A New Sample Processing Protocol for Separation and Purification Enabling Precise Analysis of Various Non-Traditional Isotopes in Geological Samples with Low Concentrations
Abstract
:1. Introduction
2. Materials and Instrumentation
2.1. Reagents and Chemicals
2.2. Samples Preparation
2.3. Instrumental Setup, Tuning, and Data Acquisition
2.4. Determination of Isotope Ratio
3. Results and Discussion
3.1. Principle of Isotopic Preparative Chromatograph (IPC)
3.2. Principle of Ultra Clean Concentrator
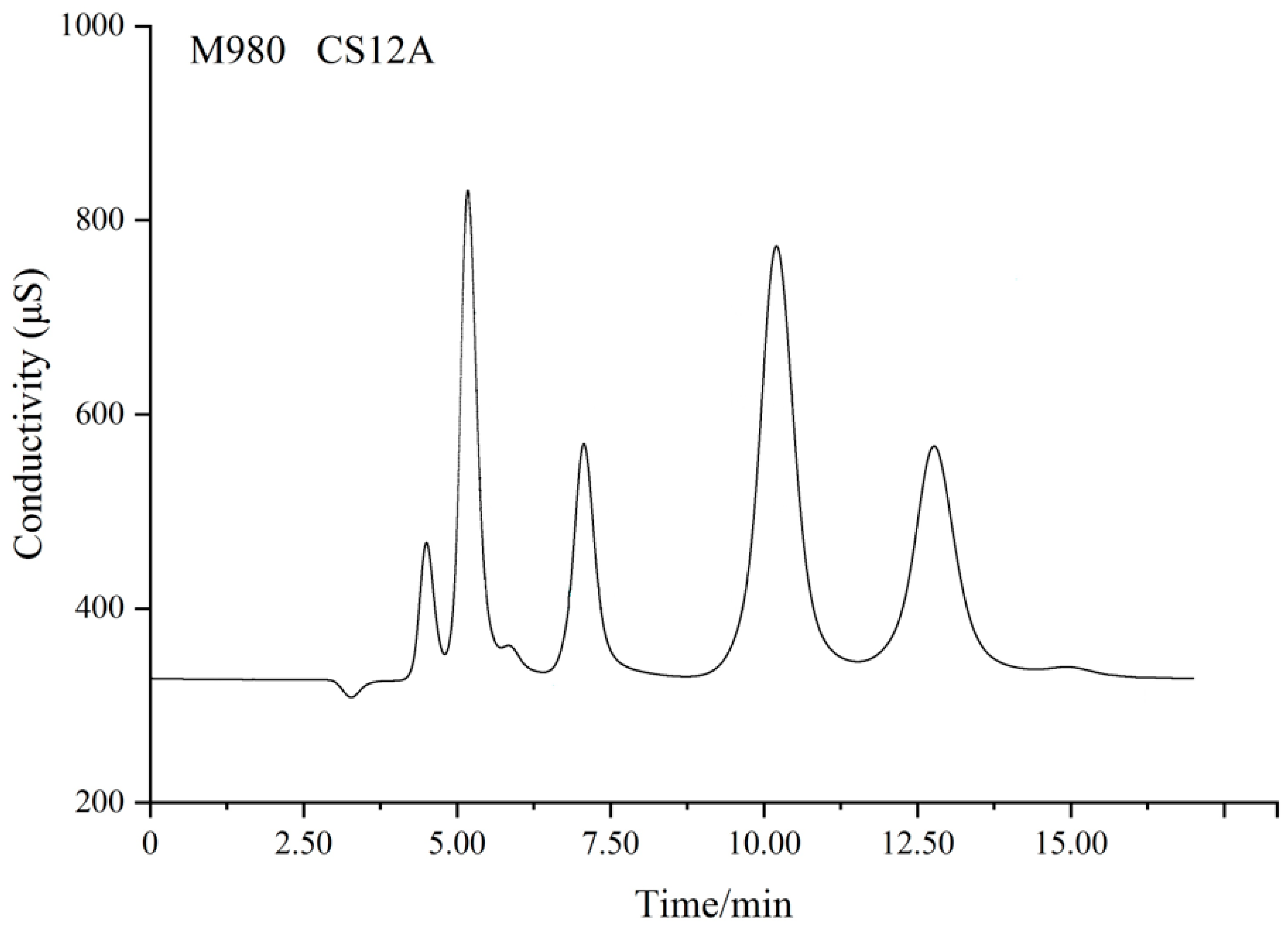
3.3. Confirming the Retention Time and Collection Time
3.4. Concentration of Samples and Times of Collections
3.5. Analytical Results of Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Han, G. One-step chromatographic purification of K, Ca, and Sr from geological samples for high precision stable and radiogenic isotope analysis by MC-ICP-MS. J. Anal. At. Spectrom. 2021, 36, 676–684. [Google Scholar] [CrossRef]
- Grigoryan, R.; Costas-Rodríguez, M.; Santens, P.; Vanhaecke, F. Multicollector Inductively Coupled Plasma–Mass Spectrometry with 1013 Ω Faraday Cup Amplifiers for Ultrasensitive Mg Isotopic Analysis of Cerebrospinal Fluid Microsamples. Anal. Chem. 2020, 92, 15975–15981. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.; Yoshimura, T.; Araoka, D.; Gamo, T.; Kawahata, H. Magnesium isotopic composition of submarine vent fluids from arc and back-arc hydrothermal systems in the western Pacific. Chem. Geol. 2020, 551, 119767. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.Y.; Chang, Y.T. Quantifying groundwater recharge and discharge for the middle reach of Heihe River of China using isotope mass balance method. J. Groundw. Sci. Eng. 2021, 9, 225. [Google Scholar]
- Feng, M.; Gui, L.W.; Hong, L.S.; Zhan, X.S. Indication of hydrogen and oxygen stable isotopes on the characteristics and circulation patterns of medium-low temperature geothermal resources in the Guanzhong Basin, China. J. Groundw. Sci. Eng. 2022, 10, 70. [Google Scholar]
- Sun, A.; Xu, Q.; Xu, S.; Shen, H.; Sun, J.; Zhang, Y. Separation and analysis of chlorine isotopes in higher plants. Chem. Geol. 2014, 381, 21–25. [Google Scholar] [CrossRef]
- Liu, L.; Mu, S.; Leng, P.; Dong, Y.; Xu, Q.; Sun, A. Extraction and analysis of hydrosoluble chlorine and its stable isotopic composition in soil using thermal ionization mass spectrometry. Chem. Geol. 2020, 558, 119993. [Google Scholar] [CrossRef]
- Rosca, C.; König, S.; Pons, M.-L.; Schoenberg, R. Improved protocols for Zn purification and MC-ICP-MS analyses enable determination of small-scale Zn isotope variations. Chem. Geol. 2021, 586, 120440. [Google Scholar] [CrossRef]
- Li, Y.; Pinto, N.G. Model for ion-exchange equilibria of macromolecules in preparative chromatography. J. Chromatogr. A 1995, 702, 113–123. [Google Scholar] [CrossRef]
- Samsonov, G.V.; El’Kin, G.E.; Atabekjan, T.V. Intraparticle and film diffusion control in the preparative ion-exchange chromatography. React. Funct. Polym. 1998, 38, 151–155. [Google Scholar] [CrossRef]
- Gallant, S.R.; Vunnum, S.; Cramer, S.M. Optimization of preparative ion-exchange chromatography of proteins: Linear gradient separations. J. Chromatogr. A 1996, 725, 295–314. [Google Scholar] [CrossRef]
- Gerberding, S.; Byers, C. Preparative ion-exchange chromatography of proteins from dairy whey. J. Chromatogr. A 1998, 808, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Arafah, R.S.; Ribeiro, A.E.; Rodrigues, A.E. Pais, Separation of nadolol racemates by high pH reversed-phase preparative chromatography. Sep. Purif. Technol. 2020, 233, 116018. [Google Scholar] [CrossRef]
- Speybrouck, D.; Howsam, M.; Lipka, E. Recent developments in preparative-scale supercritical fluid- and liquid chromatography for chiral separations. TrAC Trends Anal. Chem. 2020, 133, 116090. [Google Scholar] [CrossRef]
- Briskot, T.; Hahn, T.; Huuk, T.; Wang, G.; Kluters, S.; Studts, J.; Wittkopp, F.; Winderl, J.; Schwan, P.; Hagemann, I.; et al. Analysis of complex protein elution behavior in preparative ion exchange processes using a colloidal particle adsorption model. J. Chromatogr. A 2021, 1654, 462439. [Google Scholar] [CrossRef]
- Moreno-González, M.; Chuekitkumchorn, P.; Silva, M.; Groenewoud, R.; Ottens, M. High throughput process development for the purification of rapeseed proteins napin and cruciferin by ion exchange chromatography. Food Bioprod. Process. 2021, 125, 228–241. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, L.; Hu, Z.; Gao, S. Direct Determination of Si Isotope Ratios in Natural Waters and Commercial Si Standards by Ion Exclusion Chromatography Multicollector Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2014, 86, 9301–9308. [Google Scholar] [CrossRef]
- Martinez, M.; Garcia-Alonso, J.I.; Parat, C.; Encinar, J.R.; Hecho, I.L. Anion-Specific Sulfur Isotope Analysis by Liquid Chromatography Coupled to Multicollector ICPMS. Anal. Chem. 2019, 91, 10088–10094. [Google Scholar] [CrossRef]
- Karasinski, J.; Bulska, E.; Wojciechowski, M.; Halicz, L.; Krata, A.A. High precision direct analysis of magnesium isotope ratio by ion chromatography/multicollector-ICPMS using wet and dry plasma conditions. Talanta 2017, 165, 64–68. [Google Scholar] [CrossRef]
- Zakon, Y.; Halicz, L.; Gelman, F. Isotope Analysis of Sulfur, Bromine, and Chlorine in Individual Anionic Species by Ion Chromatography/Multicollector-ICPMS. Anal. Chem. 2014, 86, 6495–6500. [Google Scholar] [CrossRef]
- Silver, J. Overview of Analytical-to-Preparative Liquid Chromatography Method Development. ACS Comb. Sci. 2019, 21, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.A. Preparation of ion exchange columns with longitudinal stationary phase gradients. Heliyon 2021, 7, e06961. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.Y.; Zhao, G.X.; Zhang, J.; Zhang, L. A novel Isotopic Preparative Chromatography State. China Patent No. ZL 201920972768.3, 31 March 2020. [Google Scholar]
- Gui, J.Y.; Chen, X.; Han, X.; Wang, Z.; Ma, Y. Determination of stable chlorine isotopes by isotopic preparative chromatography (ipc) with positive–thermal ionization mass spectrometry (p-tims). Anal Lett. 2022, 55, 2564–2573. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, X.; Wei, H.; Li, H.; Zhao, Z. A new method for the removal of SO42− for isotopic measurement of chlorine. Chem. Geol. 2007, 238, 38–43. [Google Scholar] [CrossRef]
- Gui, J.Y.; Wang, Z.; Wang, Q.; Wang, W.; Song, H.; Huang, Y.; Mu, H.; Yan, F. A Novel Closed Concentrator for Isotope preparation. State Natural Property Administration. China Patent No. ZL 202122567714.9, 10 June 2022. [Google Scholar]

| Sample No | Type | Cl−/mg/L | NO3−/mg/L | SO42−/mg/L |
|---|---|---|---|---|
| ISL 354 | Isotope standard | 5000 ± 11 | 16.02 ± 0.65 | 3.31 ± 0.25 |
| M 354 | Mixed solution | 20 ± 3.8 | 20 ± 5.3 | 20 ± 6.3 |
| N-6 | Surface water | 5.59 ± 0.22 | 5.17 ± 0.26 | 25.69 ± 1.59 |
| N-7 | Groundwater | 19.56 ± 1.97 | 6.03 ± 0.58 | 126.7 ± 2.99 |
| N-8 | Surface water | 5.24 ± 0.19 | 4.44 ± 0.19 | 26.83 ± 1.1 |
| N-9 | Groundwater | 16.42 ± 0.52 | 13.66 ± 0.31 | 58.41 ± 2.31 |
| N-12 | Groundwater | 225.8 ± 6.22 | 66.95 ± 1.56 | 228.3 ± 5.91 |
| N-14 | Groundwater | 85.78 ± 3.11 | 11.62 ± 0.18 | 84.13 ± 3.22 |
| N-105 | Groundwater | 43.66 ± 2.64 | 7.94 ± 0.13 | 154.1 ± 3.92 |
| Sample No | Type | K+/mg/L | Na+/mg/L | Ca2+/mg/L | Mg2+/mg/L |
|---|---|---|---|---|---|
| STD-1 | Isotope standard | 0.05 ± 0.13 | 3.1 ± 0.3 | 1.02 ± 0.66 | 1000 ± 2.21 |
| M 980 | Mixed solution | 20.00 ± 1.1 | 20.00 ± 2.2 | 20.00 ± 5.5 | 20.00 ± 1.27 |
| NSBD-6 | Surface water | 2.02 ± 0.24 | 5.52 ± 0.26 | 39.85 ± 0.98 | 7.87 ± 0.28 |
| NSBD-7 | Groundwater | 2.01 ± 0.15 | 14.99 ± 1.17 | 110.80 ± 2.99 | 18.29 ± 1.11 |
| NSBD-8 | Surface water | 2.14 ± 0.22 | 5.75 ± 0.37 | 40.10 ± 1.18 | 7.90 ± 0.56 |
| NSBD-9 | Groundwater | 1.34 ± 0.03 | 12.79 ± 0.87 | 70.91 ± 2.01 | 17.41 ± 1.06 |
| NSBD-12 | Groundwater | 28.37 ± 2.12 | 113.80 ± 3.92 | 131.90 ± 4.28 | 4.23 ± 0.26 |
| NSBD-14 | Groundwater | 1.58 ± 0.19 | 105.50 ± 5.01 | 56.47 ± 2.69 | 24.62 ± 1.29 |
| NSBD-105 | Groundwater | 0.55 ± 0.22 | 40.78 ± 2.91 | 174.00 ± 5.98 | 21.49 ± 1.11 |
| Isotopic Preparative Chromatograph | Thermal Ionization Mass Spectrometer | ||
|---|---|---|---|
| Type | Pre-Isotope I | Type | TRITON |
| Column | AS19 | Accelerating field | 10 kV |
| Eluent | 30 mM NaOH | Magnetic field | 81 cm |
| Flow rate | 1 mL/min | Resolution ratio | 451 |
| Current | 80 mA | Sensitivity | ≥3 ion/100 μmol |
| Temperature | 30 °C | Measuring ions | 133Cs235Cl+/133Cs237Cl+ |
| Inject volume | 100 μL | m/z | 303/301 |
| Isotopic Preparative Chromatograph | MC-ICP-MS | ||
|---|---|---|---|
| Type | Pre-Isotope I | Type | Nu-instruments NP II |
| Column | CS12A | RF powder | 1300 W |
| Eluent | 20 mM CH3SO3H | Cooling gas | 13.0 L/min |
| Flow rate | 1 mL/min | Auxiliary gas | 0.8 L/min |
| Current | 80 mA | Accelerating voltage | 6000 V |
| Temperature | 30 °C | Nebulizer | ~37 psi |
| Inject volume | 100 μL | Resolution | Low resolution (≥200) |
| Sample No | Concentration of Cl− (mg/L) | Times of Preparation | Recovery (%) | Concentration of Mg2+ (mg/L) | Times of Preparation | Recovery (%) |
|---|---|---|---|---|---|---|
| N-6 | 5.59 | 12 | 98.2 | 7.87 | 15 | 99.5 |
| N-7 | 19.56 | 12 | 99.1 | 18.29 | 12 | 96.3 |
| N-8 | 5.24 | 12 | 97.2 | 7.90 | 15 | 96.9 |
| N-9 | 16.42 | 12 | 96.6 | 17.41 | 12 | 97.8 |
| N-12 | 225.8 | 2 | 99.1 | 4.23 | 15 | 99.2 |
| N-14 | 85.78 | 2 | 99.3 | 24.62 | 12 | 97.3 |
| N-105 | 43.66 | 12 | 98.9 | 21.49 | 12 | 96.8 |
| Sample No | Results of 3 Times | Average Value | External Accuracy | δ37Cl (‰) | SD (±) | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| N-6 | 0.319135 | 0.31903 | 0.319035 | 0.31907 | 0.00006 | 0.08 | 0.19 |
| N-7 | 0.31901 | 0.319011 | 0.319031 | 0.31902 | 0.00001 | −0.07 | 0.04 |
| N-8 | 0.319044 | 0.319155 | 0.319172 | 0.31912 | 0.00007 | 0.26 | 0.22 |
| N-9 | 0.318995 | 0.319017 | 0.319044 | 0.31902 | 0.00002 | −0.07 | 0.08 |
| N-12 | 0.318944 | 0.318932 | 0.318959 | 0.31895 | 0.00001 | −0.30 | 0.04 |
| N-14 | 0.319181 | 0.319285 | 0.319285 | 0.31925 | 0.00006 | 0.66 | 0.19 |
| N-105 | 0.318962 | 0.318993 | 0.318977 | 0.31898 | 0.00002 | −0.20 | 0.05 |
| Sample No | Results of Determination | ||||
|---|---|---|---|---|---|
| δ25Mg DSM3(‰) | SD (±) | δ26MgDSM3 (‰) | SD (±) | n | |
| N-3 | −0.42 | 0.03 | −0.56 | 0.05 | 3 |
| N-4 | −0.57 | 0.03 | −0.91 | 0.09 | 3 |
| N-5 | −0.98 | 0.05 | −1.46 | 0.12 | 2 |
| N-6 | −0.67 | 0.02 | −1.10 | 0.05 | 3 |
| N-7 | −0.97 | 0.03 | −1.59 | 0.06 | 3 |
| N-8 | −0.87 | 0.02 | −1.37 | 0.04 | 3 |
| N-9 | −0.86 | 0.02 | −1.45 | 0.07 | 2 |
| N-12 | −1.01 | 0.03 | −1.70 | 0.01 | 2 |
| N-14 | −0.98 | 0.03 | −1.75 | 0.05 | 3 |
| N-105 | −0.57 | 0.03 | −0.89 | 0.06 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, J.; Zou, W. A New Sample Processing Protocol for Separation and Purification Enabling Precise Analysis of Various Non-Traditional Isotopes in Geological Samples with Low Concentrations. Separations 2023, 10, 497. https://doi.org/10.3390/separations10090497
Gui J, Zou W. A New Sample Processing Protocol for Separation and Purification Enabling Precise Analysis of Various Non-Traditional Isotopes in Geological Samples with Low Concentrations. Separations. 2023; 10(9):497. https://doi.org/10.3390/separations10090497
Chicago/Turabian StyleGui, Jianye, and Wei Zou. 2023. "A New Sample Processing Protocol for Separation and Purification Enabling Precise Analysis of Various Non-Traditional Isotopes in Geological Samples with Low Concentrations" Separations 10, no. 9: 497. https://doi.org/10.3390/separations10090497
APA StyleGui, J., & Zou, W. (2023). A New Sample Processing Protocol for Separation and Purification Enabling Precise Analysis of Various Non-Traditional Isotopes in Geological Samples with Low Concentrations. Separations, 10(9), 497. https://doi.org/10.3390/separations10090497






