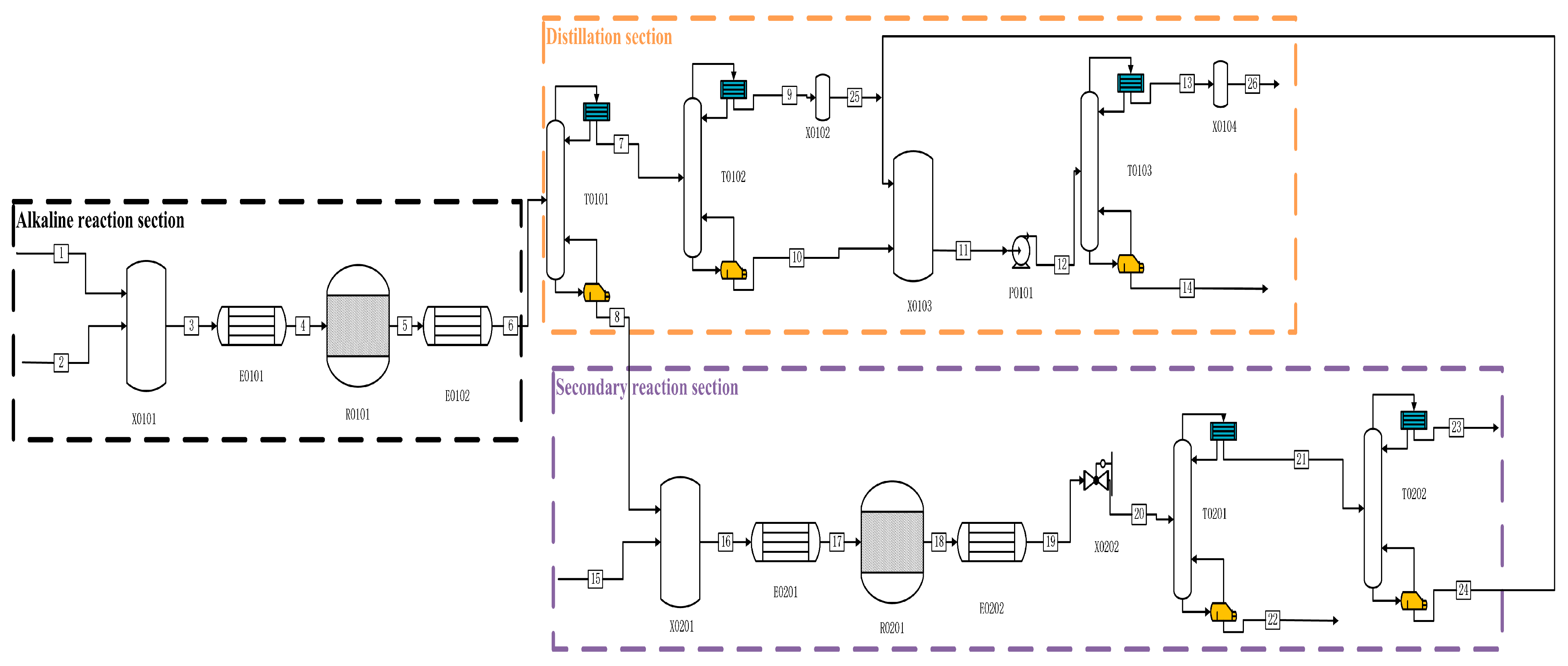
In the alkaline process shown in
Figure 2, P and C
2H
5OH enter from one stream, while NaOH aqueous solution and other impure gases enter the mixer X0101 together from two streams and one stream. Firstly, they are preheated to 60 °C by E0101 and then enter the reactor R0101 for reaction. After the reaction is complete, we preheat them to 25 °C using E0102. The whole solution enters the T0101 primary distillation tower from flow chain 6 for separation, PH
3 and other impurities enter T0102 for secondary distillation, and the kettle distillate is pressurised through P0101 and enters T0103 for final purification distillation. A high-purity PH
3 mass fraction of ≥99.9999% is distilled at the top of the tower. In the T0101 tower kettle liquid, the aqueous solution of NaOH and the resulting NaH
2PO
2 and Na
2HPO
3, as well as ethanol and H
3PO
4, are mixed and preheat exchanged to 250 °C through E0201 before entering reactor R0201 for pyrolysis. After completion, they enter E0202 for heat exchange to room temperature, and then they pass through the initial distillation tower to distil the produced PH
3 from the top of the tower. After that, they enter T0202 for pre-purification to about 99.8%, and then they flow into mixer X0102 to follow the final purification work of the tower kettle distillate from T0102, to obtain ultra-high-purity PH
3 with a yield of 93.62%.
The thermodynamic and kinetic modelling of the acidic and alkaline processes was carried out using Aspen Plus V14 software, and the Electrolyte-NRTL model was applied to describe the above process systems, with utility simulations and exergy and cost analyses also carried out in the software. In addition, the Aspen Energy Analyzer V14 software in Aspen Plus was used to describe the complex chemical and thermodynamic properties of the acidic and alkaline processes, and also for the subsequent simulation of the heat transfer network and optimisation process.
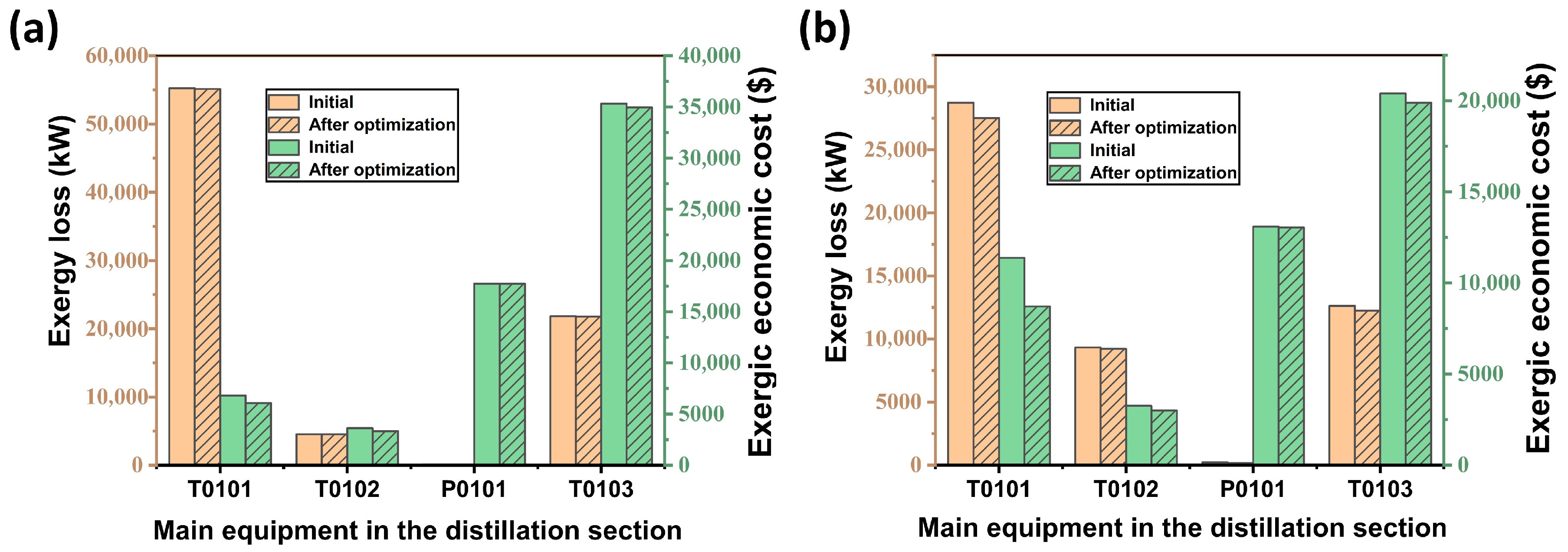
6. Conclusions
To the best of our knowledge, no prior study has conducted a comprehensive comparison of acidic and alkaline routes for ultra-high-purity PH3 production, integrating Aspen Plus V14 simulation with exergy-based multi-objective optimisation. Therefore, process flow models were established for the two routes, respectively, for simulation analysis, and the exergy losses and efficiency generated by these models were studied in combination with the NSGA-II algorithm. By establishing acidic and alkaline processes, it was found that acidic processes have greater advantages in heat transfer optimisation. They can produce high-purity PH3 with efficient equipment and recover phosphoric acid as a high-purity by-product, resulting in higher total effective energy. The NaH2PO2 and Na2HPO4 produced by the alkaline process are difficult to recycle, but by increasing phosphoric acid, the effective energy is improved to a certain extent. However, the overall increase in feed volume will still affect the overall cost. From the perspective of heat exchange, alkaline processes require higher heat exchange than acidic processes, but alkaline processes have more advantages in equipment manufacturing costs. In terms of energy loss, the equipment efficiency of acidic processes is usually higher than that of alkaline processes, but the energy loss of both processes needs to be reduced through subsequent sensitivity analysis and design to achieve optimal utilisation. Furthermore, from the perspectives of environment and safety, the use of the distillation method is simple and feasible, which is conducive to achieving large-scale production operations. Moreover, after energy is optimised through heat exchange, it can be fully utilised, providing a strong guarantee for actual production. Therefore, in the production of high-purity PH3, acidic processes have significant advantages, while alkaline processes can be considered a better choice when large quantities of PH3 production are required and corrosion-resistant equipment is available.