1. Introduction
The increasing global demand for clean water, coupled with the intensification of industrial activities, has resulted in the accumulation of complex pollutants in wastewater streams. Traditional treatment methods, while effective to a certain degree, often fail to address emerging contaminants such as pharmaceuticals, dyes, heavy metals, and pathogens. In this context, membrane-based separation technologies have emerged as a promising alternative due to their high efficiency, small footprint, and ability to selectively remove a broad spectrum of pollutants. Among these, polymeric membranes have gained significant attention due to their flexibility, cost-effectiveness, and ease of fabrication. However, limitations such as membrane fouling, low permeability, and reduced selectivity still hinder their full-scale application. To overcome these challenges, recent research has focused on incorporating nanoparticles into polymeric membranes, leading to the development of nanocomposite membranes with enhanced physicochemical and functional properties [
1,
2,
3,
4,
5].
Nanoparticles, due to their high surface area-to-volume ratio and tunable surface chemistry, play a critical role in improving membrane performance. Various types of nanoparticles—including metal oxides (e.g., TiO2, ZnO, Fe3O4), carbon-based nanomaterials (e.g., graphene oxide, carbon nanotubes), and nanoclays—have been successfully embedded into polymer matrices to fabricate hybrid membranes. These nanomaterials can improve hydrophilicity, increase mechanical strength, enhance thermal stability, and most importantly, reduce fouling by inhibiting microbial adhesion and organic accumulation on the membrane surface.
Studies are focusing on establishing the optimal concentration and the type of the polymer [
6,
7,
8,
9], the optimal concentration and the type of the nanoparticles [
10,
11,
12,
13], and the fabrication conditions [
14,
15,
16,
17,
18,
19,
20]. TiO
2 nanoparticles can degrade organic pollutants under UV radiation through photocatalysis, while silver nanoparticles provide excellent antimicrobial properties. Furthermore, carbon-based nanoparticles can enhance the permeability and selectivity of membranes due to their unique structural and electronic properties. The synergy between polymeric matrices and nanofillers offers an exciting path for tailored membrane design, where the properties can be fine-tuned for specific wastewater treatment applications such as ultrafiltration, nanofiltration, and reverse osmosis.
Despite their advantages, the large-scale implementation of nanocomposite membranes still faces several challenges. These include nanoparticle agglomeration, leaching, high production costs, and potential environmental risks associated with nanomaterials. Therefore, future research should focus on developing cost-effective and eco-friendly synthesis methods, ensuring uniform nanoparticle dispersion within the polymer matrix, and assessing the long-term performance and environmental safety of such membranes.
In conclusion, the integration of nanoparticles into polymeric membranes represents a transformative strategy in wastewater treatment technology. These advanced nanocomposite membranes show great promise for enhancing water quality, enabling the removal of both conventional and emerging contaminants with higher efficiency and lower energy input. Continued interdisciplinary research efforts, combining material science, nanotechnology, and environmental engineering, are essential to accelerate the commercial viability and sustainable use of these innovative membrane systems.
2. Materials and Methods
2.1. Materials
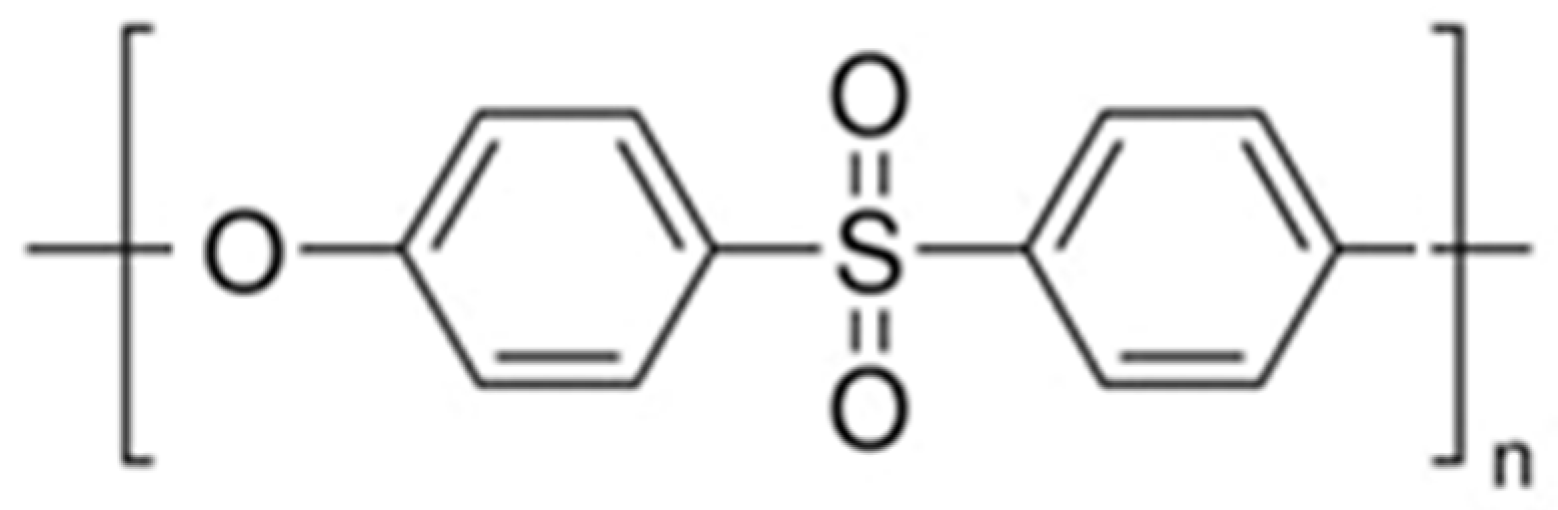
Polyethersulfone (PES),
Figure 1, from Sigma Aldrich, is a high-performance, amber-transparent thermoplastic polymer with excellent thermal stability (glass transition temperature ~220 °C, continuous use temperature up to 180 °C), high mechanical strength (tensile strength ~70–100 MPa), and good chemical resistance to acids, bases, and oxidizing agents; it also exhibits low water absorption (<0.4%), moderate hydrophilicity (contact angle ~65–75°), and maintains structural integrity in pH ranges of 2–10, making it highly suitable for membrane fabrication in water and wastewater treatment processes.
Yttrium oxide (Y2O3) nanoparticles, from Sigma Aldrich with size < 50 nm, are used for their high thermal stability, chemical resistance, and antimicrobial properties in applications such as wastewater treatment membranes, catalysts, and electronic ceramics; they have a density of approximately 5.01 g/cm3 and a molar mass of 225.81 g/mol.
2.2. Preparation of Nanocomposite Membrane
The use of nanoparticles to modify the properties of polymeric membranes can be performed both by including them in the structure of the membranes and by depositing them on the surface of the membranes. The addition of nanoparticles to the structure of polymer membranes leads to a decrease in the risk of Y2O3 elimination in the environment.
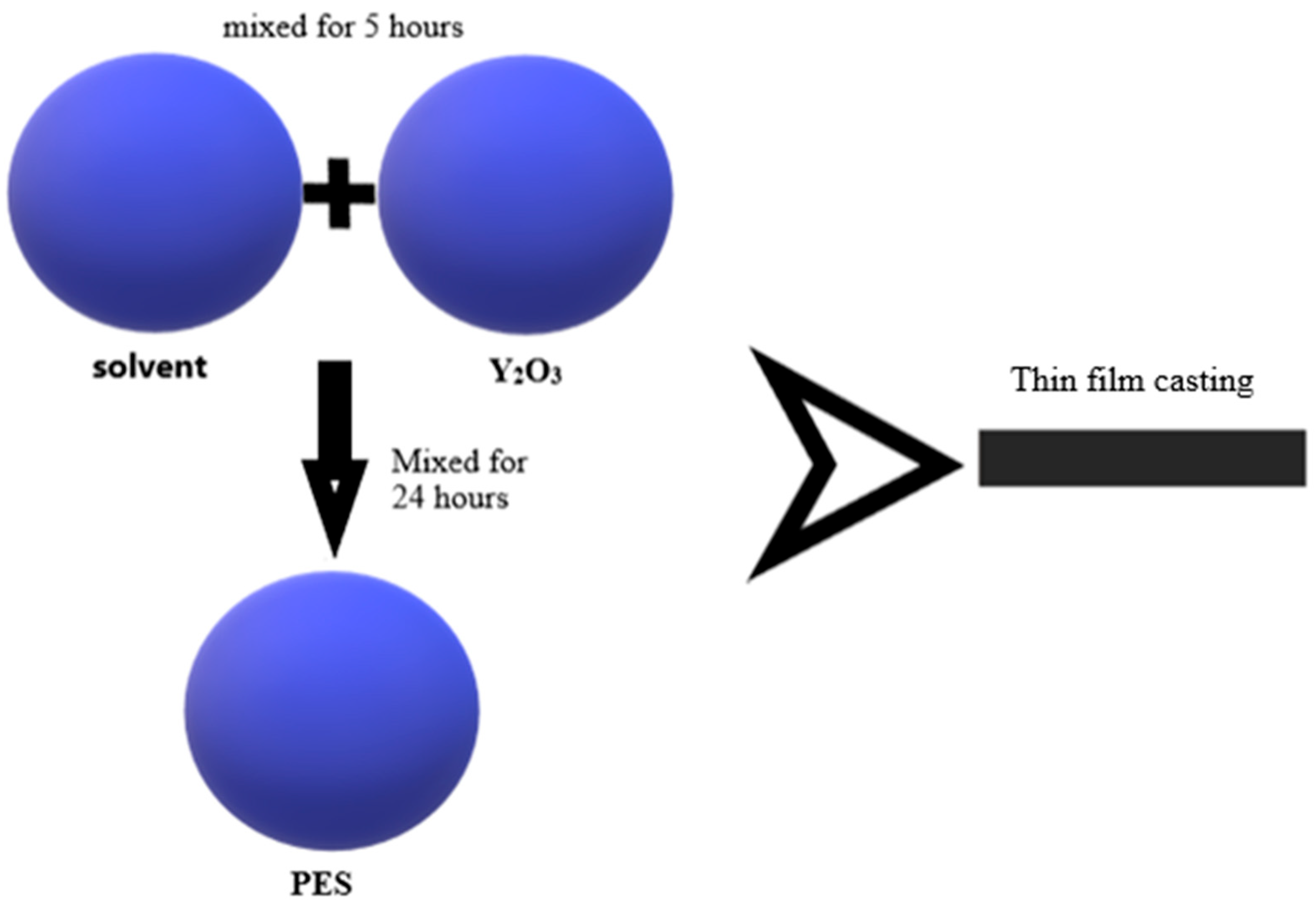
Membranes were obtained at four different concentrations of PES (21, 23, 25, and 27 wt.%) with 0.2 wt.% Y
2O
3, by the immersion precipitation method, is presented in
Figure 2.
For a better dispersion of the nanoparticles in the polymer solution, Y2O3 was added to the solvent, N-methyl-pyrrolidone (NMP), and mixed for 5 h. The polymer was added later, gradually, and mixed for 24 h.
2.3. Membrane Testing
To establish the permeation properties, 4 pieces were cut from every membrane, with an active area of 14.6 cm
2. The compaction membrane was made with pure water at a pressure of 10 bars using a dead-end installation purchased from Sterlitech Corporation, Washington DC, HP4750 Stirred Cell. All the experiments were conducted at room temperature. Pure water permeability (PWP) was determined by measuring the pure water flux (Jw) at different transmembrane pressures (ΔP) from 2 to 10 bar, using the following equation:
To compare flux decline between different membranes, relative fluxes were defined as the relation of the permeate flux to the pure water flux of the respective membrane as follows:
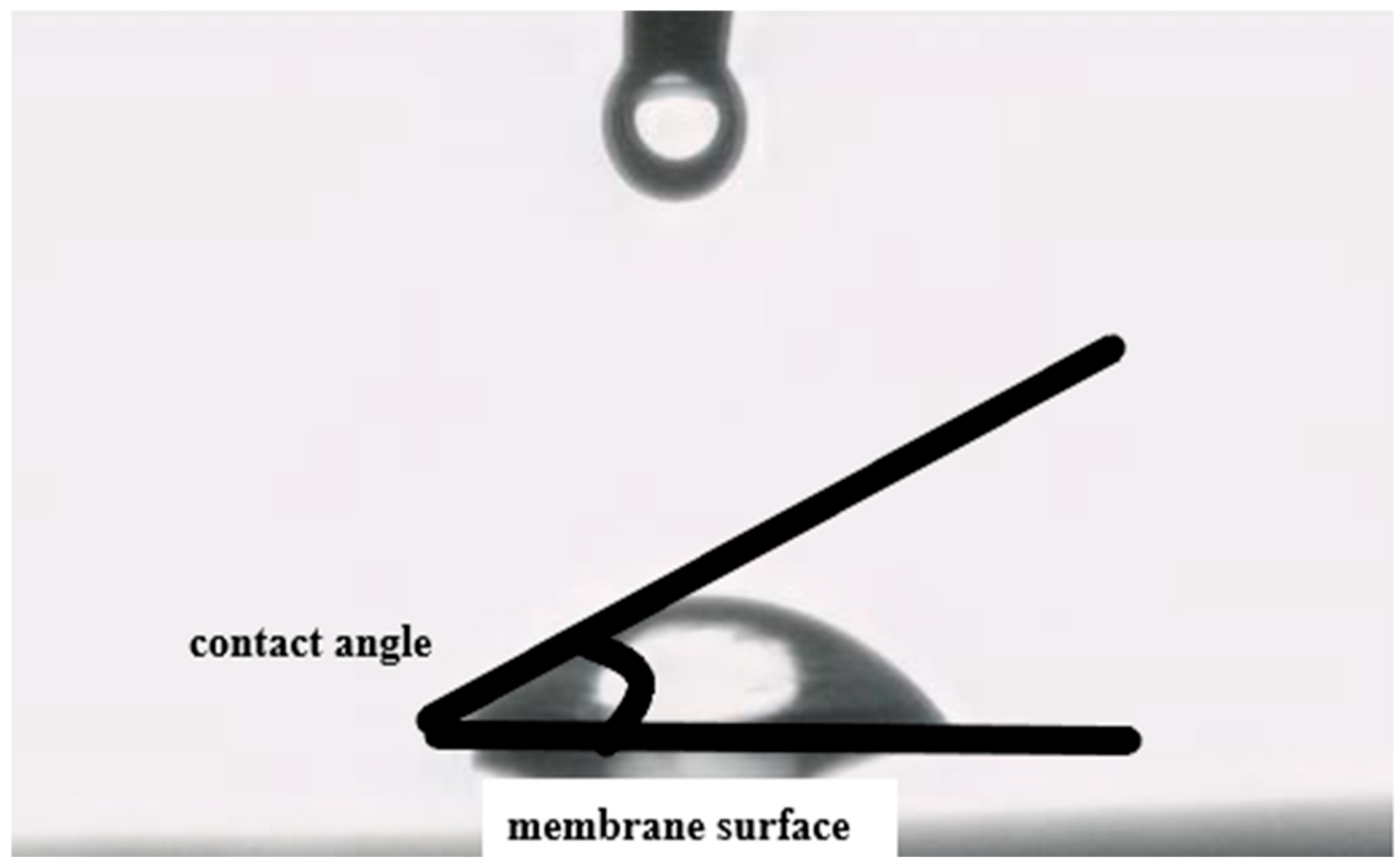
Scanning electron microscopy (SEM) with an accelerating voltage of 20 KeV was used to study the membrane structure with and without nanoparticles. To determine the influence of nanoparticles on the membrane hydrophilicity, the contact angle method was used (
Figure 3).
Using the “wet and dry” method, the membrane porosity was determined using the following equation:
where ε is the membrane porosity, W
1 is the wet membrane weight, W
2 is the dry membrane weight, A is the membrane area, t is the membrane thickness, and q is the water density. Membranes were immersed in water for 48 h to ensure that the membrane was wetted, and they were then weighed. The second step was to dry the membrane at 50 °C for 12 h and weigh it again.
3. Results and Discussions
3.1. Permeability
Permeability increases when the polymer concentration decreases, from 90 L/m
2·h for membranes with 27 wt.% PES to 107 L/m
2·h for membranes with 21 wt.% PES.
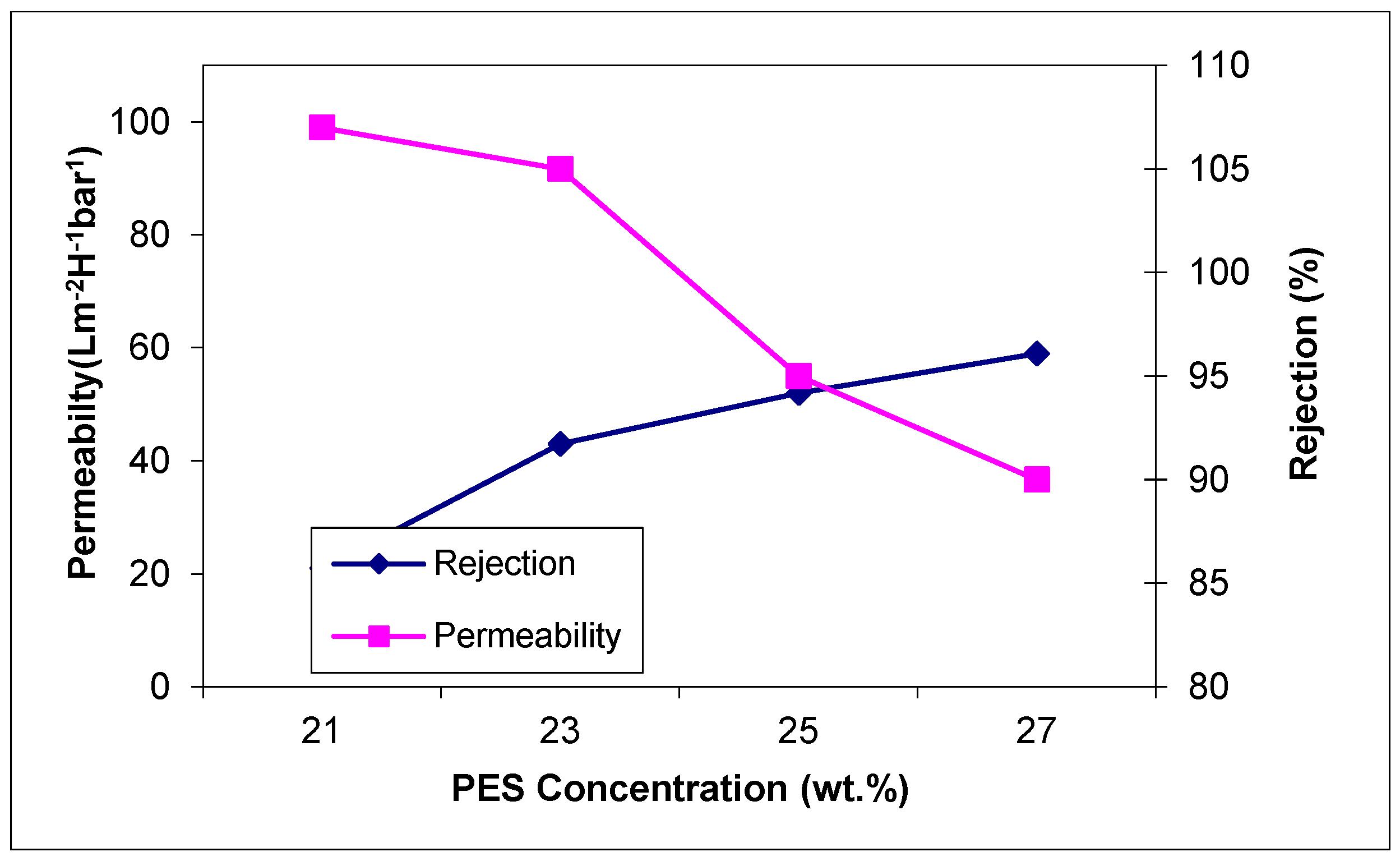
Figure 4 presents the permeability evolution at different concentrations of polymer and the influence of Y
2O
3 nanoparticles.
When adding nanoparticles in a concentration of 0.2 wt.% in the membrane structure, the permeability increases for every polymer concentration. At a concentration of 27 wt.% PES, the increase is 10%, from 90 L/m2·h bar for membranes without nanoparticles to 99 L/m2·h bar for membranes blended with Y2O3 nanoparticles.
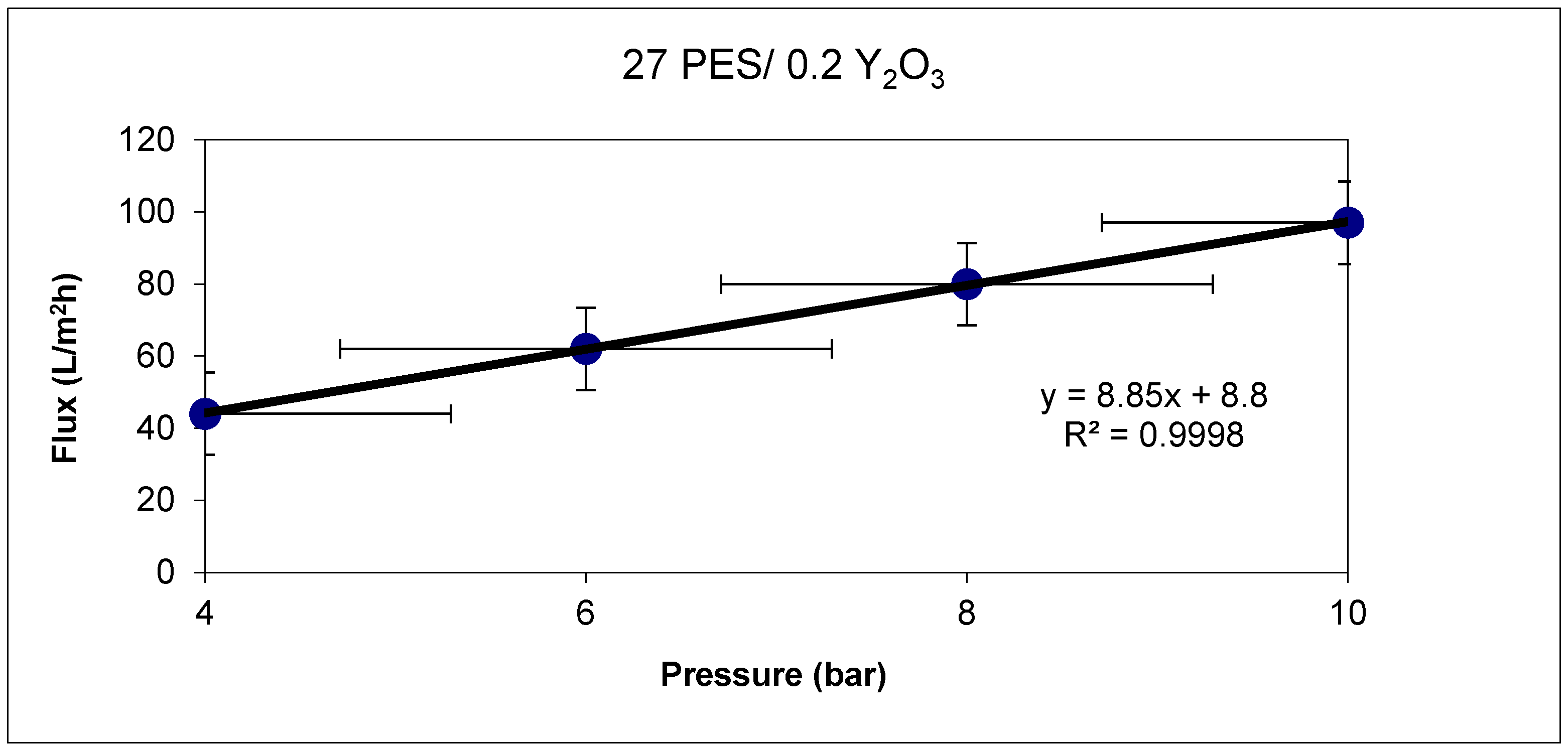
Permeability is directly related to the pressure, as is shown in
Figure 5. For membranes at 27 wt.% PES with a 0.2 wt.% Y
2O
3 nanoparticles, permeability increases from 44 L/m
2·h bar at a pressure of 4 bar to 97 L/m
2·h bar at a pressure of 10 bar.
Adding nanoparticles to the membrane structure decreases the pre-compaction time and increases stability.
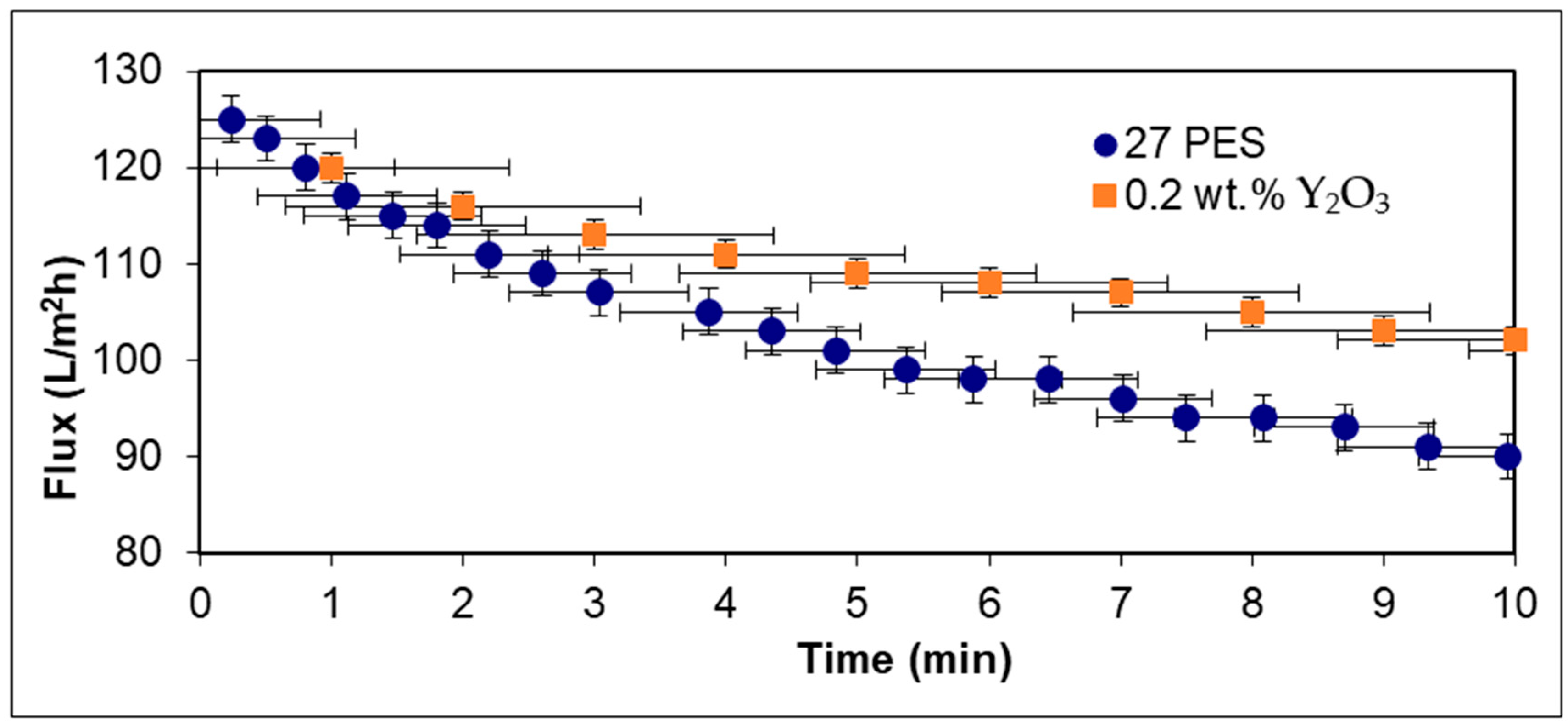
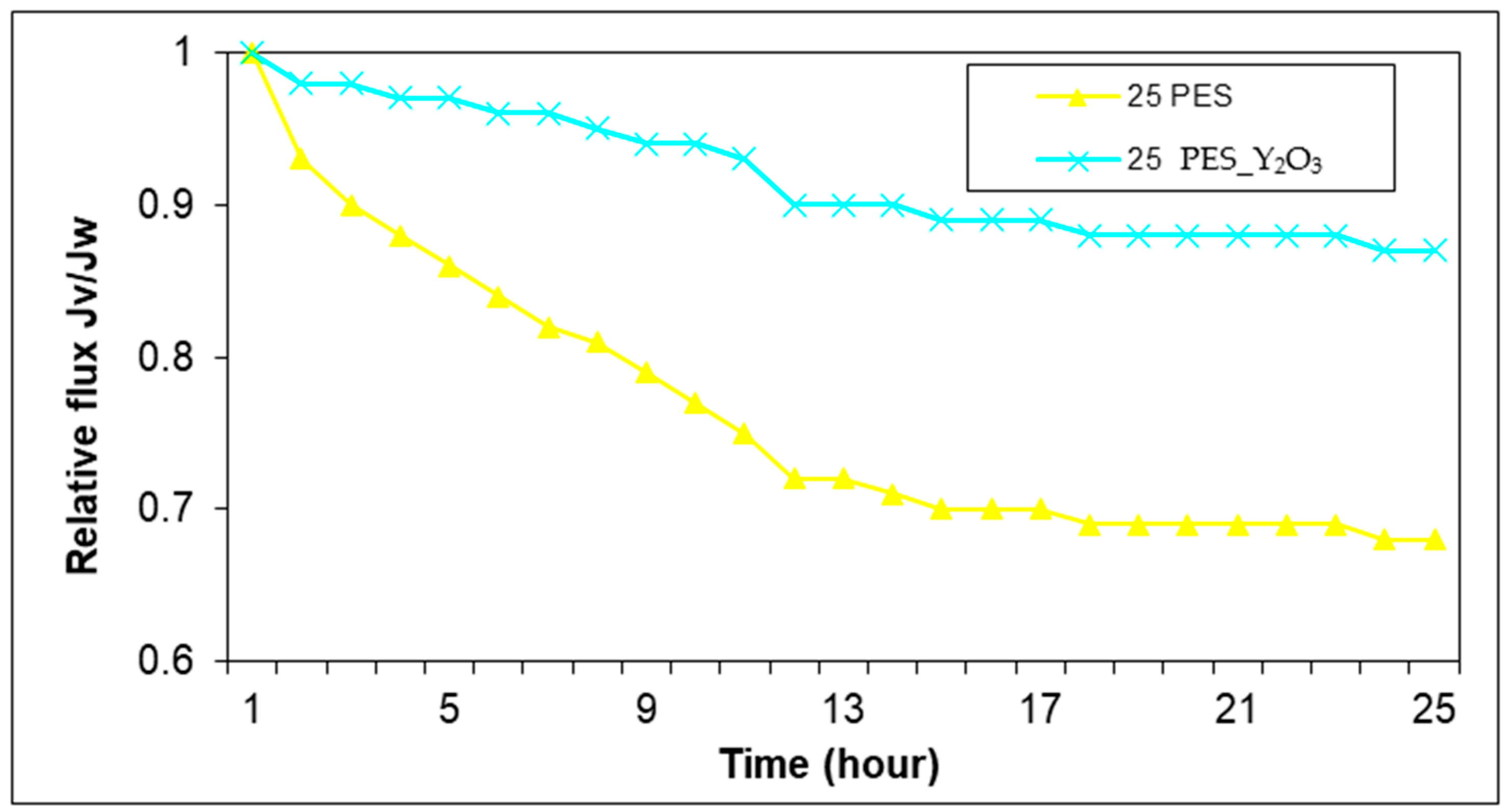
Figure 6 presents the comparison between the water flux of a membrane with 27 wt.% PES without nanoparticles and with 0.2 wt.% Y
2O
3 nanoparticles.
Additional insights into membrane performance were gained by evaluating the water flux over time for PES membranes modified with 0.2 wt.% Y2O3 at a certain polymer concentration of 27 wt.% As shown in the graph above, both types of membranes exhibited a gradual decline in flux over the 10 min testing period, primarily due to membrane fouling and compaction effects. However, the Y2O3-modified membrane maintained a higher and more stable flux throughout the test.
At the start of the experiment (t = 0 min), both membranes displayed similar initial flux values (~125 L/m2·h), indicating comparable initial permeability. As time passed, the membrane without nanoparticles showed a more pronounced decline, dropping to about 90 L/m2·h by the end of the test, while the Y2O3 modified membrane maintained a flux above 100 L/m2·h. This suggests that although Y2O3 improves membrane hydrophilicity and porosity, Y2O3 offers better long-term resistance to fouling under the same conditions.
The superior performance of Y2O3 nanoparticles can be attributed to their high surface area and strong hydrophilic character, which reduces the interaction of foulants with the membrane surface and delays the onset of pore blockage.
3.2. Hydrophilicity
The membrane surface affinity for water decreases when polymer concentration increases.
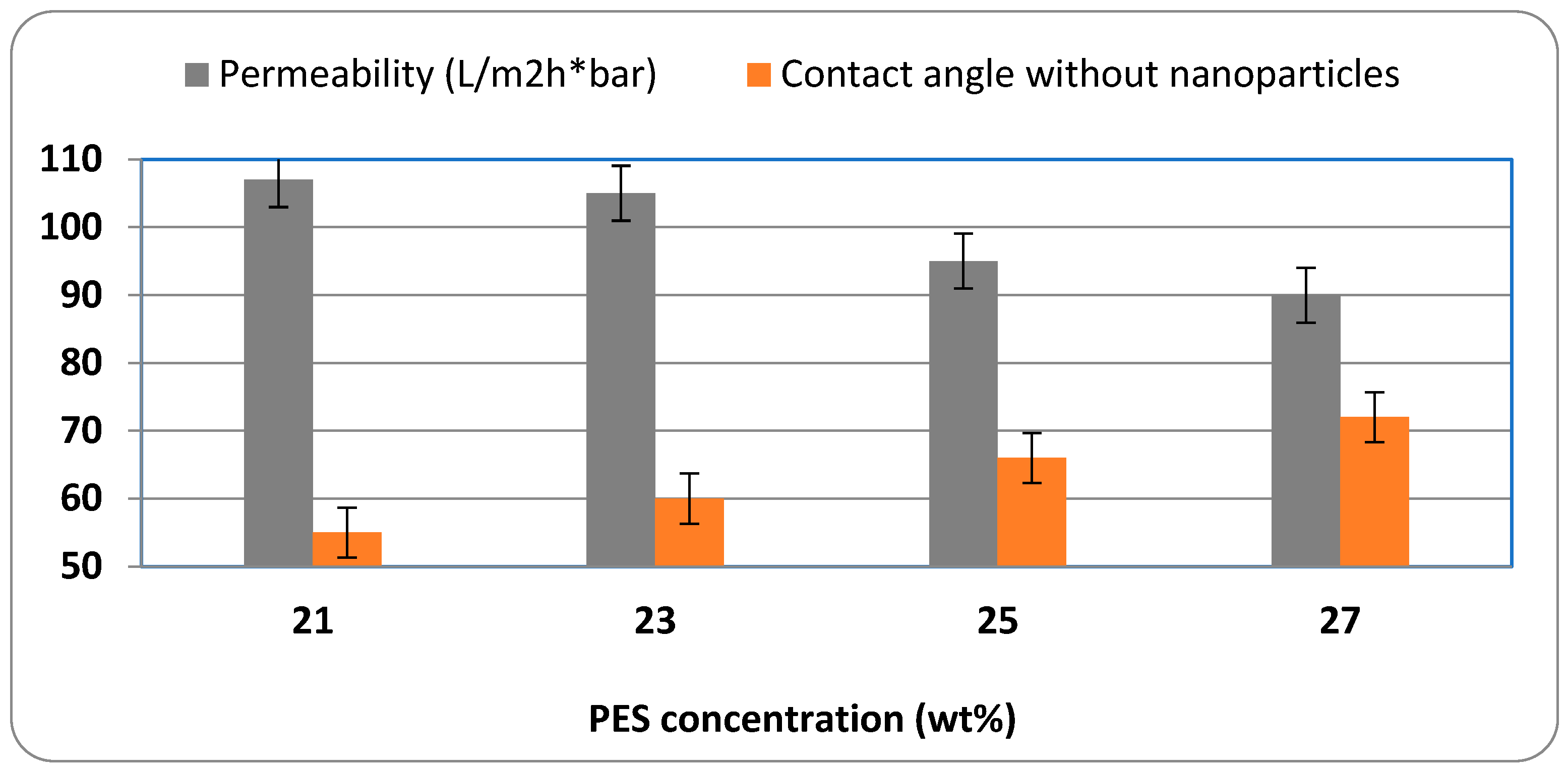
Figure 7 shows the relation between permeability and contact angle at different concentrations of PES.
Figure 7 shows that increasing the polymer concentration from 21 wt.% to 27 wt.% results in a continuous decrease in water permeability, from approximately 107 L/m
2·h at 21% to around 90 L/m
2·h at 27%. At the same time, the contact angle increases from about 54° to 72°, indicating a reduction in surface hydrophilicity. These findings support the conclusion that higher polymer content leads to a denser, more hydrophobic membrane structure with reduced pore accessibility and water transport capability.
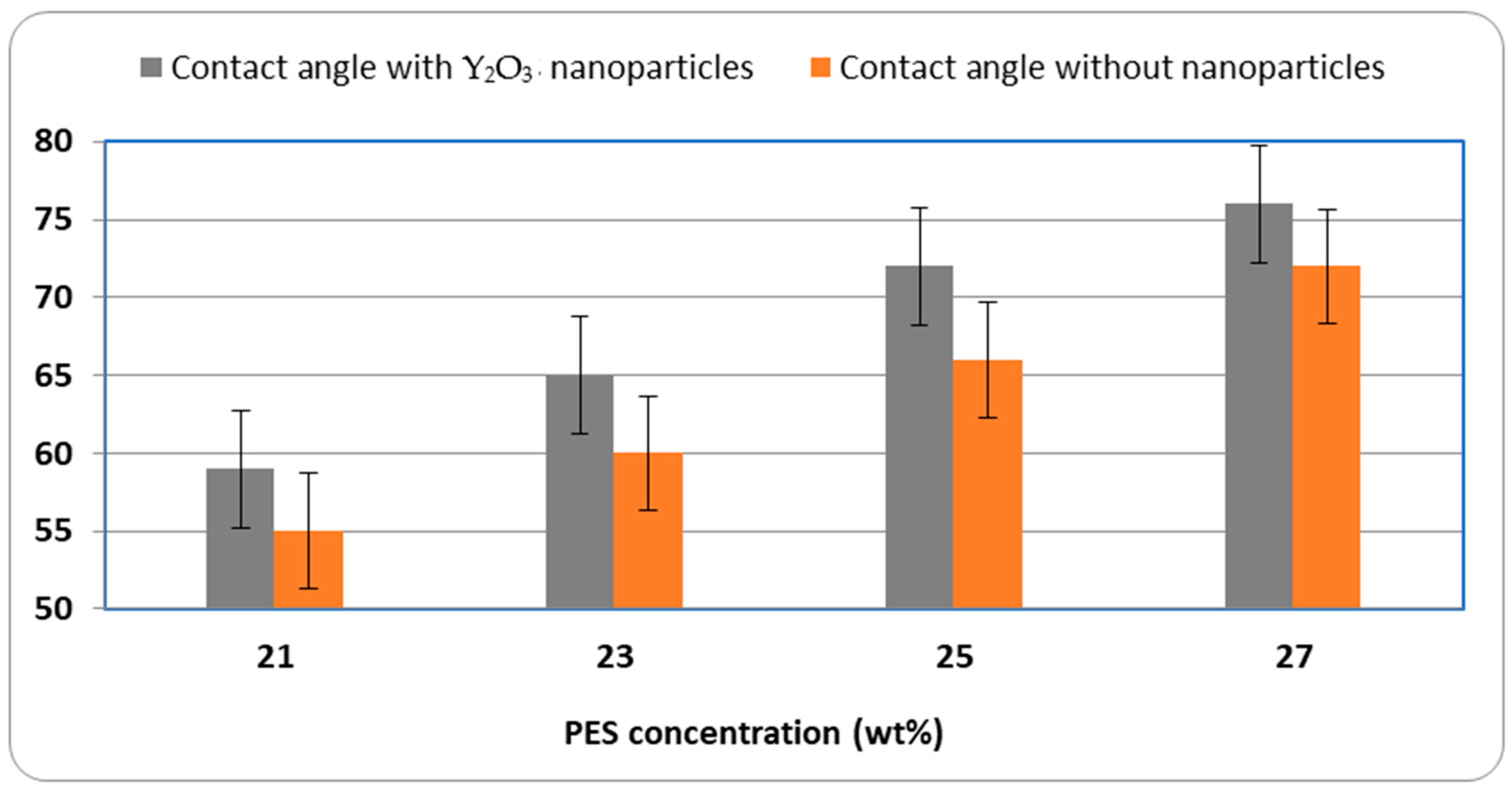
Interestingly, when Y
2O
3 nanoparticles are added to membranes of equal polymer concentrations, this negative trend can be partially reversed (
Figure 8).
The nanoparticles contribute to a notable decrease in contact angle and an improvement in water flux, even at higher polymer concentrations. Therefore, the integration of Y2O3 acts as a structural and surface modifier, restoring or enhancing hydrophilicity and permeability by modifying pore morphology and introducing polar surface characteristics.
In conclusion, while polymer concentration is a key parameter in defining membrane structure and transport properties, the incorporation of Y2O3 nanoparticles offers a viable strategy to overcome the limitations of high polymer content, yielding membranes that are both structurally robust and highly performant for water treatment applications.
3.3. Porosity
Porosity is an important property of membranes due to its influence on permeability and the compaction and fouling effects.
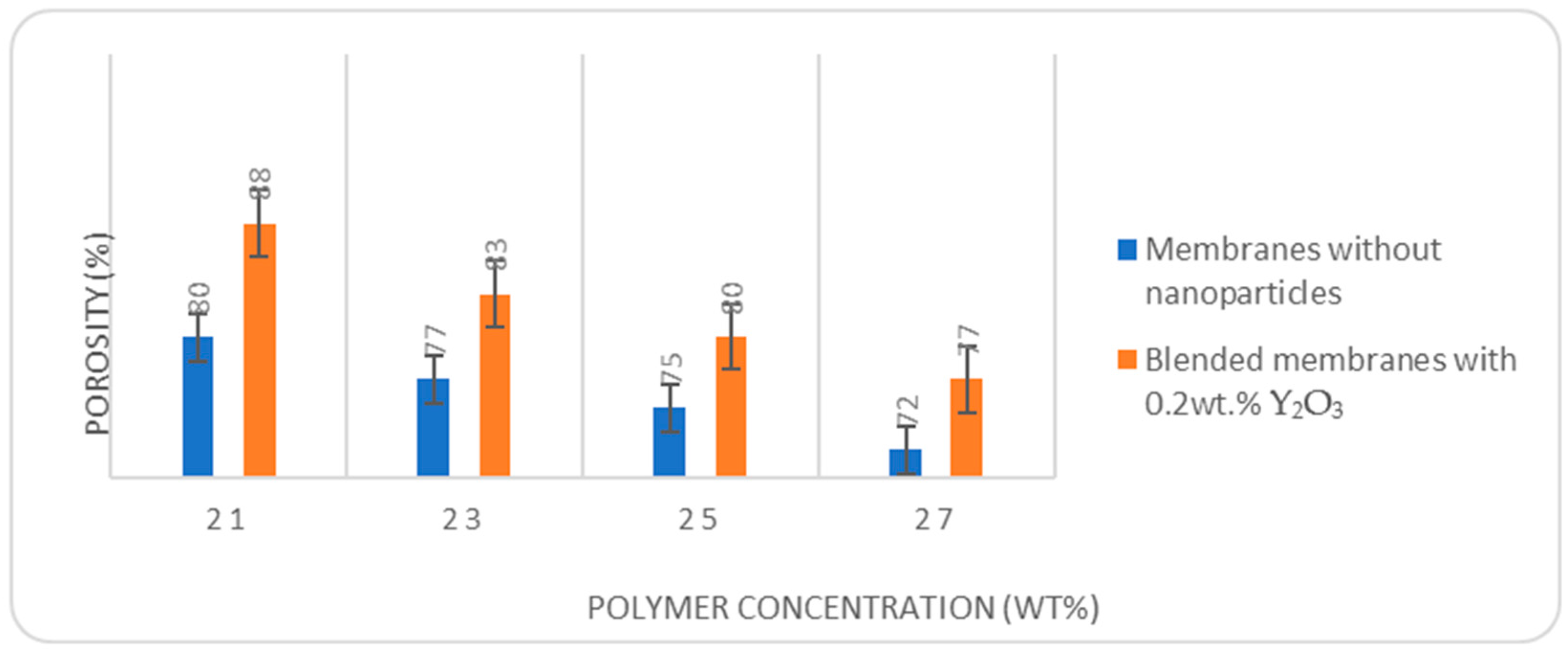
Figure 9 presents a comparison of porosity evolution at different concentrations of PES with and without Y
2O
3 nanoparticles.
Membrane porosity decreases if the polymer concentration increases the permeation properties decrease. If Y2O3 nanoparticles are added to the membrane structure, the porosity increases for every polymer concentration.
The beneficial effect of Y2O3 nanoparticles is further confirmed by porosity. At all polymer concentrations tested (21%, 23%, 25%, and 27%), the membranes containing 0.2 wt.% Y2O3 exhibited higher porosity compared to its unmodified counterparts. For instance, at 21% polymer concentration, porosity increased from 80% to 88%, while at 27%, it rose from 72% to 77%. This improvement can be attributed to the disruption of polymer chain packing and enhanced thermodynamic instability induced by the nanoparticles during the phase inversion process, which promotes pore formation.
The enhanced porosity directly correlates with improved permeability and reduced contact angle, highlighting the role of Y2O3 in tailoring membrane microstructure. These findings demonstrate that even small amounts of Y2O3 nanoparticles can significantly enhance the structural and surface properties of PES-based membranes, making them more efficient for applications in water and wastewater treatment where high flux and hydrophilicity are essential.
In conclusion, while increasing polymer concentration alone tends to reduce permeability, hydrophilicity, and porosity, the strategic incorporation of Y2O3 nanoparticles reverses these effects to a considerable extent. The result is a set of membranes with better-balanced properties—structural stability from higher polymer content combined with the high flux and surface wettability induced by nanomaterial presence.
3.4. Scanning Electron Microscopy (SEM)
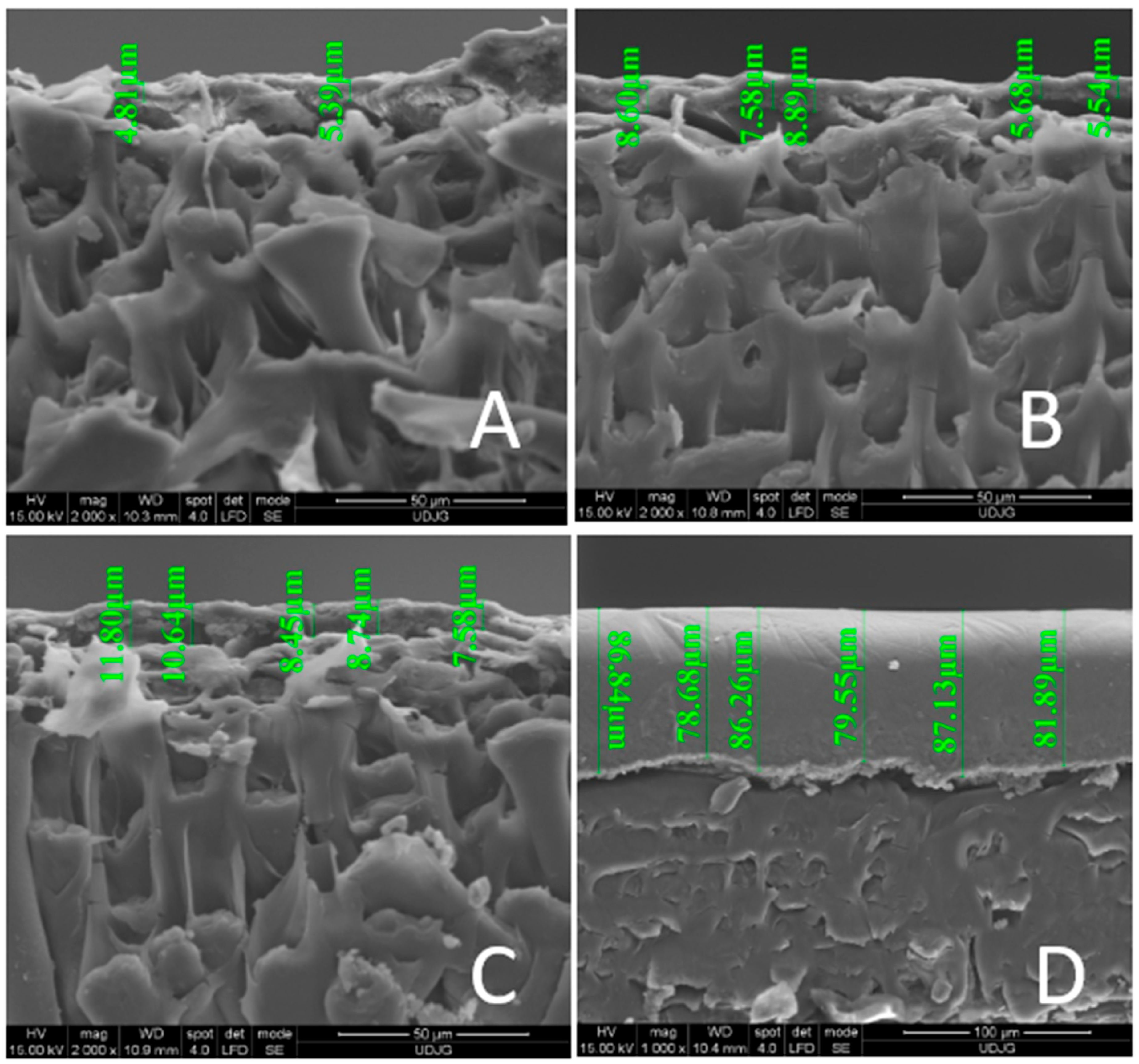
Increasing the polymer concentration also increases the viscosity of the casting solution and decreases the membrane porosity (
Figure 10).
The dense top layer increases from 4 to 5 µm for membranes with 21 wt.% PES up to 90 µm for membranes with 27 wt.% PES. Increasing PES concentration leads to decreased membrane permeability due to higher solution viscosity, reduced pore size, and denser structure formation. For applications requiring high water flux, lower PES concentrations (e.g., 21% or 23%) are preferable. However, when selectivity and structural integrity are more critical, higher concentrations (25–27%) may be more suitable. The optimal concentration should be chosen based on the specific requirements of the membrane’s intended application.
3.5. Rejection
Increasing the polymer concentration, the rejection capacity increases due to the decrease in pore size.
Figure 11 presents the relation between rejection of methylene blue and the permeability at different concentrations of PES. To study the rejection capacity, a commercial dye was used, namely methylene blue at a concentration of 5 mg·L
−1.
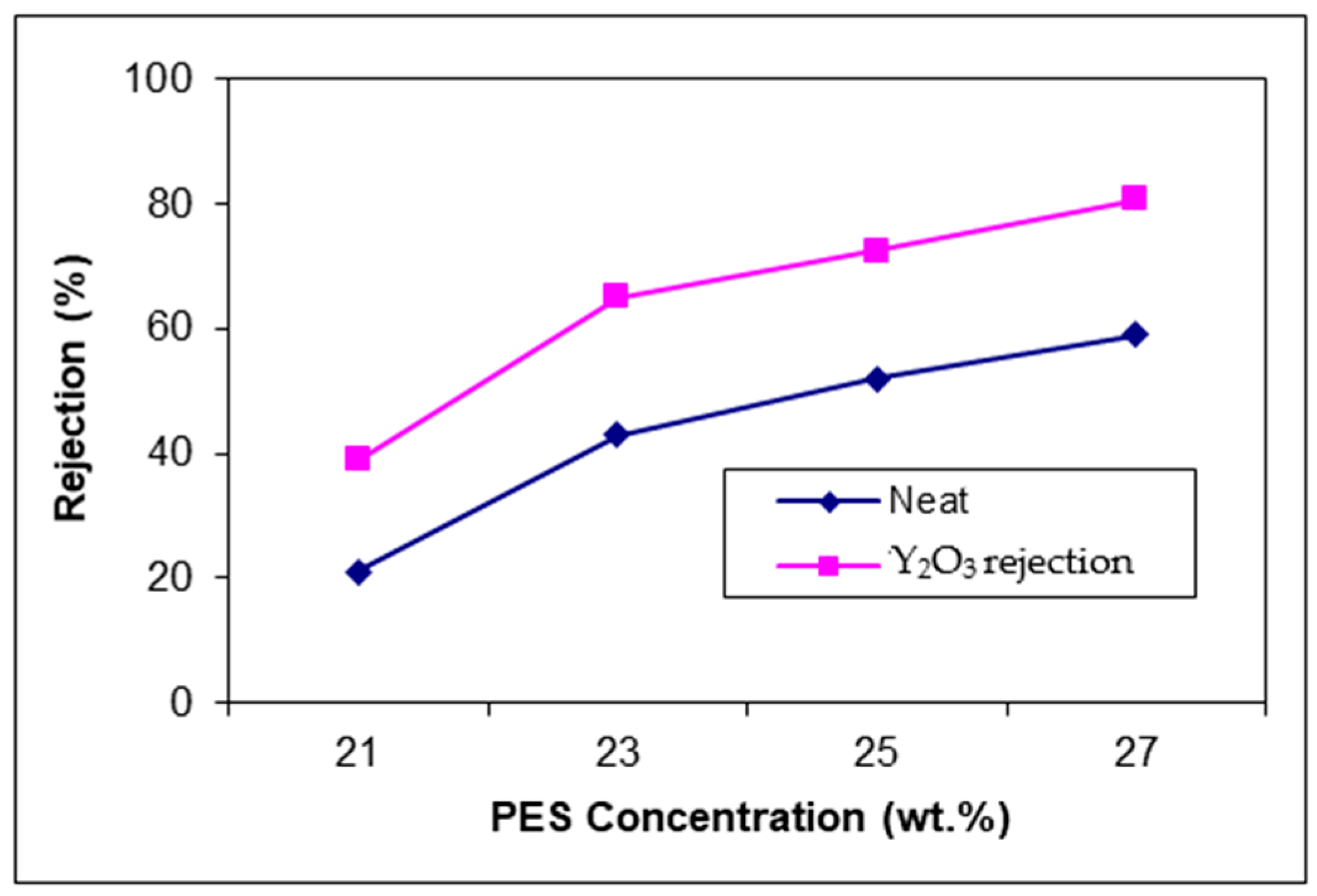
The rejection increases when the polymer concentration and permeability decrease. Adding Y
2O
3 nanoparticles, the membrane structure is modified in terms of porosity and pore size.
Figure 12 presents the methylene blue rejection of neat and blended membranes.
Membranes with Y2O3 nanoparticles have a better permeability, but an increase in terms of methylene blue rejection. This effect on both properties is possible due to the effect of nanoparticles on the porosity without increasing the pore size.
3.6. Fouling Performance
A humic acid solution at 5 mg·L
−1 was used to establish the fouling performance of membranes with and without nanoparticles. Humic acid is widely distributed in wastewater and is often used to determine membrane properties [
21].
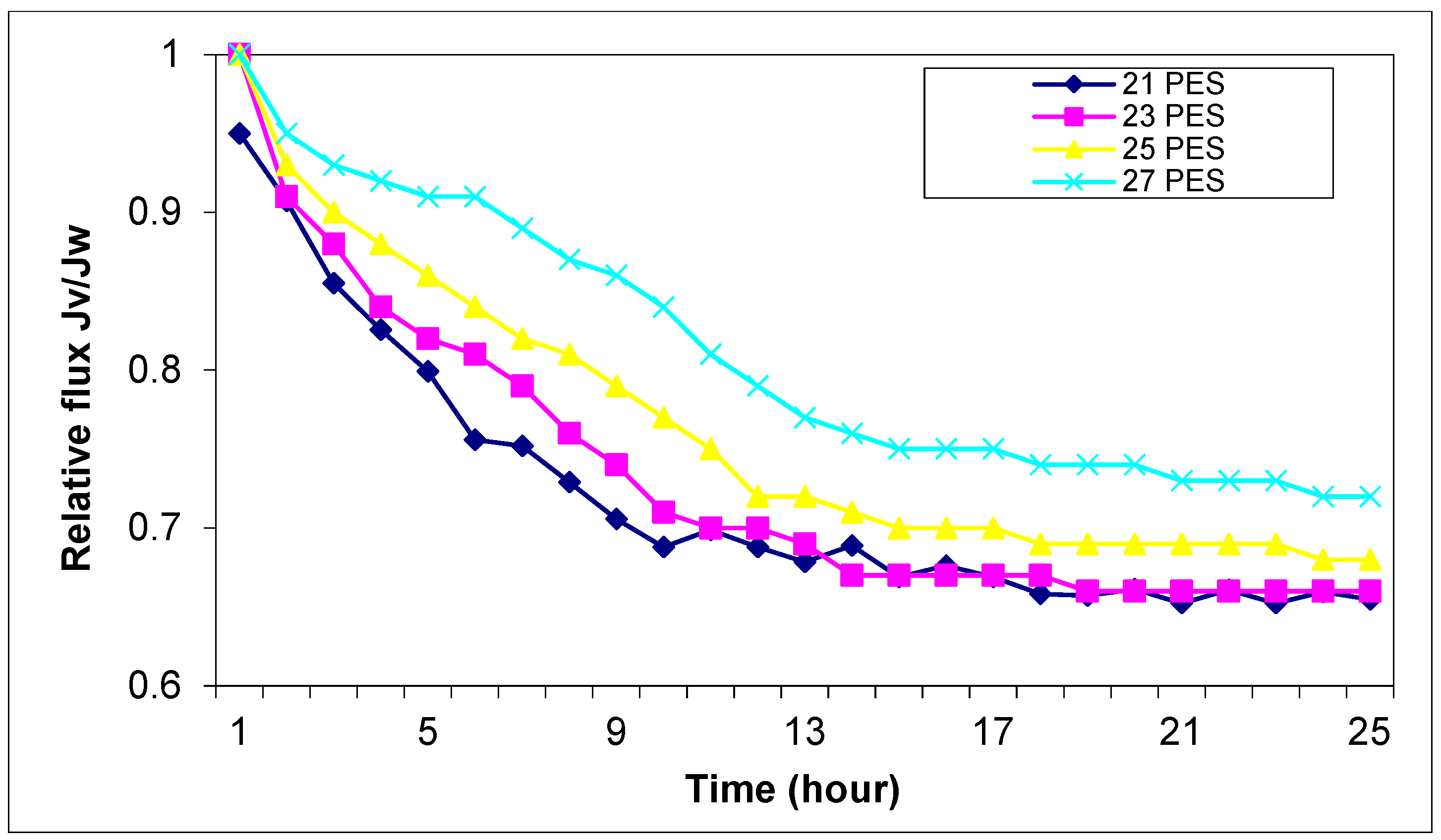
Figure 13 shows the fouling effect on the permeation properties of the membranes at different polymer concentrations.
Adding Y
2O
3 nanoparticles to the membrane structures, the relative flux is more stable in time, and the fouling decreases.
Figure 14 shows the difference between membrane flux with and without nanoparticles.
The increase in fouling performance for membranes with Y2O3 nanoparticles is due to the decreasing surface roughness. The data indicates that incorporating Y2O3 nanoparticles significantly enhances membrane performance by reducing flux decline over time. This improvement can be attributed to the nanoparticles’ ability to increase membrane hydrophilicity, decrease fouling propensity, and improve structural stability.
4. Conclusions
The concentration of polyethersulfone (PES) in the casting solution plays a crucial role in determining the final morphology and permeability of the fabricated membranes. In this study, membranes were prepared using four different PES concentrations—21%, 23%, 25%, and 27%—and their performance was evaluated in terms of water permeability.
As the PES concentration increased from 21% to 27%, a clear inverse relationship between polymer content and permeability was observed. At 21%, the membrane exhibited the highest permeability, primarily due to the formation of a highly porous and finger-like structure. The low viscosity of the casting solution at this concentration facilitates rapid phase separation during the phase inversion process, resulting in larger pore sizes and a more open pore network.
With increasing polymer concentration to 23% and 25%, the viscosity of the casting solution also increased, which slowed the phase separation rate and led to the development of a denser sponge-like structure. Consequently, pore size decreased, and water flux was moderately reduced. This transition from finger-like to sponge-like morphology is well-documented in the literature and reflects the typical behavior of thermally induced phase separation in polymeric membrane fabrication.
At the highest concentration tested (27% PES), the membrane became significantly denser, with a tightly packed structure and minimal porosity. The high viscosity of the solution restricted solvent–non-solvent exchange, limiting pore formation. As a result, water permeability was the lowest among all tested membranes. While this composition may offer advantages in terms of mechanical strength and selectivity, it compromises the overall permeability.
The addition of Y2O3 nanoparticles to the PES membrane matrix further influences the membrane’s performance, particularly in terms of surface hydrophilicity, water permeability, and methylene blue rejection. Rejection increases for membranes at 21%Pes from 21% for membranes without nanoparticles to 39% for membranes with nanoparticles. When incorporated into the casting solution at optimized concentrations, Y2O3 nanoparticles tend to disperse uniformly within the polymer matrix and migrate toward the membrane surface during phase inversion. This results in a more hydrophilic surface due to the polar nature of Y2O3, as confirmed by a noticeable decrease in contact angle values. Y2O3 can modify the surface chemistry of the membrane. Its incorporation introduces hydroxyl groups and oxygen vacancies that increase surface hydrophilicity, reducing fouling tendency and improving water affinity.
Fouling performance increases with the addition of nanoparticles; relative flux decreases by 30% for membranes without nanoparticles and by 10% for membranes with nanoparticles, both at a concentration of 25% PES.
Moreover, the presence of these nanoparticles disrupts the dense packing of polymer chains, promoting the formation of additional micro-voids and enhancing the overall porosity. Consequently, water molecules can pass through the membrane more easily, leading to a significant increase in permeability compared to unmodified PES membranes. This improvement is particularly evident at intermediate polymer concentrations (e.g., 23–25%), where a balance between structural integrity and porosity can be maintained.
In addition to improving hydrophilicity and flux, Y2O3 nanoparticles may also contribute to improved antifouling performance, further extending the membrane’s applicability in water and wastewater treatment. Therefore, incorporating Y2O3 into PES membranes represents a promising strategy for enhancing membrane performance without compromising mechanical stability.
Author Contributions
Conceptualization, Ș.B. and A.L.L.; methodology, A.L.L., A.C., G.G.I., E.D. and Ș.B.; software, A.C. and Ș.B.; validation: A.L.L., G.G.I. and E.D.; formal analysis, A.L.L., A.C., E.D. and Ș.B.; investigation, A.L.L. and A.C.; resources, A.L.L. and E.D.; writing—original draft preparation, Ș.B.; writing—review and editing, A.L.L., A.C., G.G.I., E.D. and Ş.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wei, X.; Liu, Y.; Zhang, Z.; Zhao, F.; Li, Z.; Fan, C.; Yang, Y.; Jiang, Z. 2D vermiculite nanofiltration membrane with TiO2 nanoparticles as versatile intercalator for enhanced water purification. J. Membr. Sci. 2024, 695, 122461. [Google Scholar] [CrossRef]
- Cortalezzi, M.M.; Rose, J.; Barron, A.R.; Wiesner, M.R. Characteristics of ultrafiltration ceramic membranes derived from alumoxane nanoparticles. J. Membr. Sci. 2002, 205, 33–43. [Google Scholar] [CrossRef]
- Cortalezzi, M.M.; Rose, J.; Wells, G.F.; Bottero, J.Y.; Barron, A.R.; Wiesner, M.R. Ceramic membranes derived from ferroxane nanoparticles: A new route for the fabrication of iron oxide ultrafiltration membranes. J. Membr. Sci. 2003, 227, 207–217. [Google Scholar] [CrossRef]
- Ali, M.F.; Cho, H.-S.; Bernäcker, C.I.; Albers, J.; Choi, Y.-G.; Kim, M.; Lee, J.H.; Lee, C.; Lee, S.; Cho, W.-C. A study on the effect of TiO2 nanoparticle size on the performance of composite separators in alkaline water electrolysis. J. Membr. Sci. 2023, 678, 121671. [Google Scholar] [CrossRef]
- Kim, J.; der Bruggen, V.B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Halling-Sorensen, B.; Nielsen, N.; Lanzky, P.F.; Ingerslev, F.; Holten Lutzhoft, H.C.; Jorgensen, S.E.; Occurence, S.E. fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Jafarzadeh, Y.; Yegani, R. Thermal, mechanical, and structural properties of ZnO/polyethylene membranes made by thermally induced phase separation method. J. Appl. Polym. Sci. 2015, 132, 42338. [Google Scholar] [CrossRef]
- Dong, S.; Feng, X.; Zhang, R. High-Performance Thin Film Composite Nanofiltration (NF) Membrane Constructed on Modified Polyvinylidene Fluoride (PVDF) Substrate. Membranes 2025, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Tiron, L.G.; Pintilie, Ș.C.; Lazăr, A.L.; Balta, S.; Bodor, M. Influence of polymer concentration on membrane performance in wastewater treatment. Mater. Plast. 2018, 55, 95–98. [Google Scholar] [CrossRef]
- Suresh, P.; Sibley, M.M.; Che, A.C.; Ward, L.M.; Weinman, S.T.; Duval, C.E. Functionalizing polyethersulfone membranes: Using NMR to avoid pitfalls when using UV-induced polymerization to ‘graft from’ surfaces. J. Membr. Sci. 2025, 731, 124243. [Google Scholar] [CrossRef]
- Malek, A.; Seman, A.M.N.; Johnson, D.; Hilal, N. Formation and characterization of polyethersulfone membranes using different concentrations of polyvinylpyrrolidone. Desalination 2012, 288, 31–39. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, K.; Yan, F.; Shi, Y.; Yu, P.; Yu, S.; Li, S.; Gao, C. Enhancing the performance of aromatic polyamide reverse osmosis membrane by surface modification via covalent attachment of polyvinyl alcohol (PVA). J. Membr. Sci. 2016, 501, 209–219. [Google Scholar] [CrossRef]
- Lu, S.; Zou, D. Fabrication of Green PVDF/TiO2 Composite Membrane for Water Treatment. Membranes 2025, 15, 218. [Google Scholar] [CrossRef]
- Zhu, X.; Elimelech, M. Colloidal fouling of reverse osmosis membranes: Measurements and fouling mechanisms. Environ. Sci. Technol. 1997, 31, 3654. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Han, X.; Wang, J.; Wang, S. Improved flux and anti-biofouling performances of reverse osmosis membrane via surface layer-by-layer assembly. J. Membr. Sci. 2017, 539, 403–411. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Baltă, Ș.; Kim, J.; der Bruggen, V.B. Doping of polyethersulfone nanofiltration membranes: Antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J. Mater. Chem. 2011, 21, 10311–10320. [Google Scholar] [CrossRef]
- Sun, C.; Feng, X. Enhancing the performance of PVDF membranes by hydrophilic surface modification via amine treatment. Sep. Purif. Technol. 2017, 185, 94–102. [Google Scholar] [CrossRef]
- Valchev, I.; Coraddu, A.; Kalikatzarakis, M.; Geertsma, R.; Oneto, L. Numerical methods for monitoring and evaluating the biofouling state and effects on vessels’ hull and propeller performance: A review. Ocean Eng. 2022, 251, 110883. [Google Scholar] [CrossRef]
- Baltă, S.; Buruiană, D.L.; Simionescu, C.S.; Tiron, L.G.; Bordei, M.; der Bruggen, V.B. Influence of relative air humidity and casting time on the permeation properties of PSf nanofiltration membranes. Desalin. Water Treat. 2015, 57, 13924–13929. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.; Feng, S.; Meng, J. Fluorination as a powerful tool for improving water/salt selectivity of hydrophilic polyethersulfone. J. Membr. Sci. 2023, 687, 122030. [Google Scholar] [CrossRef]
- Capasso, S.; Musmarra, D.; Iovino, P. Macromolecular Structure of a Commercial Humic Acid Sample. Environments 2020, 7, 32. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).