The Extraction Mechanism of Zirconium and Hafnium in the MIBK-HSCN System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Reagents
2.2. Experimental
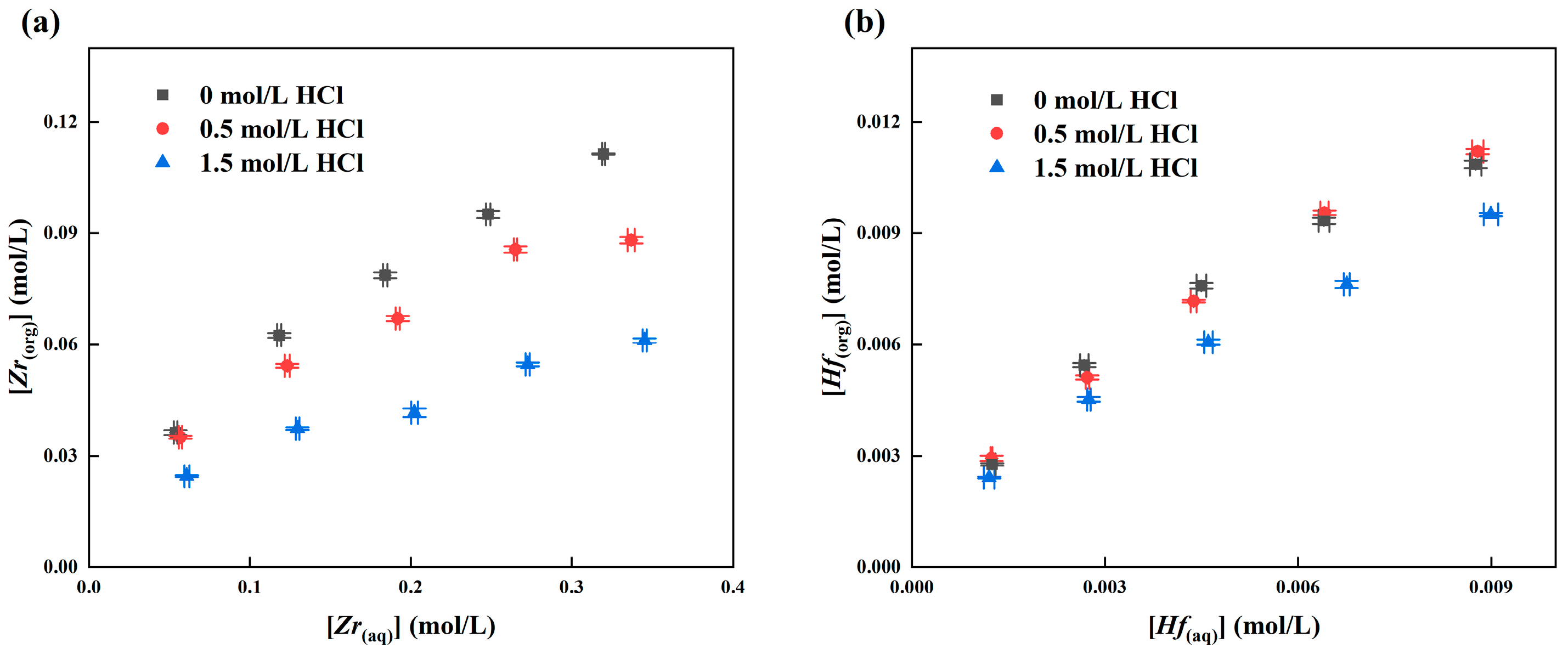
2.3. Stoichiometry and Calculation of Equilibrium Constants
3. Results and Discussion
3.1. Extraction of SCN−
3.1.1. Effect of MIBK Concentration on the SCN− Extraction
3.1.2. Effect of HCl Concentration on the SCN− Extraction
3.1.3. Stoichiometry and Equilibrium Constants
3.1.4. FT-IR and ESI-MS Analysis
3.2. Extraction of Zr and Hf
3.2.1. Effect of MIBK Concentration on the Zr and Hf Extraction
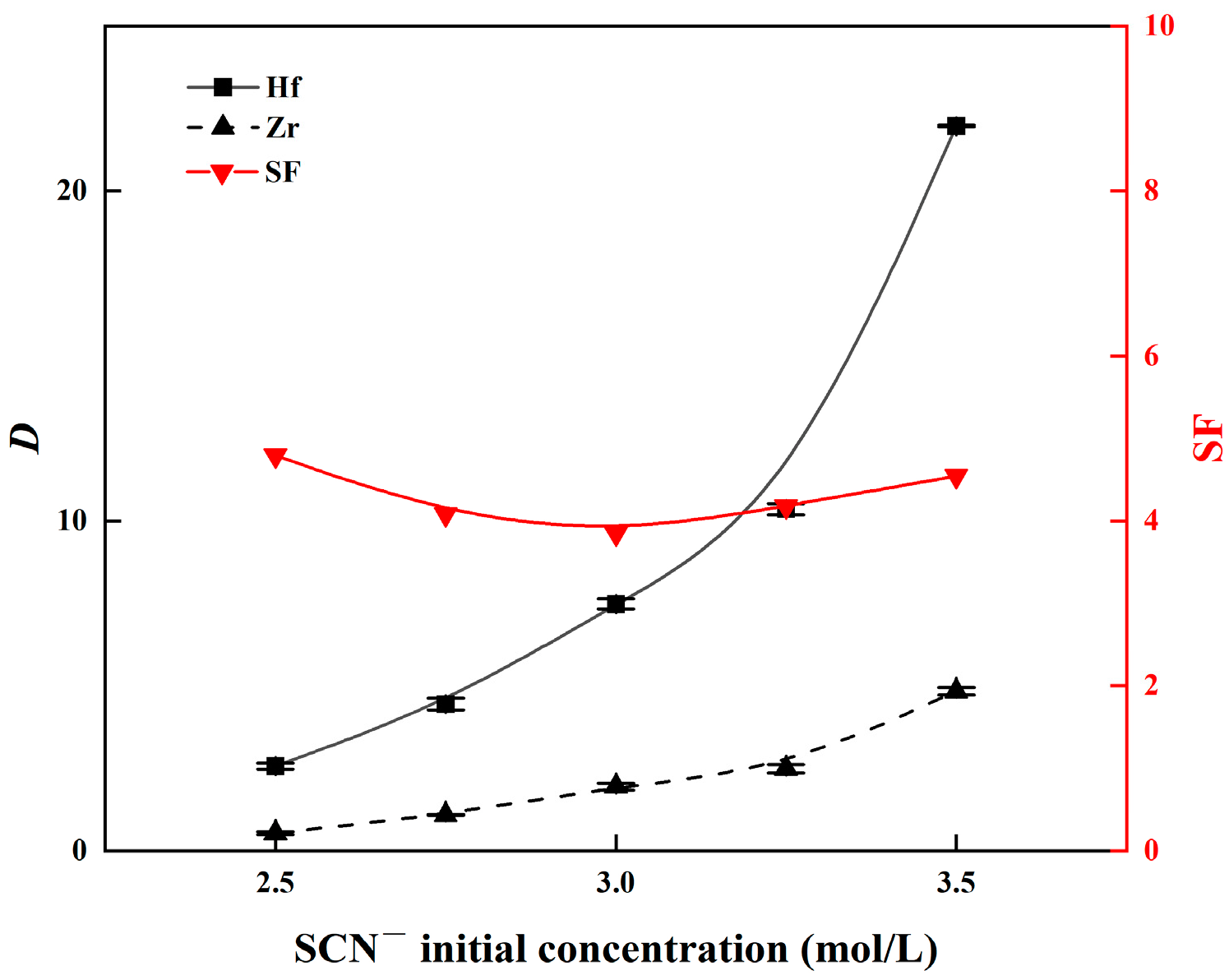
3.2.2. Effect of SCN− Concentration on the Zr and Hf Extraction
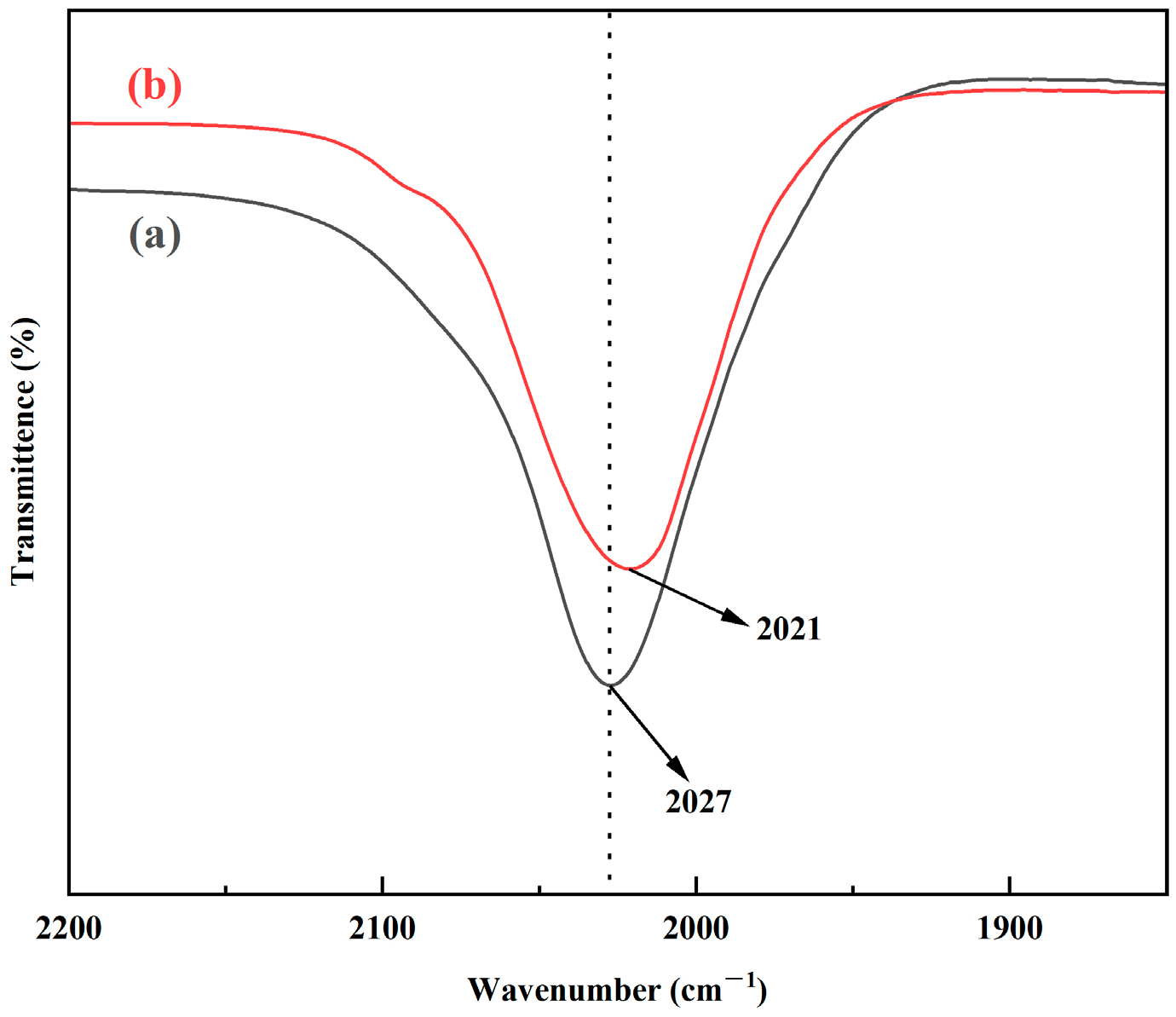
3.2.3. FT-IR Analysis
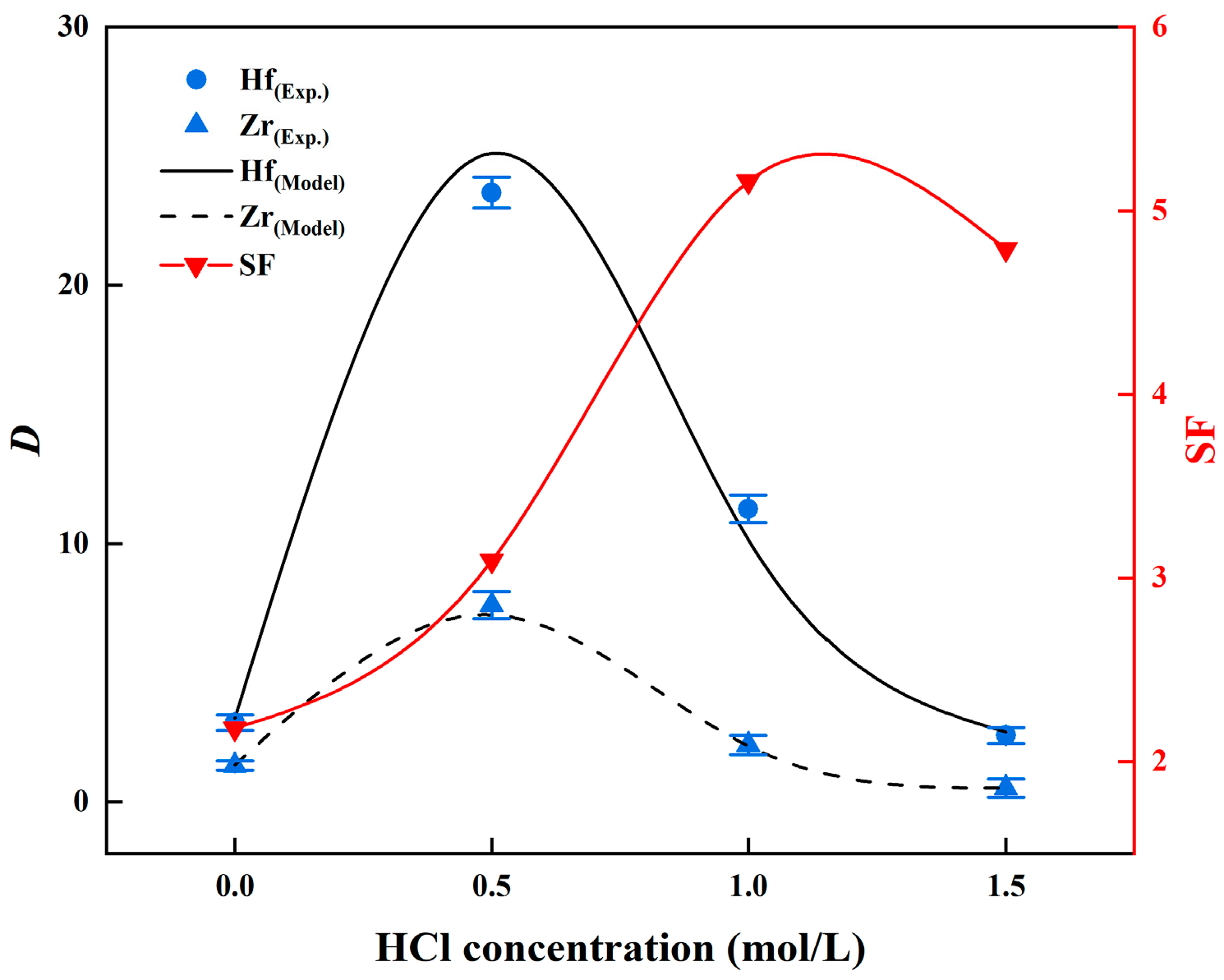
3.2.4. Effect of HCl Concentration in the Zr and Hf Extraction
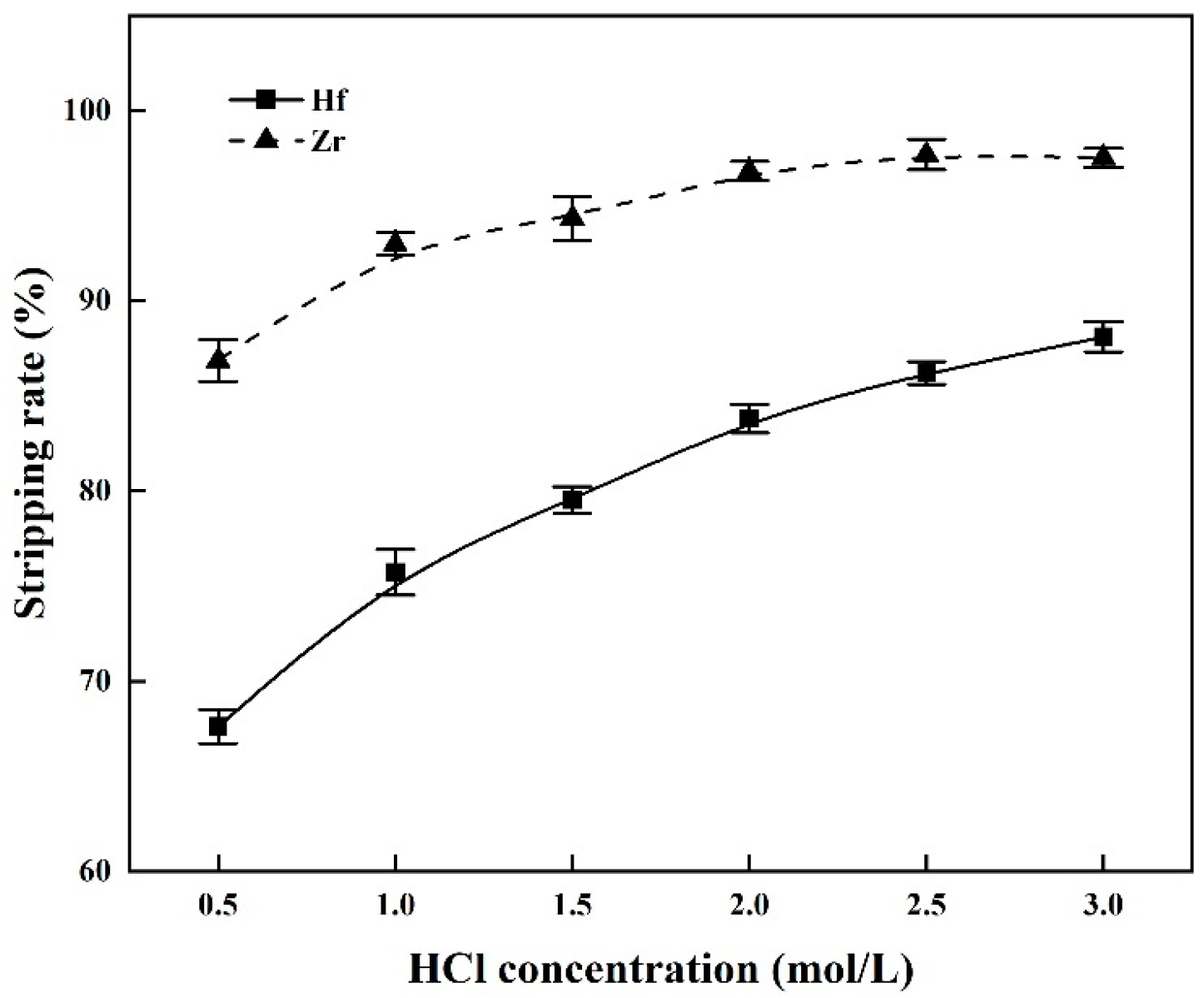
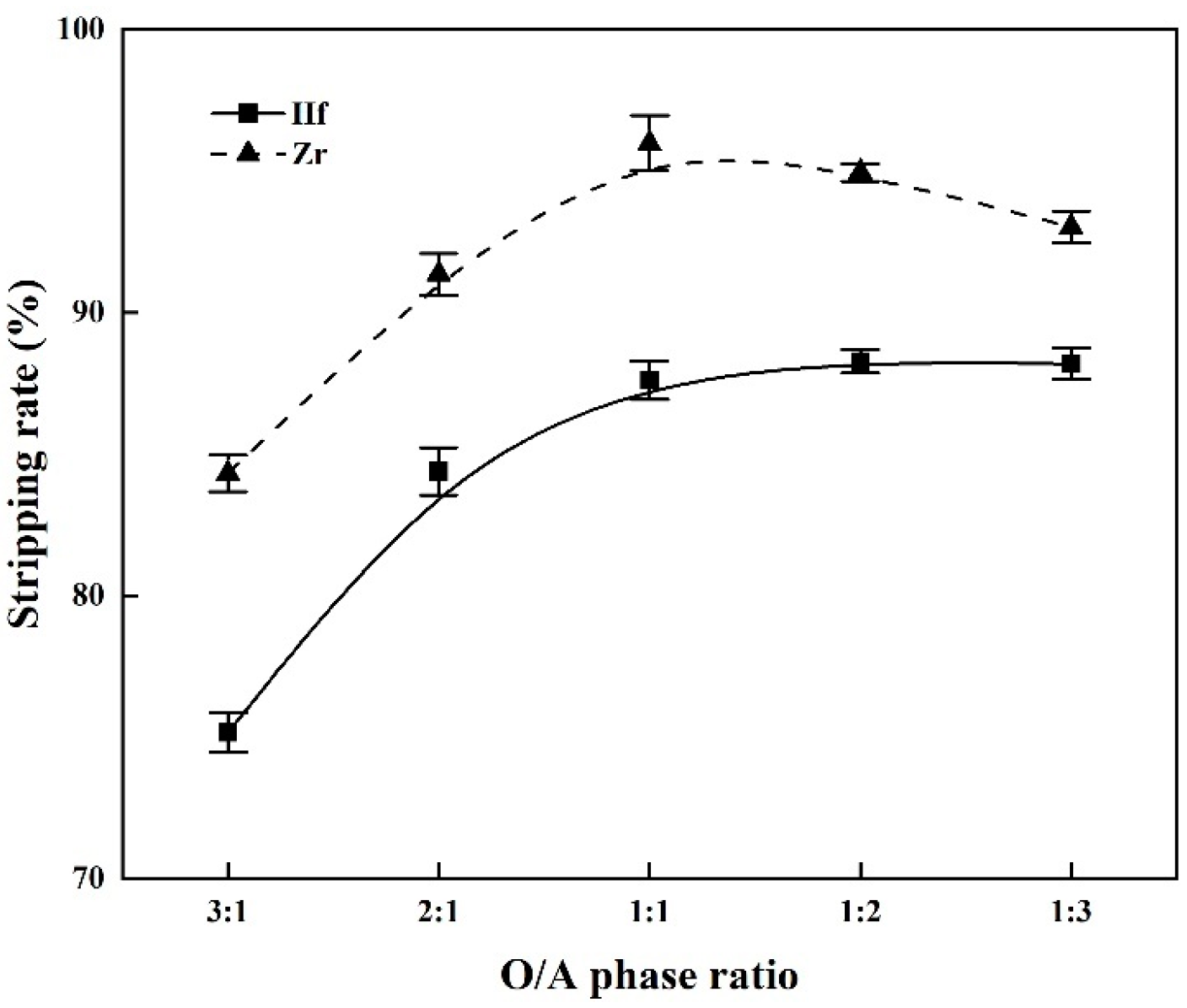
3.3. Stripping of Zr and Hf from Loaded MIBK
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benedict, M.; Pigford, T.H.; Levi, H.W. Nuclear Chemical Engineering Second Edition. Glob. Home Chem. Eng. 1981, 28, 702. [Google Scholar] [CrossRef]
- Ada, D.; Krscb, D.; Bp, A.; Smc, C.; Sm, A.; Aks, A.; Vka, D. Enhanced adsorption and separation of zirconium and hafnium under mild conditions by phosphoric acid based ligand functionalized silica gels: Insights from experimental and theoretical investigations. Sep. Purif. Technol. 2020, 239, 116518. [Google Scholar] [CrossRef]
- Crouse, P.L.; Niemand, H.F.; Postma, C.J. A theoretical approach to the sublimation separation of zirconium and hafnium in the tetrafluoride form. J. S. Afr. Inst. Min. Metall. 2015, 115, 961–965. [Google Scholar] [CrossRef]
- Banda, R.; Lee, M.S. Solvent Extraction for the Separation of Zr and Hf from Aqueous Solutions. Sep. Purif. Rev. 2015, 44, 199–215. [Google Scholar] [CrossRef]
- Felipe, E.C.B.; Ladeira, A.C.Q. Separation of zirconium from hafnium by ion exchange. Sep. Sci. Technol. 2017, 53, 330–336. [Google Scholar] [CrossRef]
- Kolon, A.J.; Smolik, M.; Jaroszek, H. The influence of the treatment of Zr(IV) and Hf(IV) sulfate solution on the ion-exchange purifying of zirconium from hafnium on Diphonix® resin. Hydrometallurgy 2013, 140, 77–81. [Google Scholar] [CrossRef]
- Xiao, Y.; Sandwijk, A.V.; Yang, Y.; Laging, V. Molten Salt Chemistry and Technology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Chaudry, M.; Malik, M.; Ahmad, M. Transport of titanium(IV) ions across di-2-ethylhexylphosphoric acid-CCl4 supported liquid membranes. J. Radioanal. Nucl. Chem. 1992, 157, 143–158. [Google Scholar] [CrossRef]
- Fischer, W.; Deierling, B.; Heitsch, H.; Otto, G.; Pohlmann, H.P.; Reinhardt, K. The Separation of Zirconium and Hafnium by Liquid-Liquid Partition of their Thiocyanates. Angew. Chem. Int. Ed. Engl. 1966, 5, 15–23. [Google Scholar] [CrossRef]
- da Silva, A.B.V., Jr.; Distin, P.A. Zirconium and hafnium separation without waste generation. CIM Bull. 1998, 91, 221–224. [Google Scholar]
- Levitt, A.E.; Freund, H. Solvent Extraction of Zirconium with Tributyl Phosphate1. J. Am. Chem. Soc. 1956, 78, 1545–1549. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Zr and Hf from sulfuric acid solutions with amine-based extractants by solvent extraction. Sep. Purif. Technol. 2015, 142, 83–89. [Google Scholar] [CrossRef]
- Otsuka, T. Zirconium and Hafnium Separation by Liquid-liquid Extraction with Hexone (Methyl isobutyl ketone). J. Min. Metall. Inst. Jpn. 1969, 85, 993–999. [Google Scholar]
- Zarpelon, L.M.C. A Contribution to the Study of the Separation of Zirconium-Hafnium in the MIBK-HSCN-HCl System; IPEN: Sao Paulo, Brazil, 1995. [Google Scholar]
- Lv, B.; Xu, Z.; Zhang, L.; Wang, L. Influence Parameters of Separation of Zirconium and Hafnium in MIBK-HSCN Systems. Chin. J. Rare Met. 2008, 32, 754–757. [Google Scholar]
- Wu, M.; Dong, P.; Wu, C.; Zhang, Z.; Chi, R.; Xu, Z. Separation of Hf(IV) from Zr(IV) in thiocyanate medium with ionic liquid Aliquat 336. Hydrometallurgy 2022, 213, 105947. [Google Scholar] [CrossRef]
- Ma, S.; Yang, F.; Tan, F.; Xie, M.; Yu, S.; Xue, L.; Li, Z.; Hu, T. Highly efficient and selective solvent extraction of zirconium and hafnium from chloride acid solution including amic acid extractant. Sep. Purif. Technol. 2021, 279, 119779. [Google Scholar] [CrossRef]
- He, H.; Xu, F.; Li, Q.; Dong, P.; Zheng, J.; Wu, C.; He, Z.; Qu, J.; Xu, Z.; Chi, R.; et al. Separation of hafnium from zirconium in HNO3 solution by solvent extraction with Cyanex572. Hydrometallurgy 2021, 202, 105600. [Google Scholar] [CrossRef]
- Amaral, J.; Morais, C.A. Equilibrium of zirconium and hafnium in the process of extraction with TBP in nitric medium—Influence in the Zr/Hf separation. Miner. Eng. 2020, 146, 106138. [Google Scholar] [CrossRef]
- Vinarov, I.V. Modern methods of separating zirconium and hafnium. Russ. Chem. Rev. 1967, 36, 522. [Google Scholar] [CrossRef]
- Krishna, G.G.; Reddy, R.S.; Raghunath, P.; Bhanuprakash, K.; Kantam, M.L.; Choudary, B.M. A Computational Study of Ligand Interactions with Hafnium and Zirconium Metal Complexes in the Liquid-Liquid Extraction Process. J. Phys. Chem. B 2004, 108, 6112–6120. [Google Scholar] [CrossRef]
- Singhal, A.; Toth, L.; Lin, J.; Affholter, K. Zirconium (IV) tetramer/octamer hydrolysis equilibrium in aqueous hydrochloric acid solution. J. Am. Chem. Soc. 1996, 118, 11529–11534. [Google Scholar] [CrossRef]
- Sommers, J.A.; Hutchison, D.C.; Martin, N.P.; Palys, L.; Amador, J.M.; Keszler, D.A.; Nyman, M. Differentiating Zr/HfIV Aqueous Polyoxocation Chemistry with Peroxide Ligation. Inorg. Chem. 2021, 60, 1631–1640. [Google Scholar] [CrossRef]
- Stern, R.D.; Kingsbury, R.S.; Persson, K.A. Aqueous Stability of Zirconium Clusters, Including the Zr(IV) Hexanuclear Hydrolysis Complex [Zr6O4(OH)4(H2O)24]12+, from Density Functional Theory. Inorg. Chem. 2021, 60, 15456–15466. [Google Scholar] [CrossRef]
- Sommers, J.A.; Palys, L.; Martin, N.P.; Fast, D.B.; Amiri, M.; Nyman, M. Oxo-Cluster-Based Zr/HfIV Separation: Shedding Light on a 70-Year-Old Process. J. Am. Chem. Soc. 2022, 144, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; He, H.; Xu, F.; Xu, Z.; Zhang, W.; He, Z.; Qu, J.; Ruan, C.; Huang, L. High-efficient and selective extraction of Hf over Zr with DIBK-P350 synergistic extraction system. Sep. Purif. Technol. 2019, 212, 255–261. [Google Scholar] [CrossRef]
- Wang, Q.; Tsunoda, K.-i.; Akaiwa, H. Extraction Chromatographie Behaviors of Zirconium and Hafnium Using Sulfoxides as Stationary Phases. Anal. Sci. 1995, 11, 909–913. [Google Scholar] [CrossRef]
- Elinson, S.V.; Petrov, K.I. Analytical Chemistry of Zirconium and Hafnium; Elsevier: Amsterdam, The Netherlands, 1965. [Google Scholar]
- Solovkin, A.S.; Tsvetkova, Z.N. The Chemistry of Aqueous Solutions of Zirconium Salts. Russ. Chem. Rev. 1962, 31, 655–669. [Google Scholar] [CrossRef]
- Larsen, E.M. Zirconium and Hafnium Chemistry. In Advances in Inorganic Chemistry and Radiochemistry; Emeléus, H.J., Sharpe, A.G., Eds.; Academic Press: Cambridge, MA, USA, 1970; Volume 13, pp. 1–133. [Google Scholar]
- Baglin, F.G.; Breger, D. Identification of the zirconium sulfate species in highly acidic aqueous solutions by Raman spectroscopy. Inorg. Nucl. Chem. Lett. 1976, 12, 173–177. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Covington, A.; Matheson, R. A Raman study of the lonization of thiocyanic acid. J. Solut. Chem. 1976, 5, 781–786. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. A review on the aqueous chemistry of Zr (IV) and Hf (IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Laubscher, A.E.; Fouché, K.F. Investigation of Zr(IV), Hf(IV) and Th(IV) thiocyanate complexes by extraction with thenoyltrifluoroacetone and dinonyl napthalene sulphonic acid. J. Inorg. Nucl. Chem. 1971, 33, 3521–3535. [Google Scholar] [CrossRef]
- Tan, B.; Chang, C.; Xu, D.; Wang, Y.; Qi, T. Modeling of the competition between uranyl nitrate and nitric acid upon extraction with tri-n-butyl phosphate. ACS Omega 2020, 5, 12174–12183. [Google Scholar] [CrossRef] [PubMed]
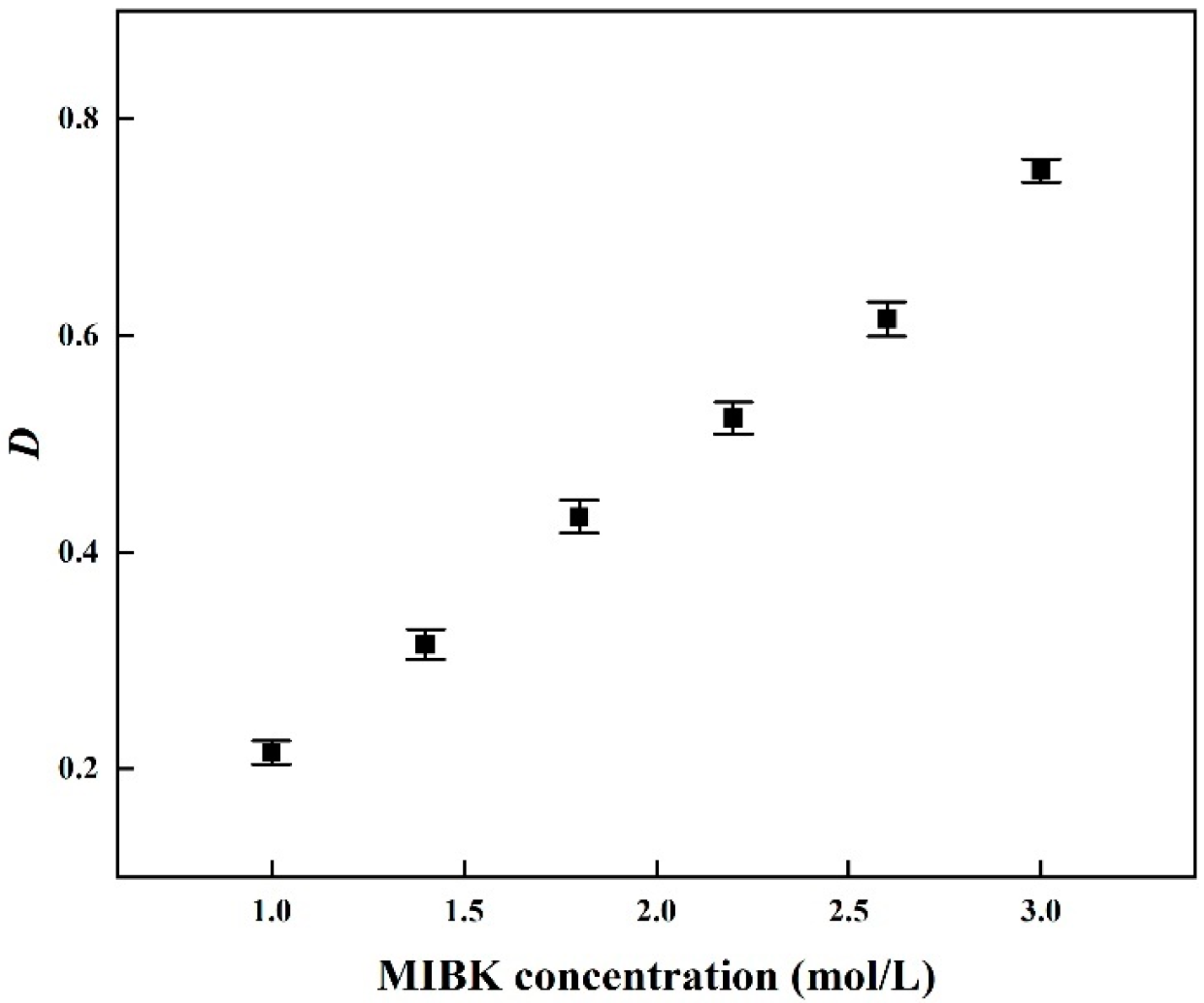
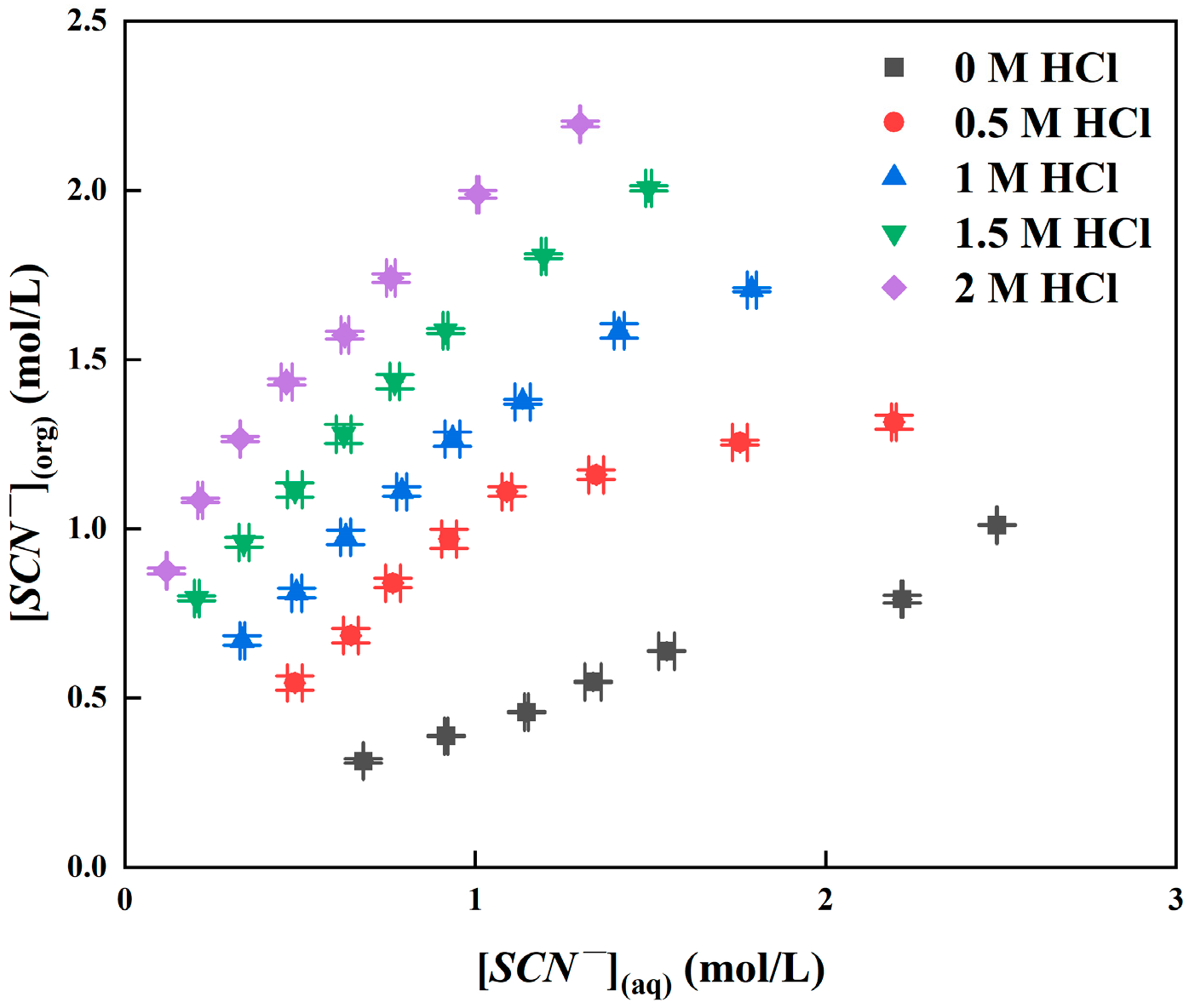

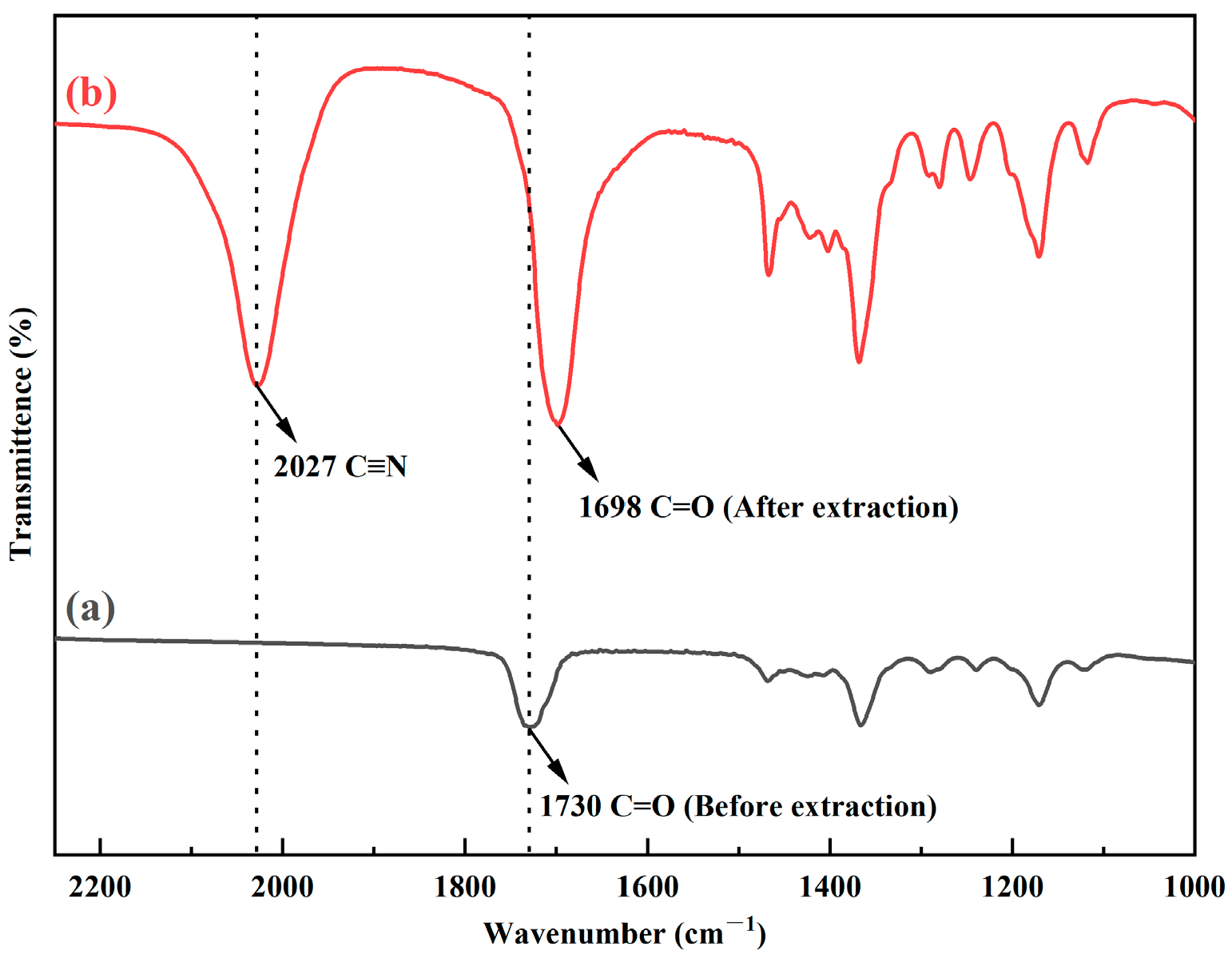
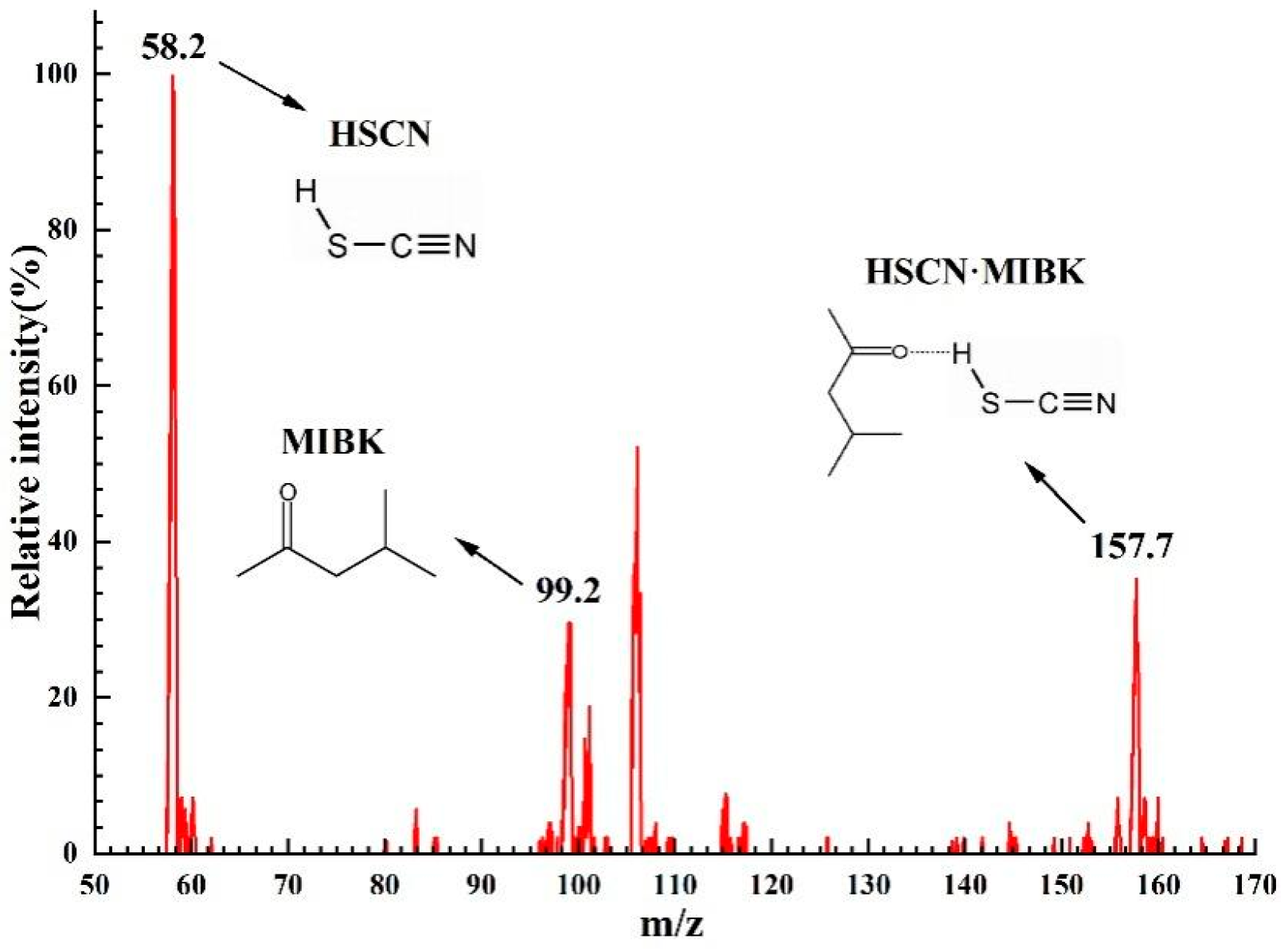



| [MIBK] | [H+(aq)] | D(SCN) |
|---|---|---|
| 1 | 1.698 | 0.194 |
| 1.4 | 1.667 | 0.288 |
| 1.8 | 1.622 | 0.383 |
| 2.6 | 1.288 | 0.614 |
| 3 | 1.259 | 0.731 |
| 4 | 1.202 | 1.064 |
| 5 | 1.072 | 1.425 |
| 8 | 1 × 10−5 | 0.011 |
| 8 | 0.055 | 0.271 |
| 8 | 0.076 | 0.319 |
| 8 | 0.132 | 0.486 |
| 8 | 0.178 | 0.663 |
| 8 | 0.191 | 0.646 |
| 8 | 0.263 | 0.874 |
| 8 | 0.363 | 1.106 |
| 8 | 0.398 | 1.289 |
| HCl Concentration (M) | δ (Zr) | δ (Hf) | K3 (Zr) | K3 (Hf) |
|---|---|---|---|---|
| 0 | 3.34 | 3.30 | 1.311 | 0.704 |
| 0.5 | 4.44 | 4.52 | 9.279 | 7.370 |
| 1 | 4.77 | 4.80 | 5.721 | 6.273 |
| 1.5 | 4.46 | 4.13 | 3.780 | 4.241 |
| 2 | 2.97 | 2.85 | 2.497 | 3.292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Li, Y.; Zhang, X.; Wang, Y.; Zhang, Y.; Qi, T. The Extraction Mechanism of Zirconium and Hafnium in the MIBK-HSCN System. Separations 2024, 11, 93. https://doi.org/10.3390/separations11040093
Xiong J, Li Y, Zhang X, Wang Y, Zhang Y, Qi T. The Extraction Mechanism of Zirconium and Hafnium in the MIBK-HSCN System. Separations. 2024; 11(4):93. https://doi.org/10.3390/separations11040093
Chicago/Turabian StyleXiong, Jing, Yang Li, Xiaomeng Zhang, Yong Wang, Yanlin Zhang, and Tao Qi. 2024. "The Extraction Mechanism of Zirconium and Hafnium in the MIBK-HSCN System" Separations 11, no. 4: 93. https://doi.org/10.3390/separations11040093
APA StyleXiong, J., Li, Y., Zhang, X., Wang, Y., Zhang, Y., & Qi, T. (2024). The Extraction Mechanism of Zirconium and Hafnium in the MIBK-HSCN System. Separations, 11(4), 93. https://doi.org/10.3390/separations11040093








