Separation and Characterization of Nickel Hydroxide from Waste Solution Using Ca(OH)2 Precipitation in Chloride Media
Abstract
1. Introduction
2. Materials and Method
2.1. Reagents and Material
2.2. Determination of Ferric (Fe3+) and Ferrous (Fe2+) Ions
2.3. Synthesis/Preparation Process of Ni(OH)2
2.4. The Effect of Precipitation
2.5. Characterization
3. Results and Discussion
3.1. The Elemental Composition of Waste Pregnant Leach
3.2. The Effect of Pre-Loading of Copper Concentration
3.3. The Effect of Precipitating Agent on Decreasing Ni2+ Ion Concentration
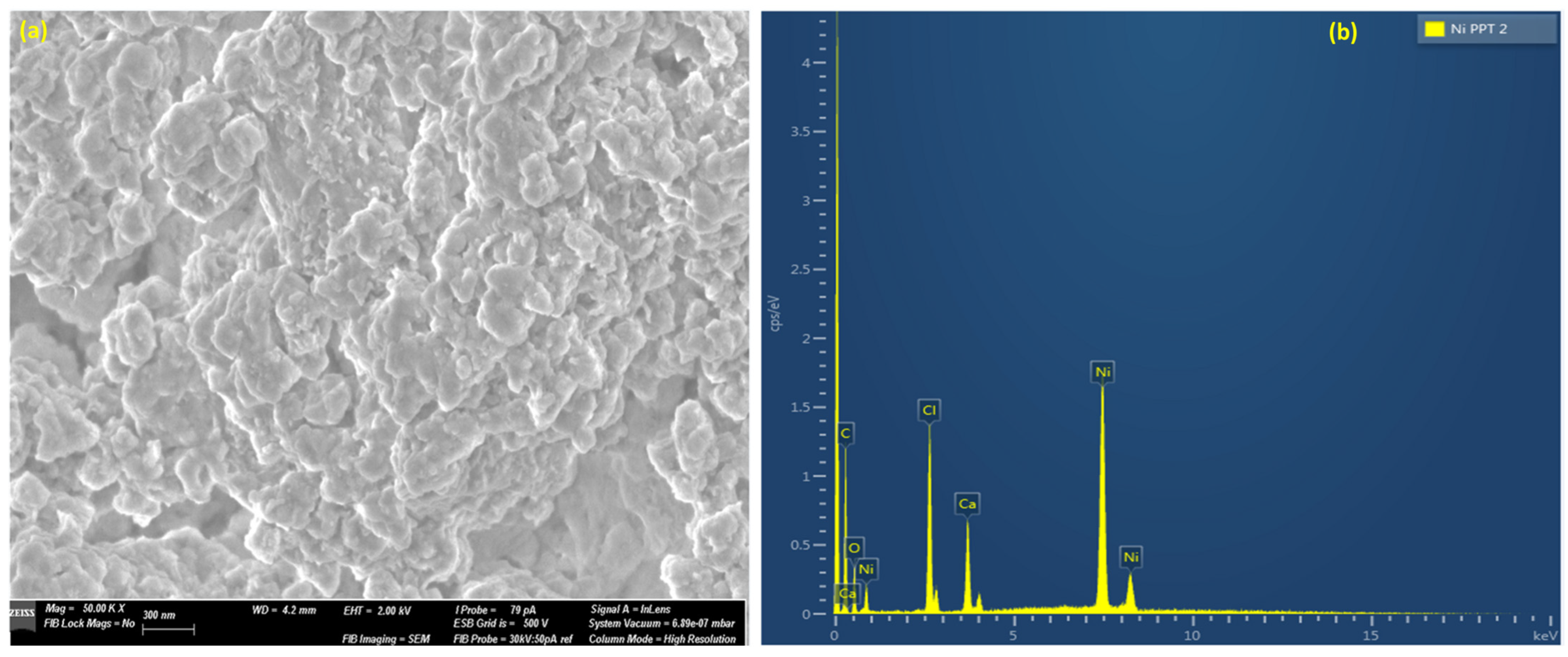
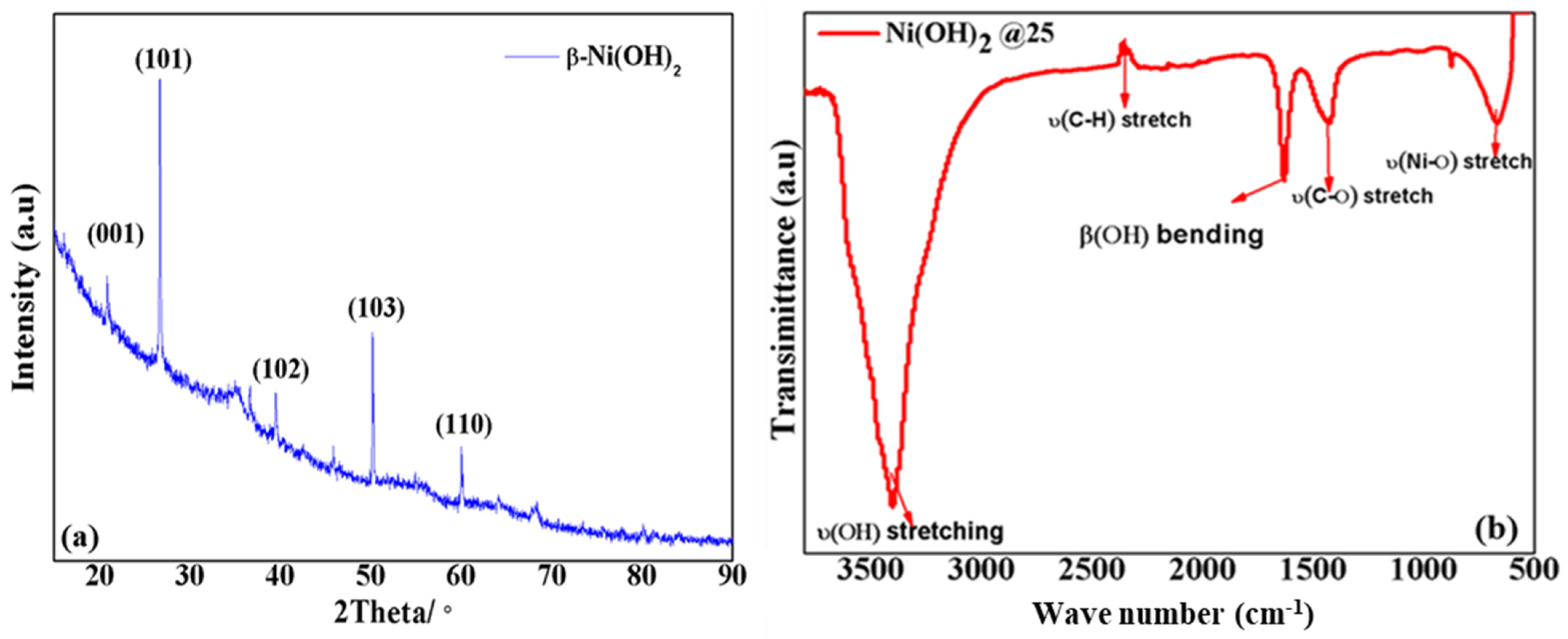
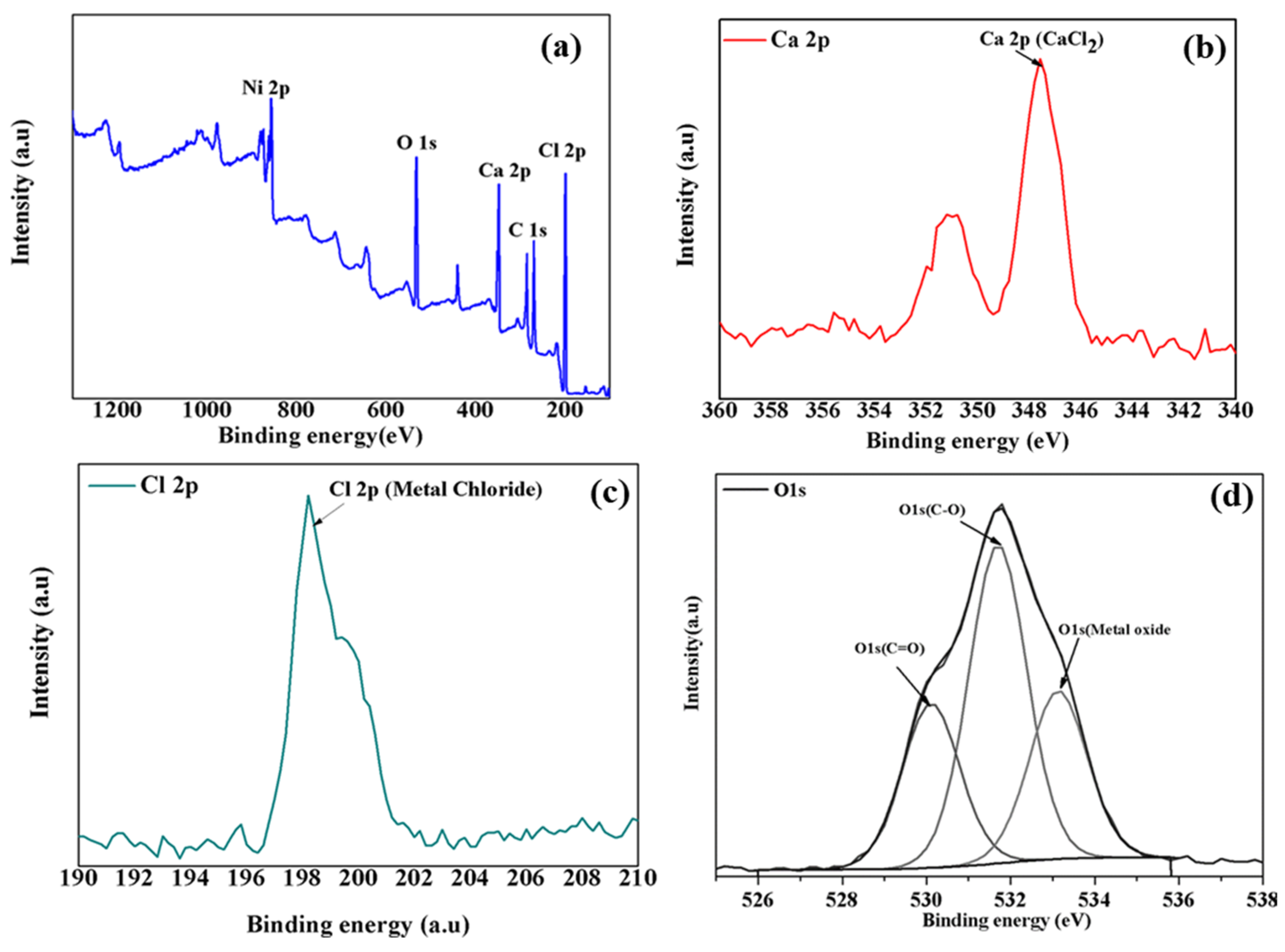
3.4. Structure–Morphology Characterization of Ni(OH)2 Precipitates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odegbemi, F.; Idowu, G.A.; Adebayo, A.O. Nickel recovery from spent nickel-metal hydride batteries using LIX-84I-impregnated activated charcoal. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100452. [Google Scholar] [CrossRef]
- Watanabe, K.; Kikuoka, T.; Kumagai, N. Physical and electrochemical characteristics of nickel hydroxide as a positive material for rechargeable alkaline batteries. J. Appl. Electrochem. 1995, 25, 219–226. [Google Scholar] [CrossRef]
- Avena, M.J.; Vazquez, M.V.; Carbonio, R.E.; De Pauli, C.P.; Macagno, V.A. A simple and novel method for preparing Ni(OH)2 Part I: Structural studies and voltammetric response. J. Appl. Electrochem. 1994, 24, 256–260. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The Influence of Nickel-Hydroxide Composition and Microstructure on the High-Temperature Performance of Nickel Metal Hydride Batteries. J. Electrochem. Soc. 2006, 153, A492. [Google Scholar] [CrossRef]
- Ramesh, T.N.; Kamath, P.V. Synthesis of nickel hydroxide: Effect of precipitation conditions on phase selectivity and structural disorder. J. Power Sources 2006, 156, 655–661. [Google Scholar] [CrossRef]
- Cabanas-Polo, S.; Suslick, K.S.; Sanchez-Herencia, A.J. Effect of reaction conditions on size and morphology of ultrasonically prepared Ni(OH)2 powders. Ultrason. Sonochem. 2011, 18, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Solomane, N.; Ajibade, P.A.; Omondi, B. Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2). Open Access 2019, 234, 605–607. [Google Scholar] [CrossRef]
- Matthews, J.A. Chemical Precipitation. Encycl. Environ. Chang. 2014, 3, 141–142. [Google Scholar] [CrossRef]
- Song, Q.; Tang, Z.; Guo, H.; Chan, S.L.I. Structural characteristics of nickel hydroxide synthesized by a chemical precipitation route under different pH values. J. Power Sources 2002, 112, 428–434. [Google Scholar] [CrossRef]
- Ludwig, R.D.; McGregor, R.G.; Blowes, D.W.; Benner, S.G.; Mountjoy, K. A permeable reactive barrier for treatment of heavy metals. Ground Water 2002, 40, 59–66. [Google Scholar] [CrossRef]
- Endo, M.; Yoshikawa, E.; Tamaki, Y.; Hara, A.; Hikichi, K.; Sasaki, A. Variations in the Concentration of Dissolved Metal Ions and their Buffering Effect in an Acidified River Environment. J. Water Environ. Technol. 2012, 10, 463–471. [Google Scholar] [CrossRef][Green Version]
- Wanta, K.C.; Tanujaya, F.H.; Putra, F.D.; Susanti, R.F.; Gemilar, G.P.; Astuti, W.; Petrus, H.T.B.M. Synthesis and Characterization of Nickel Hydroxide From Extraction Solution of Spent Catalyst. Metalurgi 2020, 35, 111. [Google Scholar] [CrossRef]
- Hidmi, L.; Edwards, M. Role of temperature and pH in Cu(OH)2 solubility. Environ. Sci. Technol. 1999, 33, 2607–2610. [Google Scholar] [CrossRef]
- Asakai, T.; Suzuki, T. Reliability in Standardization of Iron(III) and Titanium(III) Solutions in Volumetric Analysis. ACS Omega 2021, 6, 21147–21152. [Google Scholar] [CrossRef] [PubMed]
- Mabowa, H.M.; Mkhohlakali, A.; Mokoena, S.; Tshilongo, J.; Chimuka, L. Removal of Nickel from Nickel Sulfite-Fire Assay Dissolution Filtrate Through Precipitation. ACS Omega 2023, 9, 5592–5600. [Google Scholar] [CrossRef]
- Nozari, I.; Azizi, A. An Investigation into the Extraction Behavior of Copper from Sulfate Leach Liquor Using Acorga M5640 Extractant: Mechanism, Equilibrium, and Thermodynamics. Min. Metall. Explor. 2020, 37, 1673–1680. [Google Scholar] [CrossRef]
- Younas, M.; Druon-Bocquet, S.; Romero, J.; Sanchez, J. Experimental and Theoretical Investigation of Distribution Equilibria and Kinetics of Copper(II) Extraction with LIX 84 I and TFA. Sep. Sci. Technol. 2015, 50, 1523–1531. [Google Scholar] [CrossRef]
- Basturkcu, H.; Acarkan, N. Selective nickel-iron separation from atmospheric leach liquor of a lateritic nickel ore using the para-goethite method. Physicochem. Probl. Miner. Process. 2017, 53, 212–226. [Google Scholar] [CrossRef]
- Kettaf, S.; Guellati, O.; Harat, A.; Kennaz, H.; Momodu, D.; Dangbegnon, J.; Manyala, N.; Guerioune, M. Electrochemical measurements of synthesized nanostructured β-Ni(OH)2 using hydrothermal process and activated carbon based nanoelectroactive materials. SN Appl. Sci. 2019, 1, 34. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, Y.; Huang, X.; Shen, W.; Yu, J.; Li, J. A Facile Synthesis of Three Dimensional β-Ni(OH)2 Composed of Ultrathin Nanosheets for High Performance Pseudocapacitor. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2089–2097. [Google Scholar] [CrossRef]
- Narayan, R.T. Effect of Crystallinity of β- and β bc-Nickel Hydroxide Samples on Chemical Cycling. Indian J. Mater. Sci. 2015, 2015, 820193. [Google Scholar] [CrossRef]


| Element Composition | Al | Ca | K | Mn | Na | Co | Pb | S | Zn | Cu | Fe | Cl | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (ppm) | 7.3 | 4.53 | 22.5 | 6.7 | 210 | 9.8 | 10 | 162 | 73.9 | 3900 | 4720 | 80,000 | 50,130 |
| Solution Sample | |||||
|---|---|---|---|---|---|
| Element | Precursor Solution | pH 2.5 | Wash | pH 6.5 | |
| Cu | ppm | 801 | 501 | 23.3 | 95.7 |
| Fe | 2197.5 | 2.3 | 1.3 | 0 | |
| Ni | 50,130 | 3700 | 1800 | 600 | |
| Co | 43 | 26.4 | 1.5 | 0.7 | |
| Ca | 0 | 47,400 | 2300 | 57,200 | |
| Al | 1 | 10.8 | 1.2 | 1.1 | |
| Zn | 72.7 | 40.2 | 2.2 | 0 | |
| S | 134.1 | 446 | 19.7 | 456.3 | |
| Mg | 0 | 461.9 | 23.9 | 375 | |
| Pb | 14 | 8 | 0.2 | 0 | |
| Element | Peak BE (eV) | Atomic % |
|---|---|---|
| C 1s | 285.2 | 36.1 |
| O 1s | 531.7 | 23.5 |
| Cl 2p | 199.3 | 22.4 |
| Ni 2p | 856.2 | 11.8 |
| Ca 2p | 348.1 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabowa, M.H.; Mkhohlakali, A.; Chimuka, L.; Tshilongo, J. Separation and Characterization of Nickel Hydroxide from Waste Solution Using Ca(OH)2 Precipitation in Chloride Media. Separations 2024, 11, 96. https://doi.org/10.3390/separations11040096
Mabowa MH, Mkhohlakali A, Chimuka L, Tshilongo J. Separation and Characterization of Nickel Hydroxide from Waste Solution Using Ca(OH)2 Precipitation in Chloride Media. Separations. 2024; 11(4):96. https://doi.org/10.3390/separations11040096
Chicago/Turabian StyleMabowa, Mothepane Happy, Andile Mkhohlakali, Luke Chimuka, and James Tshilongo. 2024. "Separation and Characterization of Nickel Hydroxide from Waste Solution Using Ca(OH)2 Precipitation in Chloride Media" Separations 11, no. 4: 96. https://doi.org/10.3390/separations11040096
APA StyleMabowa, M. H., Mkhohlakali, A., Chimuka, L., & Tshilongo, J. (2024). Separation and Characterization of Nickel Hydroxide from Waste Solution Using Ca(OH)2 Precipitation in Chloride Media. Separations, 11(4), 96. https://doi.org/10.3390/separations11040096






