1. Introduction
Maize (
Zea mays L.), also known as corn or maize, is used by a significant portion of the global population in different dishes, and is subjected to various post-harvest handling and storage conditions that can significantly affect its quality [
1,
2]. The total cultivated area of cereals increased by 2.5% in 2019 compared to 2018 in the EU. Common wheat and spelt yielded a harvest of 131.8 million tons, barley yielded 55.6 million tons, and cereal maize and corn-cob mix yielded 70.1 million tons [
3,
4]. The harvested production of cereals in the EU increased by 9.2% in 2019, with increases in common wheat and spelt (+14.0%), grain maize and corn-cob mix (+1.6%), and barley (+10.0%). Real (deflated) cereal prices were lower in 2019 (when compared to 2018): wheat and spelt prices across the EU were down 1.9%, barley prices were down 6.3%, and grain maize and corn-cob mix prices were down 3.1%. Winter commodities in the EU include rapeseed, wheat, rye, and triticale, while summer crops include maize, sunflowers, rice, soybeans, potatoes, and sugar beets [
3,
4]. In 2019, the EU-27 harvested 70,1 million tons of cereal maize and corn-cob combination, an increase of 1.1 million tons from 2018 [
3,
4]. Higher production levels in the majority of Member States compensated for the comparatively steep decline (6.6%) in Romania, which remained the leading producer of this cereal and accounted for a quarter of the EU’s harvested production [
3,
4].
Grain losses are caused by the grain’s cellular respiration and the resulting spontaneous heating. This procedure is dependent on the moisture content and temperature of the grain. There are several types of maize storage methods, each with its unique impact on the quality of the stored maize. Traditional storage methods include the use of cribs, silos, and sacks. More modern methods involve the use of hermetic bags and metal silos. These storage methods differ in their effectiveness in preserving the quality of maize, with factors such as temperature, humidity, and pest control playing significant roles [
5,
6,
7,
8,
9]. The storage conditions, including temperature, humidity, and pest management strategies, can lead to changes in the physical and chemical properties of the maize, impacting its nutritional value and safety for consumption [
10]. One of the major challenges in maize storage is the infestation by pests, such as weevils (
Sitophilus zeamais), which can cause substantial grain damage and weight loss [
10]. Additionally, improper storage conditions can lead to the growth of fungi, resulting in the production of mycotoxins that pose serious health risks to both humans and animals [
11].
Given the importance of maintaining maize quality during storage, there is a need for reliable and comprehensive methods to evaluate the impact of storage conditions on maize quality. Chromatographic methods, due to their sensitivity and specificity, have been widely used in the analysis of food quality, including the detection of mycotoxins in cereals [
12].
Vitamin B1 (thiamine) and B3 (niacin) are essential nutrients found in maize. These vitamins play crucial roles in energy metabolism and are vital for maintaining good health [
13,
14,
15]. However, their concentration can be influenced by storage conditions, potentially leading to a decrease in nutritional value [
16]. Similarly, α-tocopherol, a form of vitamin E, and β-carotene, a precursor of vitamin A, are potent antioxidants present in maize [
17,
18,
19]. They contribute to the prevention of various diseases by neutralizing harmful free radicals in the body [
20,
21]. Aflatoxins, particularly aflatoxin B1, are toxic compounds produced by certain fungi, such as
Aspergillus flavus and
Aspergillus parasiticus, which can contaminate maize during storage [
5,
22,
23,
24]. Consumption of aflatoxin-contaminated maize can lead to serious health problems, including liver damage and cancer [
25,
26,
27,
28]. Therefore, monitoring aflatoxin levels during storage is crucial for ensuring food safety [
5,
29]. In addition to these micronutrients and contaminants, the carbohydrate and protein content of maize, which contribute to its caloric and nutritional value, can also be affected by storage conditions [
30,
31]. Changes in these macronutrients can impact the overall quality and nutritional value of the stored maize. Chromatographic methods, such as high-performance liquid chromatography (HPLC), have been widely used for the analysis of these parameters due to their high sensitivity, precision, and reliability [
32,
33,
34].
This study utilizes these methods to provide a comprehensive evaluation of the impact of storage on maize quality, offering valuable insights for improving storage practices and ensuring the nutritional quality and safety of stored maize.
2. Materials and Methods
2.1. Materials
Solvents: acetonitrile CHROMASOLV, gradient grade, for HPLC, ≥99.9%; methanol CHROMASOLV, for HPLC, ≥99.9%; 2-propanol CHROMASOLV, for HPLC, 99.9%; acetone CHROMASOLV, for HPLC, ≥99.8% from Honeywell (Seelze, Germany); n-Hexane ≥97%; HiPerSolv CHROMANORM for HPLC; ethyl acetate ≥99.8%; HiPerSolv CHROMANORM for HPLC from VWR Chemicals (Sidney, BC, USA); ethanol, gradient grade, for liquid chromatography; and LiChrosolv from MERCK (Darmstadt, Germany).
Standards: (±)-α-tocopherol, synthetic, ≥96% (HPLC); rac-β-tocopherol, (+)-γ-tocopherol ≥96% (HPLC); 100MG, δ-tocopherol, β-carotene synthetic, ≥93% (UV); and thiamine hydrochloride pharmaceutical secondary standard. Certified reference materials: niacinamide pharmaceutical secondary standard from Sigma-Aldrich (Schnelldorf, Germany); BIOPURE Mycotoxin Mix 1 (aflatoxins), in acetonitrile, 5 mL; aflatoxin B1 2.00 µg/mL; aflatoxin B2 0.503 µg/mL; aflatoxin G1 2.00 µg/mL; and aflatoxin G2 0.506 from Romer Labs (Getzersdorf, Austria).
The Evoqua Ultrapure Water System Ultra ClearTM/Integra UV UF and UV UF TM (Günzburg, Germany) was used to obtain ultra-pure water (Günzburg, Germany).
Twenty samples of maize (
Zea mays L.) hybrid DK5092 were collected from September 2019 to January 2020 from a large farm situated in the west of Romania, in Timisoara County. The maize was harvested in September and was dried and cooled using the Frigor Tec KK 280 AHY model (Amtzell, Germany). The maize was stored at a temperature of 13 °C and a moisture content of less than 14% in a 2500 t silo. The samples were collected on a weekly basis according to Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs [
35] (
Appendix A). The SiloDrill from Burkle (Bad Bellingen, Germany) was used for sampling with the following characteristics: stainless steel AISI 304, standard length 150 cm, spiral screw diameter 90 mm, chamber diameter 40 mm, and volume 400 mL. The SiloDrill was introduced 3 times in different directions using the extension. All the collected corn was mixed together to form a representative sample. The samples were refrigerated until they were processed for analysis. All samples were ground using a laboratory mill (Seris II RomerLab, Getzersdorf, Austria).
Romania has a generally continental climate, with cold winters and hot summers [
36]. The average air temperature and relative humidity in West Romania from September 2019 through January 2020 are presented in
Table 1 [
37].
2.2. Methods
2.2.1. Vitamin B Analysis
A modified version of the method used by Suri et al. was applied for the determination of vitamin B content [
38]. At room temperature, 0.5 g of powdered sample was extracted with 1 milliliter of ultra-pure H
2O for 20 min in an ultrasonic bath (SONOREX, Bandelin, RK 103, Berlin, Germany). The samples were centrifuged at 11,000 rpm for 2 min using a Microcentrifuge Hettich D-78532 (Kirchlengern, Germany) and the supernatant was filtered through a 0.45 m cellulose filter. Thiamine (B1) and niacin (B3) were analyzed using the UHPLC Vanquisher H from Dionex, Thermo Fisher Scientific (Germering, Germany), which was equipped with a DAD detector. The gradient mobile phase was composed of ultra-pure water containing 1% acetic acid and MeOH at a flow rate of 0.3 mL/min. The chromatographic column used was a Thermo Fisher Accucore aQ 100 × 2.1 mm
2, 2.6 m, kept at 25 °C. The detector was set to 270 nm and the injection volume was 8 l. Individual and mixed standard stock solutions of 1 mg/mL of each compound were prepared in a mobile phase and stored in amber-colored vials at 5 °C prior to use. Working standard solutions of B1 (10, 25, 50, 100 µg/mL) and B3 (10, 25, 50, 100 µg/mL) were prepared by diluting the stock solution with the mobile phase to the appropriate concentrations. The results were expressed as micrograms per gram of dry weight (DW).
2.2.2. Tocopherols Analysis
The modified version of the methodology employed by Bao et al. was used for the analysis [
39]. A quantity of 10 g of the ground sample was subjected to extraction with 25 mL of isopropanol for a duration of 20 min in an ultrasonic bath at ambient temperature. The residue was redissolved in 1 mL of mobile phase. The samples were then filtered through glass microfiber filter paper (Whatman, 110 mm, 1.6 μm, Maidstone, UK). The filtered samples were diluted 1:10 with isopropanol, filtered again through a 45 µm syringe filter (Chromafil Xtra RC, Macherey, Nagel, France), and then injected into the instrument. The determination of tocopherols (α, β, γ, δ) was carried out using a Perkin Elmer 200 Series high-performance liquid chromatograph (HPLC) equipped with a fluorescence detector. The mobile phase was composed of 50% acetonitrile (ACN), 45% methanol (MeOH), and 5% water (H
2O), operating in an isocratic mode with a flow rate of 0.75 mL/min. The separation of compounds was achieved using a Poroshell 120, EC-C18, 3.0 × 150 mm, 2.7 µm chromatographic column from Agilent, Santa Clara, CA, USA, which was maintained at a temperature of 30 °C. A volume of 5 µL of the sample was analyzed using the fluorescence detector, which was set at an excitation wavelength of 290 nm and an emission wavelength of 330 nm. Individual standard stock solutions of 500 ng/mL of each compound were prepared in the mobile phase and stored in amber-colored vials at 5 °C prior to use. For the calibration curves, the concentrations used were 5, 10, 25, and 50 µg/l in the mobile phase. The results are expressed as µg/g DW.
2.2.3. β-Carotene Analysis
A methodology similar to Kimura et al. was used for the determination of β- carotene [
40]. A quantity of 10 g of the ground sample was subjected to extraction with 25 mL of extraction mixture (hexane/acetone/ethanol 2:1:1) for a duration of 20 min in an ultrasonic bath at ambient temperature. After extraction, 5 mL of saturated NaCl solution was added to separate the phases. The hexane layer was then recovered and dried under a stream of nitrogen. The residue was redissolved in 1 mL of mobile phase. A UHPLC Vanquisher H from Dionex (Thermo Fisher Scientific, Rommerskirchen, Germany) equipped with a DAD detector was utilized for β-carotene analysis. The separation column was Acclaim C30 5 um 4.6 × 150 (Thermo Scientific, Waltham, MA USA) set at 40 °C. The volume of the injection was 8 µL. At a flow rate of 2 mL/min, the mobile phase consisted of 30% acetonitrile (ACN) and 70% methanol (MeOH) in isocratic mode. The DAD detector was set at 460 nm. A standard stock solution of 1 mg/mL of mobile phase was prepared in amber-colored vials at 5 °C prior to use. The solutions for the calibration curve were prepared at concentrations of 10, 25, 50, and 100 g/mL in the mobile phase. The results are expressed as µg/g DW.
2.2.4. Aflatoxin Analysis
The determination of aflatoxins B1, B2, G1, and G2 from maize was achieved using high-performance liquid chromatography with post-column derivatization and immunoaffinity column purification according to EN ISO 16050:2011 [
41]. We extracted 25 g of homogenized ground sample with 125 mL of extraction mixture (MeOH and H
2O 7:3) and 5 g of NaCl for 2 min at high speed in a blender (Oster, Model 6808-051, McMinnville, TN, USA). The samples were then filtered through glass microfiber filter paper (Whatman, 110 mm, 1.6 μm, Maidstone, UK). Then, 8 mL of the filtrate was diluted with 20 mL of ultra-pure water. The purification was performed according to the immunoaffinity (IA) column manufacturer’s instructions (AflaStar R Immunoaffinity columns, 3 mL, RomerLab, Getzersdorf, Austria). The diluted sample volume passed through the IA column at a speed no faster than 1–3 mL/min. After it passed completely, the IA column was washed with 2 × 10 mL of deionized water. After all the liquid was removed from the column, the samples were eluted with 3 × 0.5 mL of MeOH. The samples were then analyzed using a high-performance liquid chromatograph (HPLC Perkin Elmer 200 Series, Waltham, MA, USA) with a fluorescence detector and post-column derivatization (SH-Romer Derivatization Unit, RomerLab, Getzersdorf, Austria). Chromatographic conditions were as follows: the mobile phase was a mixture of H
2O, MeOH, and ACN 3:1:1 (isocratic) at a flow of 0.85 mL/min; the separation column was Tracer Excel 120 ODS-B 5 μm 15 cm × 0.46 cm (TEKNOKROMA, Barcelona, Spain) at 30 °C; and the injection volumes were 40 µL, wavelength 360 nm with 440. The aflatoxin B1, B2, G1, and G2 stock solution was obtained by diluting the aflatoxin mix solution in the mobile phase to a concentration of 500 ng/mL of aflatoxin B1 and G1, and 125 ng/mL of aflatoxin B2 and G2. By transferring 25, 50, 100, 200, and 400 L of the aflatoxin stock solution into 5 mL volumetric flasks and diluting it with the mobile phase, a series of multi-element standard solutions with varied concentrations were prepared. The results are expressed as µg/kg DW.
2.2.5. Other Parameters with Nutritional Value Analysis
A modified version of Chadalavada et al. was used for FT-NIR analysis of cereals [
42]. On the Bruker USA Tango, ground samples were measured directly, without extraction. The method parameters are 92 s of measurement time, 16 cm-1 resolution, and a rotating scan type. The results were compared to the calibration curves for cereal matrices supplied by Bruker. The results are expressed as a percentage.
2.2.6. Quality Control of Analytical Methods
Method recovery is an essential parameter used to assess the accuracy and reliability of an analytical method for quantifying analytes in a sample matrix. Known amounts of the analyte of interest are added (spiked) to representative portions of the ground maize samples. These spiked samples undergo the same sample preparation procedure as the original samples. After preparation, the spiked samples are analyzed using the appropriate HPLC system. The measured concentration of the analyte in the spiked samples was then compared to the known spike concentration. The method recovery is calculated as the percentage of the measured concentration relative to the spike concentration.
Repeatability was also performed by analyzing six independent replicates at 2 concentration levels of spiked samples.
All of the samples were analyzed three times to ensure the reliability of the results.
2.2.7. Statistical Evaluation
Using the ANOVA method, the results were represented as means standard deviation (SD). The Pearson correlation matrix and r2 coefficient were employed to investigate the relationship between the parameters. The Minitab program for Windows version 17.0 (Minitab LLC, State College, PA, USA) was utilized for every statistical analysis.
3. Results and Discussion
Our paper presents novel insights into the nutritional and safety quality of maize by considering micronutrient content (liposoluble and water-soluble vitamins), macronutrients (carbohydrates and proteins), and food safety issues (aflatoxin). This dual-focus approach provides a more thorough understanding of the quality of maize as a food source and the impact that storage has on the quality. In contrast to conventional sampling methodologies, we collected maize over a period of five months using a longitudinal strategy. Instead of acquiring a snapshot of the quality of maize at a specific period, we collected 20 samples of maize from a farm in Western Romania between September 2019 and January 2020. This novel approach enabled us to capture the temporal variations in nutritional and safety parameters, casting light on the impact of seasonal changes and storage conditions on the quality of maize.
The HPLC methods demonstrated good performance with high recovery percentages ranging from 80.9% to 89.3% for various parameters, indicating accurate measurement of the analytes. The linearity (r
2) values were close to 1, indicating a strong linear relationship between the analyte concentrations and the instrument response. The intraday variability, represented by the RSD, was low, with values ranging from 1.2% to 1.9%.(
Table 2) This indicates good precision and reproducibility of the HPLC methods, as the measurements within the same day showed minimal variability. The results show that the HPLC methods used for the complex evaluation of the parameters in the study are reliable, accurate, and precise, providing confidence in the analytical data obtained.
The results obtained in the 5 months are presented as a monthly average and SD in
Table 3.
The aflatoxins were under the detection limit for all samples except sample 20 from January that had aflatoxin B1 at the quantification limit of 0.3 µg/kg, but was well below the maximum permitted level set by Commission Regulation (EU) No 165/2010, which is 20 µg/kg [
43].
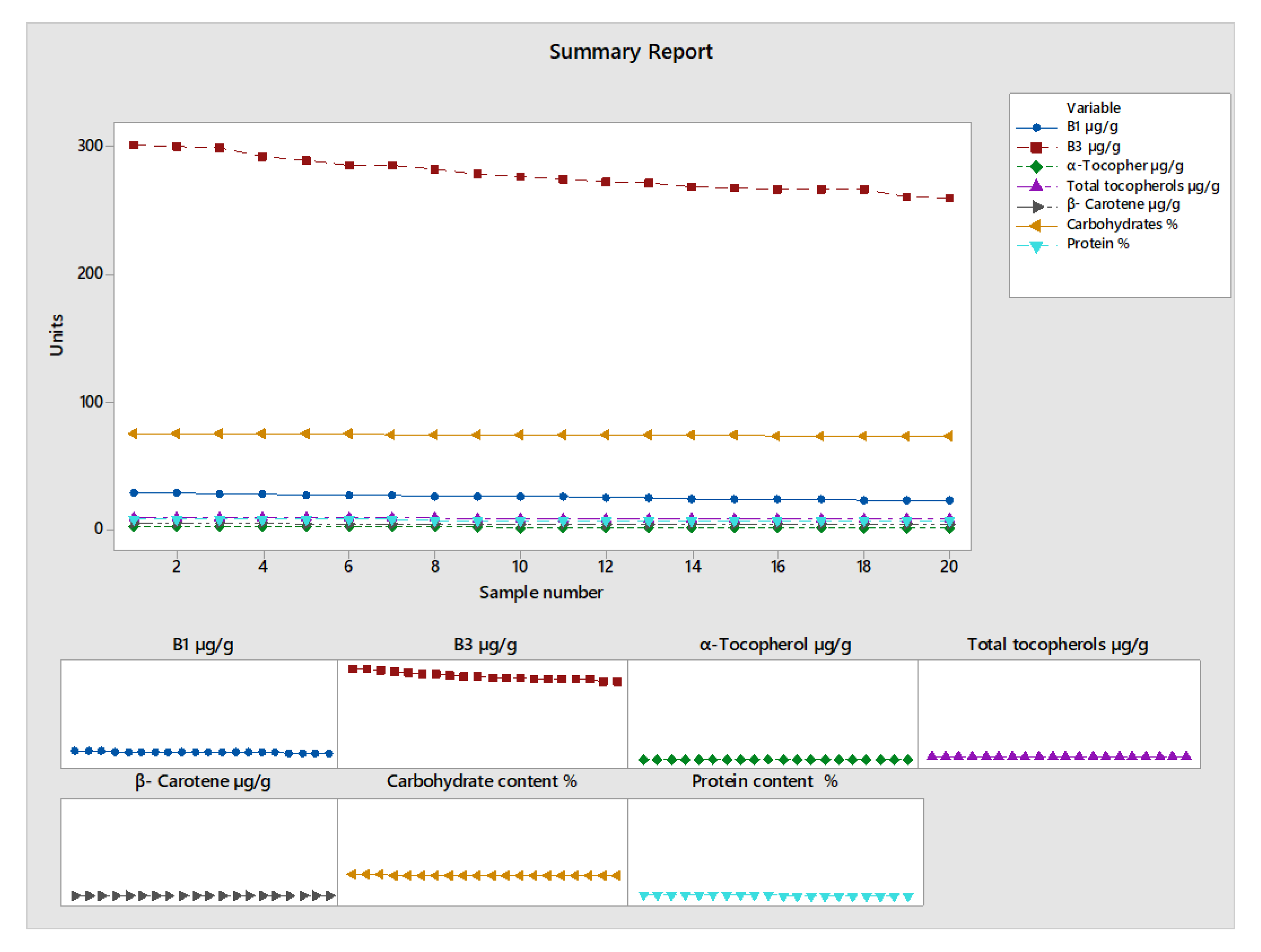
All measured parameters showed a general decline over the period from September to January. This could be due to the drying and cooling process after harvesting and during storage. Vitamins B1 and B3 exhibit a downward trend over time, which could be associated with the drying and storage process. The vitamin B1 concentration drops from 29.43 µg/g in September to 23.85 µg/g in January. Vitamin B3 follows a similar trend, decreasing from 300.6 µg/g to 263.93 µg/g during the same period. Khamila et. al. found results that also prove that the vitamins analyzed were significantly affected by storage conditions (temperature and relative humidity) [
44]. The data reflects a decrease in the concentration of α-tocopherol and total tocopherols over the months. The α-tocopherol decreases from 2.78 µg/g to 2.35 µg/g, while total tocopherols drop from 10.06 µg/g to 8.82 µg/g. The level of α-tocopherol is similar to the one found by Muzhingi et al.: 2.6–19.5 µg g
−1 DW [
45]. We could not locate any research on the impact of frigid storage on tocopherols. We noted that β-carotene also declines over the period, decreasing from 5.59 µg/g in September to 5.16 µg/g in January. The results are similar to the ones found by Mugode et al., wherein most of the β-carotene degradation in biofortified maize hybrids occurred during storage [
46], but the total number lost is much higher in their study: ~28%. The carbohydrate content shows a slight decline from 76% in September to 74.18% in January. Protein content also decreases over the period, decreasing from 8.76% in September to 7.96% in January. Shobha et al. also studied the influence of storage conditions on protein maize, proving that protein quality depends on appropriate storage conditions [
47]. The percentage lost for each month is between 2.52 to 16.96. The biggest loss is in α-tocopherol, while the smallest loss is in carbohydrate content (
Table 4).
The observations in the present study corroborate with extant literature demonstrating the decline in both micro- and macronutrient content in maize during storage. The nutrient degradation patterns observed echo the results of a previous investigation by da Silva Timm et al., emphasizing the influence of storage on maize’s nutritional composition [
48]. Notably, the diminishing levels of vitamins B1 and B3 align with the results obtained by Zhang et al., underscoring that these vitamins are particularly prone to degradation under unfavorable storage conditions, such as exposure to high temperature and humidity [
49]. These findings highlight the necessity for optimized storage conditions to maintain these vital nutrients. The documented reduction in α-tocopherol and total tocopherols during storage is significant, though limited research exists on the effects of storage on tocopherols specifically. Previous research indicates tocopherols as potent antioxidants, with stability contingent on environmental conditions like temperature and light [
50]. In the case of β-carotene, the reduction corresponds to the findings of Borba et al., in that significant degradation occurs during storage [
51]. Nevertheless, the current study presents a relatively smaller loss, hinting at more favorable storage conditions. The marginal decline in carbohydrate and protein content is consistent with previous literature; specifically, the work of Wang et al. supports that these macronutrients are susceptible to changes resulting from storage conditions [
52]. From a food safety perspective, the delayed detection of aflatoxins and the low aflatoxin B1 value, significantly below the maximum permitted level, in January is encouraging. The study underscores the profound impact of effective maize storage management on nutritional content, as evidenced by the significant correlation between the decrease of all analyzed parameters.
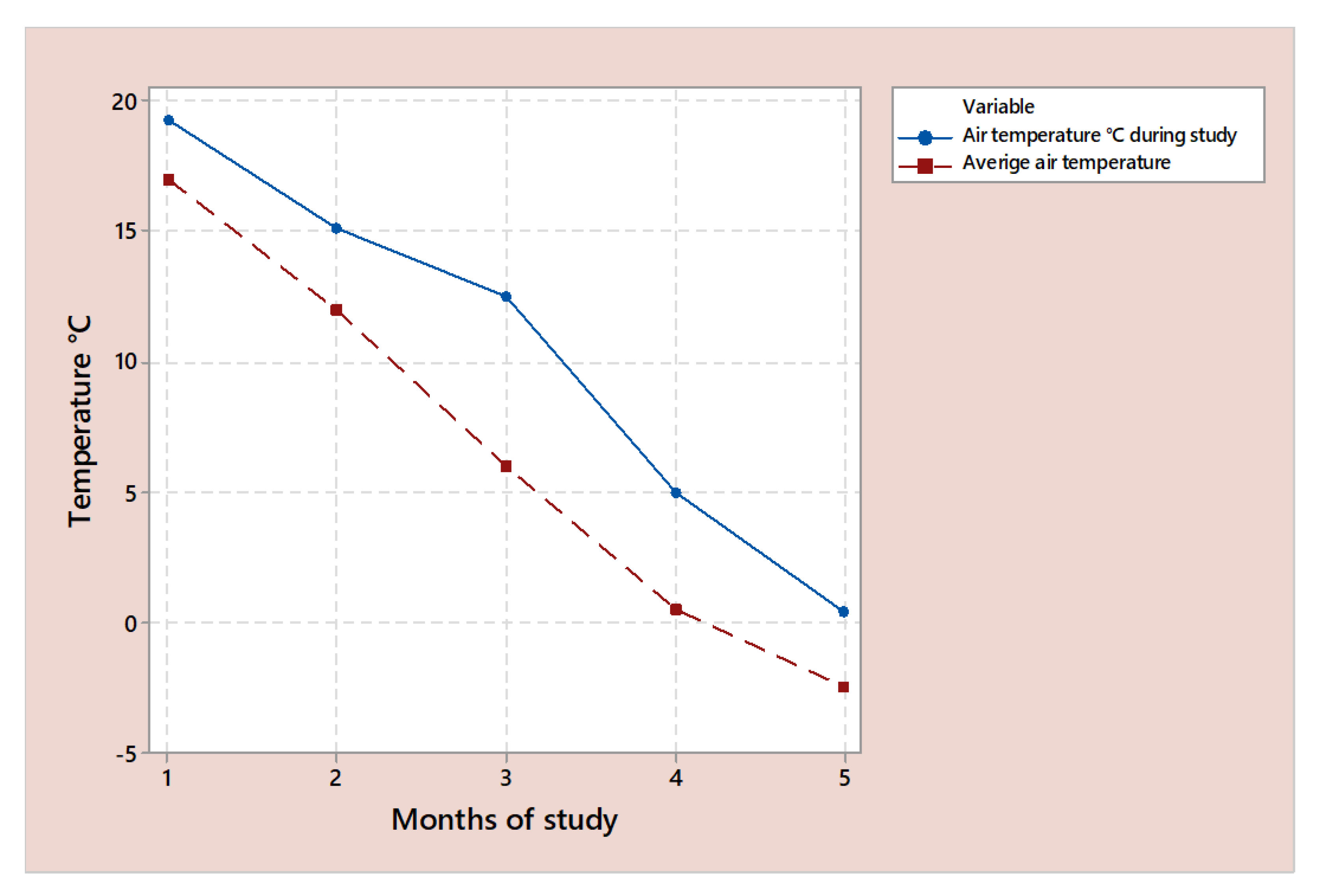
The cooling of the maize immediately after drying assured that no significant loss of valuable vitamins occurred in the first 10 to 15 days, which is common in storage conditions where cereals are put directly into silos after drying. After the drying stage, the maize temperature can reach up to 30 °C in order to achieve the low humidity needed for storage [
53] (
Figure 1). If no cooling operation is applied, then the farmer must rely on the weather to lower the temperature of the crop. From
Figure 2, we can see that in the period of the study, the registered temperature was above the average temperature in Romania.
There is a high positive correlation (0.94–0.95) between the loss of all analyzed parameters; this is due to the fact that the same factors influence the degradation of these compounds (
Figure 3,
Table 5 and
Table 6).
Based on the results obtained, it is evident that the nutritional and safety quality of maize is significantly influenced by seasonal changes throughout the studied period. The analysis reveals distinct variations in the concentrations of vitamins, antioxidants, and other essential components, highlighting the dynamic nature of maize composition in response to the shifting seasons. These findings emphasize the importance of considering seasonal factors when evaluating maize quality, and underscore the need for tailored storage and preservation strategies to maintain optimal nutritional value year-round. Furthermore, the study proves that HPLC is a versatile technique that can be tailored to specific analytical needs by selecting appropriate chromatographic columns, mobile phases, and detection methods, allowing for the determination of a wide range of anilities with little sample preparation.