Abstract
Methods for obtaining high-purity perrhenic acid (with metallic impurities content below 100 ppm) of a high concentration > 200 g/dm3 and entirely from secondary raw materials were compared. Comparative analyses of three methods were performed: electrodialysis, solvent extraction (research carried out directly as part of the Small Grant project acronym RenMet), and ion-exchange (developed as part of previous projects implemented by Łukasiewicz-IMN). The basic process parameters were selected as comparative indicators: efficiency and selectivity of the process, purity of the obtained product, availability and consumption of raw materials and reagents, equipment necessary to carry out the process, the profitability of the technology, and the ecological aspects, i.e., the possibility of managing the generated solid waste and post-production solutions. Analysis of the verified indicators allowed us to select the most economically and ecologically advantageous method of obtaining high-purity perrhenic acid from secondary raw materials. Its preparation using the ion-exchange method emphasizes the product’s purity and the process’s simplicity, using readily available waste materials and renewable ion-exchange resin, and is based on a sustainable circular economy.
1. Introduction
Perrhenic acid is an inorganic chemical compound, that is a colorless or slightly yellow liquid, highly soluble in water and organic solvents [1,2]. It has weak oxidizing properties and forms numerous salts, such as perrhenate salts with metals such as Cu [3], Ni [4], Co [5], Pb [6], Zn [4], and Li [7] or Na [8], K [9], and Ca [10], as well as with the ammonium cation NH4+ [11]. Perrhenic acid, ammonium perrhenate, and metallic rhenium are among the most frequently produced rhenium products and constitute the substrate for the production of other rhenium-containing components with specific desired properties often used in niche industries, e.g., catalysis, pharmacy, and medicine [12,13,14]. The application of perrhenate acid requires its use as a reagent with high catalytic purity and a high concentration in the solution.
The production of perrhenic acid is a troublesome process, not only due to the possibility of significant losses of rhenium but primarily due to the possibility of its contamination, mainly with cations such as NH4+, Na, Mg, Al, K, Ca, Ni, Co, Fe, Zn, Cu, and Cr. The production of perrhenic acid with higher concentrations, most often carried out by concentration, also causes an increase in the number of impurities in the finished product. Such perrhenic acid usually does not meet the applicable quality standards.
Many methods of obtaining perrhenic acid differ in the initial substrate, purity and concentration of the product, and the techniques used.
One of the common methods for the production of perrhenic acid involves the direct dissolution of metallic rhenium or its oxides, most often in solutions of hydrochloric acid with the addition of a strong oxidant, e.g., nitric acid or hydrogen peroxide [15], directly in concentrated nitric acid [16] or in H2O2 solutions [17].
As-obtained perrhenic acid solution has a low concentration; therefore, to obtain commercial products, it is required to concentrate it and remove the excess acids used. There is also a method consisting first of the oxidative roasting of rhenium-bearing materials, such as rhenium sulfide, and then the production of volatile rhenium(VII) oxide, which, after adsorption in water, forms perrhenate acid [18], or the direct dissolution of rhenium(VII) oxide in water [19].
These methods require the use of expensive and complex equipment that, in the case of metallic rhenium, are produced by reducing ammonium perrhenate. The purity of the perrhenic acid obtained in this way is determined by the purity of the Re2O7 substrate, which is a very hygroscopic substance that is difficult to obtain and store [20].
Perrhenic acid can also be obtained by solvent extraction [21,22,23]. Literature reports on the preparation of perrhenic acid by solvent extraction are scarce. Most of them are based on the extraction of rhenium and then the conversion of it into the form of ammonium perrhenate by re-extraction with ammonia solutions, not on the direct preparation of perrhenic acid.
Methods for obtaining perrhenic acid directly by solvent extraction often use acidic ammonium perrhenate solutions, from which perrhenate ions are extracted using selected, dedicated extractants. Most often, a 50% solution of tributyl phosphate (TBP) in toluene is used as an extractant [24,25], but tri-n-octylamine, bis-iso-dodecyl amine, pyridine, Aliquat 336, trioctylphosphine oxide, cyclohexanone, ethyl, xanthate, and mesityl oxide [26,27], as well as tetraphenylphosphonium chloride or triphenylbenzylphosphonium chloride, can also be used [28]. To re-extract rhenium from organic-phase acid, basic solutions are used, i.e., HCl, H2SO4, and perrhenates are extracted with NH4OH when ammonium perrhenate is produced or NaOH and KOH when the products are NaReO4 and KReO4, respectively [17]. The production of high-purity perrhenic acid by solvent extraction needs to be further condensed. The process of perrhenic acid concentration causes an increase in the content of impurities in proportion to the degree of its concentration as well. A disadvantage of these methods is often the large number of operations, which generate losses of rhenium, increase the possibility of contamination of the final product, and extend the time needed to obtain it.
There are also known methods of obtaining perrhenic acid using the electrodialysis process [28,29,30]. Most often, the process of concentrated perrhenic acid production by electrodialysis is carried out using solutions of rhenium salts: ammonium or potassium perrhenate, as well as sodium with a rhenium concentration of 35–40 g/dm3. The process is carried out in multi-chamber electrodialysis with alternating anion-exchange (MA-40) and cation-exchange (MK-40) membranes, a graphite or platinum anode, and a stainless steel or platinum cathode. The most commonly used electrode solutions in the anode chamber are hydrochloric acid at a concentration of 10 g/dm3 [28,29] or perrhenic acid solutions [30,31] with a concentration of about 1 g/dm3 [28,29,32], while in the cathode chamber, 0.1 M potassium hydroxide solution [30] and a sodium hydroxide solution with a concentration of 100 g/dm3 are used [31]. By using these methods, it is possible to obtain a concentration of up to 600 g/dm3 of perrhenic acid; however, due to the reduction in the selectivity of the process and the mechanical stability of the membranes as well as the current efficiency of the process (increasing electricity consumption), the production of perrhenic acid with a concentration higher than 350 g/dm3 is unprofitable. The disadvantage of this method is not only the use of many expensive ion-exchange membranes to construct one membrane cell but also their rapid wear and destruction of and reduction in selectivity.
There is a method of HReO4 production by electrodialysis that limits the process inconveniences described in the abovementioned publications, which eliminates losses of rhenium [33]. Using bipolar membranes (BMEDs) enables the production of acid and base from solutions of their salts. The process is carried out in an electrodialyser of two main production streams (diluate and concentrate) and two near-electrode streams (catholyte and anolyte). The stream solutions circulate in a closed circuit and are separated from each other by cation-exchange, anion-exchange, and bipolar membranes. Ammonium perrhenate solution with an initial rhenium ions concentration of 5–150 g/dm3 circulates as a diluate, and the concentrate solution is perrhenic acid at a concentration of about >5 g/dm3. Under the influence of electric voltage, perrhenate ions migrate selectively through the membranes. This allows for obtaining a concentrate enriched in ReO4− ions and thus perrhenic acid, with a rhenium ion concentration of up to 130 g/dm3. In contrary to numerous advantages, the disadvantage of this process is the insufficient purity of the obtained product of perrhenic acid. It does not meet the quality standards for HReO4 required by customers, i.e., the content of total impurities < 100 ppm, including the content of each impurity ≤ 10 ppm.
Another group of methods for obtaining perrhenic acid are techniques based on the ion-exchange process using ion-exchange resins. This has been extensively researched and described and is most often used in industry [34,35,36]. The method involves passing an ammonium perrhenate solution through a strongly acidic cation resin bed, primarily prepared in hydrogen form. During sorption, ammonium, potassium, or sodium ions are sorbed. The process is carried out at a temperature of 30–50 ℃. The effluent from the column is a HReO4 solution containing up to 20 g/dm3 Re, which is then directed to concentration by evaporation under reduced pressure to obtain acid of the desired concentration. For further purification of perrhenic acid, active carbon is used [34].
The disadvantage of all these methods of perrhenic acid production by ion-exchange is low solubility of substrates such as ammonium, potassium, or sodium perrhenate in water [37,38,39]. The low concentration of rhenium in the starting solutions influences the low concentration of perrhenic acid obtained. Thus, further concentration under pressurized conditions by evaporation is necessary, and it requires the use of evaporators that contain large amounts of energy.
Since all known and used methods for obtaining perrhenic acid have both advantages and disadvantages, it cannot be indicated which is the best and which allows for the production of perrhenic acid at both a high concentration and high purity. Also, using rhenium-bearing waste as a source of rhenium requires verification of all three methods to select the most economically and ecologically advantageous one for the production of high-purity perrhenic acid from secondary raw materials. Thus, in this article, a comprehensive analysis of production methods of high-purity and high-concentration perrhenic acid only from secondary materials is attempted.
2. Materials and Methods
Ammonium perrhenate used in the research was obtained by leaching superalloy scraps (with the main compositions shown in Table 1) using a mixture of H2SO4 and HCl acids with the addition of oxidants (H2O2 and HNO3) as the leaching agent [35,37,40]. A solution containing 1.1 g/dm3 Re, mainly of nickel, cobalt, chromium, and aluminum, was obtained. This solution was directed to the sorption of rhenium using a weakly basic ion-exchange resin A170 (Purolite, King of Prussia, PA, USA, hydroxide form). Next, it was directed to the recovery of valuable metal components (Ni, Co). Rhenium adsorbed by resin was eluted with an aqueous ammonia solution (25%, Chempur, Piekary Śląskie, Poland, p.a.). The ammonium perrhenate was crystallized from the obtained ammoniacal eluate. The ammonium perrhenate was obtained with the following composition: min. 64.4% Re, Al, Cu, Fe, Mg, Mo, Na, Ni, Pb, Zn < 0.0005%, Ag, Ba, Ca, K, Cd, Co, Cr, Pd, Rh, Ru, Sb, Sn, Tl < 0.001%, As, Se, Si, W < 0.002%, V, P, Ir < 0.005%, Hg < 0.0001%, and Cl2 < 0.05%. This compound was used in all the described and tested methods for obtaining perrhenic acid.

Table 1.
Concentration of main elements in superalloys used in research.
In the process of the production of perrhenic acid by the electrodialysis method, the starting concentrate and near-electrode solutions of perrhenic acid concentrations of 40 g/dm3 and 30 g/dm3 HReO4, respectively, were obtained from a solution of perrhenic acid with a concentration of 120 g/dm3 containing the following: <1 ppm Ca, <10 ppm K, <1 ppm Mg, <1 ppm Cu, <1 ppm Na, <1 ppm Mo, <1 ppm Ni, <1 ppm Pb, <1 ppm Fe, <5 ppm NH4+, <1 ppm Co, <1 ppm Sign. This acid was obtained from secondary raw materials—superalloy scrap waste during previous projects realized in Łukasiewicz—IMN (projects: InRen and NanoRen) [35,38,41].
In all cases, vacuum evaporators were used to concentrate the solutions. First was a glass vacuum evaporator from Kavalier with an efficiency of 5 dm3/h (Figure 1b) with a temperature of ~80 °C and operating pressure of 400 ÷ 500 mbar. Second was the Hei-VAP rotary evaporator from Heidolph with parameters of 130 °C and 400 mbar, respectively. The choice of the evaporator depended on the volume of the concentrated solution and the assumed final concentration of perrhenic acid.
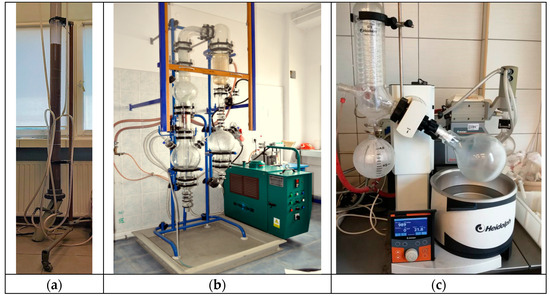
Figure 1.
Equipment for production of perrhenic acid by ion-exchange: (a) ion column to sorption and elution of ammonium ions; (b) and (c) evaporators to concentration of postsorption solutions.
The most favorable process parameters were used in all the presented methods for obtaining high-purity perrhenic acid. These process parameters and the proposed process diagrams for obtaining rhenic acid by electrodialysis and solvent extraction were selected based on research results carried out as part of the Small Grant RenMet project. The process based on ion-exchange was based on the previous experience of scientists from Łukasiewicz—IMN.
Figure 1 shows the laboratory equipment used in the research to obtain perrhenic acid, while Figure 2 presents the methodology for the production of perrhenic acid by ion-exchange. Ammonium perrhenate was dissolved in water and directed to the sorption of ammonium ions using a strongly acidic C160 cation-exchange resin (Purolite, King of Prussia, PA, USA, hydrogen form) [35,37]. The postsorption solution, containing perrhenic acid, was sent to the concentration stage.
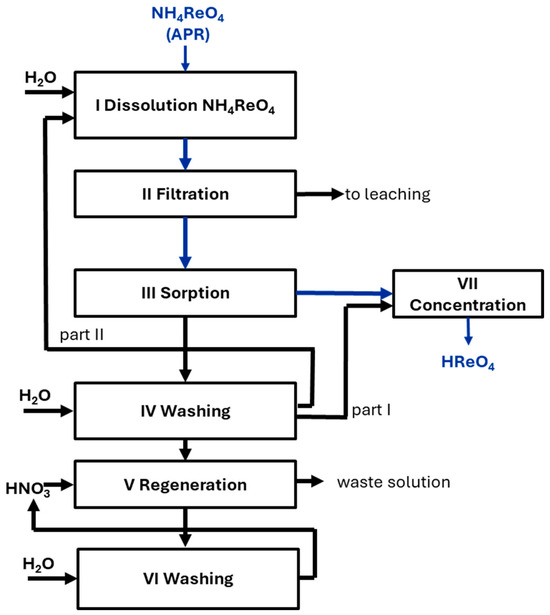
Figure 2.
Scheme for production of perrhenic acid by ion-exchange.
Perrhenic acid obtained by solvent extraction is presented in the diagram in Figure 3, and Figure 4 shows the laboratory equipment used in the research on its preparation. For further purification and concentration of perrhenic acid, the equipment consisted of an ion column, and evaporators were used (Figure 1).
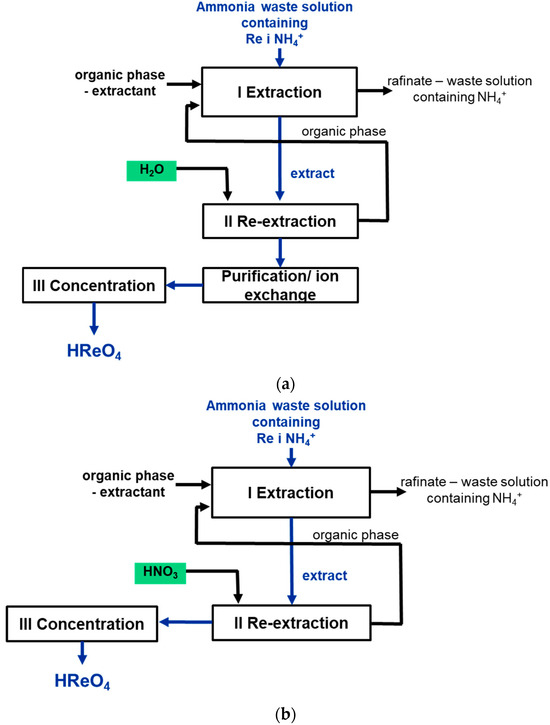
Figure 3.
Scheme of obtaining perrhenic acid using the solvent extraction process: (a) extraction by 50% TBP Exxsol 80 solution; (b) extraction by 10% TOA Exxsol 80+ 20% TBP solution and 5% Cyphos IL Exxsol 80 solution + 20% TBP.
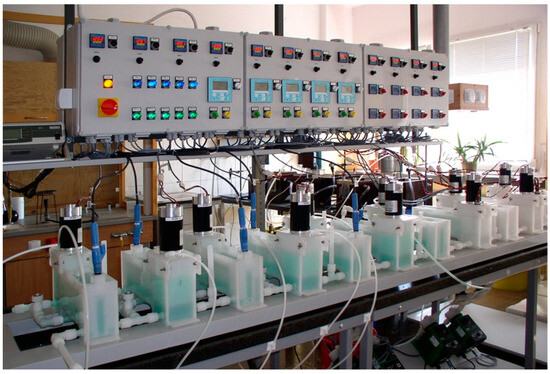
Figure 4.
Equipment for obtaining perrhenic acid using the solvent extraction process.
The following extractant mixtures were used in the tests: 30% trioctylamine, 20% tributyl phosphate and 50% Exxsol D80 (V/V), 50% tributyl phosphate and 50% Exxsol D80 (V/V), 5% Cyphos IL 101, 20% tributyl phosphate, and 75% Exxsol D80 (V/V). Water was used for re-extraction in the case of 30% trioctylamine, 20% tributyl phosphate, and a dilute solution of nitric acid for 50% tributyl phosphate and 5% Cyphos IL 101.
Perrhenic acid obtained by electrodialysis using bipolar membranes is shown in the diagram in Figure 5, and Figure 6 shows the laboratory equipment used in the research on its preparation. Figure 7 and Figure 8 show the electrodialyser structure and ion-exchange diagram in this process, respectively.
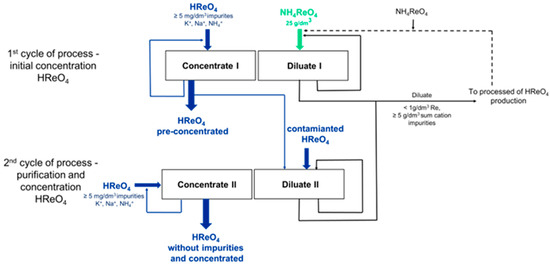
Figure 5.
Scheme of obtaining perrhenic acid by electrodialysis.
Figure 6.
Set for electrodialysis test.
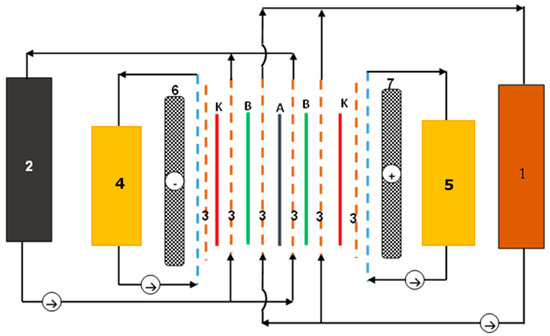
Figure 7.
Scheme of the electrodialyser construction: 1—concentrate flow; 2—diluate flow; 3—spacer; 4—catholyte flow; 5—anolyte flow; 6—cathode; 7—anode; A—anionite membrane; K—cationite membrane; B—bipolar membrane.
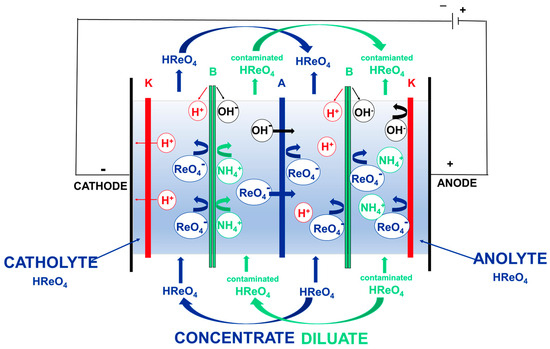
Figure 8.
Scheme of ion-exchange for high-purity perrhenic acid production using the electrodialysis process and bipolar membranes: A—anion-exchange membrane; K—cation-exchange membrane; B—bipolar membrane.
The process of perrhenic acid production using electrodialysis is carried out in the electrodialyser shown in Figure 6 and Figure 7, in which anion-exchange membranes (PC SA) are mounted alternately with bipolar membranes (PC bip), and both electrode chambers are separated by the cation-exchange membranes (PC SK). The cathode is made of acid-resistant steel, and the DSA anode is made of titanium covered with iridium and ruthenium oxides. The active surface of ion-selective membranes was 64 cm2. The process should be carried out cyclically, with the first cycle consisting of the initial concentration of the perrhenic acid solution and the second cycle being the purification and concentration of the perrhenic acid solution. Perrhenic acid solutions with a concentration of 30 g/dm3 HReO4 are used as electrode solutions (anolyte and catholyte) in both cycles. A solution of ammonium perrhenate of a concentration equal to 40 g/dm3 NH4ReO4 is used as the starting diluate in the first cycle, and the concentrate is a solution of perrhenic acid of a concentration of 40 g/dm3 HReO4. The starting diluate in II is a solution of purified perrhenic acid, with a rhenium concentration higher than about 15 g/dm3 (concentration of acid 20 g/dm3), and the concentrate is a solution of high-purity perrhenic acid, in which the content of each of the cationic impurities does not exceed 5 mg/dm3. If the concentration of any cationic impurity in this concentrate exceeds 10 mg/dm3, it is withdrawn from the process and replaced with a fresh starting concentrate. This withdrawn concentrate is returned to the second cycle of the process as a starting diluate. The process of obtaining perrhenic acid by electrodialysis is carried out cyclically until the required concentration of perrhenic acid is obtained in the solution using the concentrate obtained in the previous cycles of the process as the diluate starting solution. In both cycles, diluate is withdrawn from the process if the rhenium concentration drops below 1 g/dm3, and the withdrawn solution is replaced with fresh diluate. The withdrawn diluate, after supplementing with an appropriate amount of NH4ReO4, should be returned to the first cycle of the process as a starting diluate. The solutions of all the streams circulate through the electrodialyser in a closed circuit. After applying an electric voltage, the ions contained in the solutions migrate selectively through the membranes, the concentrate solution is enriched with ReO4− ions, and the diluate solution is depleted in perrhenates ions. The process efficiency was calculated using formula:
where C0, C1—concentration of diluate stream at the beginning and finish process, respectively, mol/dm3.
3. Results and Discussion
3.1. Production of High-Purity Perrhenic Acid by Ion-Exchange
In the ion-exchange column, 1.0 dm3 of C160 ion exchanger previously conditioned in a 32% HNO3 solution was introduced. Then, five trials to produce perrhenic acid were performed. In the first step, 3.0 dm3 of demineralized water was introduced into the glass reactor, where 65.0 g of ammonium perrhenate was dissolved. After filtering on a bag filter, the obtained solution was passed through an ion-exchange column; after the solution was exhausted, water was added. The solution and water were passed by the column at a flow rate of no more than 7.5 BVs (Bed Volumes). The effluent from the column was collected in portions of 250 cm3. The pH of the effluent was also monitored. A sample was taken from each portion, and the rhenium concentration was determined. After obtaining the analysis results, the first four portions, with a total volume of 1 BVs, were directed to the waste. The next portions of the effluents, with a total volume of 3.5 BVs, were combined and sent for concentration. Subsequent portions of the effluent, with a total volume of 2 BVs, were retained and used to dissolve further portions of NH4ReO4. Then, the ion exchanger was regenerated by passing a 32% HNO3 solution with a volume of 1.5 BVs through the bed. The excess acid was washed out by passing 25.0 dm3 of water through the column. The regenerated bed was used in the next four sorption tests, carried out in accordance with the methodology used in the first test. Table 2 contains a summary of the volumes of solutions intended for sorption and the concentrations of rhenium in these solutions. Figure 9 shows how the concentration of rhenium in the column effluent changed during the sorption of ammonium ions.

Table 2.
Solutions of NH4ReO4 used in experiments to produce perrhenic acid using the ion-exchange method.
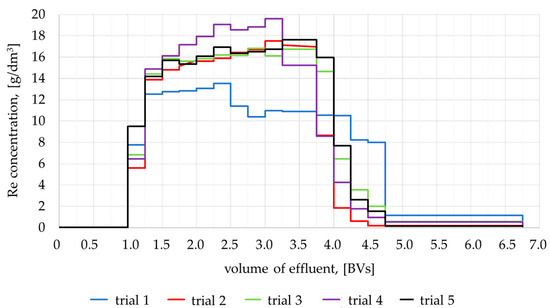
Figure 9.
Changes in rhenium concentration in the effluent.
The first part of the effluent, with a volume equal to 1 BVs, did not contain rhenium, and only water remained in the bed from washing after ion exchanger regeneration. This part of the effluent was removed. In the following portions of the effluent with a volume of 3 BVs, the rhenium concentration was close to the concentration in the feed. This part of the leakage contained the main product of the installation and was directed to concentration. In subsequent portions of the effluent (0.5 BVs), the concentration of rhenium decreased, but these solutions did not contain ammonium, so they were also sent for concentration. The last portions of the leak, ~2 BVs, already contained residual amounts of rhenium, so they were used to dissolve fresh portions of ammonium perrhenate. Table 3 lists the solutions collected in the samples, which were combined and sent for concentration.

Table 3.
Effluents to concentration.
A total of 17.5 dm3 of the column effluents were concentrated on a vacuum evaporator to a volume of 0.7 dm3. The concentration of rhenium in the concentrated solution was 300.1 g/dm3. Then, half of this solution was concentrated to a volume of 0.116 dm3; the concentration of rhenium in this solution was 905.6 g/dm3. Consequently, two high-concentration solutions of perrhenic acid were obtained, containing the following:
- (1)
- A total of 300.1 g/dm3 Re and <0.0001% Ca, <0.0001% K, <0.0001% Mg, <0.0001% Cu, <0.0001% Na, <0.0001% Mo, <0.0001% Ni, <0.0001% Pb, <0.0001% Fe, <0.0001% NH4+, <0.0001% Bi, <0.0001% Zn, <0.0001% W, <0.0001% As and <0.0001% Al.
- (2)
- A total of 905.6 g/dm3 Re and <0.0001% Ca, <0.0005% K, <0.0001% Mg, <0.0001% Cu, <0.0001% Na, <0.0001% Mo, <0.0001% Ni, <0.0001% Pb, <0.0001% Fe, <0.0002% NH4+, <0.0001% Bi, <0.0001% Zn, <0.0001% W, <0.0001% As and <0.0001% Al.
Both of these solutions of perrhenic acid were high-purity solutions in which the sum of the metallic impurities was ˂100 ppm, and none of the impurities exceeded 10 ppm, the exception being the Fe of acid with a concentration above 900 g/dm3.
3.2. Production of High-Purity Perrhenic Acid By Solvent Extraction
As part of the research carried out in the Small Grant RenMet project, the possibility of obtaining perrhenic acid extractive was checked using selected, commercially available organic solvents, i.e., tributyl phosphate, trioctylamine, and Cyphos IL 101 ionic liquid. An ammonia solution was used as the research material waste, with Re and NH4+ concentrations of 13.5 and 43.7 g/dm3, respectively. This solution came from the process of rhenium recovery from superalloy scraps.
The results of the preliminary tests showed that all three selected extractants demonstrate the high extraction efficiency of ReO4− ions. For each of the extractants, a series of tests were carried out regarding the extraction and re-extraction conditions, the influence of pH, the composition of organic phases, phase ratios, contact time, and the type and temperature of the re-extractant used on the efficiency and selectivity of both processes. This research allowed us to determine the extraction and re-extraction conditions that led to the production of perrhenic acid. It has been established that there are at least two possible procedures for obtaining perrhenic acid by solvent extraction:
- (1)
- By extraction with a 50% (V/V) solution of tributyl phosphate in Exssol D80, re-ex traction with demineralized water at an elevated temperature of about 50 °C, and then the concentration of the obtained re-extract, preferably under reduced pressure;
- (2)
- By extraction using three-component organic mixtures containing 5% (V/V) of Cyphos IL 101 ionic liquid and 20% (V/V) tri-butyl phosphate in Exssol D80 or 10% trioctylamine and 20% tributyl phosphate in ExssoluD80, re-extraction with once-diluted nitric acid, and then stripping of nitrogen oxides.
Although the developed methods allow for obtaining perrhenic acid with the Re concentration required to be >100 g/dm3 in the project, with an efficiency exceeding 90% and the content of the sum of metal impurities <100 ppm, this technology is quite time-consuming and requires the use of many consecutive stages.
In the first method, to obtain the assumed efficiency, it is necessary to use at least two extraction stages with a phase ratio 1:1, followed by repeated re-extraction with demineralized water at a temperature of 50 °C. Despite obtaining high extraction and re-extraction efficiencies, the second method requires stripping the nitrogen oxides, which involves a high-energy input and compliance with appropriate health and safety procedures, which is particularly important in using the described methods on a scale larger than the laboratory. Moreover, for all three methods, including those selected as the most preferred, the insufficient selectivity of the extraction and re-extraction of ReO4− ions in relation to ammonium ions was observed. It is therefore recommended to carry out an additional stage of purification of the obtained rhenic acid (VII) using other techniques, e.g., the sorption of NH4+ ions using strongly acidic Purolite C160 cation-exchange resins.
The necessity of using an additional purification step to eliminate NH4+ ions, namely applications in the solvent extraction process connected with the ion-exchange method using selected ion exchangers, even while maintaining an amount below 10 ppm for selected single pollutants (Na, Al, K, Ca, Fe, and Zn <10 mg/dm3 and Mg, Cu, Ni, and Co <5 mg/dm3), disqualifies this method as recommended for use in practice.
3.3. Production of High-Purity Perrhenic Acid by Electrodialysis
In all experiments for the production of perrhenic acid by electrodialysis using bipolar membranes, the process was carried out at the intensity of the process streams flowing through the electrodialyser of 3 dm3/h, current density of 200 A/m2, and the temperature of all the process streams maintained in the range of 40 °C.
3.3.1. Preliminary Concentration of Perrhenic Acid
In the first cycle of the preliminary concentration of perrhenic acid in test I-1, four process solutions were fed to the electrodialyser at a constant flow rate of process streams. As a concentrate, 1 dm3 of a solution containing 40 g/dm3 HReO4 and less than 1 mg/dm3 NH4+, <1 mg/dm3 Na, and <1 mg/dm3 K was introduced. As a starting diluate, 2 dm3 of a solution of ammonium perrhenate of a concentration of 40 g/dm3 was used. Finally, as a near-electrode solution, a solution of perrhenic acid of a concentration of 30 g/dm3 HReO4 in a volume of 1 dm3 of each solution was introduced. After 185 min, a sudden increase in voltage was noticed and the process was stopped. The concentration of rhenium in the diluate decreased to 1.55 g/dm3. The determined concentration of rhenium in the concentrate was 82.1 g/dm3 (110 g/dm3 HReO4), and the impurity content was 89.2 mg/dm3 NH4+, <1 mg/dm3 Na, and <1 mg/dm3 K. The process efficiency calculated for the diluate was 94.4%, and the concentration of perrhenic acid in the concentrate increased 2.75 times. The concentration of rhenium in the electrode solutions did not change.
The process was repeated three more times (trials from I-2 to I-4) using the concentrate obtained from the previous process as the starting concentrate. A fresh solution of ammonium perrhenate with a concentration of 40 g/dm3 NH4ReO4 was used as the starting dilute. The test conditions were the same, with the difference that in the last fourth test (I-4), 1 dm3 of dilute was used.
Table 4 shows the results obtained in all four tests of the first cycle of preliminary concentration of the perrhenic acid solution. The process efficiency calculated for diluate from samples I-2, I-3, and I-4 and was 96.5%, 95.9%, and 90.4%, respectively. The concentration of perrhenic acid in the concentrate increased 1.68, 1.39, and 1.11 times, respectively. The concentration of rhenium in the solutions at the electrodes did not change or change very slightly, ranging from 2 to 5%.

Table 4.
Concentration of rhenium and impurities in diluate before and after I cycle purification and concentration of perrhenic acid in electrodialysis process.
The results obtained in the first cycle of the preliminary concentration of perrhenic acid by the electrodialysis process using bipolar membranes in four consecutive tests showed the possibility of concentrating the concentrate from the acid solution of 40 g/dm3 to 283.3 g/dm3 HReO4, simultaneously contaminating it with ammonium ions up to 275.0 mg/dm3. In all tests performed in the first electrodialysis cycle, the efficiency of the process exceeded 90%. Despite the contamination with NH4+ ions appearing in the concentrates, these were successfully concentrated and enriched in rhenium while simultaneously reducing the rhenium concentration in the diluates from 27.5 g/dm3 to values in the range of 0.98 to 2.67 g/dm3 Re. Diluates after the process can be enriched in ReO4− ions by adding an amount of NH4ReO4 and returning it to the process as a starting diluate. The concentrate solution contaminated with NH4+, K, and Na cations was sent to the second electrodialysis cycle, which included the purification and concentration of perrhenic acid.
3.3.2. Purification and Concentration of Perrhenic Acid >100 g/dm3
The concentrate obtained in the first cycle of the electrodialysis of the initial concentration (after trial I-4) with a concentration of 283.3 g/dm3 HReO4 and contaminated with NH4+ and Na and K ions (Section 3.3.1) was sent for a purification test using electrodialysis in a two-stream system to reduce the concentration of NH4+ ions and Na and K to <10 mg/dm3. All tests of the second electrodialysis cycle were carried out using the exact solutions at the electrodes and other process conditions, except the test duration, as in the first cycle of the electrodialysis process described in Section 3.3.1.
In the first trial of the second cycle (II-1), 1 dm3 of perrhenic acid solution to a rhenium concentration of about 29.6 g/dm3 (40.0 g/dm3 HReO4) containing <1 mg/dm3 NH4+, <1 mg/dm3 Na, and <1 mg/dm3 K was introduced into the electrodialyser as a concentrate. As a diluate, a solution volume of about 1 dm3 of concentrate obtained in the first cycle of the process was introduced, and it was the solution of perrhenic acid with a rhenium concentration of 210 g/dm3 (283.3 g/dm3 HReO4) containing impurities in the amount of 275 mg/dm3 NH4+, 15 mg/dm3 Na, and 35 mg/dm3 K. After 8 h, the process was completed. After the process, the concentration of rhenium in the diluate decreased to 51.2 g/dm3. The process efficiency calculated for the diluate was 75.6%. The concentration of rhenium in the electrode solutions did not change. The determined concentration of rhenium in the concentrate was 179.5 g/dm3 (242.2 g/dm3 HReO4), and the impurities were 52 mg/dm3 NH4+, 3.1 mg/dm3 Na, and 6.1 mg/dm3 K. The concentration of perrhenic acid in the concentrate increased more than six times. Still, due to the excess of impurities, this solution was sent as a starting diluate for the following process of the second electrolysis cycle.
In the second test carried out as part of the second cycle of the electrodialysis process (II-2), the concentrate from the previous test was used as the dilute at a volume of about 1 dm3 of perrhenic acid solution with a rhenium concentration of about 179.5 g/dm3 (242.2 g/dm3 HReO4) and contents of impurities of 52.0 mg/dm3 NH4+, 3.1 mg/dm3 Na, and 6.1 mg/dm3 K. As a concentrate, there was 1 dm3 of solution with a rhenium concentration of about 85.2 g/dm3 (115 g/dm3 HReO4) containing <1 mg/dm3 NH4+, <1 mg/dm3 Na, and <1 mg/dm3 Na. After completing the process, which lasted 8 h, the concentration of rhenium in the diluate decreased to 20.5 g/dm3. The process efficiency calculated for diluate was 88.6%. The determined concentration of rhenium in the concentrate was 239.8 g/dm3 (329.5 g/dm3 HReO4), and the contents of impurities were 6.1 mg/dm3 NH4+, 1.1 mg/dm3 Na, and 2.2 mg/dm3 K. The concentration of perrhenic acid in the concentrate increased over 2.8 times. The concentration of rhenium in the electrode solutions did not change.
Since the concentration of rhenium in the diluate after trial II-2 was high 20.5 g/dm3, this solution was used in the subsequent trial of the second cycle (II-3) as the starting diluate. In trial II-3, the same concentrate was used as in the previous trial II-2; it was solution with a volume of 1 dm3 and rhenium concentration of 85.2 g/dm3 (115 g/dm3 HReO4). The conditions of the trials were the same. After 60 min, a sudden increase in voltage was noticed and the process was stopped. After completing the process, the concentration of rhenium in the diluate decreased to 0.41 g/dm3. The process efficiency calculated for diluate was 98.0%. The determined concentration of rhenium in the concentrate was 102.4 g/dm3 (142.1 g/dm3 HReO4), and the impurity content was <2.6 mg/dm3 NH4+, <1 mg/dm3 Na, and <1 mg mg/dm3 K. The concentration of rhenium in the electrolyte solutions has not changed.
Table 5 and Table 6 present the results obtained in all three tests of the second cycle of the purification and concentration of the perrhenic acid solution.

Table 5.
Concentration of rhenium and impurities in diluate before and after II cycle purification and concentration of perrhenic acid in electrodialysis process.

Table 6.
Concentration of rhenium and impurities in concentrate before and after II cycle purification and concentration of perrhenic acid in electrodialysis process.
The results obtained in the second cycle of the purification and concentration of perrhenic acid by the electrodialysis process using bipolar membranes showed the possibility of concentrating the concentrate from the acid solution from 40 g/dm3 to 329.5 g/dm3 HReO4, simultaneously reducing its contamination with NH4+, K, and Na to the required level below 10 mg/dm3 of each pollutant. After the process, the diluates can be combined with purified perrhenic acid; the starting diluate is returned to the process or enriched in ReO4− ions by adding an amount of NH4ReO4 and used as a diluate in the first cycle of the initial HReO4 concentration.
4. Conclusions
Methods for obtaining high-purity perrhenic acid (with metallic impurities concentration below 100 ppm) with a high concentration 100–200 g/dm3 were briefly characterized entirely from secondary raw materials. The obtained perrhenic acid should have a specific composition to meet quality and customer requirements or other standards based on future applications, i.e., 100–900 g/dm3 Re and <1 ppm Ca, <10 ppm K, <1 ppm Mg, <1 ppm Cu, <1 ppm Na, <1 ppm Mo, <1 ppm Ni, <1 ppm Pb, <1 ppm Fe, <1 ppm Co, <1 ppm Zn, and <5 ppm NH4+. Two of the three methods presented—electrodialysis and solvent extraction—were the subject of research carried out directly as part of the Small Grant project with the acronym RenMet: “Innovative hydrometallurgical technologies for the production of rhenium compounds from recycled waste materials for catalysis, electromobility, aviation and defense industry”, and the ion-exchange method was developed as part of previous projects implemented by Łukasiewicz-IMN. Table 7 below shows the advantages and disadvantages of the methods for producing high-purity and high-concentration perrhenic acid. The basic process parameters criteria were selected: efficiency and selectivity of the process, purity of the obtained product, availability and consumption of raw materials and reagents, equipment necessary to carry out the process, and the profitability of the technology and ecological aspects, i.e., the possibility of managing the generated solid waste and post-production solutions. In order to fully determine which of the methods is the most sustainable in terms of costs and environmental impact, it would be worth focusing in future work on conducting a life cycle assessment of these processes. To support the conclusions, the results of a performed multi-criteria decision analysis (MCDA) are outlined in Table 7.

Table 7.
Comparative parameters for obtaining perrhenic acid using various techniques.
Factors for selecting the most economically and ecologically advantageous method for high-purity perrhenic acid production from secondary raw materials were analyzed. The main disadvantages of using the electrodialysis process to obtain perrhenic acid are as follows:
- -
- Necessity use of many expensive ion-exchange membranes and their rapid wear and destruction;
- -
- Carrying out the process in two cycles: the first cycle is the initial concentration of the perrhenic acid solution, and the second cycle is the process of the purification of the perrhenic acid.
Each of these cycles requires carrying out many stages until the required purity and concentration of the obtained product perrhenic acid are achieved.
The main disadvantage that eliminates the use of the solvent extraction method to obtain perrhenic acid is the low selectivity of the extraction and re-extraction process and the need to use an additional purification operation to remove NH4+ ions using, e.g., the ion-exchange method.
High-purity and high-concentration, above 100 g/dm3, perrhenic acid was obtained using the ion-exchange method. The key element of this method is the product’s purity and the process’s simplicity, easily available waste materials, and renewable ion-exchange resin. It is, therefore, based on a sustainable circular economy.
Author Contributions
Conceptualization, D.K., K.L.-S. and G.B.; methodology, D.K. and P.K.; software, P.K. and A.G.; validation, D.K., K.L.-S., G.B. and P.D.; formal analysis, K.L.-S., D.K. and P.D.; investigation, D.K., J.M., P.K., K.P. and K.G.; resources, K.L.-S., J.M., P.K., K.P. and K.G.; data curation, D.K. and A.G.; writing—original draft preparation, D.K., P.K., S.O. and M.C.; writing—review and editing, K.L.-S., J.M., M.C., D.B., S.O. and P.D.; visualization, D.K.; M.C., S.O. and D.B.; supervision, G.B. and P.D.; project administration, K.L.-S.; funding acquisition, K.L.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Norwegian Financial Mechanism 2014–2021—Small Grant 2020 NOR/SGS//RenMet/0049/2020-00 (11/PE/0146/21), entitled “Innovative hydrometallurgical technologies for the production of rhenium compounds from recycled waste materials for catalysis, electromobility, aviation and defense industry”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patent application and project contract.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savitskii, E.M.; Tulkina, M.A.; Povarova, K.B. Rhenium Alloys; Israel Program for Scientific Translations: Jerusalem, Israel, 1970. [Google Scholar]
- Kloprogge, J.T.; Ponce, C.P.; Loomis, T. The Periodic Table: Nature’s Building Blocks: An Introduction to the Naturally Occurring Elements, Their Origins and Their Uses; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128215388. [Google Scholar]
- Varfolomeev, M.B.; Zemenkova, A.N.; Chrustalev, V.N.; Stručkov, J.T.; Lunk, H.J.; Ziemer, B. Crystal Structure of Copper Perrhenate Tetrahydrate, Cu(ReO4)2·4H2O. J. Alloys Compd. 1994, 215, 339–343. [Google Scholar] [CrossRef]
- Butz, A.; Miehe, G.; Paulus, H.; Strauss, P.; Fuess, H. The Crystal Structures of Mn(ReO4)2·2H2O and of the Anhydrous Perrhenates M(ReO4)2 of Divalent Manganese, Cobalt, Nickel, and Zinc. J. Solid State Chem. 1998, 138, 232–237. [Google Scholar] [CrossRef]
- Reiff, W.M.; Dodrill, B.C.; Torardi, C.C. New Insulating Layered Network 3d-Ferromagnets Composed of the Divalent Metal Perrhen Ates: Fe(ReO4)2, Co(ReO4)2 And Ni(ReO4)2. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 274, 137–143. [Google Scholar] [CrossRef]
- Picard, J.; Baud, G.; Besse, J.; Chevalier, R. Structure Cristalline Du Perrhenate de Plomb Pb(ReO4)2. J. Less Common Met. 1984, 96, 171–176. [Google Scholar] [CrossRef]
- Abakumov, A.M.; Rozova, M.G.; Shpanchenko, R.V.; Mironov, A.V.; Antipov, E.V.; Bramnik, K.G. Synthesis and Crystal Structure of the Lithium Perrhenate Monohydrate LiReO4·H2O. Solid State Sci. 2001, 3, 581–586. [Google Scholar] [CrossRef]
- Atzesdorfer, A.; Range, K.J. Sodium Metaperrhenate, NaReO4: High Pressure Synthesis of Single Crystals and Structure Refinement. Z. Naturforsch. Sect. B J. Chem. Sci. 1995, 50, 1417–1418. [Google Scholar] [CrossRef]
- Müller, A. Das Infrarotspektrum von KReO4 Und NH4ReO4. Z. Naturforsch. A 1965, 20, 745–746. [Google Scholar] [CrossRef]
- Baur, W.H.; Kassner, D. The Crystal Structure of Ca(ReO4)2 2H2O. J. Solid State Chem. 1992, 100, 166–169. [Google Scholar] [CrossRef]
- Salvati, L.; Hercules, D.M.; Vogt, H. Laser Microprobe Mass Analysis of NH4ReO4, AgReO4 and Al(ReO4)3. Spectrosc. Lett. 1980, 13, 243–251. [Google Scholar] [CrossRef]
- Roskill. Rhenium: Outlook to 2029, 11th ed.; Roskill Information Services Ltd.: London, UK, 2019; ISBN 978-1-910-92279-8. [Google Scholar]
- Huang, M.; Zhu, J. An Overview of Rhenium Effect in Single-Crystal Superalloys. Rare Met. 2016, 35, 127–139. [Google Scholar] [CrossRef]
- Rahman, U.U.H.M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as Emerging Materials: Synthesis, Properties, and Applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Druce, J.G.F.; Druce, G. Rhenium: Dvi-Manganese, the Element of Atomic Number 75; Cambridge University Press: Cambridge, UK, 1948; ISBN 9781107693241. [Google Scholar]
- Brauer, G. Handbook of Preparative Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 1965; ISBN 978-0-12-395590-6. [Google Scholar]
- Colton, R. The Chemistry of Rhenium and Technetium; Interscience Publishers: Geneva, Switzerland, 1965; ISBN 9780470166505. [Google Scholar]
- Sumida, I.; Kawano, Y.; Hamamoto, M. Method for Producing Aqueous Solution of Perrhenic Acid from Rhenium Sulfide. US9624561B2, 18 April 2017. [Google Scholar]
- Leddicotte, G. The Radiochemistry of Rhenium; Subcommittee on Radiochemistry, National Academy of Sciences, National Research Council: Washington, DC, USA, 1961. [Google Scholar]
- Ho, T.; Fieser, M.; Fieser, L. Rhenium(VII) Oxide. In Fieser and Fieser’s Reagents for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2013; p. 417. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Krompiec, S.; Chmielarz, A.; Anyszkiewicz, K.; Gotfryd, L. Synthesis of Perrhenic Acid Using Solvent Extraction. Hydrometallurgy 2009, 95, 325–332. [Google Scholar] [CrossRef]
- Hong, T.; Liu, M.; Ma, J.; Yang, G.; Li, L.; Mumford, K.A.; Stevens, G.W. Selective Recovery of Rhenium from Industrial Leach Solutions by Synergistic Solvent Extraction. Sep. Purif. Technol. 2020, 236, 116281. [Google Scholar] [CrossRef]
- Zhan-fang, C.; Hong, Z.; Zhao-hui, Q. Solvent Extraction of Rhenium from Molybdenum in Alkaline Solution. Hydrometallurgy 2009, 97, 153–157. [Google Scholar] [CrossRef]
- George, K.; Masters, A.J.; Livens, F.R.; Sarsfield, M.J.; Taylor, R.J.; Sharrad, C.A. A Review of Technetium and Zirconium Extraction into Tributyl Phosphate in the PUREX Process. Hydrometallurgy 2022, 211, 105892. [Google Scholar] [CrossRef]
- Pruett, D.J. The Solvent Extraction off Heptavalent Technetium and Rhenium by Tributyl Phosphate; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1984. [Google Scholar]
- Anderson, C.D.; Taylor, P.R.; Anderson, C.G. Extractive Metallurgy of Rhenium: A Review. Miner. Metall. Process 2013, 30, 59–73. [Google Scholar] [CrossRef]
- Francoise, T.S.M.; Michel, B.M. Process for the Extraction of Rhenium and Analogous Elements. US2634280A, 7 April 1953. [Google Scholar]
- Agapova, L.Y.; Ponomareva, E.I.; Abisheva, Z.S. Production of Concentrated Rhenium Acid by Electrodialysis of Rhenium Salts Solutions. Hydrometallurgy 2001, 60, 117–122. [Google Scholar] [CrossRef]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Agapova, L.Y.; Sharipova, A.S. Purification of Crude Ammonium Perrhenate from Potassium by Recrystallization, Sorption, and Membrane Electrodialysis. Theor. Found. Chem. Eng. 2019, 53, 841–847. [Google Scholar] [CrossRef]
- Palant, A.A.; Bryukvin, V.A.; Levin, A.M.; Reshetova, O.V. Electrodialysis Synthesis of Concentrated Solutions of Perrhenic Acid. Russ. Metall. 2011, 2011, 185–187. [Google Scholar] [CrossRef]
- Kuznetsova, O.G.; Levin, A.M.; Sevost’yanov, M.A.; Tsybin, O.I.; Bol’shikh, A.O.; Bol’shikh, M.A. Improved Electrodialysis Synthesis of Perrhenic Acid from the Electrolytes of Processing the Wastes of Tungsten–Rhenium Alloys. Russ. Metall. 2020, 2020, 71–76. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Agapova, L.Y.; Ponomareva, E.I.; Abdrakhmanova, Z.T. Application of Electrodialysis Method for High Purity Metal Rhenium Obtaining. In Proceedings of the 7th International Symposium on Technetium and Rhenium—Science and Utilization, Moscow, Russia, 4–8 July 2011; pp. 247–252. [Google Scholar]
- Leszczynska-Sejda, K.; Benke, G.; Chmielarz, A.; Pietek, G.; Dubrawski, M. Sposób Otrzymywania Kwasu Renowego(VII). PL228984B1, 30 May 2018. [Google Scholar]
- Shuichi, O.; Tsutomu, T. Production of Perrhenic Acid. JPS62123019A 1987, 9 May 1990. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Krompiec, S.; Michalik, S.; Krompiec, M. Synthesis of Perrhenic Acid Using Ion Exchange Method. Hydrometallurgy 2007, 89, 289–296. [Google Scholar] [CrossRef]
- Lisic, E.; Callahan, A.; Mirzadeh, S.; Knapp, F. A New Tandem Generator/Ion Exchange System Providing Carrier-Free Rhenium-188-Perrhenic Acid. In Proceedings of the 204th American Chemical Society National Meeting, Anaheim, CA, USA, 2–6 April 1995. [Google Scholar]
- Benke, G.; Anyszkiewicz, K.; Hac, D.; Litwinionek, K.; Leszczyńska-Sejda, K. Progress in the Methods of Recovering Rhenium from Copper Metallurgy. Przem. Chem. 2006, 85, 793–797. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A. Hydrometallurgical Methods for Production of Nickel(II) and Cobalt(II) Perrhenates—Semi-Products for Manufacture of Re-Ni, Re-Co Alloy Powders. Erzmetall 2013, 66, 267–273. [Google Scholar]
- Mehlig, J.P. The Perchlorate method for potassium. J. Chem. Educ. 1927, 4, 1537. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Drzazga, M.; Ciszewski, M. Application of Ion Exchange for Preparation of Selected Metal Perrhenates—Precursors for Superalloy Production. Metals 2019, 9, 201. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Anyszkiewicz, K.; Satora, W.; Kozub, K. Hydrometallurgical Methods for Production of Ni(ReO4)2 and Co(ReO4)2. In Proceedings of the European Metallurgical Conference EMC, Weimar, Germany, 23–26 June 2013; pp. 885–898. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


