Influence of Organic Carbon from Weathered Sediments on Triclocarban Distribution in Environmental Aqueous Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Natural Samples
| Water | pH | C.e. µS/cm | Na+, mg/L | K+, mg/L | Ca2+, mg/L | HCO3− mg/L | Mg2+, mg/L |
|---|---|---|---|---|---|---|---|
| 1 | 7.3 | 290 | 2.68 ± 0.25 | 1.79 ± 0.14 | 56.1 ± 0.35 | 212 ± 20.5 | 11.5 + 0.09 |
| 2 | 7.1 | 500 | 5.46 + 0.28 | 1.55 ± 0.09 | 40 ± 0.29 | 209 ± 18.2 | 13.9 + 0.11 |
| 3 | 6.3 | 950 | 10.96 ± 0.55 | 3.93 ± 0.25 | 287 ± 20.5 | 1305 ± 100.5 | 89.0 + 0.07 |
| 4 | 6.7 | 700 | 25.06 + 5.0 | 3.46 ± 0.20 | 192 ± 15.2 | 914 ± 80.7 | 32.7 + 0.02 |
| 5 | 5.9 | 1350 | 55.85 + 9.2 | 8.22 ± 0.74 | 130 ± 9.7 | 1044 ± 101.6 | 29.7 + 0.025 |
3.2. Characterization of the Weathered Sediments
| Weathered Sediment | Specific Surface 1 SBET, m2∙g−1 | pHPZC 2 | Carboxylic Groups Conc. (mmol ∙g−1) | Lactone Groups Conc. (mmol∙g−1) | Phenolic Groups Conc. (mmol∙g−1) |
|---|---|---|---|---|---|
| At the beginning | 110 | 8.5 | - | - | - |
| After one month | 135 | 8.9 | 0.05 | 0.03 | 0.02 |
| After three months | 174 | 5.7 | 4.5 | 1.20 | 0.65 |
| After 6 months | 208 | 2.8 | 5.44 | 1.40 | 1.72 |
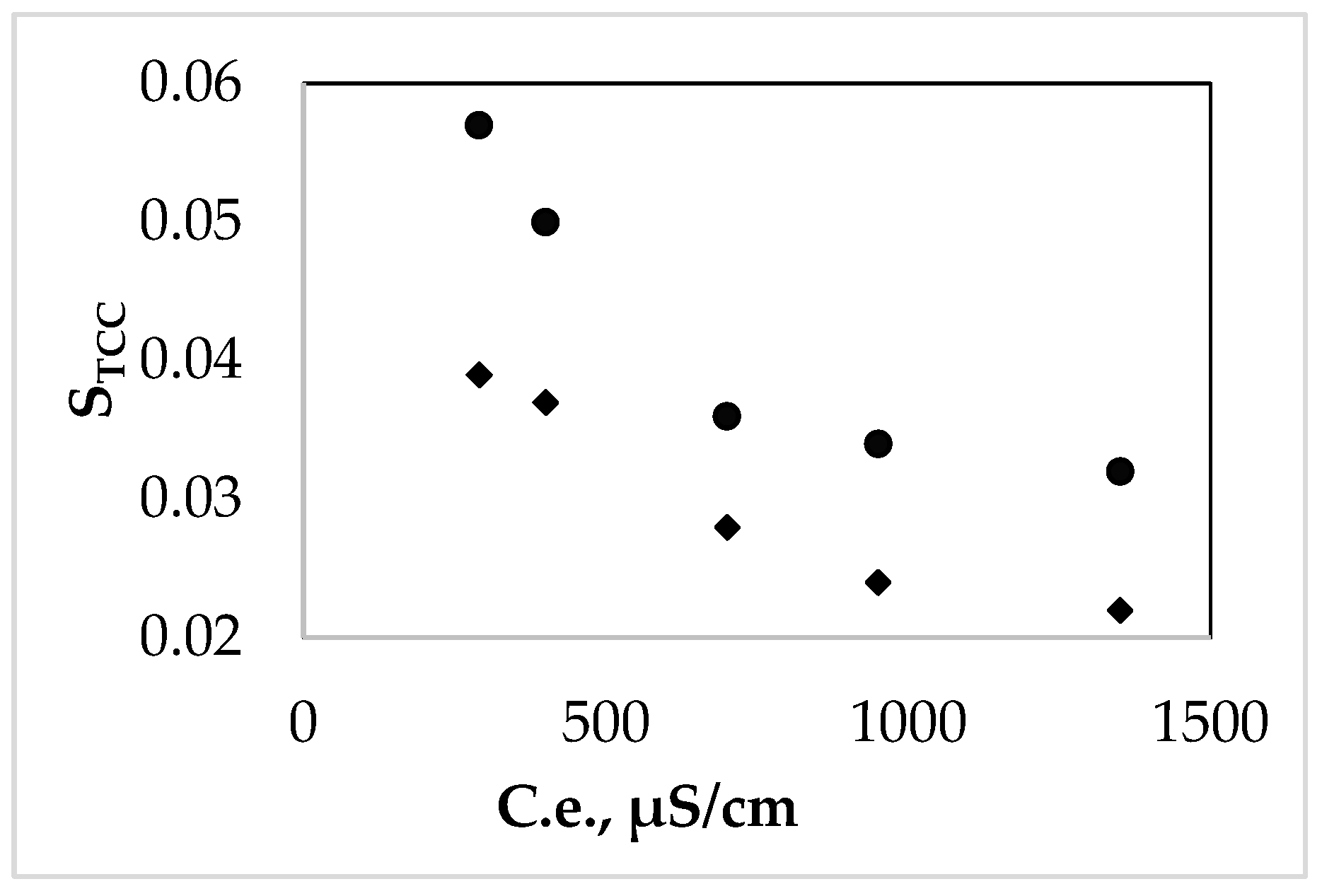
3.3. Influence of the Chemical Matrix of the Natural Water over TCC Solubility
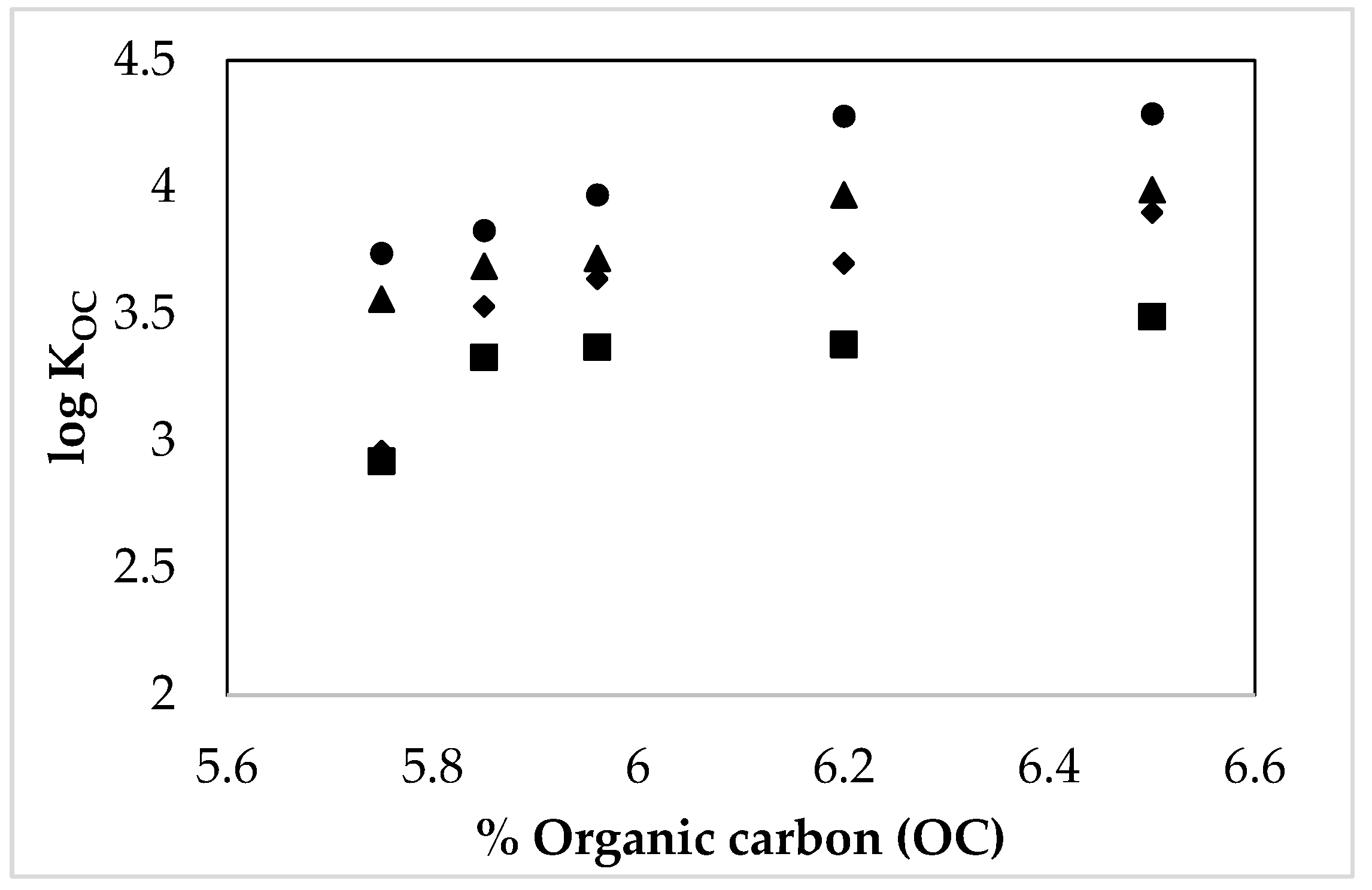
3.4. Influence of the Environmental Parameters over the TCC Solubility and Distribution in the Studied Natural Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gunasekara, A.S.; Troung, T. Environmental Fate of Fipronil; Environmental Monitoring Branch; California Environmental Protection Agency: Sacramento, CA, USA, 2005.
- Halden, R.U.; Paul, D.H. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 39, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, K.; Seethapan, S.; Pugazhendhi, A. Fate of triclocarban in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pes, K.; Friese, A.; Cox, C.J.; Laize, V.; Fernanadez, I. Biochemical and molecular responses of the Mediterranean mussel to short-term exposure to three commonly prescribed drugs. Mar. Environ. Res. 2021, 168, 105309. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lin, C.; Lei, K.; Xin, M.; Wang, B.; Ouyang, W.; Liu, X.; He, M. Endocrine disrupting chemicals in a typical urbanized bay of Yellow Sea, China: Distribution, risk assessment and identification of priority pollutants. Environ. Pollut. 2021, 287, 117588. [Google Scholar] [CrossRef]
- Schenker, U.; Macleod, M.; Scheringer, M.; Hungerbuhler, K. Improving data quality for environmental fate models a least squares adjustement procedure for harmonizing physico chemical properties of organic compounds. Environ. Sci. Technol. 2005, 39, 8434–8441. [Google Scholar] [CrossRef]
- Razzaque, M.M.; Gratwohl, P. Predicting organic carbon-water patitioning of hydrophobic organic chemicals in soils and sediments based on water solubility. Water Res. 2008, 42, 3775–3780. [Google Scholar] [CrossRef]
- Qian, Y.; Xia, S.; Ma, P. Octanol/water partition coefficient of substituted benzene derivatives containing halogens and carboxyl-determination using the shake-flask method and estimation using fragment method. J. Chem. Eng. Data 2008, 53, 280–282. [Google Scholar]
- Niederer, C.; Goss, K.U. Effect of ortho-chlorine substitution on the partition behavior of chlorophenols. Chemosphere 2008, 71, 697–702. [Google Scholar] [CrossRef]
- Ion, I.; Senin, R.M.; Ivan, G.R.; Radulescu, I.; Ion, A.C. Effect of ionic strength and natural organic matter in aqueous solutions over the sorption of organic contaminants on irradiated and pristine carbon nanomaterials. U.P.B. Sci. Bull. Ser. B Chem. Mater. Sci. 2018, 80, 17–30. [Google Scholar]
- Ivan, G.R.; Capra, L.; Ion, I.; Ion, A.C. Effects of pH, temperature, ionic strength and organic matter on triclocarban solubility. J. Environ. Eng. Landsc. Manag. 2021, 29, 244–250. [Google Scholar] [CrossRef]
- Capra, L.; Manolache, M.; Ion, I.; Ion, A.C. Validation of a method for determination of antimony in drinking water by ICP-OES. U.P.B. Sci. Bull. Ser. B Chem. Mater. Sci. 2016, 78, 103–112. [Google Scholar]
- Ion, A.C.; Ion, I.; Antonisse, M.M.G.; Snellink-Ruel, B.H.M.; Reinhoudt, D.N. Characteristics of fluoride-selective electrode with uranyl salophen receptors in aqueous solutions. Russ. J. Gen. Chem. 2001, 71, 159–161. [Google Scholar] [CrossRef]
- Ion, I.; Culetu, A.; Costa, J.; Luca, C.; Ion, A.C. Polyvinyl chloride-based membranes of 3,7,11-tris (2-pyridylmethyl)-3,7,11,17-tetraazabicyclo [11.3.1] heptadeca-1(17),13,15-triene as a Pb(II)-selective sensor. Desalination 2010, 259, 38–43. [Google Scholar] [CrossRef]
- Capra, L.; Manolache, M.; Ion, I.; Stoica, R.; Stinga, G.; Doncea, S.M.; Alexandrescu, E.; Somoghi, R.; Calin, M.R.; Radulescu, I.; et al. Adsorption of Sb (III) on oxidized exfoliated graphite nanoplatelets. Nanomaterials 2018, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Droppo, I.G.; Ongley, E.D. The state of suspended sediments in the fresh water fluvial environment—A method of analysis. Water Res. 1992, 26, 65–72. [Google Scholar] [CrossRef]
- Philips, J.M.; Russel, M.A.; Walling, D. Time-integrated sampling of fluvial suspended sediment: A simple methodology for small catchments. Hydrol. Process. 2000, 14, 2589–2602. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Product Properties Test Guidelines, OPPTS 830:7840, Water Solubility: Column Elution Method: Shake Flask Method; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1996.
- Walling, D.E. Physical and chemical properties of sediment: The quality dimension. Int. J. Sediment Res. 1989, 4, 27–39. [Google Scholar]
- Phillips, J.M.; Walling, D.E. The particle size characteristics of fine-grained channel deposits in the river Exe Basin, Devon UK. Hydrol. Process. 1999, 13, 1–19. [Google Scholar] [CrossRef]
- McLeod, P.B.; Van Den Heuvel-Greve, M.J.; Allen King, M.R.; Luoma, S.N.; Luthy, G.R. Effects of particulate carbonaceous matter on the bioavailability of benzopyrene and 2,2’,5,5’-tetrachlorobiphenyl to the clam, Macoma balthica. Environ. Sci. Technol. 2000, 34, 1729–1736. [Google Scholar]
- Ghosh, U. In-situ sorbent amendments: A new direction in contaminated sediment management. Environ. Sci. Technol. 2011, 45, 1163–1168. [Google Scholar] [CrossRef]
- Calin, M.R.; Radulescu, I.; Ion, A.C.; Sirbu, F. Radiochemical investigations on natural mineral waters from Bucovina region, Romania. Rom. J. Phys. 2016, 61, 1051–1066. [Google Scholar]
- Quach, N.K.N.; Yang, W.D.; Chung, Z.J.; Tran, H.L. The influence of the activation temperature on the structural properties of the activated carbon xerogels and their electrochemical performance. Ann. Mater. Sci. Eng. 2017, 9, 8308612. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent advances in carbon-nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Snyder, E.H. Fate, Transport and Risk Assessment of Biosolids-Borne Triclocarban (TCC). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2009. [Google Scholar]
- Yousefi, N.; Lu, X.; Elimelech, M. Environmental performance of of graphene-based 3D nanostructures. Nat. Nanotechnol. 2019, 14, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spongberg, A.L.; Witter, J.D. Adsorption and degradation of triclosan and triclocarban in soils and biosolids-amended soils. J. Agric. Food Chem. 2009, 57, 4900–4905. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Lin, X.; Yang, K.; Lin, D. The role of humic acid in stabilizing fullerene C60 suspensions. J. Zhejiang Univ.—Sci. A Appl. Phys. Eng. 2014, 15, 634–642. [Google Scholar] [CrossRef]
- Duncan, L.K.; Jinscheck, J.R.; Vikesland, P.J. C60 colloid formation in aqueous systems: Effects of preparation method on size, structure and surface charge. Environ. Sci. Technol. 2008, 42, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, N.; Yang, K.; Xing, B.; Wu, F. Different stabilities of multiwalled carbon nanotubes in fresh surface water samples. Environ. Pollut. 2010, 158, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Li, Q.L. Characterizing photochemical transformation of aqueous nC60 under environmentally relevant conditions. Environ. Sci. Technol. 2010, 44, 3008–3013. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Influence of humic acid on the aggregation kinetics of fullerene C60 nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 2007, 309, 126–134. [Google Scholar] [CrossRef]

| Component | Value |
|---|---|
| Humidity (105 °C), % | 5.48 ± 0.02 |
| Inorganic Carbon IC, % | 2.47 ± 0.16 |
| Organic Carbon TOC, % | 5.75 ± 0.15–6.75 ± 0.17, weathered in time |
| Ca % | 5.19 |
| Mg % | 2.04 |
| K % | 1.31 |
| Fe % | 2.11 |
| Contact Time (Stirring), h | TCC Concentration, µg/L | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples 1 | Samples 2 | Samples 3 | Samples 4 | Samples 5 | ||||||
| 25 °C | 5 °C | 25 °C | 5 °C | 25 °C | 5 °C | 25 °C | 5 °C | 25 °C | 5 °C | |
| 1 | 23.4 | 22.3 | 9.39 | 4.7 | 23.4 | 9.8 | 10.4 | 7.5 | 21.6 | 31 |
| 3 | 25.8 | 42.9 | 26.2 | 13.5 | 34.9 | 16.6 | 32.8 | 11.4 | 34.7 | 53.3 |
| 4 | 27.3 | 57.3 | 36.6 | 14.7 | 33.2 | 23.2 | 36.3 | 19.0 | 38.6 | 54.6 |
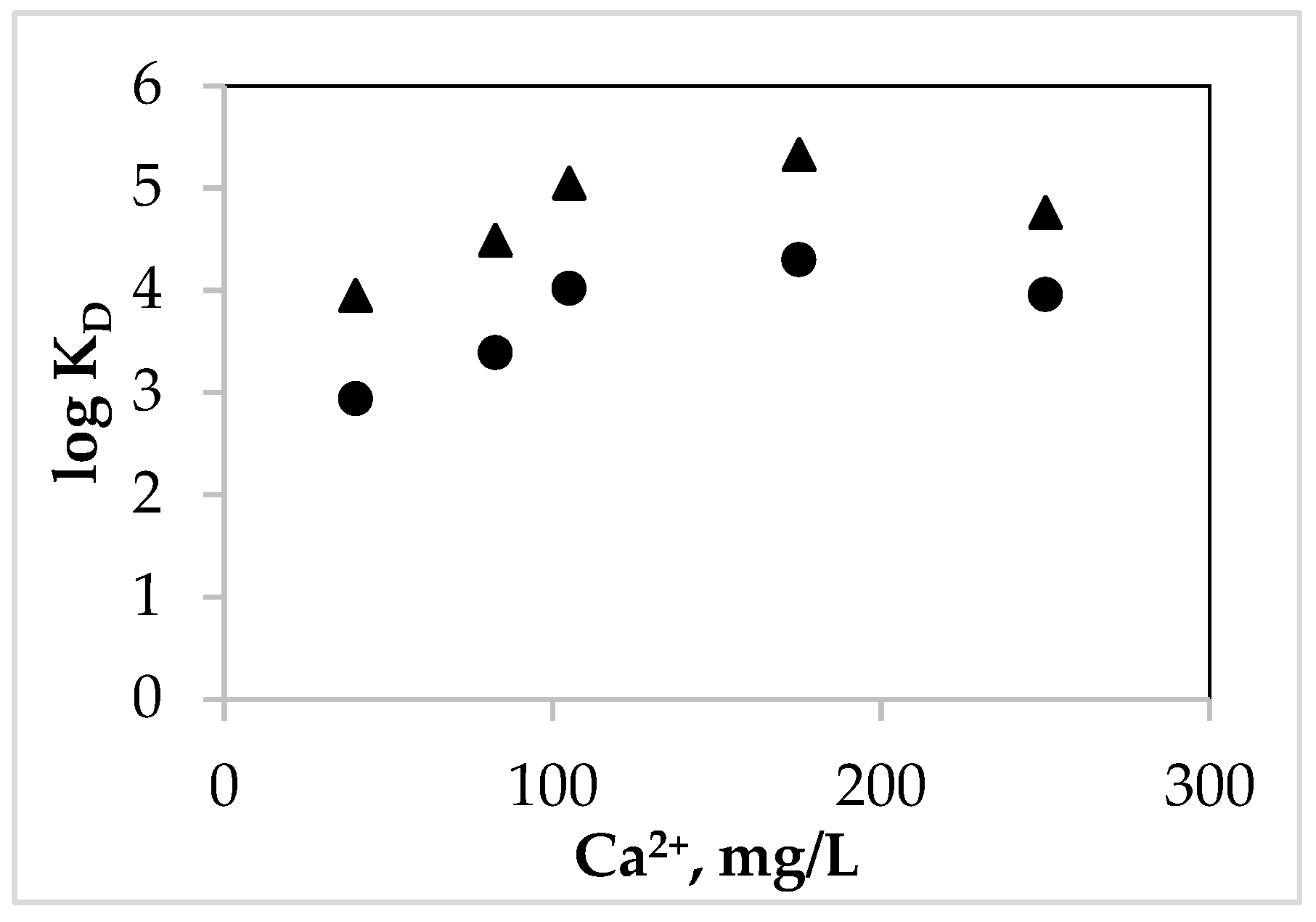
| Na+ mg/L | Ca2+ mg/L | Log KD, S 1 | Log KD, S 2 |
|---|---|---|---|
| 2.50 | 40.00 | 2.94 ± 0.26 | 3.90 + 0.32 |
| 4.10 | 82.00 | 3.39 ± 0.24 | 4.49 + 0.30 |
| 7.15 | 250.00 | 3.96 ± 0.19 | 4.76 + 0.30 |
| 95.00 | 105.00 | 4.02 ± 0.20 | 5.05 + 0.26 |
| 50.60 | 175.00 | 4.27 ± 0.19 | 5.33 + 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, I.; Senin, R.M.; Ion, A.C. Influence of Organic Carbon from Weathered Sediments on Triclocarban Distribution in Environmental Aqueous Systems. Separations 2024, 11, 255. https://doi.org/10.3390/separations11090255
Ion I, Senin RM, Ion AC. Influence of Organic Carbon from Weathered Sediments on Triclocarban Distribution in Environmental Aqueous Systems. Separations. 2024; 11(9):255. https://doi.org/10.3390/separations11090255
Chicago/Turabian StyleIon, Ion, Raluca Madalina Senin, and Alina Catrinel Ion. 2024. "Influence of Organic Carbon from Weathered Sediments on Triclocarban Distribution in Environmental Aqueous Systems" Separations 11, no. 9: 255. https://doi.org/10.3390/separations11090255
APA StyleIon, I., Senin, R. M., & Ion, A. C. (2024). Influence of Organic Carbon from Weathered Sediments on Triclocarban Distribution in Environmental Aqueous Systems. Separations, 11(9), 255. https://doi.org/10.3390/separations11090255





