Rapid and Simultaneous Extraction of Bisabolol and Flavonoids from Gymnosperma glutinosum and Their Potential Use as Cosmetic Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. UPLC and GC Coupled to Mass Spectrometry Analysis
2.3. NMR Spectroscopy
3. Results
3.1. Isolation and Standardization of the Method of Extraction
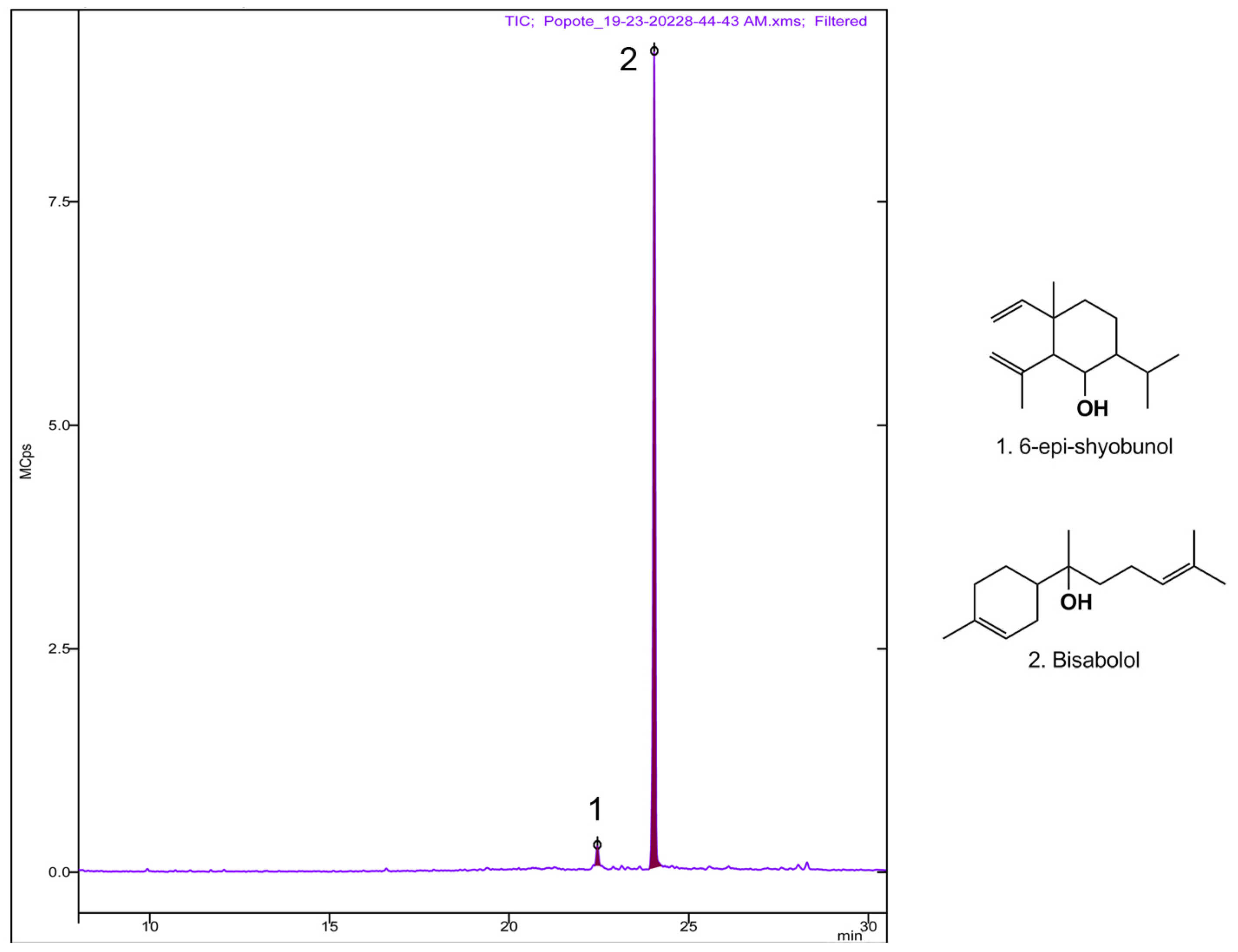
3.2. Chemical Composition Assay by GC-MS of the CHCl3 Fc1
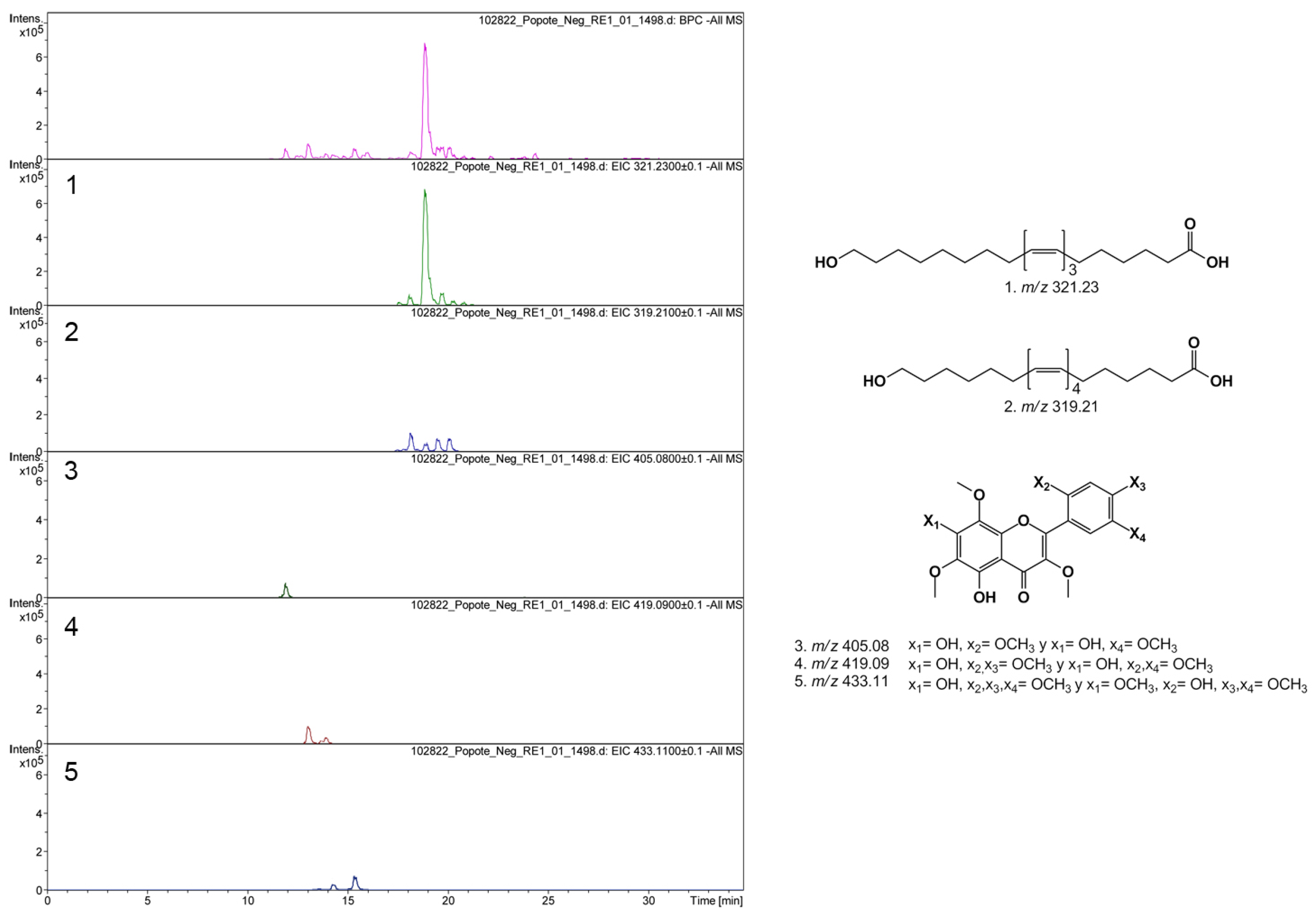
3.3. Chemical Composition by UPLC-MS Assay of MeOH Fc1
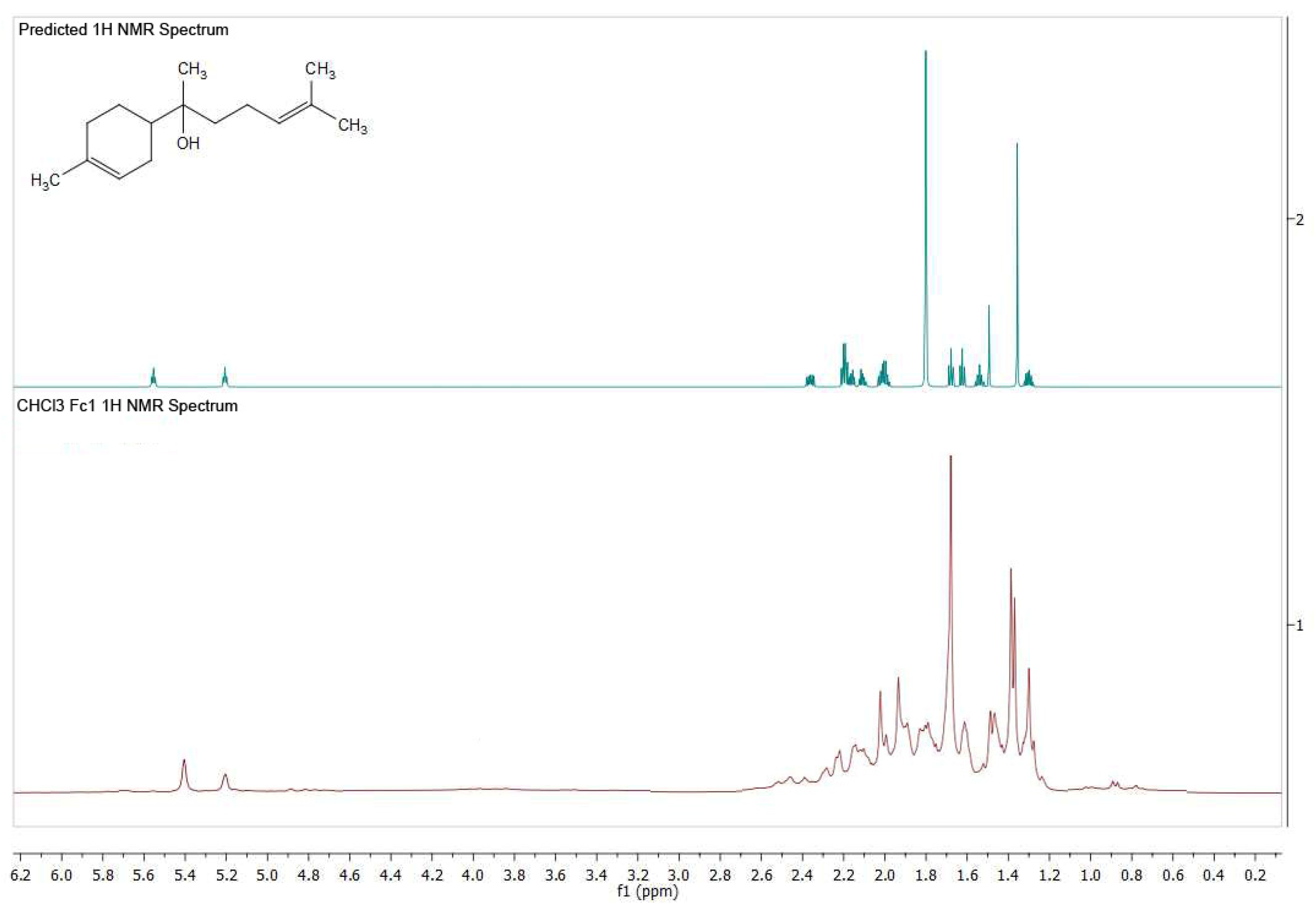
3.4. NMR Analysis of the Chloroform Extract of G. glutinosum
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bos, J.D.; Kapsenberg, M.L. The Skin Immune System Its Cellular Constituents and their Interactions. Immunol. Today 1986, 7, 235–240. [Google Scholar] [CrossRef]
- Bos, J.D.; Kapsenberg, M.L. The Skin Immune System: Progress in Cutaneous Biology. Immunol. Today 1993, 14, 75–78. [Google Scholar] [CrossRef]
- Erarslan, Z.B.; Ecevit-genç, G.; Kültür, Ş. Medicinal plants traditionally used to treat skin diseases in turkey—Eczema, psoriasis, vitiligo. J. Fac. Pharm. Ank. Univ. 2020, 44, 137–166. [Google Scholar]
- Bodeker, G.; Ryan, T.J.; Volk, A.; Harris, J.; Burford, G. Integrative skin care: Dermatology and traditional and complementary medicine. J. Altern. Complement. Med. 2017, 23, 479–486. [Google Scholar] [CrossRef]
- Weiss, R.F.; Fintelmann, V. Herbal Medicine; Thieme Medicinal Publishers: Stuttgart, Germany; New York, NY, USA, 2000; pp. 293–314. [Google Scholar]
- Gurib-Fakim, A. Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.P. Current and future status of herbal medicines. Veter-World 2008, 1, 347–350. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Dawid-Pać, R. Medicinal plants used in treatment of inflammatory skin diseases. Adv. Dermatol. Allergol. 2013, 3, 170–177. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Rocha-Filho, P.A.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In Vitro and In Vivo Evaluation of Nanoemulsion Containing Vegetable Extracts. Cosmetics 2017, 4, 32. [Google Scholar] [CrossRef]
- Ajjoun, M.; Kharchoufa, L.; Merrouni, I.A.; Elachouri, M. Moroccan medicinal plants traditionally used for the treatment of skin diseases: From ethnobotany to clinical trials. J. Ethnopharmacol. 2022, 297, 115532. [Google Scholar] [CrossRef]
- Selwyn, A.; Govindaraj, S. Study of plant-based cosmeceuticals and skincare. S. Afr. J. Bot. 2023, 158, 429–442. [Google Scholar] [CrossRef]
- Cotton, C.M. Ethnobotany: Principles and Applications; John Wiley and Sons: London, UK, 1996; p. 434. [Google Scholar]
- Argueta, V.A.; Cano, A.J. Atlas de las Plantas de la Medicina Tradicional Mexicana; Instituto Nacional Indigenista: Mexico City, México, 1994; pp. 1318–1319. [Google Scholar]
- Arias, T.A.A.; Valverde, V.M.T.; Reyes, S.J. Las plantas de la región de Zapotitlán Salinas, Puebla; Instituto Nacional de Ecología: Mexico City, México, 2000; pp. 8–9, 22. [Google Scholar]
- Hernández, T.; Canales, M.; Avila, J.G.; Durán, A.; Caballero, J.; Romo de Vivar, A.; Lira, R. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México). J. Ethnopharmacol. 2003, 88, 181–188. [Google Scholar] [CrossRef]
- Canales, M.; Hernández, T.; Caballero, J.; Romo de Vivar, A.; Avila, G.; Duran, A.; Lira, R. Informat consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef]
- Canales, M.; Hernández, T.; Serrano, R.; Hernández, L.B.; Duran, A.; Ríos, V.; Sigrist, S.; Hernández, H.L.H.; Garcia, A.M.; Angeles-López, O.; et al. Antimicrobial and general toxicity activities of Gymnosperma glutinosum: A comparative study. J. Ethnopharmacol. 2007, 110, 343–347. [Google Scholar] [CrossRef]
- Serrano, R.; Hernández, T.; Canales, M.; García-Bores, A.M.; Romo De Vivar, A.; Céspedes, C.L.; Avila, J.G. Ent-labdane type diterpene with antifungal activity from Gymnosperma glutinosum (Spreng.) Less. (Asteraceae). Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2009, 8, 412–418. [Google Scholar]
- Morado-Castillo, R.; Quintanilla-Licea, R.; Gomez-Flores, R.; Blaschek, W. Total Phenolic and Flavonoid Contents and Flavonoid Composition of Flowers and Leaves from the Mexican Medicinal Plant Gymnosperma glutinosum (Spreng.) Less. Eur. J. Med. Plants 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; González-Chávez, M.M.; Zapata-Morales, J.R.; Verdinez-Portales, A.K.; Sánchez-Recillas, A.; Ortiz-Andrade, R.; Isiordia-Espinoza, M.; Martínez-Gutiérrez, F.; Ramírez-Morales, M.A.; Domínguez, F.; et al. Antinociceptive Activity of Ent-Dihydrotucumanoic Acid Isolated from Gymnosperma glutinosum Spreng Less. Drug Dev. Res. 2017, 78, 340–348. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Arana-Argáez, V.E.; Deveze-Alvarez, M.A.; Chan-Zapata, I.; Torres-Romero, J.C.; Carranza-Álvarez, C.; Luna-Rubio, S.; González-Chávez, M.M.; Zapata-Morales, J.R.; Aragón-Martínez, O.H.; et al. Anti-inflammatory and diuretic effects of the diterpene ent-dihydrotucumanoic acid. Drug Dev. Res. 2019, 80, 800–806. [Google Scholar] [CrossRef]
- González-Chávez, M.M.; Arana-Argáez, V.; Zapata-Morales, J.R.; Ávila-Venegas, A.K.; Alonso-Castro, A.J.; Isiordia-Espinoza, M.; Martínez, R. Pharmacological evaluation of 2-angeloyl ent-dihydrotucumanoic acid. Pharm. Biol. 2017, 55, 873–879. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Miwa, T.; Shibuya, M.; Yamamoto, Y.; Nagino, M. The Anticancer Effects of Novel alpha-Bisabolol Derivatives Against Pancreatic Cancer. Anticancer. Res. 2017, 37, 589–598. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Cariño-Cortés, R.; Ponce-Monter, H.A.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Pharmacological interaction of -bisabolol and diclofenac on nociception, inflammation, and gastric integrity in rats. Drug Dev. Res. 2018, 79, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, H.A.O.; Silva-Filho, S.E.; Wiirzler, L.A.M.; Cardia, G.F.E.; Uchida, N.S.; Silva-Comar, F.M.S.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of (−)-alpha-Bisabolol on the Inflammatory Response in Systemic Infection Experimental Model in C57BL/6 Mice. Inflammation 2020, 43, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1370. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.K.; Yoon, P.K.S. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Fact. 2016, 15, 185. [Google Scholar] [CrossRef]
- Sharma, R.R.; Deep, A.; Abdullah, S.T. Herbal products as skincare therapeutic agents against ultraviolet radiation-induced skin disorders. J. Ayurveda Integr. Med. 2022, 13, 100500. [Google Scholar] [CrossRef]
- Gaspar, A.L.; Gaspar, A.B.; Contini, L.R.; Silva, M.F.; Chagas, E.G.; Bahú, J.O.; Concha, V.O.; Carvalho, R.A.; Severino, P.; Souto, E.B.; et al. Lemongrass (Cymbopogon citratus)-incorporated chitosan bioactive films for potential skincare applications. Int. J. Pharm. 2022, 628, 122301. [Google Scholar] [CrossRef] [PubMed]


| Material Collected | Yield | CHCl3 Fc1 | MeOH Fc1 |
|---|---|---|---|
| 1 June 2022 (250 g) | 18.9 g (7.56%) 1 | 17.1 g | 1.6 g |
| 2 December 2022 (250 g) | 19.5 g (7.80%) 1 | 17.6 g | 1.80 g |
| Material Collected | Yield | CHCl3 Fc2 | MeOH Fc2 |
|---|---|---|---|
| 1 June 2022 (250 g) | 28.3 g (11.32%) 1 | 5.1 g | 20.4 g |
| 2 December 2022 (250 g) | 27.5 g (11.00%) 1 | 7.6 g | 19.8 g |
| Rt | Formula | [M-H]− MWdetected | [M-H]− MWexact | % Relative |
|---|---|---|---|---|
| 11.9 | C19H18O10 | 405.0804 | 405.0816 | 7.0 |
| 13.1 | C20H20O10 | 419.0977 | 419.0972 | 11.0 |
| 13.6 | C20H20O10 | 405.0809 | 405.0816 | 1.1 |
| 13.9 | C20H20O10 | 419.0967 | 419.0972 | 4.1 |
| 14.3 | C21H22O10 | 433.1136 | 433.1129 | 3.0 |
| 15.4 | C21H22O10 | 433.1143 | 433.129 | 7.9 |
| 17.5 | C20H32O3 | 319.2197 | 319.2267 | 0.2 |
| 17.6 | C20H34O3 | 321.2393 | 321.2424 | 0.7 |
| 18.1 | C20H32O3 | 319.2195 | 319.2267 | 5.1 |
| 18.9 | C20H34O3 | 321.2399 | 321.2424 | 44.4 |
| 19.5 | C20H32O3 | 319.2191 | 319.2267 | 3.4 |
| 19.7 | C20H34O3 | 321.2394 | 321.2424 | 3.0 |
| 20.1 | C20H32O3 | 319.2197 | 319.2267 | 6.2 |
| 20.3 | C20H34O3 | 321.2395 | 321.2424 | 0.8 |
| 20.8 | C20H34O3 | 321.2398 | 321.2424 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Patiño, M.B.; Leyva Pérez, J.P.; Alcibar Muñoz, M.M.; Arzate-Vázquez, I.; Arrieta-Baez, D. Rapid and Simultaneous Extraction of Bisabolol and Flavonoids from Gymnosperma glutinosum and Their Potential Use as Cosmetic Ingredients. Separations 2023, 10, 406. https://doi.org/10.3390/separations10070406
Gómez-Patiño MB, Leyva Pérez JP, Alcibar Muñoz MM, Arzate-Vázquez I, Arrieta-Baez D. Rapid and Simultaneous Extraction of Bisabolol and Flavonoids from Gymnosperma glutinosum and Their Potential Use as Cosmetic Ingredients. Separations. 2023; 10(7):406. https://doi.org/10.3390/separations10070406
Chicago/Turabian StyleGómez-Patiño, Mayra Beatriz, Juan Pablo Leyva Pérez, Marcia Marisol Alcibar Muñoz, Israel Arzate-Vázquez, and Daniel Arrieta-Baez. 2023. "Rapid and Simultaneous Extraction of Bisabolol and Flavonoids from Gymnosperma glutinosum and Their Potential Use as Cosmetic Ingredients" Separations 10, no. 7: 406. https://doi.org/10.3390/separations10070406
APA StyleGómez-Patiño, M. B., Leyva Pérez, J. P., Alcibar Muñoz, M. M., Arzate-Vázquez, I., & Arrieta-Baez, D. (2023). Rapid and Simultaneous Extraction of Bisabolol and Flavonoids from Gymnosperma glutinosum and Their Potential Use as Cosmetic Ingredients. Separations, 10(7), 406. https://doi.org/10.3390/separations10070406