Abstract
Puerariae Radix is one of the most widely used ancient traditional Chinese medicines and is also consumed as food, which has rich edible and medicinal value. Puerariae Radix can be divided into Puerariae Lobatae Radix (PL) and Puerariae Thomsonii Radix (PT). These two medicinal materials are very similar, and they are often mixed up or misused. In this study, gas chromatography–ion migration spectrometry (GC-IMS) was used to analyze the volatile organic compounds (VOCs) of PL and PT, and the differences in VOCs were analyzed using fingerprint, principal component analysis (PCA), and orthogonal partial least squares discriminant analysis (OPLS-DA). The results showed that a total of 173 VOCs were obtained from PL and PT, and 149 were qualitatively identified, including 38 aldehydes, 22 alcohols, 22 ketones, 19 esters, 13 esters, 10 acids, 10 pyrazines, 6 terpenes, 4 furans, and 2 pyridines. The characteristic VOCs of PL and PT were clarified by constructing GC-IMS fingerprints. PL and PT can be effectively distinguished, and five characteristic VOCs were screened using PCA and OPLS-DA analysis methods. This study identified and evaluated the types and differences in VOCs in PL and PT. The established method is simple, rapid, accurate, and sensitive, and it provides theoretical guidance for the identification, tracing, and quality evaluation of PL and PT.
1. Introduction
Puerariae Lobatae Radix (PL) and Puerariae Thomsonii Radix (PT) are traditional medicinal materials. They are included in the “List of Items Both Food and Drugs” by the Ministry of Health of China, detailing their widespread use in medicine, health products, food, and so on [1,2]. In order to improve their taste, efficacy, and convenience, they are usually eaten in powder form. Although China has formulated systems and regulations for the application of powdered traditional Chinese medicine and food, the application of powdered Chinese medicine faces obstacles. Furthermore, powdered Chinese medicine does not display the obvious characteristics of conventional Chinese medicine, so it is relatively difficult to identify. The authenticity of the powder of traditional Chinese medicine directly affects the safety and efficacy of traditional Chinese medicine, so the scientific identification of the powder of Chinese medicine is very important. PL and PT are derived from the dried roots of leguminous plants Pueraria lobata (Willd.) Ohwi and Puerariathomsonii Benth., respectively. They can release muscles and subside fever, encourage the production of body fluids, induce eruptions, and elevate spleen yang to arrest diarrhea [3], and they both contain flavonoids, starch, dietary fiber, and a variety of trace elements and other components. Flavonoids such as puerarin, daidzin, and daidzein have significant effects on improving microcirculation and lowering blood pressure [4]. Dietary fibers such as cellulose and lignin have anti-cancer effects and regulate blood sugar [5]. Starch is the main component of PL and PT, which contains trace isoflavone compounds, which are rich in calcium, phosphorus, potassium, iron, zinc, and other mineral elements essential for the human body [6] and is often used as raw material for new health food [7]. Although PL and PT contain similar components, the content of pueraria is greatly different due to the influence of the growing environment and variety. The 2020 edition of the Chinese Pharmacopoeia stipulates that the contents of puerarin in PL and PT are not less than 2.4% and 0.3%, respectively. Puerarin is a special isoflavone compound in PL and PT that can be used to treat cardiovascular and cerebrovascular diseases [8], diabetes and complications of diabetes [9], osteonecrosis [10], Parkinson’s disease [11], Alzheimer’s disease [12], endometriosis [13], cancer [14], etc.
Most of the original plants of PL are wild, while most of the original plants of PT are cultivated. At present, there are many cultivated varieties of PL in various places, and the sources of artificially cultivated varieties are complex, resulting in an uneven quality and yield of medicinal materials. In addition, low-cost PT and PL are often mixed and sold to obtain higher profits [15], which seriously affects the safety and effectiveness of drugs [16]. At present, the identification studies of PL and PT mostly adopt trait identification, microscopic identification, high-performance liquid fingerprints [17], gene sequencing [18], etc. Although they are provided more choices for identification, there are also some limitations, such as the character identification is subjective, the microscopic identification is not specific, the HPLC identification operation is cumbersome and time-consuming, and the gene sequencing technology is relatively difficult. Therefore, an efficient, rapid, and intuitive method is urgently needed for the analysis and identification of PL and PT.
Volatile organic compounds (VOCs) are important indicators for the identification and quality evaluation of traditional Chinese medicine. At present, gas chromatography–ion migration spectrometry (GC-IMS) is a new analysis technology for VOC detection that has been widely used in the separation, identification, and quantification of VOCs. It has a high separation capability for GC and fast response, high resolution, and high sensitivity for IMS [19]. In the process of substance analysis, the sample requires limited pretreatment to retain the sample’s smell to the maximum extent, and via signal integration in the spectrum, the visualization of flavor substances can be realized, and the types of VOCs in the sample can be rapidly analyzed [20,21,22,23]. It has been widely used in the analysis of food flavor [24,25,26,27,28,29]. He Jia used HS-GC-IMS technology to analyze VOCs in Ophiopogon from different producing areas, and these characteristics could effectively identify Ophiopogon from Sichuan and Zhejiang, as well as the two traditional main producing areas of Cixi City and Sanmen County, providing a scientific basis for the identification of Ophiopogon origin [30]. Zhen–Zhou Wang used GC-IMS technology to identify Ginseng Radix ET Rhizoma Rubra, Panacis Quniquefolii Radix, and Ginseng, and realized the origin traceability of Ginseng via a gas-phase ion migration system combined with data analysis software, providing reference for the identification of Ginseng and clinical use accuracy [31]. Fengliu Guo used GC-IMS to identify Fritillariae Cirrhosae Bulbus and other Fritillaria, providing new ideas and data support for the rapid authenticity identification of Fritillariae Cirrhosae Bulbus [32].
At present, there are almost no reports on the identification of PL and PT using GC-IMS. Therefore, in this study, we analyzed and identified the VOCs of PL and PT using GC-IMS technology and established the fingerprints of VOCs. Additionally, the differences between the VOCs of PL and PT were explored by combining principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA), which provides technical support for VOCs’ rapid analysis and the identification of variety for PL and PT.
2. Materials and Methods
2.1. Materials
The powders of Puerariae Lobatae Radix were purchased from the National Institutes for Food and Drug Control, Beijing, China (No. 121551-201805, named PL); Puerariae Lobatae Radix is the dried root of Pueraria lobata (Willd.) Ohwi, commonly known as “ye ge”.
The powders of Puerariae Thomsonii Radix were purchased from Yifeng Pharmacy, Changsha, China (No. 220801, named PT). Puerariae Thomsonii Radix is the dried root of Pueraria thomsonii Benth., commonly known as “fen ge”.
2.2. Analysis Using GC–IMS
PL and PT were ground into powders. After passing through a 65-mesh sieve, 1 g of the powders was accurately weighed into a 20 mL headspace bottle. Then, they were incubated at 80 °C for 20 min, and the samples were injected. Three parallel samples were included for each group.
Headspace sampling conditions: the sample incubation temperature was 80 °C, the incubation speed was 500 r/min, the incubation time was 20 min, the injection volume was 500 µL, the needle temperature was 85 °C, and splitless injection was performed.
Chromatographic conditions: FlavourSpec® gas-phase ion mobility spectrometer (G.A.S., Dortmund, Germany); CTC-PAL 3 static headspace automatic sampling device (CTC Analytics AG, Zwingen, Switzerland); 20 mL headspace bottle (Shandong Haineng Scientific Instrument Co., Ltd., Jinan, China); the chromatographic column was MXT-WAX capillary chromatography column (15 m × 0.53 mm × 1 μm, Restek Company of the United States, Bellefonte, PA, USA); temperature: 60 °C; carrier gas: high-purity nitrogen (purity ≥ 99.999%); programmed pressure increase: initial flow rate of 2.00 mL/min maintained for 2 min, linearly increased to 10.00 mL/min, linearly increased to 100.00 mL/min within 10 min, and maintained for 40 min. Chromatography running time: 60 min; injection port temperature: 80 °C.
IMS conditions: drift tube temperature was 45 °C, drift gas was N2, and drift gas velocity was 75 mL/min.
2.3. Data Analysis
The software configured by GAS company and the built-in NIST gas chromatography retention index database and IMS migration time database were used to characterize the VOCs in the sample. The plug-in of VOCal data processing software, such as Reporter, Gallery Plot, and Dynamic PCA, was used to generate the 3D spectrum, 2D spectrum, difference spectrum, fingerprints, and PCA map of VOCs, respectively, to compare VOCs between samples. SIMCA software was used for OPLS-DA to calculate the projected importance of variables (VIP).
3. Results
3.1. GC-IMS Analysis of VOCs in PT and PL
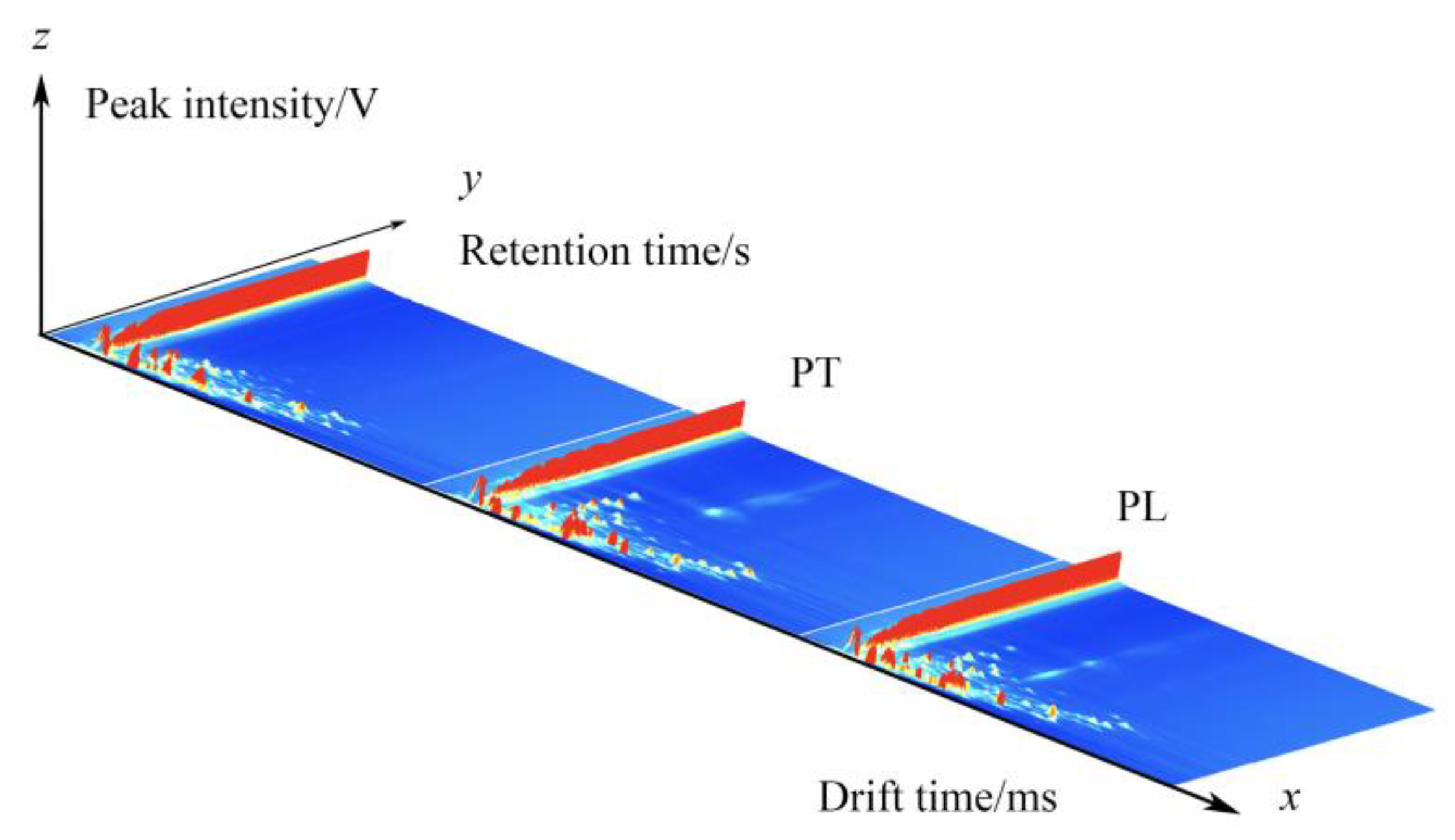
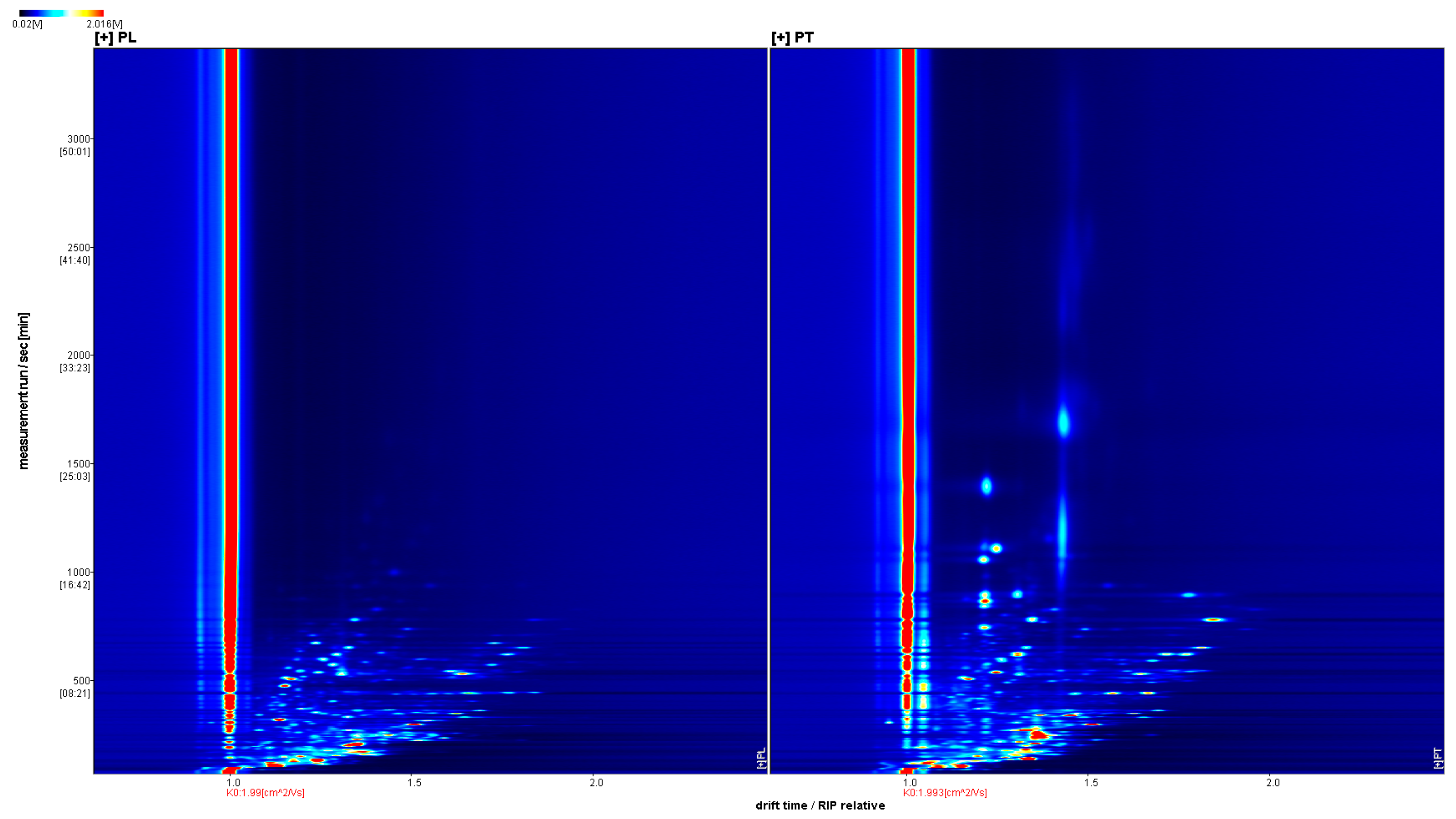
GC-IMS was used to analyze the VOCs of PL and PT, and a three-dimensional spectrum was obtained, in which the X axis represents the ion drift time, the Y axis represents the retention time of the gas chromatograph, and the Z axis represents the peak intensity used for quantification, as shown in Figure 1. We can observe the difference in VOCs in the PL and PT samples. To facilitate observation, the following two-dimensional top view is used for comparison. As shown in Figure 2, the horizontal coordinate is ion drift time, the red vertical line at 1.0 is the normalized reactive ion peak (RIP peak), and the vertical coordinate is the retention time of gas chromatography. Each point on either side of the RIP represents a volatile organic compound. The color represents the peak strength of the substance. From blue to red, the darker the color, the greater the peak intensity. There are certain differences in VOCs in PT and PL samples, as can be seen in Figure 2.
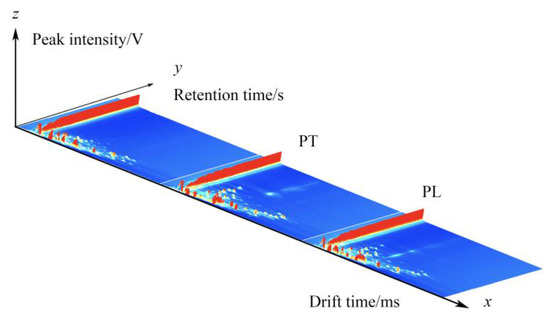
Figure 1.
GC-IMS three-dimensional spectrum of PL and PT.
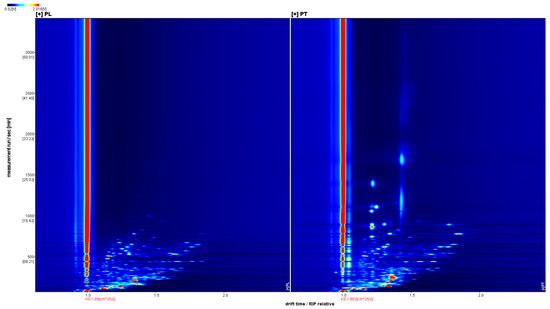
Figure 2.
GC-IMS two-dimensional spectrum of PL and PT.
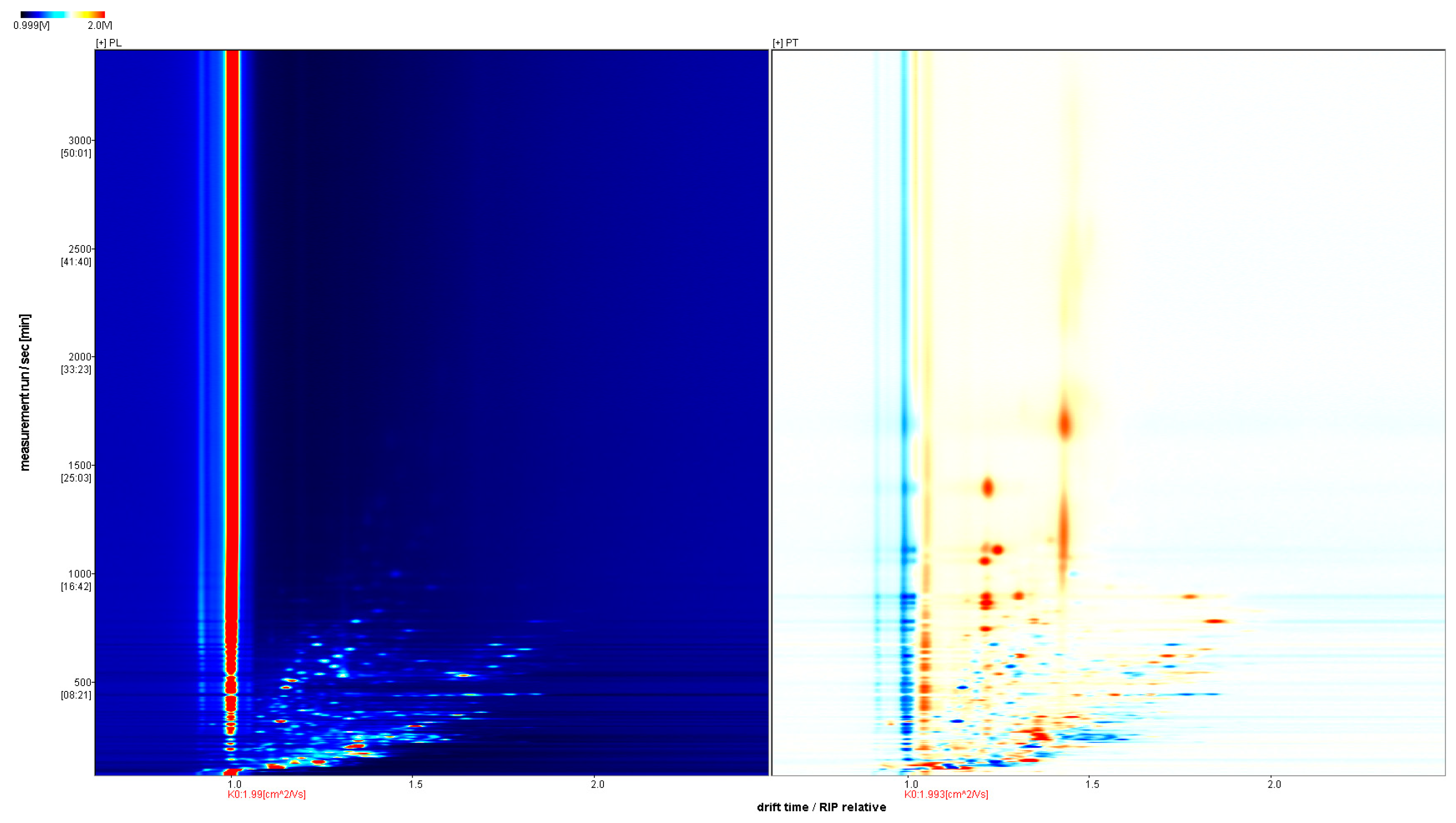
In order to further visually compare the differences in VOCs, the spectra of PL samples were selected as the reference, and the spectra of the PT samples were deducted from the reference ratio to obtain the difference comparison diagram of different samples, as shown in Figure 3. If the two volatile substances are consistent, the deducted background is white, while red means that the concentration of the substance is higher than the reference, and blue means that the concentration of the substance is lower than the reference. It is easier to distinguish the difference between two samples using contrast atlases.
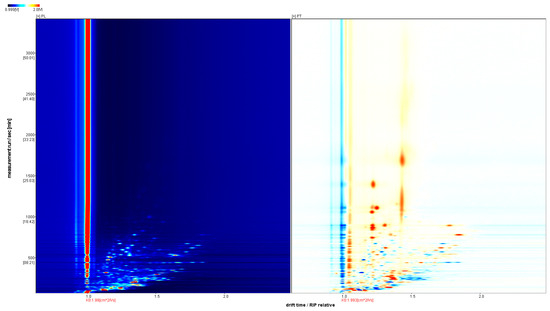
Figure 3.
Comparison of GC-IMS differences between PL and PT.
3.2. Fingerprints of VOCs in PL and PT
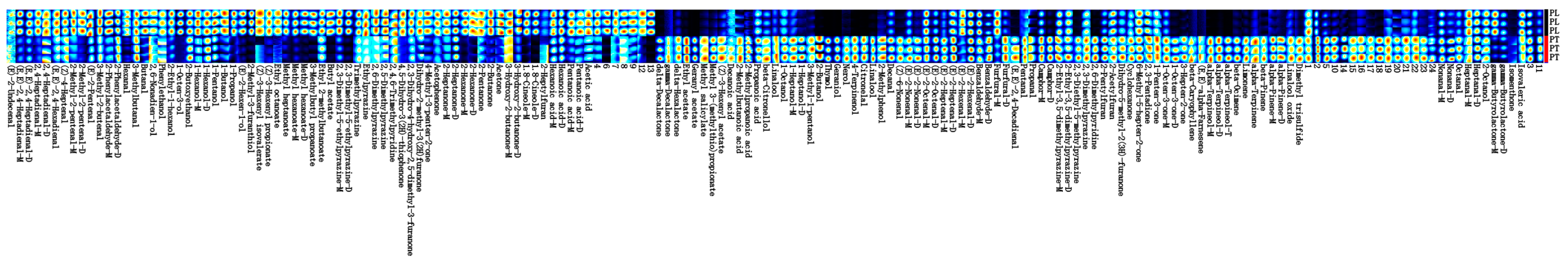
The construction of characteristic flavor fingerprints of PL and PT can provide an effective means for quality evaluation the and identification of the variety. To find the exact difference in VOCs between samples of PL and PT, the GC-IMS results of the two samples were further analyzed using the Gallery Plot plug-in, and the VOCs detected in each sample were selected for a fingerprint comparison (Figure 4). Each row in the diagram represents all of the selected signal peaks in the sample, and each column represents the signal peaks of the same volatile organic compound in a different sample. Some substances are followed by _M, D, and T, which are monomers, dimers, and trimers of the same substance, respectively. The numbers are unidentified peaks, and the darker the color of each bright spot, the greater the compound content. The complete volatile information for each sample and the differences in volatiles between the samples are outlined in Figure 4.

Figure 4.
Fingerprints of VOCs in PL and PT.
A comprehensive analysis of Figure 4 showed that the contents of VOCs such as 3-methylbutyraldehyde, 1-octene-3-ol, e-2-hexene-1-ol, isovalerate leaf alcohol ester, butyl acetate, 2,3-dimethyl-5-ethylpyrazine, 2-hexanone, and 1,8-cineulin were high in PL. The contents of VOCs such as delta-decalactone, citronellal, Z-6-nonenal, (E, E)-2, 4-decadienal, camphor, α-terpinol, and α-pinene were high in PT.
3.3. Identification of VOCs in Different PL and PT
A total of 173 VOCs were detected from PL and PT using GC-IMS analysis, as shown in Table 1. A total of 149 VOCs (monomers, dimers, or trimers) were identified by comparing the NIST2020 vapor phase retention index database built into the practical Vocal software with the IMS migration time database of G.A.S. Among them, there were 38 aldehydes, 22 alcohols, 22 ketones, 19 esters, 13 terpenes, 10 acids, 10 pyrazines, 6 terpenes, 4 furans, and 2 pyridines. In addition, the peak areas of PL and PT show significant differences in the content of VOCs, such as 3-methylbutyraldehyde, 1-octene-3-ol, e-2-hexene-1-ol, isovalerate leaf alcohol ester, butyl acetate, 2,3-dimethyl-5-ethylpyrazine, 2-hexanone, 1,8-cineole, delta-decenolactone, citronellal, Z-6-nonenal, (E, E)-2,4-decenal, camphor, alpha-terpinol, α-pinene, etc.

Table 1.
Results of VOC analysis of PL and PT.
3.4. Chemometrics Analysis
3.4.1. PCA of VOCs in Samples
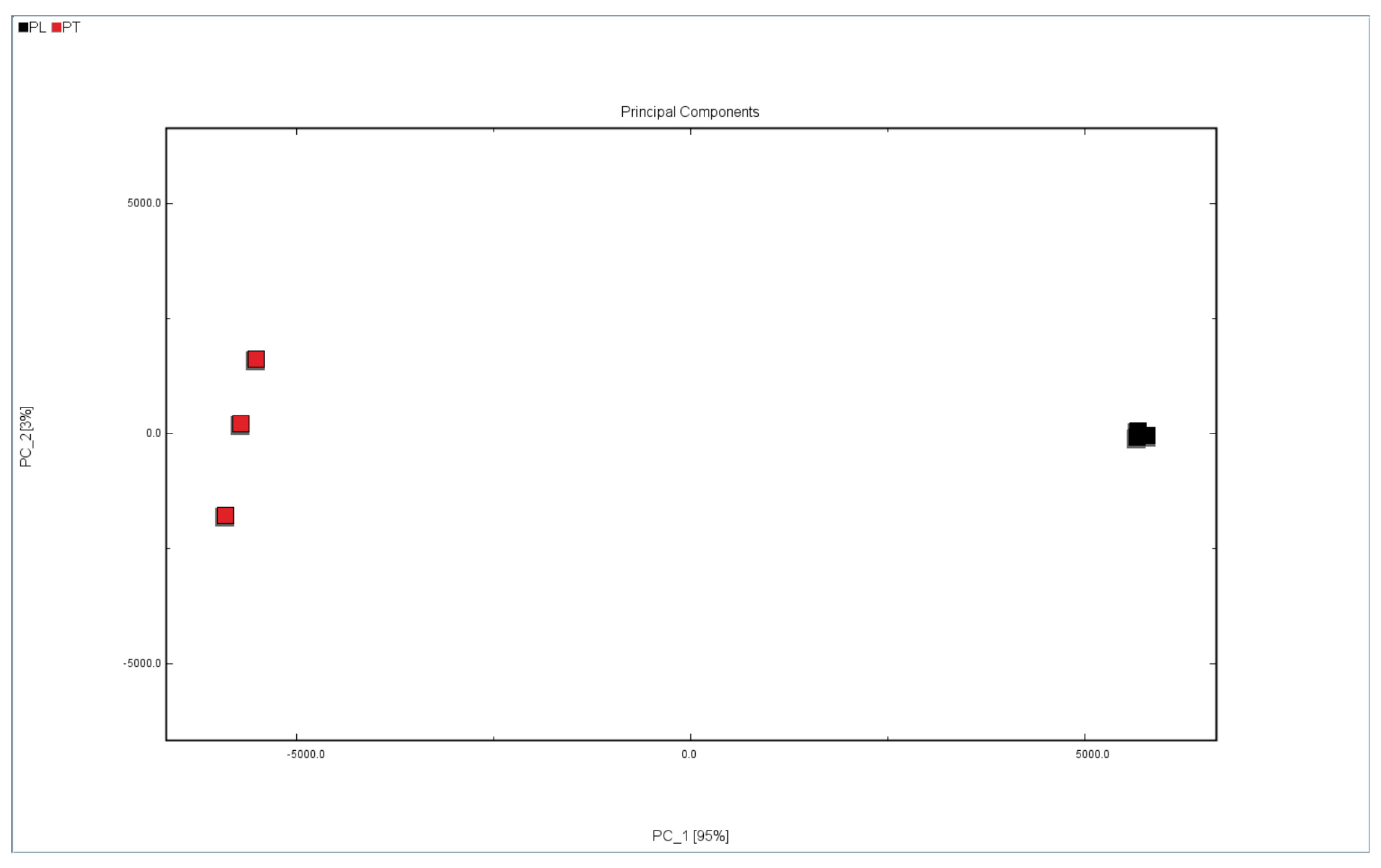
Principal component analysis (PCA) is a multi-variable data analysis tool that converts and reduces the dimensions of the information collected by the sensor to obtain the most important factor with the largest contribution rate, and it reflects the difference in the test samples on the PCA diagram [33]. In order to distinguish the difference between PL and PT, PCA was performed on all samples of PL and PT. As shown in Figure 5, there are clear differences between PL and PT. If the distance between the samples is close then the difference is small. If the distance is long then the difference is obvious. As can be seen from Figure 5, the distance between PL and PT is very long, which means that the VOC contents of them are significantly different.
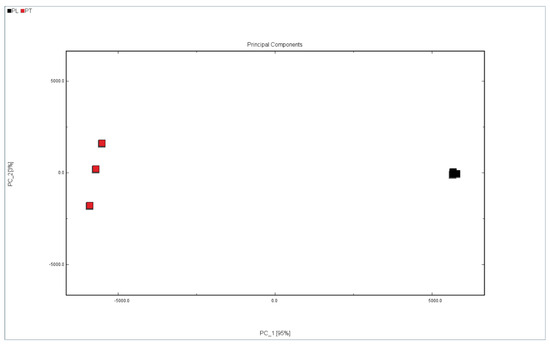
Figure 5.
PCA analysis of PL and PT.
3.4.2. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
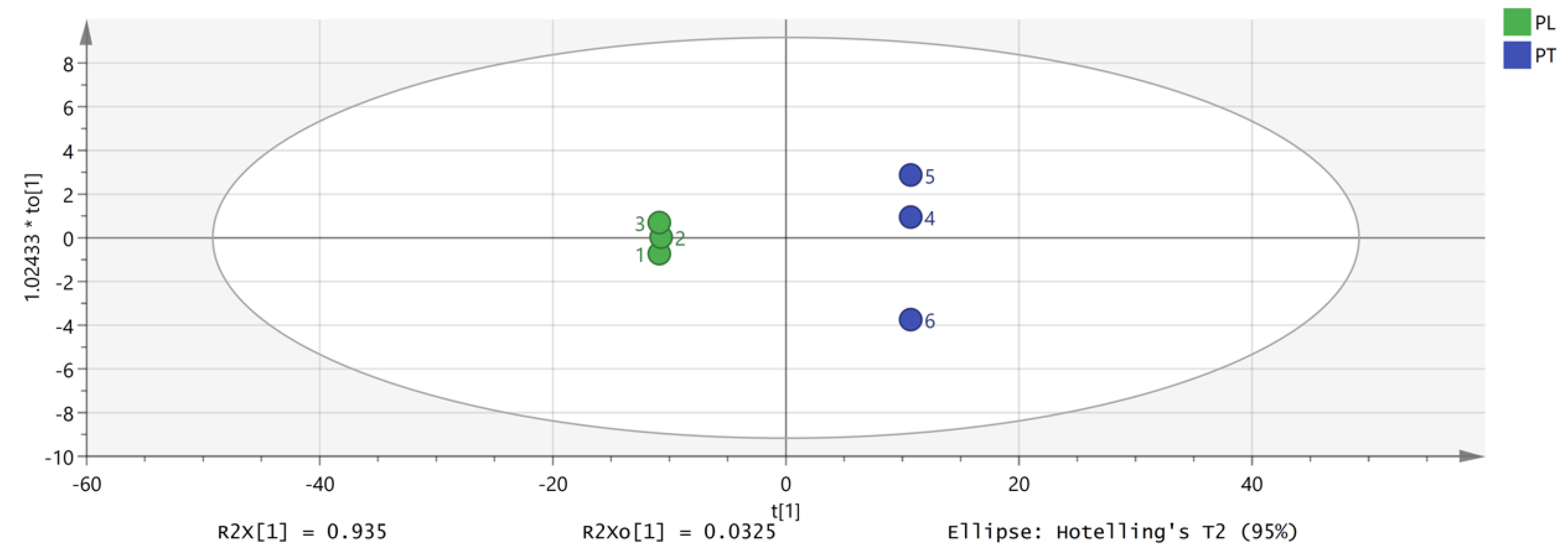
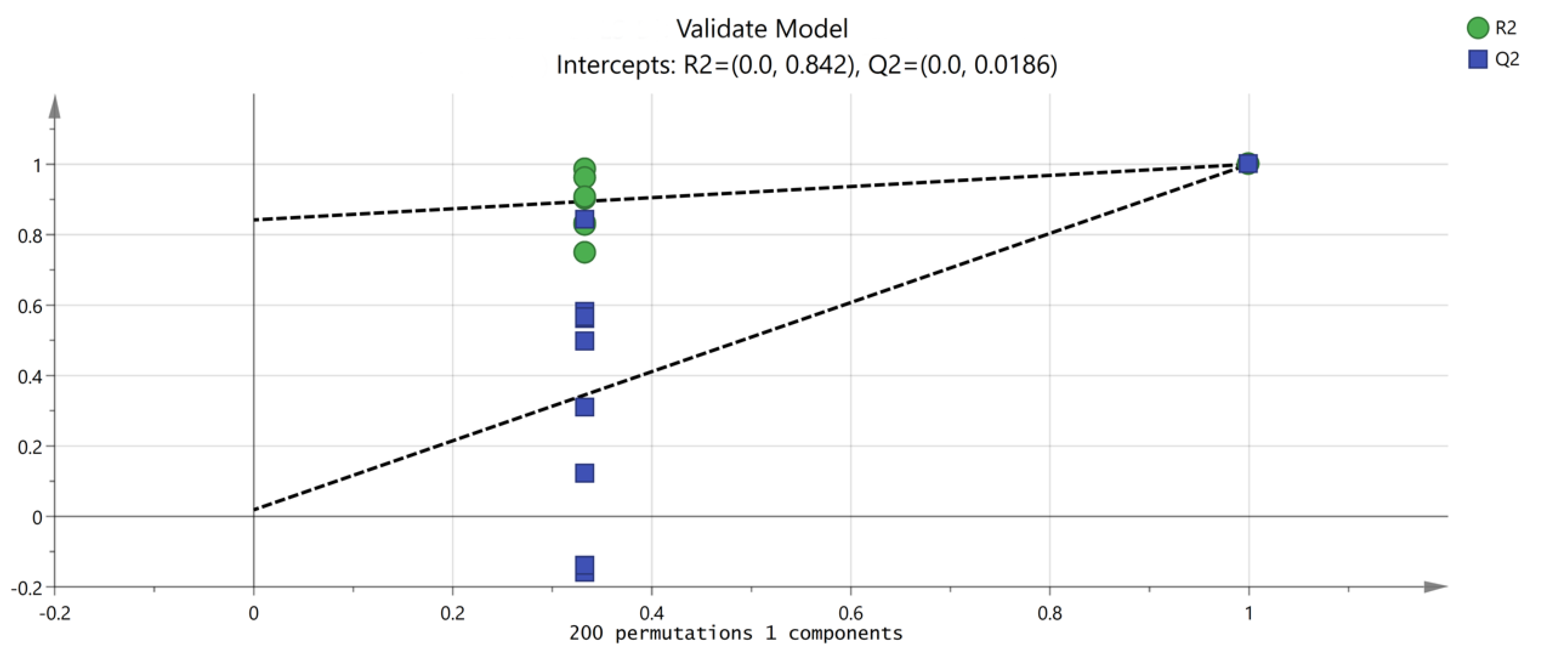
PCA focuses on describing the classification trend of samples. Unlike PCA analysis, OPLS-DA is a supervised analysis that can statistically analyze complex data dimensionality reduction, visualize the data, and then build a model to predict the data. In order to further explore and judge the differences and accuracy of VOCs in PL and PT, we further evaluated the feasibility of GC-IMS technology for rapid authenticity identification. The peak volume of 149 VOCs with large differences in selected content was taken as a variable, and the OPLS-DA scores were obtained with partial least squares discriminant analysis. The results are shown in Figure 6, which are consistent with the results of PCA, and different Pueraria samples are clearly distinguished. According to the data processed by SIMCA software, the model can relatively accurately summarize, explain, and predict; the VOC composition of PL and PT is identifiable according to this study; and different varieties can be distinguished to clarify the differences between PL and PT. Figure 6 shows the verification of the OPLS-DA model by using permutation testing. It can be seen from Figure 7 that R2 intersects the vertical axis (0, 0.842), Q2 intersects the vertical axis (0, 0.0186), and the slope of the two regression lines is large. It was confirmed that the model could be used to study the classification and discrimination of VOCs in two different varieties of PL and PT via verification.
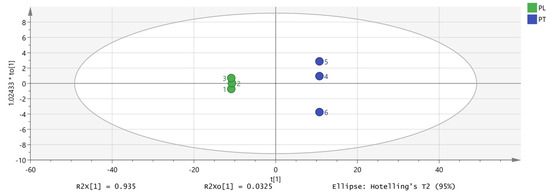
Figure 6.
OPLS-DA analysis of VOCs in PT and PL.
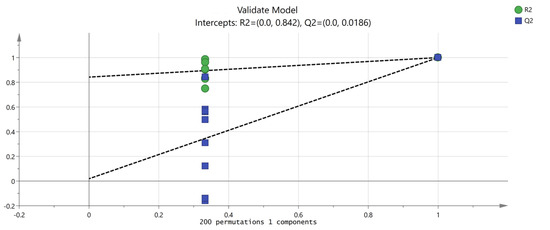
Figure 7.
Permutation test results of VOCs in PT and PL.
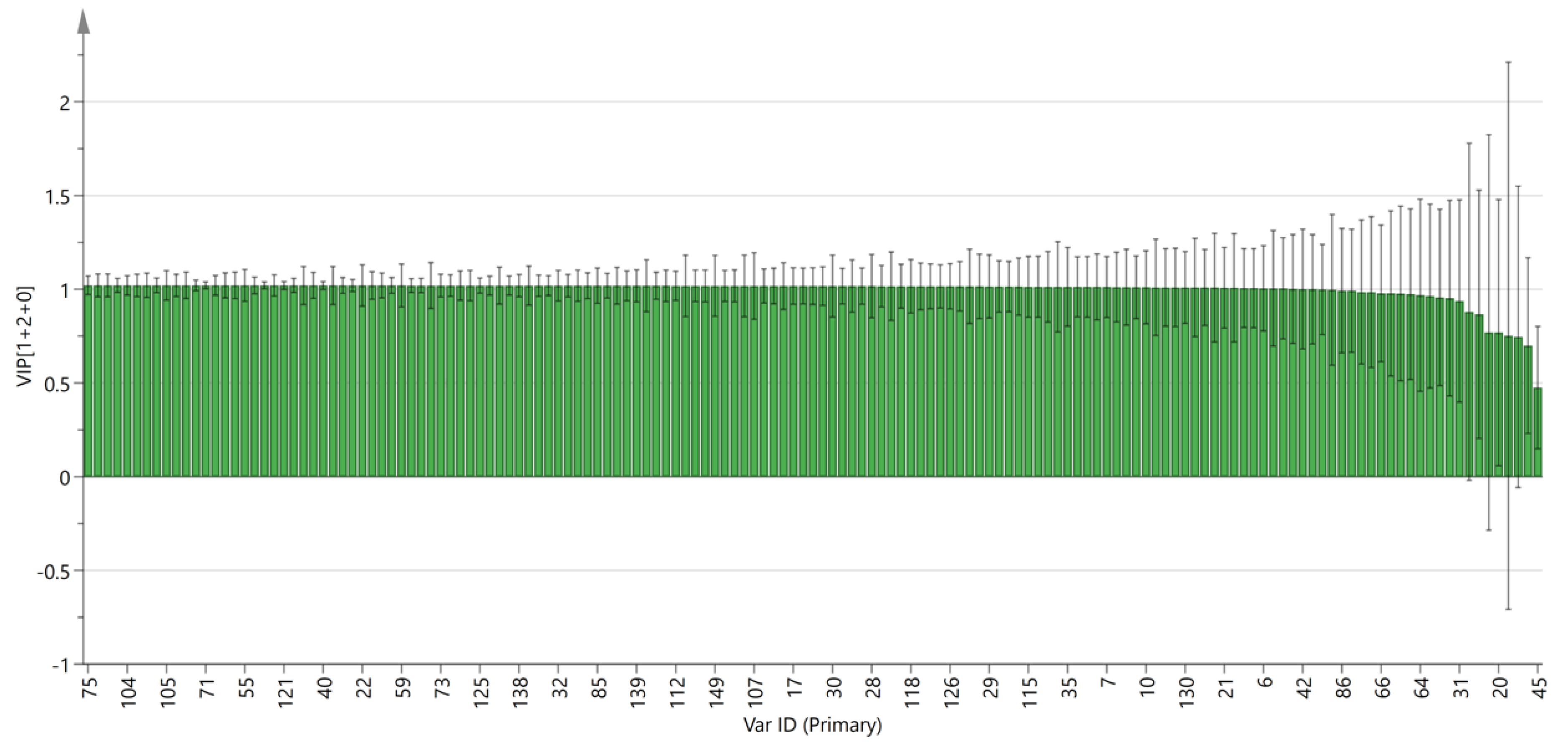
The variable importance projection (VIP) of the OPLS-DA model with different peak volumes of VOCs is highlighted in Figure 8. The larger the VIP value, the more significant the difference. By observing the VIP value, potential markers can be analyzed. The results showed that there were five VOCs with a VIP value > 1 and p < 0.05, including 2-methyl-3-furanthiol, 1-propanol, ethyl acetate, gamma-butyrolactone-M, and methyl hexanoate-D. The above five VOCs are important indicators for the classification and identification of PL and PT, and they can provide a reference for the rapid authenticity identification of the two pieces.
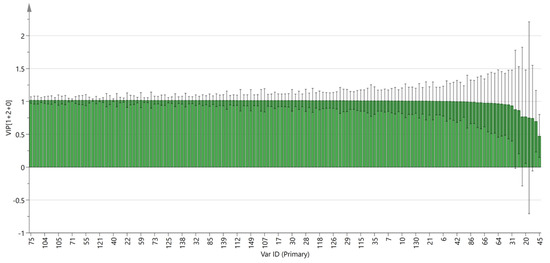
Figure 8.
VIP value of the characteristic variables.
4. Discussion
In this study, we used GC-IMS for the first time to analyze and identify VOCs in PL and PT. A total of 173 VOCs were detected, and 149 of them were identified, mainly including aldehydes, alcohols, ketones, lipids, and other components, by rapidly comparing the types and contents of VOCs in PL and PT by observing the size and color changes in the sample points representing compound information. By constructing GC-IMS fingerprints, it was shown that the VOCs of PL and PT have extremely high similarity, but the content differences between the groups are obvious. Using principal component analysis and partial least squares discrimination, the distribution of VOCs of PL and PT samples occupies a relatively independent space in the diagram, which can be easily distinguished. Then, the VIP value and p-value were used to identify five different markers of PL and PT, which provided a scientific basis for rapid identification. Compared with traditional analytical methods such as enthrone colorimetry and high-performance liquid chromatography for the identification of PL and PT, GC-IMS technology has great room for the development of identifying the origin of Chinese medicinal materials and counterfeit and shoddy materials. Not only can the composition differences in VOCs of Chinese medicinal materials be analyzed, but samples with similar compositions of VOCs can also be accurately classified according to the content differences in characteristic volatile substances. The experimental results of this study show that GC-IMS can effectively analyze and identify the VOCs in PL and PT, detect the difference between PL and PT, and reach scientific judgments. Moreover, this method requires less sample dosage and is simple in the process of drug pretreatment, which has great application potential, and it provides a scientific basis for the research and development of PT and PL identification in the future.
5. Conclusions
The rapid identification of traditional Chinese medicines based on “odor” information is an important part of the traditional identification method of traditional Chinese medicines [34]. For example, Houttuynia Cordata has a strong fishy smell, and Xiangjiapi has a special fragrance. Experienced pharmacists can directly and quickly identify authenticity and even evaluate quality based on the unique smell and odor thickness of traditional Chinese medicine. With its fast and convenient advantages, up until now, this method has spread as a traditional identification approach. However, for some decoction pieces with insufficient odor information or even weak odor, it may be difficult to quickly realize the identification of traditional Chinese medicine using the traditional “sniffing” method. As a trace detection technology for VOCs, GC-IMS technology cleverly combines the advantages of the rapid identification of traditional traits with the accuracy and quantification of modern instrument analysis. It can be used to quickly and accurately detect information on VOCs in traditional Chinese medicine to allow the inheritance and development of traditional skills. At present, this technology is widely used in food, agriculture, medicine, and other fields. It is mainly used for the rapid detection and characterization of VOCs in samples, as well as the comparative analysis of the differences in VOCs in different samples, and many studies have shown that GC-IMS technology can be used for the identification or classification of two/multiple types of samples [35,36].
Author Contributions
Conceptualization, Y.M. and L.Z. (Lingfeng Zhu); methodology, Y.M. and L.Z. (Lingfeng Zhu); software, F.F. and L.Z. (Lijun Zhu); validation, J.C. and J.L.; formal analysis, Y.M. and L.Z. (Lingfeng Zhu); investigation, L.Z. (Lijun Zhu); resources, J.C.; data curation, J.L.; writing—original draft preparation, Y.M. and L.Z. (Lingfeng Zhu); writing—review and editing, C.L.; visualization, Y.M. and L.Z. (Lingfeng Zhu); supervision, F.F.; project administration, D.H.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hunan Agricultural Science and Technology Innovation Fund Project, Hunan Academy of Agricultural Sciences (No. 2023CX30) and the First-Class Discipline Project on Chinese Medicine of Hunan University of Chinese Medicine, Hunan University of Chinese Medicine (No. 2023).
Data Availability Statement
This published article includes all data generated or analyzed during this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gudeta, W.F.; Igori, D.; Belete, M.T.; Kim, S.E.; Moon, J.S. Complete genome sequence of pueraria virus A, a new member of the genus Caulimovirus. Arch. Virol. 2022, 6, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef] [PubMed]
- Committee for the National Pharmacopoeia of P.R. China. Pharmacopoeia of P.R. China, Part I; Beijing Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Wu, W.T.; Guo, Z.L.; Ge, S.C.; Kuang, W.L.; Li, W.D.; Wang, S.D.; Liu, P.; Zhou, Z.W.; Zhu, W.F. Pharmacokinetics, pharmacodynamics, and tissue distribution of oral co-loaded puerarin/daidzein mixed micelles in rats. Chin. J. Chin. Mater. Med. 2023, 18, 5068–5077. [Google Scholar] [CrossRef]
- Ding, Z.E.; Wan, X.C. Extraction and separation of dietary fiber from Pueraria. J. Cereals Oils 2004, 4, 38–41. [Google Scholar] [CrossRef]
- Liu, D.; Ma, L.; Zhou, Z.; Liang, Q.; Xie, Q.; Ou, K.; Liu, Y.; Su, Y. Starch and mineral element accumulation during root tuber expansion period of Pueraria thomsonii Benth. Food Chem. 2021, 343, 128445. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, M.Q.; JIN, L.B.; Yang, H.L. Research progress of pueraria starch. Guangxi J. Light Ind. 2010, 2, 4–5. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 7, 961–975. [Google Scholar] [CrossRef]
- Bai, Y.L.; Han, L.L.; Qian, J.H.; Wang, H.Z. Molecular Mechanism of Puerarin Against Diabetes and its Complications. Front. Pharmacol. 2022, 12, 780419. [Google Scholar] [CrossRef]
- Tang, W.; Xiao, L.; Ge, G.; Zhong, M.; Zhu, J.; Qin, J.; Feng, C.; Zhang, W.; Bai, J.; Zhu, X.; et al. Puerarin inhibits titanium particle-induced osteolysis and RANKL-induced osteoclastogenesis via suppression of the NF-κB signaling pathway. J. Cell Mol. Med. 2020, 20, 11972–11983. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Wu, S.; Li, X.; Li, Q. Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease model in mice. Phytother. Res. 2014, 2, 179–186. [Google Scholar] [CrossRef]
- Hong, X.P.; Chen, T.; Yin, N.N.; Han, Y.M.; Yuan, F.; Duan, Y.J.; Shen, F.; Zhang, Y.H.; Chen, Z.B. Puerarin Ameliorates D-Galactose Induced Enhanced Hippocampal Neurogenesis and Tau Hyperphosphorylation in Rat Brain. J. Alzheimers. Dis. 2016, 2, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, L.; Zhang, D.Y.; Zhai, D.X.; Shen, W.; Bai, L.L.; Liu, Y.Q.; Cai, Z.L.; Li, J.; Yu, C.Q. The effects and possible mechanisms of puerarin to treat endometriosis model rats. Evid. Based Complement. Altern. Med. 2015, 2015, 269138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Lin, L.; Liang, S.; Yan, J. Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol. Rep. 2018, 4, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, W.; Yan, H.; Shi, Y.; Zhang, W.J.; Zhang, P.; Cheng, X.L.; Ma, S.C. Analysis of quality and related problems of Chinese medicinal materials and decoction pieces. Chin. J. Pharm. Sci. 2015, 4, 277–283. [Google Scholar] [CrossRef]
- Xiang, G.; Zhou, L.P.; Gui, J.X.; Zhu, J.X. Research progress and prospect of near infrared spectroscopy in consumer products. Infrared 2017, 12, 1–5+12. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Lam, H.M.; Yam, W.S.; Leung, L.K.; Choi, K.L.; Waye, M.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J. Ethnopharmacol. 2005, 96, 133–138. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Li, Y.; Jiang, M.; Liu, C.; He, M.; Wu, B. Chloroplast genomes of two Pueraria DC. species: Sequencing, comparative analysis and molecular marker development. FEBS Open Bio 2022, 12, 349–361. [Google Scholar] [CrossRef]
- He, S.; Zhang, B.; Dong, X.; Wei, Y.; Li, H.; Tang, B. Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry. Molecules 2023, 9, 3874. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, D.; Zhou, Y.; Duan, H.; He, Y.; Jiang, Y.; Yan, W. Characteristic Volatile Organic Compound Analysis of Different Cistanches Based on HS-GC-IMS. Molecules 2022, 27, 6789. [Google Scholar] [CrossRef]
- Augustini, A.L.R.M.; Borg, C.; Sielemann, S.; Telgheder, U. Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis. Molecules 2023, 28, 6574. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; He, Y.; Ji, X.J.; Tang, H.; Dong, Y.M.; Yan, W.J. HS-GC-IMS detection of volatile organic compounds in Acacia honey powders under vacuum belt drying at different temperatures. Food Sci. Nutr. 2021, 9, 4085–4093. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 15, 126158. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Cao, S.; Liu, Z.; Li, N.; Xu, D.; Mo, H.; Hu, L. Monitoring of Volatile Compounds of Ready-to-Eat Kiwifruit Using GC-IMS. Foods 2023, 12, 4394. [Google Scholar] [CrossRef]
- Calle, J.L.P.; Vázquez-Espinosa, M.; Barea-Sepúlveda, M.; Ruiz-Rodríguez, A.; Ferreiro-González, M.; Palma, M. Novel Method Based on Ion Mobility Spectrometry Combined with Machine Learning for the Discrimination of Fruit Juices. Foods 2023, 12, 2536. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Salatti-Dorado, J.A.; Cardador, M.J.; Frizzo, L.; Jordano, R.; Arce, L.; Medina, L.M. Relationship between Volatile Organic Compounds and Microorganisms Isolated from Raw Sheep Milk Cheeses Determined by Sanger Sequencing and GC–IMS. Foods 2023, 12, 372. [Google Scholar] [CrossRef]
- Wu, D.; Xia, Q.; Cheng, H.; Zhang, Q.; Wang, Y.; Ye, X. Changes of Volatile Flavor Compounds in Sea Buckthorn Juice during Fermentation Based on Gas Chromatography–Ion Mobility Spectrometry. Foods 2022, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Duan, W.; Zhao, Y.; Liu, X.; Wen, G.; Zeng, F.; Liu, G. Development of a Flavor Fingerprint Using HS-GC-IMS for Volatile Compounds from Steamed Potatoes of Different Varieties. Foods 2023, 12, 2252. [Google Scholar] [CrossRef]
- He, J.; Ye, L.; Li, J.; Huang, W.; Huo, Y.; Gao, J.; Liu, L.; Zhang, W. Identification of Ophiopogonis Radix from different producing areas by headspace-gas chromatography-ion mobility spectrometry analysis. J. Food Biochem. 2022, 6, e13850. [Google Scholar] [CrossRef]
- Huang, J.; Ding, S.; Xiong, S.; Liu, Z. The Mediating Effects of Diabetes Distress, Anxiety, and Cognitive Fusion on the Association Between Neuroticism and Fear of Hypoglycemia in Patients with Type 2 Diabetes. Front. Psychol. 2021, 12, 697051. [Google Scholar] [CrossRef]
- Hapunda, G.; Abubakar, A.; Pouwer, F.; Vijver, F. Correlates of fear of hypoglycemia among patients with type 1 and 2 diabetes mellitus in outpatient hospitals in Zambia. Int. J. Diabetes Dev. Ctries. 2020, 4, 619–626. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.C.; Gu, S.Q.; Zhu, S.C.; Zhou, X.X.; Ding, Y.T. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y. Research on Rapid Identification of TCM Decoction Pieces Based on “Odor” Information Analysis; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2017. [Google Scholar]
- Liang, T.Y.; Juan, Y.; Hao, D.; Yu, L.M.; Bing, H.; Zeng, X.F. Identification of volatile flavor compounds in Different years of Xinhui orange peel based on GC-IMS. Chin. Condiments 2020, 4, 168–173. [Google Scholar] [CrossRef]
- Yang, B.Y.; Yao, L.; Ji, H.Y.; Jing, Y.Y.; Chen, G.L.; Gang, Z.; Yan, Y.G.; Hu, B.X.; Liang, P. Analysis on the difference of volatile organic compounds VOCs of coltsl nectar before and after moxibustion based on HS-GC-IMS technique. Chin. Herb. Med. 2022, 6, 1854–1861. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).