Irradiated Gao Miao Zi Bentonite for Uranium Retention: Performance and Mechanism
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Irradiation and Preparation of Samples
2.3. Characterization
2.4. Batch Adsorption Experiments
3. Results and Discussion
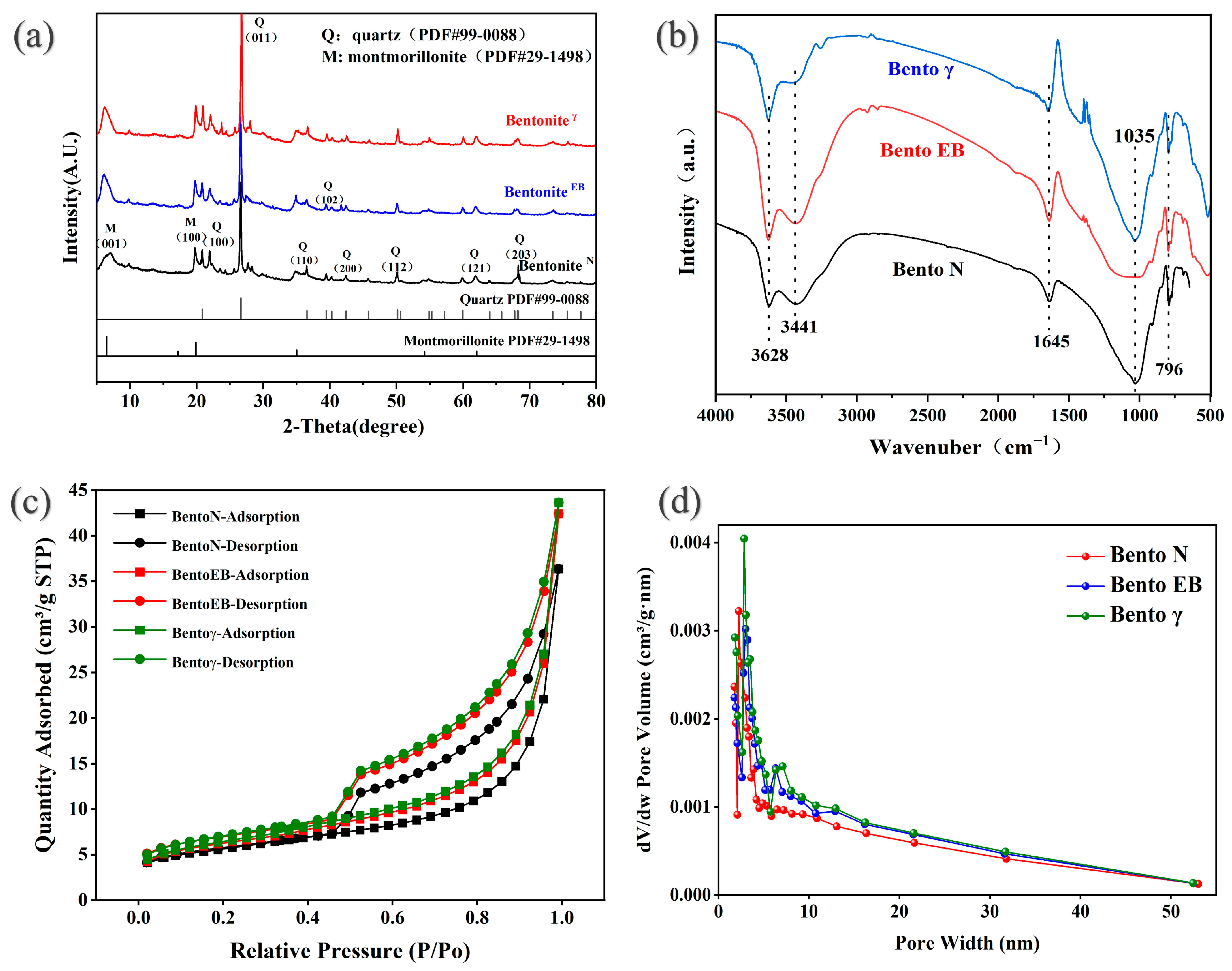
3.1. Structural Characterization of GMZ Bentonite
3.2. Batch Adsorption Studies
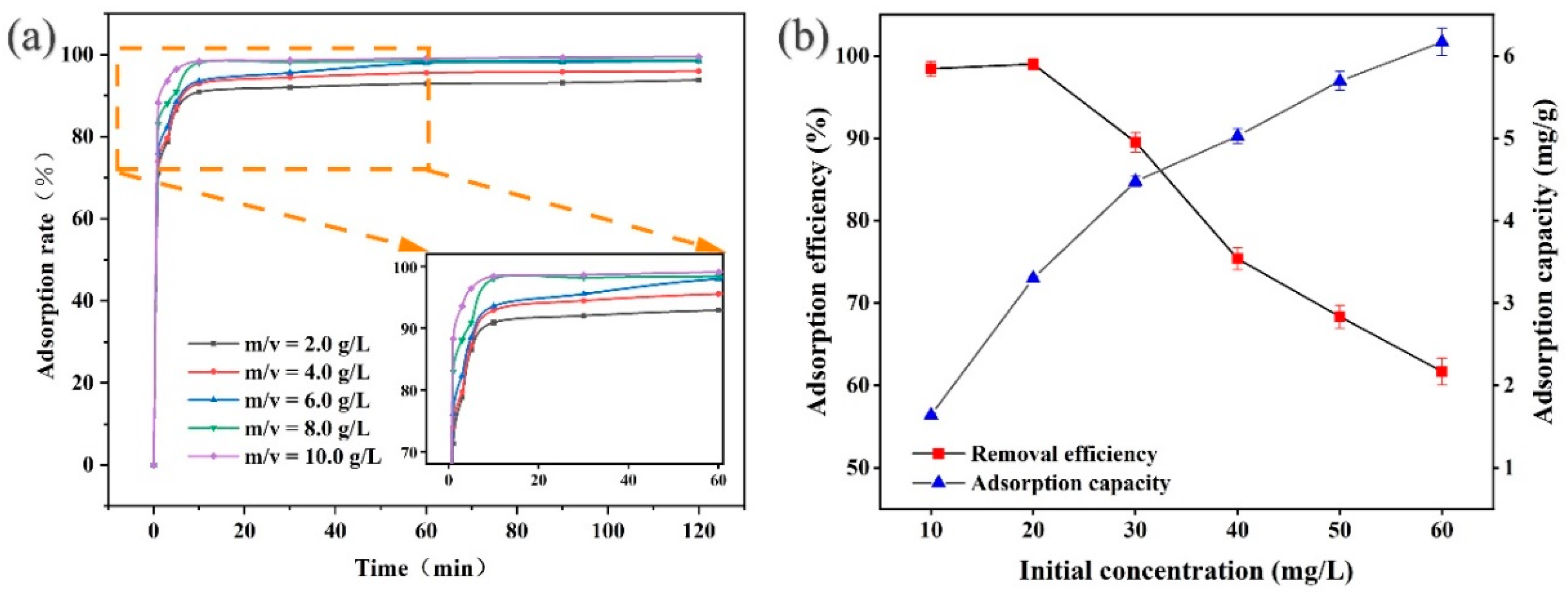
3.2.1. Effect of Dosage, Contact Time, and Initial Concentration
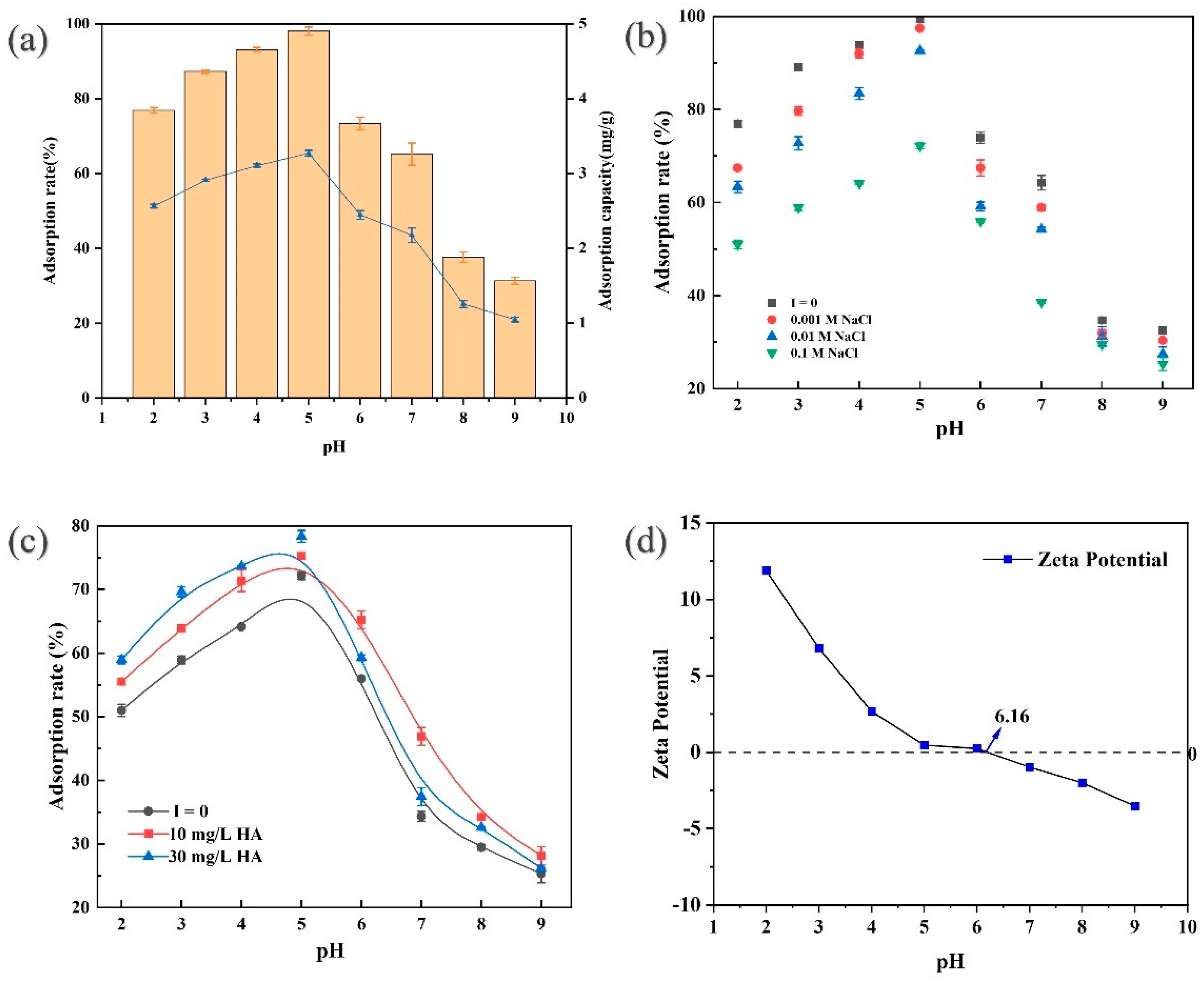
3.2.2. Effect of pH
3.2.3. Effect of Ionic Strength
3.2.4. Effect of Natural Organic Matter
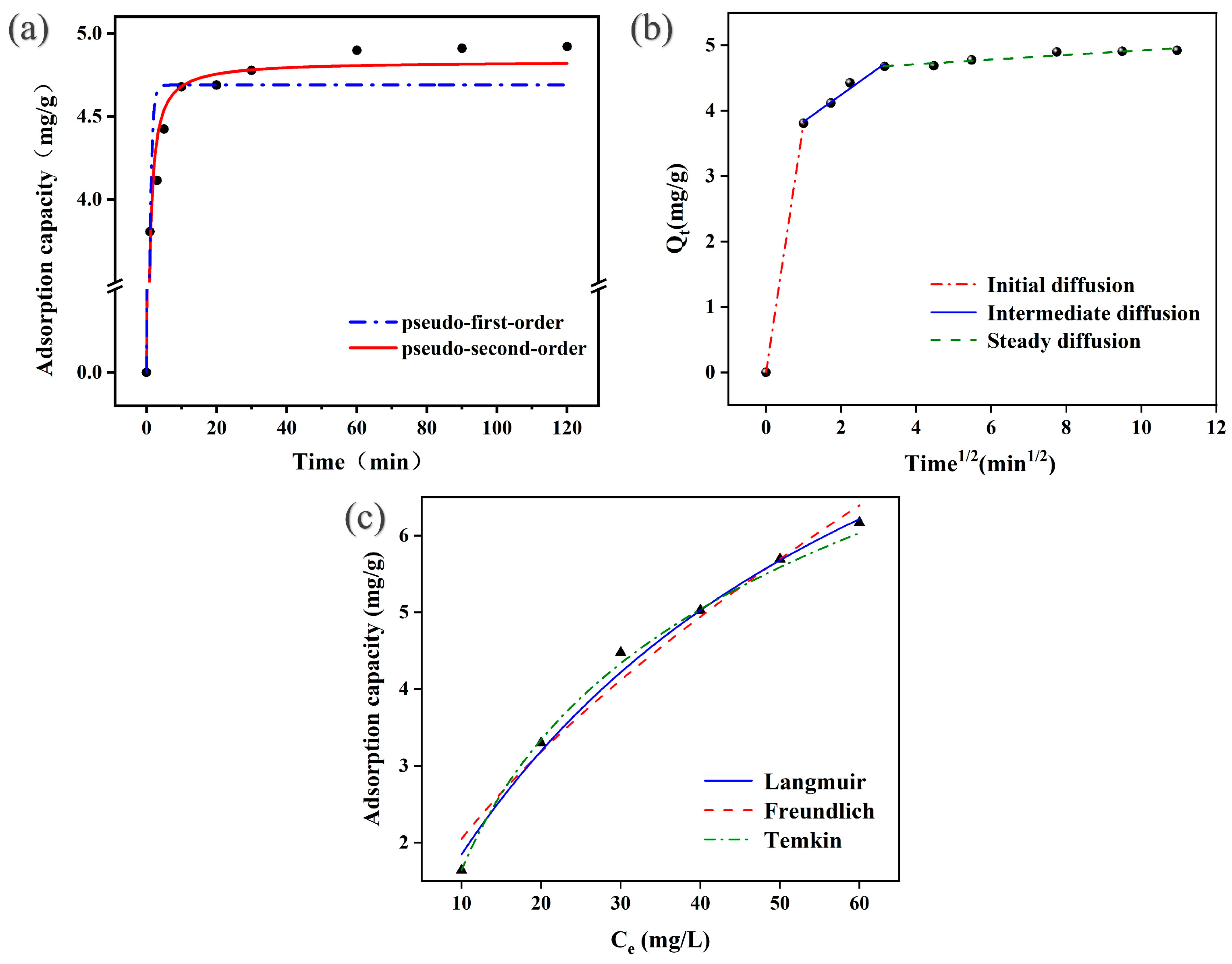
3.2.5. Kinetics and Isotherm Analysis
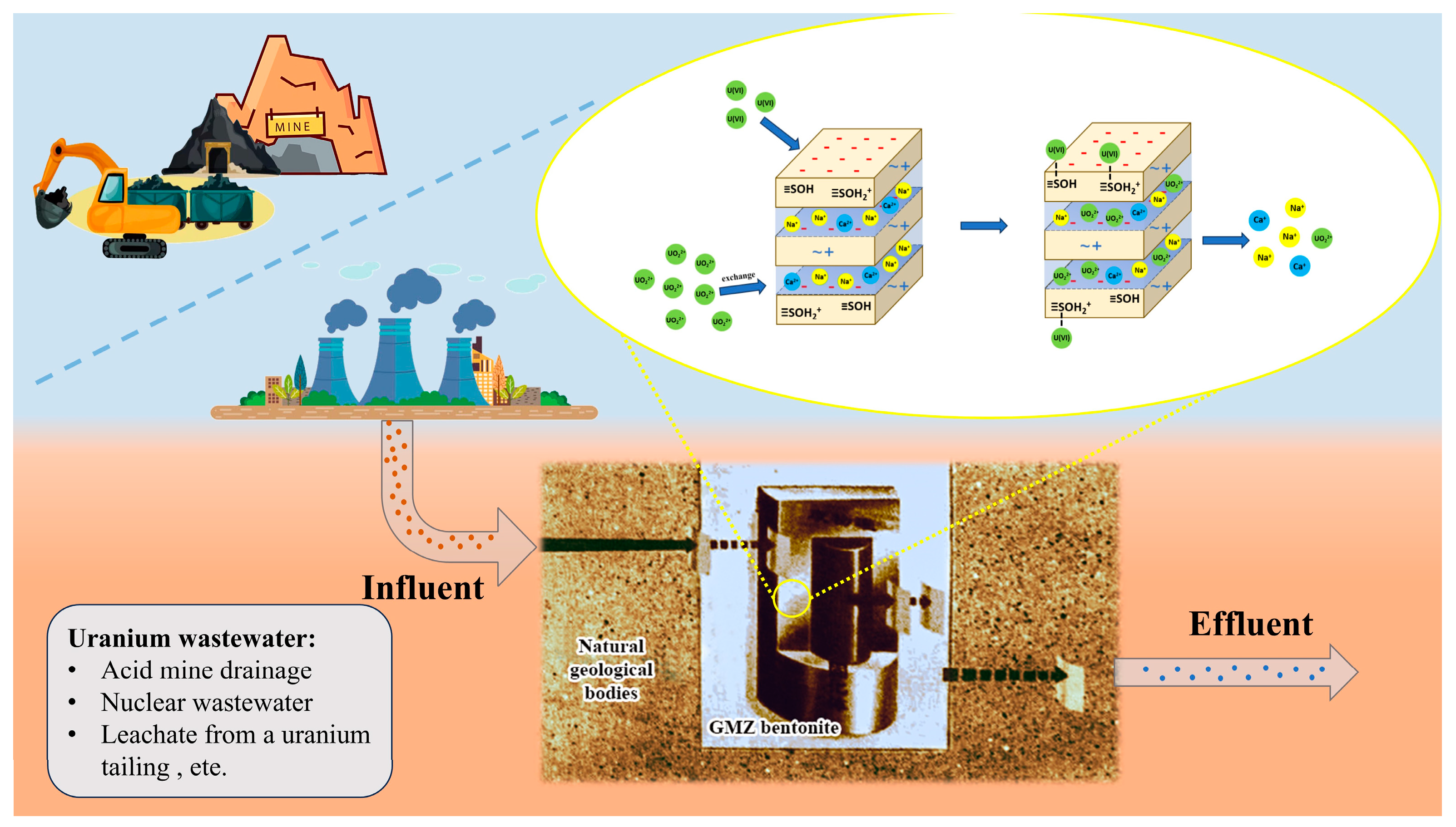
3.2.6. Adsorption Mechanism
- (1)
- (2)
- Ion exchange (taking sodium ion as an example)
- (3)
- The hydrolysis of U(VI) in solution
- (4)
- exchange with hydrolyzed species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, T.; Li, Q.; Li, K.; Zhang, Y.; Zhu, W. Construction of novel magnesium oxide aerogel for highly efficient separation of uranium(VI) from wastewater. Sep. Purif. Technol. 2022, 295, 121296. [Google Scholar] [CrossRef]
- Su, M.; Liu, Z.; Wu, Y.; Peng, H.; Ou, T.; Huang, S.; Song, G.; Kong, L.; Chen, N.; Chen, D. Graphene oxide functionalized with nano hydroxyapatite for the efficient removal of U(VI) from aqueous solution. Environ. Pollut. 2021, 268, 115786. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Kavasi, N.; Sahoo, S.K.; Omori, Y.; Sorimachi, A.; Takahashi, H.; Aono, T. Measurement of uranium isotope ratios in Fukushima-accident contaminated soil samples using multi collector inductively coupled plasma mass spectrometry. J. Environ. Radioact. 2021, 232, 106568. [Google Scholar] [CrossRef]
- Xiong, T.; Jia, L.; Li, Q.; Zhang, Y.; Zhu, W. Efficient removal of uranium by hydroxyapatite modified kaolin aerogel. Sep. Purif. Technol. 2022, 299, 121776. [Google Scholar] [CrossRef]
- Rathod, A.M.; Verpaele, S.; Kelvin, M.; Sullivan, K.V.; Leybourne, M.I. Uranium: An overview of physicochemical properties, exposure assessment methodologies, and health effects of environmental and occupational exposure. Environ. Geochem. Health 2023, 45, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.; Mane, M.; Ravindranathan, S.; Das, A. Polymer Nanorings with Uranium Specific Clefts for Selective Recovery of Uranium from Acidic Effluents via Reductive Adsorption. ACS Sens. 2020, 5, 3254–3263. [Google Scholar] [CrossRef]
- Sheng, G.; Shao, X.; Li, Y.; Li, J.; Dong, H.; Cheng, W.; Gao, X.; Huang, Y. Enhanced removal of uranium(VI) by nanoscale zerovalent iron supported on Na-bentonite and an investigation of mechanism. J. Phys. Chem. A 2014, 118, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ou, T.; Su, M.; Peng, H.; Song, G.; Kong, L.; Chen, D. U(VI) sequestration by Al-rich minerals: Mechanism on phase dependence and the influence of natural organic matter. Chem. Eng. J. 2021, 415, 128858. [Google Scholar] [CrossRef]
- Chen, T.; Shi, P.; Zhang, J.; Li, Y.; Duan, T.; Dai, L.; Wang, L.; Yu, X.; Zhu, W. Natural polymer konjac glucomannan mediated assembly of graphene oxide as versatile sponges for water pollution control. Carbohydr. Polym. 2018, 202, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, Z.; Yuvaraja, G.; Ou, T.; Huang, Y.; Hu, X.; Chen, D. The influence of particle size and natural organic matter on U(VI) retention by natural sand: Parameterization and mechanism study. Sci. Total Environ. 2020, 741, 140292. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Sun, J.; He, H.; Liu, J.; Zhong, Q.; Zeng, Q.; Huang, X.; Wang, J.; Wu, Y.; Chen, D. Uranium re-adsorption on uranium mill tailings and environmental implications. J. Hazard. Mater. 2021, 416, 126153. [Google Scholar] [CrossRef] [PubMed]
- Neiva, A.M.R.; Antunes, I.M.H.R.; Carvalho, P.C.S.; Santos, A.C.T. Uranium and arsenic contamination in the former Mondego Sul uranium mine area, Central Portugal. J. Geochem. Explor. 2016, 162, 1–15. [Google Scholar] [CrossRef]
- Singh, S.P.; Hendry, M.J. Solid-Phase Distribution and Leaching Behaviour of Nickel and Uranium in a Uranium Waste-Rock Piles. Water Air Soil Pollut. 2012, 224, 1360. [Google Scholar] [CrossRef]
- Haakonde, T.; Yabe, J.; Choongo, K.; Chongwe, G.; Islam, M.S. Preliminary Assessment of Uranium Contamination in Drinking Water Sources Near a Uranium Mine in the Siavonga District, Zambia, and Associated Health Risks. Mine Water Environ. 2020, 39, 735–745. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, B.; Qin, Z.; Tang, X.; Feng, Y.; Lv, M.; Miao, S.; Li, H.; Chen, Y.; Chen, F.; et al. Electrochemical removal and recovery of uranium: Effects of operation conditions, mechanisms, and implications. J. Hazard. Mater. 2022, 432, 128723. [Google Scholar] [CrossRef]
- Yuan, T.G.; Chen, D.Y. Experimental Study on the Treatment of Uranium-Contaminated Mine Wastewater Using Zero-Valent Iron Nanoparticles. Appl. Mech. Mater. 2013, 316–317, 516–519. [Google Scholar] [CrossRef]
- Rossiter, H.M.A.; Graham, M.C.; Schäfer, A.I. Impact of speciation on behaviour of uranium in a solar powered membrane system for treatment of brackish groundwater. Sep. Purif. Technol. 2010, 71, 89–96. [Google Scholar] [CrossRef]
- Campbell, K.M.; Gallegos, T.J.; Landa, E.R. Biogeochemical aspects of uranium mineralization, mining, milling, and remediation. Appl. Geochem. 2015, 57, 206–235. [Google Scholar] [CrossRef]
- Coelho, E.; Reis, T.A.; Cotrim, M.; Mullan, T.K.; Correa, B. Resistant fungi isolated from contaminated uranium mine in Brazil shows a high capacity to uptake uranium from water. Chemosphere 2020, 248, 126068. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Bermudez, S.; Villa-Alfageme, M.; Mas, J.L.; Alba, M.D. Comparison of solvent extraction and extraction chromatography resin techniques for uranium isotopic characterization in high-level radioactive waste and barrier materials. Appl. Radiat. Isot. 2018, 137, 177–183. [Google Scholar] [CrossRef]
- Verma, P.K.; Semenkova, A.S.; Krupskaya, V.V.; Zakusin, S.V.; Mohapatra, P.K.; Romanchuk, A.Y.; Kalmykov, S.N. Eu(III) sorption onto various montmorillonites: Experiments and modeling. Appl. Clay Sci. 2019, 175, 22–29. [Google Scholar] [CrossRef]
- Zhu, S.; Tan, Z.; Wei, X.; Tian, Q.; Zhu, Z.; Yang, F.; Henderson, M.J.; Yan, M. Interaction between Gaomiaozi bentonite colloid and uranium. Nucl. Anal. 2022, 1, 100048. [Google Scholar] [CrossRef]
- Neurauter, C.M.J.; Srikanthan, N.; Tong, H.; Behazin, M.; Simpson, M.J. Characterization of natural organic matter in Wyoming-type bentonites irradiated at varied moisture levels. Appl. Geochem. 2024, 170, 106083. [Google Scholar] [CrossRef]
- Zong, P.; Wu, X.; Gou, J.; Lei, X.; Liu, D.; Deng, H. Immobilization and recovery of uranium(VI) using Na-bentonite from aqueous medium: Equilibrium, kinetics and thermodynamics studies. J. Mol. Liq. 2015, 209, 358–366. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Shao, D.; Li, Y.; Xu, Z.; Cheng, C.; Asiri, A.M.; Marwani, H.M.; Hu, S. Adsorption of U(VI) on bentonite in simulation environmental conditions. J. Mol. Liq. 2017, 242, 678–684. [Google Scholar] [CrossRef]
- Dong, L.; Tao, W.; Wei, L.I.U.; Zhongtian, Y. The Influence of γ-Rays Irradiation and Thermal Sequence Aging on the Mineral Composition of GMZ Sodium Bentonite. J. Phys. Conf. Ser. 2020, 1676, 012061. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, R.; He, P.; Pan, Y.; Leng, Y.; Wang, Y.; Liu, Y.; Zhu, M.; Tuo, X. Effect of high-dose γ-ray irradiation on the structural stability and U(VI) adsorption ability of bentonite. J. Radioanal. Nucl. Chem. 2022, 331, 339–352. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Jiang, Q.; Xie, J.; Cao, S.; Zhang, Q.; Gu, R. Effects of γ-ray irradiation on the stability and U(VI) adsorption performance of bentonite colloids. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134318. [Google Scholar] [CrossRef]
- Shi, S.; Liu, J.; Shu, J.; Wu, P.; Zhao, C.; Liu, N.; Lan, T. Uranium(VI) adsorption from carbonate solutions using cetyltrimethylammonium bromide modified purified-bentonite-MOF composite. Appl. Clay Sci. 2023, 241, 106986. [Google Scholar] [CrossRef]
- Houhoune, F.; Nibou, D.; Chegrouche, S.; Menacer, S. Behaviour of modified hexadecyltrimethylammonium bromide bentonite toward uranium species. J. Environ. Chem. Eng. 2016, 4, 3459–3467. [Google Scholar] [CrossRef]
- Hui, J.; Wang, Y.; Liu, Y.; Cao, X.; Zhang, Z.; Dai, Y.; Liu, Y. Effects of pH, carbonate, calcium ion and humic acid concentrations, temperature, and uranium concentration on the adsorption of uranium on the CTAB-modified montmorillonite. J. Radioanal. Nucl. Chem. 2019, 319, 1251–1259. [Google Scholar] [CrossRef]
- Sahu, S.; Mallik, L.; Pahi, S.; Barik, B.; Sahu, U.K.; Sillanpää, M.; Patel, R.K. Facile synthesis of poly o-toluidine modified lanthanum phosphate nanocomposite as a superior adsorbent for selective fluoride removal: A mechanistic and kinetic study. Chemosphere 2020, 252, 126551. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, C.; Tu, H.; Yang, J.; Li, F.; Li, D.; Liao, J.; Yang, Y.; Tang, J.; Liu, N. U(VI) adsorption onto cetyltrimethylammonium bromide modified bentonite in the presence of U(VI)-CO3 complexes. Appl. Clay Sci. 2017, 135, 64–74. [Google Scholar] [CrossRef]
- Parab, H.; Mahadik, P.; Sengupta, P.; Vishwanadh, B.; Kumar, S.D. A comparative study on native and gamma irradiated bentonite for cesium ion uptake. Prog. Nucl. Energy 2020, 127, 103419. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhu, F.; Mao, P.; Wu, Z.; Hong, K. Dispersing Bentonite by Electron Beam Irradiation and Its Adsorption Performance of Cr(VI) in the Aqueous Solution. Water Air Soil Pollut. 2022, 233, 503. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xie, S.-B.; Xia, L.-S.; Tang, Q.; Kang, X.; Huang, F. Study on adsorptive property of bentonite for cesium. Environ. Earth Sci. 2016, 75, 148. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, F.; Chang, L.; Wu, D. Sodium modification of low quality natural bentonite as enhanced lead ion adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129753. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, H.; Li, X.; Jia, Q. Experimental study on sodium modification and purification of GMZ bentonite. Constr. Build. Mater. 2023, 367, 130060. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Ren, P.; Hua, R.; Wu, H.; Xu, M.; Tong, Y. Through-diffusion study of Se(IV) in γ-irradiated bentonite and bentonite–magnetite. J. Radioanal. Nucl. Chem. 2019, 322, 801–808. [Google Scholar] [CrossRef]
- Wm, Y. Uranium Adsorption from Aqueous Solution Using Sodium Bentonite Activated Clay. J. Chem. Eng. Process Technol. 2017, 8, 4. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Huang, Z.; Du, L.; Tan, Z.; Fu, Y.; Wang, X. Sorption and desorption of uranium(VI) on GMZ bentonite: Effect of pH, ionic strength, foreign ions and humic substances. J. Radioanal. Nucl. Chem. 2015, 308, 877–886. [Google Scholar] [CrossRef]
- Yuan, F.; Sun, Z.; Li, C.; Tan, Y.; Zhang, X.; Zheng, S. Multi-component design and in-situ synthesis of visible-light-driven SnO(2)/g-C(3)N(4)/diatomite composite for high-efficient photoreduction of Cr(VI) with the aid of citric acid. J. Hazard. Mater. 2020, 396, 122694. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, S.; Liao, J.; Yan, T.; Liu, Y.; Tang, X. Novel graphene oxide/bentonite composite for uranium(VI) adsorption from aqueous solution. J. Radioanal. Nucl. Chem. 2018, 317, 1349–1360. [Google Scholar] [CrossRef]
- Salah, B.A.; Gaber, M.S.; Kandil, A.h.T. The Removal of Uranium and Thorium from Their Aqueous Solutions by 8-Hydroxyquinoline Immobilized Bentonite. Minerals 2019, 9, 626. [Google Scholar] [CrossRef]
- Nwosu, F.O.; Ajala, O.J.; Owoyemi, R.M.; Raheem, B.G. Preparation and characterization of adsorbents derived from bentonite and kaolin clays. Appl. Water Sci. 2018, 8, 195. [Google Scholar] [CrossRef]
- Bello, O.S.; Owojuyigbe, E.S.; Babatunde, M.A.; Folaranmi, F.E. Sustainable conversion of agro-wastes into useful adsorbents. Appl. Water Sci. 2016, 7, 3561–3571. [Google Scholar] [CrossRef]
- Tu, J.; Peng, X.; Wang, S.; Tian, C.; Deng, H.; Dang, Z.; Lu, G.; Shi, Z.; Lin, Z. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci. Total Environ. 2019, 677, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zhang, B.; Liu, Q.; Li, S.; Yan, H.; Liu, L. Synthesis of a hydrotalcite-like compound from oil shale ash and its application in uranium removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 129–137. [Google Scholar] [CrossRef]
- Song, S.; Yin, L.; Wang, X.; Liu, L.; Huang, S.; Zhang, R.; Wen, T.; Yu, S.; Fu, D.; Hayat, T.; et al. Interaction of U(VI) with ternary layered double hydroxides by combined batch experiments and spectroscopy study. Chem. Eng. J. 2018, 338, 579–590. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Wang, H.; Xie, Y.; Wakeel, M.; Wahid, A.; Zhang, X. Gamma-ferric oxide nanoparticles decoration onto porous layered double oxide belts for efficient removal of uranyl. J. Colloid Interface Sci. 2019, 535, 265–275. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Xiao, C.; Shao, D.; Xu, Z.; Wang, J.; Hu, S.; Li, X.; Wang, W. Polyaniline (PANI) modified bentonite by plasma technique for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2017, 411, 331–337. [Google Scholar] [CrossRef]
- Fan, Q.H.; Hao, L.M.; Wang, C.L.; Zheng, Z.; Liu, C.L.; Wu, W.S. The adsorption behavior of U(vi) on granite. Environ. Sci. Process. Impacts 2014, 16, 534–541. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, Z.; Xiao, Z. U(VI) sorption on illite: Effect of pH, ionic strength, humic acid and temperature. J. Radioanal. Nucl. Chem. 2014, 303, 867–876. [Google Scholar] [CrossRef]
- Ivanov, P.; Griffiths, T.; Bryan, N.D.; Bozhikov, G.; Dmitriev, S. The effect of humic acid on uranyl sorption onto bentonite at trace uranium levels. J. Environ. Monit. 2012, 14, 2968–2975. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Zhao, W.; Xu, J. Sorption behavior of U(VI) onto Chinese bentonite: Effect of pH, ionic strength, temperature and humic acid. J. Mol. Liq. 2013, 188, 178–185. [Google Scholar] [CrossRef]
- Medhi, H.; Bhattacharyya, K.G. Retracted Article: Kinetic and mechanistic studies on adsorption of Cu(ii) in aqueous medium onto montmorillonite K10 and its modified derivative. New J. Chem. 2017, 41, 13533–13552. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W. Evaluating the adsorption of Shanghai silty clay to Cd(II), Pb(II), As(V), and Cr(VI): Kinetic, equilibrium, and thermodynamic studies. Environ. Monit. Assess. 2021, 193, 131. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Shakil, N.A.; Dutta, A.; Kumar, J.; Saini, M.K. Sorption kinetics and isotherm modelling of imidacloprid on bentonite and organobentonites. J. Environ. Sci. Health B 2017, 52, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.; Wu, Y.; Han, W.; Kong, L.; Song, G.; Chen, D.; Su, M. Synthesis of thickness-controllable polydopamine modified halloysite nanotubes (HNTs@PDA) for uranium (VI) removal. J. Hazard. Mater. 2022, 424, 127208. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Kamboh, M.A.; Arian, S.S.; Alrefaei, A.F.; Alkhamis, H.H.; Albeshr, M.F.; Cho, J.; Barghi, A.; Amiri, I.S. Nitrile-calixarene grafted magnetic graphene oxide for removal of arsenic from aqueous media: Isotherm, kinetic and thermodynamic studies. Chemosphere 2021, 268, 129348. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, Z.; Guo, Y.; Zhang, Y.; Zhao, Y.; Xin, Y. Adsorption Characteristics And Mechanism of U(Vi) In Water By Dopamine Hydrochloride Modified Bentonite. Water Air Soil Pollut. 2024, 235, 353. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Lekshmi, G.S.; Shainy, F. Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J. Colloid Interface Sci. 2019, 534, 248–261. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, D.; Kong, L.; Tsang, D.C.W.; Su, M. Rapid and effective removal of uranium (VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J. Hazard. Mater. 2019, 371, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.; Rodríguez-Castellón, E.; Algarra, M.; Rocha, F.; Bobos, I. Influence of pH, layer charge location and crystal thickness distribution on U(VI) sorption onto heterogeneous dioctahedral smectite. J. Hazard. Mater. 2016, 317, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ouyang, J.; Liu, Z.; Huang, G.; Wang, Y.; Li, Z.; Adesina, A.A. Highly efficient sorption of U(VI) from aqueous solution using amino/amine-functionalized magnetic mesoporous silica nanospheres. J. Radioanal. Nucl. Chem. 2019, 319, 987–995. [Google Scholar] [CrossRef]
- Song, W.; Liu, M.; Hu, R.; Tan, X.; Li, J. Water-soluble polyacrylamide coated-Fe3O4 magnetic composites for high-efficient enrichment of U(VI) from radioactive wastewater. Chem. Eng. J. 2014, 246, 268–276. [Google Scholar] [CrossRef]
- Guimarães, V.; Rodríguez-Castellón, E.; Algarra, M.; Rocha, F.; Bobos, I. Kinetics of uranyl ions sorption on heterogeneous smectite structure at pH4 and 6 using a continuous stirred flow-through reactor. Appl. Clay Sci. 2016, 134, 71–82. [Google Scholar] [CrossRef]
- Xie, J.; Gu, R.; Jiang, Q.; Cao, S.; Zhang, Q.; Luo, H.; Cheng, J. Highly efficient removal of uranium (VI) from aqueous solutions by amino functionalized bentonite. J. Radioanal. Nucl. Chem. 2024, 333, 1301–1314. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, J.; Shehzad, H.; Le, Z.; Li, Z.; Adesina, A.A. Adsorption of U(VI) onto the carboxymethylated chitosan/Na-bentonite membranes: Kinetic, isothermic and thermodynamic studies. J. Radioanal. Nucl. Chem. 2018, 317, 1377–1385. [Google Scholar] [CrossRef]
- Missana, T.; Alonso, U.; García-Gutiérrez, M. Evaluation of component additive modelling approach for europium adsorption on 2:1 clays: Experimental, thermodynamic databases, and models. Chemosphere 2021, 272, 129877. [Google Scholar] [CrossRef]



| Country | Source | Concentration of Uranium (mg/L) |
|---|---|---|
| China | Uranium mine wastewater in western [16] | 10.00 |
| A mine wastewater in Shaoguan [17] | 2.68 | |
| Leachate from a uranium tailing | 19.80 | |
| Australia | A uranium-contaminated groundwater [18] | 0.29 |
| United States | Uranium wastewater from the Crow Butte ISR sites [19] | 12.20 |
| Brazil | Mine drainage from the Osam Usumi mine [20] | 1.05–4.46 |
| Material | SBET (m2/g) | Aperture (nm) | Hole Capacity (cm3/g) |
| Bento N | 19.9368 | 8.9857 | 0.0562 |
| Bento EB | 22.4455 | 9.0902 | 0.0656 |
| Bento γ | 23.0011 | 9.0032 | 0.0675 |
| Kinetic Parameters | Parameter | Numeric |
|---|---|---|
| Pseudo-first-order | K1 (1/min) | 1.5966 |
| qe (mg/g) | 4.69 | |
| R2 | 0.9713 | |
| Pseudo-second-order | K2 (g/(mg·min)) | 0.6414 |
| qe (mg/g) | 4.83 | |
| R2 | 0.9927 | |
| Particle diffusion model | K1 (mg/(kg·min0.5)) | 3.8068 |
| K2 (mg/(kg·min0.5)) | 0.4111 | |
| R32 | 0.8797 |
| Isotherm Parameters | Parameter | Numeric |
|---|---|---|
| Langmuir | qm (mg/g) | 11.7984 |
| KL (L/mg) | 0.0186 | |
| R2 | 0.9947 | |
| Freundlich | KF (mg·g−1) (Lm·g−1)1/n | 0.4745 |
| 1/n | 0.6352 | |
| R2 | 0.9948 | |
| Temkin | KT (L/mg) | 0.1955 |
| BT (KJ/mol) | 1.0110 | |
| R2 | 0.9804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Song, G.; Mo, Y.; Wang, S.; Chen, D.; Su, M. Irradiated Gao Miao Zi Bentonite for Uranium Retention: Performance and Mechanism. Separations 2025, 12, 1. https://doi.org/10.3390/separations12010001
Zhang Y, Song G, Mo Y, Wang S, Chen D, Su M. Irradiated Gao Miao Zi Bentonite for Uranium Retention: Performance and Mechanism. Separations. 2025; 12(1):1. https://doi.org/10.3390/separations12010001
Chicago/Turabian StyleZhang, Yushan, Gang Song, Yujie Mo, Shuwen Wang, Diyun Chen, and Minhua Su. 2025. "Irradiated Gao Miao Zi Bentonite for Uranium Retention: Performance and Mechanism" Separations 12, no. 1: 1. https://doi.org/10.3390/separations12010001
APA StyleZhang, Y., Song, G., Mo, Y., Wang, S., Chen, D., & Su, M. (2025). Irradiated Gao Miao Zi Bentonite for Uranium Retention: Performance and Mechanism. Separations, 12(1), 1. https://doi.org/10.3390/separations12010001