Decoration of Pt–Ni Alloy on Molten Salt Etched Halloysite Nanotubes for Enhanced Catalytic Reduction of 4-Nitrophenol
Abstract
1. Introduction
2. Experiments and Characterization
2.1. Materials
2.2. Characterization
2.3. Preparation of rHNTs
2.4. Preparation of Pt–Ni/rHNTs and Pt–Ni/HNTs
2.5. Catalytic Reduction of 4-NP
3. Results and Discussion
3.1. Fabrication Process of rHNTs and Pt–Ni/rHNTs
3.2. Characterization of Pt–Ni/rHNT Nanocatalysts
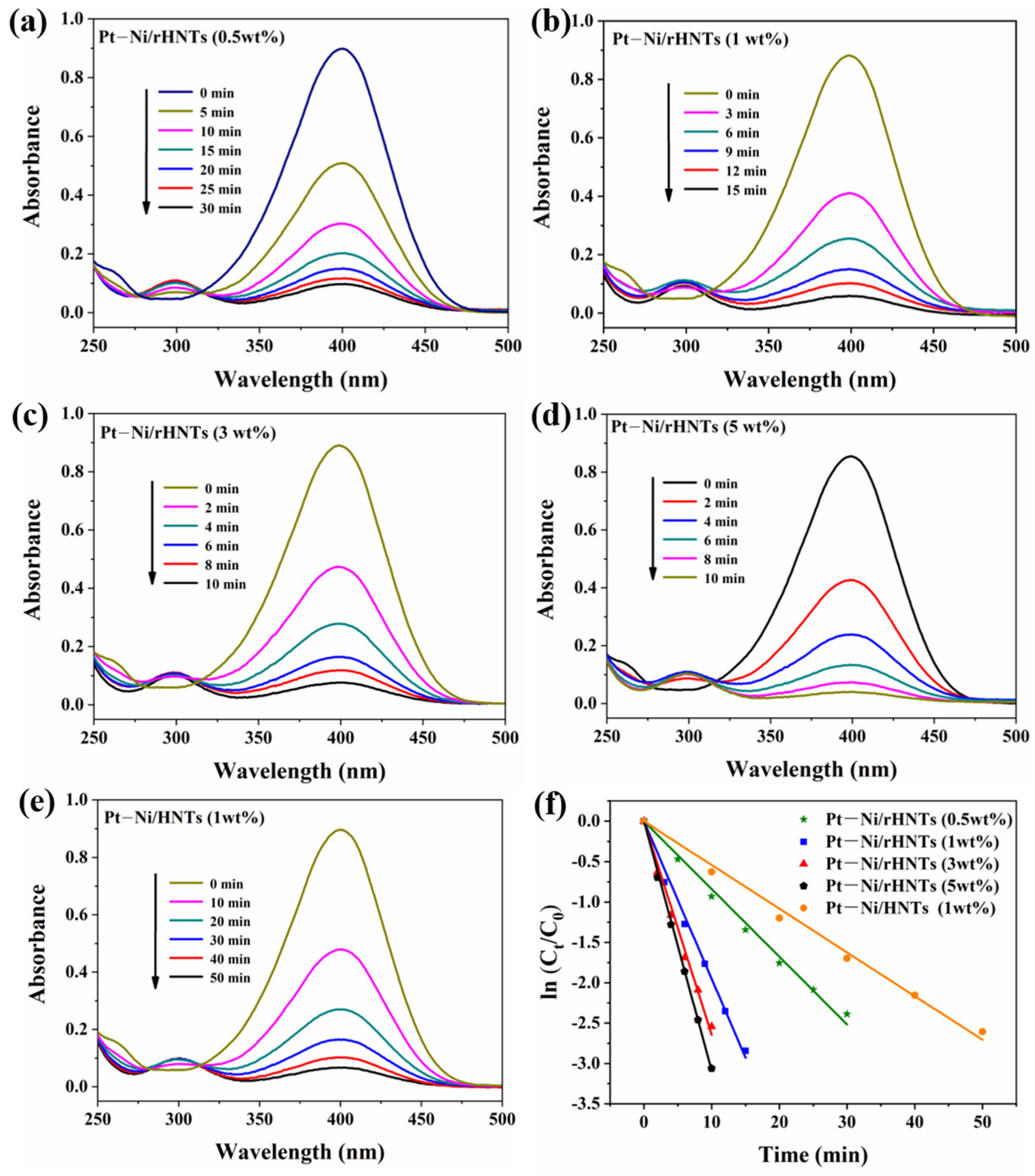
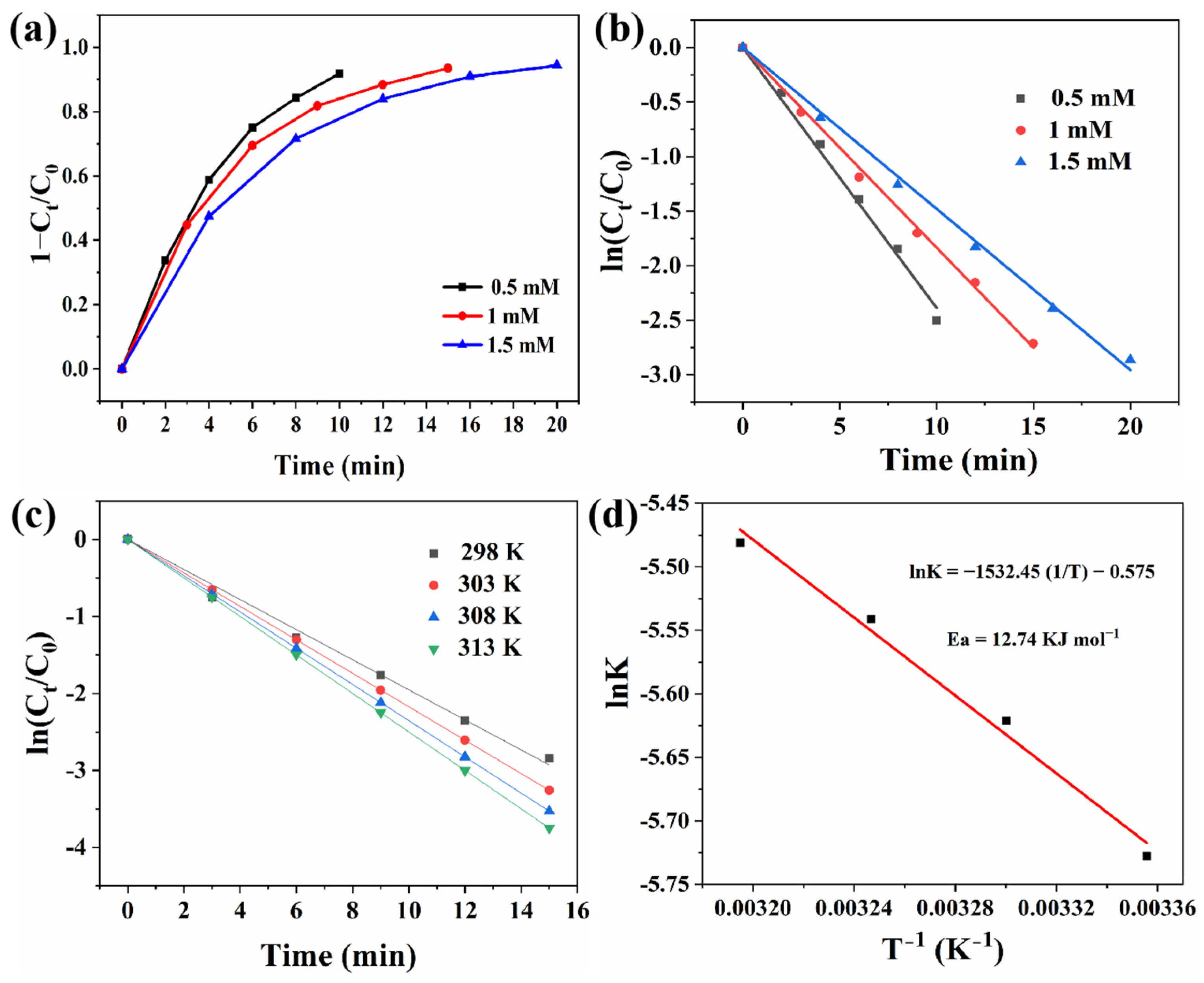
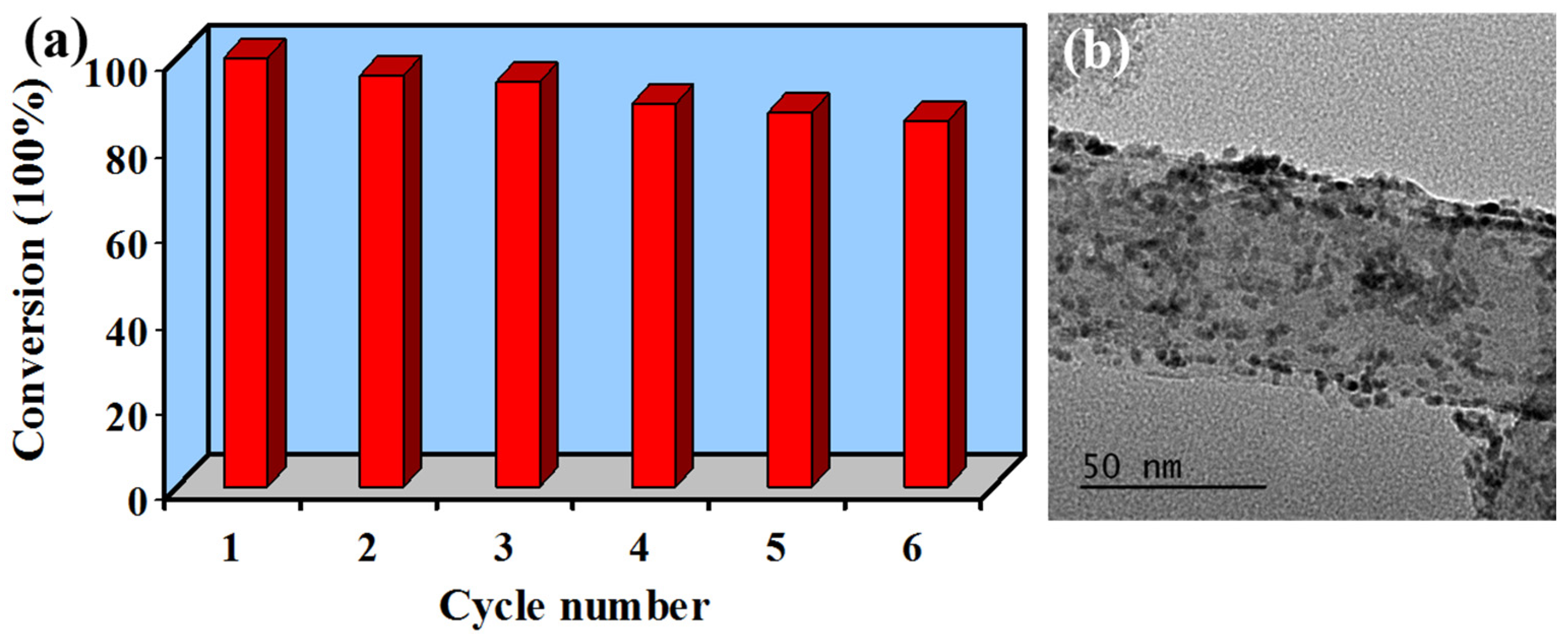
3.3. Catalytic Reduction of 4-NP on Pt–Ni/rHNT Nanocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Quan, X.; Hu, C.; Li, C. Effective catalytic reduction of 4-nitrophenol to 4-aminophenol over etched halloysite nanotubes@α-Ni(OH)2. ACS Appl. Energy Mater. 2020, 3, 4756–4766. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, Z.; Du, F.; Liu, H.; Zhang, Q.; Zhang, Y. Recyclable Ag/halloysite nanotubes/polyvinyl alcohol sponges for enhanced reduction of 4-nitrophenol. Appl. Clay Sci. 2022, 223, 106510. [Google Scholar] [CrossRef]
- Wi-Afedzi, T.; Kwon, E.; Tuan, D.D.; Lin, K.A.; Ghanbari, F. Copper hexacyanoferrate nanocrystal as a highly efficient non-noble metal catalyst for reduction of 4-nitrophenol in water. Sci. Total Environ. 2020, 703, 134781. [Google Scholar] [CrossRef]
- Zhang, M.; Su, X.; Ma, L.; Khan, A.; Wang, L.; Wang, J.; Maloletnev, A.S.; Yang, C. Promotion effects of halloysite nanotubes on catalytic activity of Co3O4 nanoparticles toward reduction of 4-nitrophenol and organic dyes. J. Hazard. Mater. 2021, 403, 123870. [Google Scholar] [CrossRef]
- Yu, J.; Niedenthal, W.; Smarsly, B.M.; Natile, M.M.; Huang, Y.; Carraro, M. Au nanoparticles supported on piranha etched halloysite nanotubes for highly efficient heterogeneous catalysis. Appl. Surf. Sci. 2021, 546, 149100. [Google Scholar] [CrossRef]
- Hu, R.; Xu, R.; Wang, Z.; Wang, J.; Zhou, S. Enhanced catalytic nitrophenol reduction via highly porous hollow silica nanospheres stabilized Au nanoclusters. Chem. Eng. J. 2023, 471, 144780. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Tan, W.; Zhong, C.; Tu, Y.; Song, H.; Du, L.; Liao, S.; Cui, Z. Amorphous TiOx stabilized intermetallic Pt3Ti nanocatalyst for methanol oxidation reaction. Nano Lett. 2023, 23, 5187–5193. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Na, J.; Hossain, M.S.A.; Lin, J.; Kaneti, Y.V.; Iqbal, M.; Jiang, B.; Bando, Y.; Asahi, T.; Yamauchi, Y. Block copolymer-templated electrodeposition of mesoporous Au-Ni alloy films with tunable composition. Appl. Mater. Today 2020, 18, 100526. [Google Scholar] [CrossRef]
- Swain, S.; Kandathil, V.; Karim, G.M.; Maiti, U.N.; Patil, S.A.; Samal, A.K. Computational and experimental design of the octahedral PdFe alloy nanocatalyst for hiyama cross-coupling and environmental pollutant degradation. ACS Appl. Nano Mater. 2023, 6, 3254–3267. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Chen, H.; Yang, Y.; Wang, D.; Shang, H.; Zhang, B. Phosphorus and sulfur co-doped nickel molybdate with rich-oxygen vacancies for efficient water splitting. J. Colloid Interface Sci. 2025, 677, 167–177. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Gao, X.; Zhao, Y. Constructing ultrafine monodispersed Co2P/(0.59-Cu3P) on Cu doped CoZn-ZIF derived porous N-doped carbon for highly efficient dehydrogenation of ammonia borane. Nano Res. 2023, 16, 6687–6700. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Xue, X.; Wang, D.; Shang, H.; Zhao, Y.; Zhang, B. Interface engineering of three-phase nickel–cobalt sulfide/nickel phosphide/iron phosphide heterostructure for enhanced water splitting and urea electrolysis. J. Colloid Interface Sci. 2024, 665, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Orfei, E.; Fasolini, A.; Abate, S.; Dimitratos, N.; Basile, F. Layered-double hydroxides and derived oxide as CRM-free highly active catalysts for the reduction of 4-nitrophenol. Catal. Today 2023, 419, 114153. [Google Scholar] [CrossRef]
- Xiong, C.; Zhang, X.; Lei, Y.; Zhang, L.; Shang, H.; Zhang, B.; Zhao, Y. One-pot synthesis of ultrafine Ni0.13Co0.87P nanoparticles on halloysite nanotubes as efficient catalyst for hydrogen evolution from ammonia borane. Appl. Clay Sci. 2021, 214, 106293. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, W.; Jin, Z.; Fu, Y.; Wang, W.; Zhang, Y.; Liu, J.; Zhang, B. Storing solar energy within Ag-paraffin@halloysite microspheres as a novel self-heating catalyst. Appl. Energy 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Zhao, Y.; Zhang, B.; Niu, Y.; Xiang, X.; Chen, R. Fabricating roughened surfaces on halloysite nanotubes via alkali etching for deposition of high-efficiency Pt nanocatalysts. CrystEngComm 2015, 17, 3110. [Google Scholar] [CrossRef]
- Ma, X.; Yang, C.; Feng, X.; Shang, H.; Zhao, Y.; Zhang, B. Halloysite-based aerogels for efficient encapsulation of phase change materials with excellent solar energy storage and retrieval performance. Appl. Energy 2023, 341, 121101. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Xue, X.; Xiong, C.; Gao, X.; Zhang, B. Halloysite nanotubes supported Co2P bridged by carbon dots for enhanced hydrogen evolution from ammonia borane. Chem. Eng. J. 2024, 483, 149332. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Guo, L.; Mi, A.; Xue, X.; Wu, Z.; Gao, H.; Shang, H.; Wang, D.; Zhao, Y.; Zhang, B. Enhanced hydrophilicity and stability of carbon-based aerogel with assembling of halloysite nanotubes and silica fibers for efficient solar seawater evaporation and desalination. Sep. Purif. Technol. 2024, 342, 126966. [Google Scholar] [CrossRef]
- Mi, A.; Guo, L.; Yan, Y.; Wang, D.; Shang, H.; Zhao, Y.; Zhang, B. A solid-system strategy for controlled hydrolytic release of hydrogen by encapsulation of ammonia borane in cobalt decorated halloysite aerogel. ACS Sustain. Chem. Eng. 2024, 12, 5716–5725. [Google Scholar] [CrossRef]
- Chen, P.; Tang, Y.; Gao, Y.; Zhang, Y.; Liu, L. Design and structure optimization of coal-based hierarchical porous carbon by molten salt method for high-performance supercapacitors. J. Power Sources 2023, 580, 233334. [Google Scholar] [CrossRef]
- Arole, K.; Blivin, J.W.; Saha, S.; Holta, D.E.; Zhao, X.; Sarmah, A.; Cao, H.; Radovic, M.; Lutkenhaus, J.L.; Green, M.J. Water-dispersible Ti3C2Tz MXene nanosheets by molten salt etching. iScience 2021, 24, 103403. [Google Scholar] [CrossRef]
- Sun, G.; Liu, Y.; Dong, S.; Wang, J. Study on novel molten salt-ceramics composite as energy storage material. J. Energy Storage 2020, 28, 101237. [Google Scholar] [CrossRef]
- Zhao, F.; Kong, W.; Hu, Z.; Liu, J.; Zhao, Y.; Zhang, B. Tuning the performance of Pt–Ni alloy/reduced graphene oxide catalysts for 4-nitrophenol reduction. RSC Adv. 2016, 6, 79028–79036. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Wang, L.; Yang, J.; Xiang, X.; Zhang, B.; Li, F. Highly active supported Pt nanocatalysts synthesized by alcohol reduction towards hydrogenation of cinnamaldehyde: Synergy of metal valence and hydroxyl groups. Chem. Asian J. 2015, 10, 1561–1570. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Wei, Y.; Li, B.; Yan, W.; Yin, L.; Wu, D.; Liu, P.; Zhang, P. Cotton-derived carbon fiber-supported Ni nanoparticles as nanoislands to anchor single-atom Pt for efficient catalytic reduction of 4-nitrophenol. Appl. Catal. A General 2022, 643, 118734. [Google Scholar] [CrossRef]
- Zhao, H.; Pang, X.; Huang, Y.; Bai, Y.; Ding, J.; Bai, H.; Fan, W. Ag/Ni–MOF heterostructure with synergistic enrichment and activation properties for electrocatalytic reduction of 4-nitrophenol. Chem. Commun. 2022, 58, 13499–13502. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, Q.; Zhang, L.; Zhao, Y.; Zhang, B. NiCo alloy decorated on porous N-doped carbon derived from ZnCoZIF as highly efficient and magnetically recyclable catalyst for hydrogen evolution from ammonia borane. Renew. Energy 2021, 173, 273–282. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Wang, X.; Tan, F.; Wang, W.; Qiao, X.; Qiu, X.; Chen, J. Anchoring of silver nanoparticles on graphitic carbon nitride sheets for the synergistic catalytic reduction of 4-nitrophenol. Chemosphere 2017, 172, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, L.; Shen, Q.; Sun, N.; Wei, W. Surfactant-free synthesis of Ag nanoparticles loaded ZIF-8 as a catalytic filter device for continuous reduction of 4-nitrophenol. Catal. Lett. 2023, 153, 453–459. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R. Enhanced catalytic reduction of nitrophenols by sodium borohydride over highly recyclable Au@graphitic carbon nitride nanocomposites. Appl. Catal. B Environ. 2019, 240, 337–347. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, Y.; Zhu, W.; Lin, Z.; Tsai, M. In-situ fabrication of Ag nanoparticles decorated magnetic N-doped graphene heterogeneous catalyst for the catalytic reduction of 4-nitrophenol. J. Taiwan Inst. Chem. Eng. 2024, 163, 105524. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, W.; Yeh, J.; Tsai, M. In-situ synthesis of Au/carbonized botanical leaf functionalized-electroactive polyimide nanocomposites and their application in enhanced catalytic activity for reduction 4-nitrophenol. J. Environ. Chem. Eng. 2024, 12, 111455. [Google Scholar] [CrossRef]





| Catalysts | Pt–Ni/rHNTs | Pt–Ni/HNTs | |||
|---|---|---|---|---|---|
| Theoretical Pt–Ni loading (wt %) | 0.5 | 1 | 3 | 5 | 1 |
| Measured actual Pt–Ni loading (wt %) | 0.36 | 0.80 | 2.63 | 4.10 | 0.32 |
| Sample | K (min−1) | R2 | TOF (×1017) Molecules g−1 s−1 |
|---|---|---|---|
| Pt–Ni/rHNTs (0.5 wt %) | 0.0841 | 0.9902 | 611.2 |
| Pt–Ni/rHNTs (1 wt %) | 0.1953 | 0.9915 | 764.1 |
| Pt–Ni/rHNTs (3 wt %) | 0.2645 | 0.9903 | 317.3 |
| Pt–Ni/rHNTs (5 wt %) | 0.3088 | 0.9986 | 240.8 |
| Pt–Ni/HNTs (1 wt %) | 0.0541 | 0.9923 | 187.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Zhao, Y.; Zhai, Z.; Chen, S.; Zhang, B. Decoration of Pt–Ni Alloy on Molten Salt Etched Halloysite Nanotubes for Enhanced Catalytic Reduction of 4-Nitrophenol. Separations 2024, 11, 305. https://doi.org/10.3390/separations11110305
Duan J, Zhao Y, Zhai Z, Chen S, Zhang B. Decoration of Pt–Ni Alloy on Molten Salt Etched Halloysite Nanotubes for Enhanced Catalytic Reduction of 4-Nitrophenol. Separations. 2024; 11(11):305. https://doi.org/10.3390/separations11110305
Chicago/Turabian StyleDuan, Jingmin, Yafei Zhao, Zhuhe Zhai, Shengqiang Chen, and Bing Zhang. 2024. "Decoration of Pt–Ni Alloy on Molten Salt Etched Halloysite Nanotubes for Enhanced Catalytic Reduction of 4-Nitrophenol" Separations 11, no. 11: 305. https://doi.org/10.3390/separations11110305
APA StyleDuan, J., Zhao, Y., Zhai, Z., Chen, S., & Zhang, B. (2024). Decoration of Pt–Ni Alloy on Molten Salt Etched Halloysite Nanotubes for Enhanced Catalytic Reduction of 4-Nitrophenol. Separations, 11(11), 305. https://doi.org/10.3390/separations11110305





