1. Introduction
In both human and veterinary medicine, fluoroquinolones (FQs) are medications that are used to treat a variety of bacterial illnesses. They successfully combat anaerobes, gram-positive, gram-negative, and mycobacterium bacteria. The bacterial enzymes DNA gyrase and topoisomerase IV are inhibited by FQs, which results in their bactericidal activity. The World Health Organization (WHO) stated that this class of antibiotics is the first-line treatment for complicated urinary tract infections and bacterial diarrhea, as well as the second-line therapeutic intervention for tuberculosis patients who have developed resistance to the first-line anti-tuberculosis drug. They can be used to treat osteomyelitis, various wound infections, and respiratory infections in an efficient manner. Many sexually transmitted infections are also treated with FQs [
1,
2].
Ciprofloxacin (CPF), 1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-quinoline-3-carboxylic acid, is a FQ used to treat a number of infections, notably those that affect the respiratory, urinary, and gastrointestinal tracts [
2].
The photo-stability of drugs describes how light affects the stability of medicinal substances and/or finished products. Thus, photo-stability is concerned with how light affects the stability of pharmaceutical compounds. Both natural and artificial light sources, such as fluorescent light, can affect medications that are sensitive to light. Sunlight can trigger interactions between the medication’s molecule and the endogenous substrates, which can transform the drug into a hazardous by-product or result in the production of reactive oxygen species. It is not always necessary for photosensitive materials to be exposed to light very severely; even a small amount might cause serious issues. Obviously, the loss of product potency is the most significant negative impact of photo-degradation [
3].
A crucial aspect of quality control (QC) and pharmacotherapeutics for each drug, particularly FQs, which are known to be vulnerable to distinct degradation, is focusing on the quantification of the active pharmaceutical ingredients (APIs) in the presence of their degradation products [
4]. Regarding the photo-degradation of CPF, 7-amino-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid (MDP) is the predominant degradant following both artificial and natural light exposure. After being exposed to a high-pressure mercury lamp in aqueous acidic solutions for more than 5 h, this is still the dominant chemical [
5].
Figure 1 shows the chemical structures of both CPF and its main photo-degradation product (MDP).
CPF has been determined by spectroscopy [
6,
7,
8], liquid chromatography (LC) [
9,
10,
11,
12,
13], capillary electrophoresis (CE) [
14,
15], and electrochemistry [
16,
17,
18] in a variety of sample formats. For the selective quantification of the investigated medication in the presence of its various degradation products, various approaches have been used. Several spectrophotometric techniques, including ratio derivative, ratio difference, mean centering, and dual wavelength, have been utilized to measure CPF in the presence of the product of its acid-induced degradation [
19]. Stability-indicating quantification of the investigated medication in solid dosage form was performed using high-performance liquid chromatography (HPLC) [
20]. The same analytical technique was applied to investigate the degradation process and quantify CPF in the presence of its acidic, basic, oxidative, light, and thermal degradation products [
21]. Additionally, HPLC was used for the stability-indicating determination of CPF in mixtures with other pharmaceuticals including tinidazole [
22,
23], dexamethasone [
24], and metronidazole [
25].
CE can be regarded as a crucial analytical method for the determination of FQs due to its high separation efficiency, minimal sample and reagent usage, short analysis time, and application to a wider range of samples [
26]. The existence of a permanent charge on the FQs at all pH values constitutes a major reason for the preference for the use of CZE in quantification, because the ionized state is a beneficial property in CZE, where separation is based on differences in the electrophoretic mobilities of the analytes [
27].
The term “ultra-performance liquid chromatography” (UPLC) refers to a chromatographic technique that uses a combination of 1.7 µm-sized reversed-phase packing materials with operating pressures between 6000 and 15,000 psi. In addition to having several advantages over traditional HPLC, it is more sensitive, as evidenced by a higher signal to noise (S/N) ratio, since zone broadening is reduced. Better chromatographic peak resolution and faster analytical times are further characteristics [
28].
After a thorough search of the literature, it was found that no publications have yet been published that directly applied CZE or UPLC for the stability-indicating quantification of CPF in the presence of its major photo-degradant.
The major goal of this research was to optimize and validate the CZE and UPLC methods for quantifying CPF in the presence of its main photo-degradant through a comparative approach between the suggested methodologies.
2. Materials and Methods
2.1. Chemicals, Reagents, Dosage Form, and Standard Solutions
The solvents and chemicals were of HPLC and analytical grades, respectively. Acetonitrile (ACN), methanol (MeOH), potassium dihydrogen phosphate, o-phosphoric acid, sodium hydroxide (NaOH), and boric acid (H3BO3) were bought from Merck (Darmstadt, Germany). The background electrolyte (BGE) was boric acid solution at a concentration of 40 mM. It was adjusted to pH 8.5 using NaOH. The Milli-Q Plus system, manufactured by Millipore, Bedford, MA, USA, was used to supply the pure water utilized in this work.
CPF (CAS no. 85721-33-1) and MDP (CAS no. 105674-91-7) pure materials were purchased from Cymit Quimica S.L (Barcelona, Spain). A certificate declared purities of 99.95% and 99.93% for CPF and MDP, respectively. Ciprobay® tablets claiming to contain 500 mg ciprofloxacin per tablet were purchased. An intravenous (I.V.) solution, Cipro® I.V. solution, labeled as containing 2 mg ciprofloxacin per mL was purchased.
The necessary quantity of each chemical was dissolved in a mixture of ACN:H2O (1:1, v/v) and held at 4 °C in the dark in order to make stock solutions (200 µg/mL) of each. Stock solutions were diluted with ACN:H2O (1:1, v/v) to prepare working standard solutions.
2.2. Instrumentation
The Agilent 7100 CE apparatus from Waldbronn, Germany, which was used for the CZE measurements has a UV-Vis detector and an automated injector. For separation, a fused silica capillary with an inner diameter of 75 µm and a length of 64.5 cm, obtained from Polymicro Technologies in Phoenix, AZ, USA, was used. Agilent Chem-Station software was used to measure peak areas, migration times, and other data. Utilizing a pH meter from Mettler Toledo (Greifensee, Switzerland), the liquid pH was adjusted.
The WatersTM Acquity system (Milford, CT, USA) was used for UPLC, and the column dimensions were 100 × 2.1 mm, 1.7 µm. The detector was a UV-visible detector (Waters, 2489, Milford, CT, USA).
A photo-degradation cabinet with a high-pressure mercury lamp to provide stable UV-irradiation was used to irradiate the dosage forms.
2.3. Methods’ Development
2.3.1. CZE Method
A suggested preconditioning protocol developed by Agilent technologies (Wald-bronn, Germany) for capillaries was followed. The preconditioning process was performed by flushing fresh capillaries for 20 min with NaOH solution at a concentration of 1 M, 20 min with NaOH solution at a concentration of 0.1 M, 2 min with deionized water, and finally, 30 min with a BGE solution. The capillary was washed on the following day with water for the first 2 min, 0.1 M NaOH solution for 20 min, 1 M NaOH solution for 5 min, and BGE for 30 min. After cleaning the capillary ends in water for 20 min each day, they were left in water for the whole night.
A voltage of 16 kV was employed at 25 °C in order to conduct the best separation of the materials under study. For 10 s, the sample solution was hydrodynamically injected at a pressure of 60 mbar. At 280 nm, the analytes under study were detected.
2.3.2. UPLC Method
To attain an optimal separation pattern for the investigated analytes with a reasonable resolution, the chromatographic conditions were adjusted. Many attempts were carried out to reach the optimum stationary phase/mobile phase match that ensured excellent separation.
2.4. Method Validation
The ICH-Q2B specifications were used to validate the proposed methods [
29].
2.4.1. Linearity
Different aliquots of the examined analyte (5–500 µg) were transferred to a number of 10 mL measuring flasks. Then, a mixture of ACN:BGE (1:3, v/v) (CZE technique) and ACN:H2O (1:1, v/v) (UPLC method) was added to each flask’s volume to obtain a concentration of 0.5–50 µg/mL. For the electrophoretic examination of the samples, an elution liquid consisting of ACN:H2O:MeOH (2:1:1, by volumes) was utilized. On the other hand, UPLC separation was carried out using a stationary phase of the WatersTM column (100 × 2.1 mm, 1.7 µm) and a developing system made up of potassium dihydrogen phosphate buffer:acetonitrile (7:3, v/v). Its pH was adjusted to 3.0 using o-phosphoric acid, and its flow rate was adjusted to 0.4 mL/min.
2.4.2. Accuracy
The percentage of an analyte recovered from a given quantity can be used to express the accuracy [
29]. The results of nine samples at concentrations of 5, 10, and 30 µg/mL CPF were examined using the technique under linearity.
2.4.3. Precision
In a number of statistically significant trials, the method was demonstrated to have both intra- and inter-day precision, represented as the recovery % ± relative standard deviation (RSD). Three CPF concentrations (5, 10, and 30 µg/mL) were examined three times using the specified methods, either on the same day (intra-day) or on three different days (inter-day).
2.4.4. LOD and LOQ
The LOD is the least amount of the drug that can be quantified from the background, while the LOQ is the least amount of the drug that can be perfectly and precisely quantified. Equations (1) and (2) were used for their calculation [
29].
(σ represents the standard deviation, and s represents the slope).
2.4.5. Robustness
The methods’ robustness can be evaluated by examining the effects of slight variations in the cited methods’ performance. For this, either the CZE method or the UPLC method’s acetonitrile concentration (±1%) in the elution liquid was changed. A slight adjustment to the mobile phase’s pH (UPLC method) or the BGE’s pH (CZE method) was performed.
2.5. Applications
2.5.1. Quantification of CPF in the Presence of Its Main Photo-Degradant (MDP)
A series of volumetric flasks (10 mL capacity) were used to combine complementary volumes of CPF and MDP stock solutions (200 µg/mL). In order to prepare various laboratory-prepared mixes comprising 90% to 10% of intact CPF and 10% to 90% of MDP, the capacity of each flask was then filled with an ACN:BGE (1:3,
v/
v) mixture (CZE-method) and an ACN:H
2O (1:1,
v/
v) mixture (UPLC-method). The process was carried out as described in
Section 2.4.1.
2.5.2. Irradiation of CPF Dosge Forms to UV-Light
Twenty Ciprobay® tablets and Cipro® I.V. solution were placed in the photo-degradation cabinet and subjected to UV-irradiation from a high-pressure mercury lamp for six hours.
2.5.3. Determination of CPF in Pharmaceutical Preparations (UV-Irradiated and Non-Irradiated)
Twenty Ciprobay® tablets were weighed and ground. A tablet powder, corresponding to 20 mg CPF, was then transferred to a 100 mL measuring flask. Sixty milliliters of ACN:H2O (1:1, v/v) was added. A total time period of 15 min was spent sonicating the contents of the volumetric flask. The obtained solution’s volume was increased to 100 mL using the same solvent mixture. A Whatman filter paper was used for the filtration process. A suitable aliquot from the filtrate was properly diluted using aliquots of the ACN:BGE (1:3, v/v) mixture (CZE-method) and ACN:H2O (1:1, v/v) mixture (UPLC-method) to reach a claimed concentration of 10 µg/mL.
Regarding the Cipro® I.V. solution, 0.5 mL of the container’s content was diluted to reach a final volume of 100 mL using the mentioned diluting mixtures for both the CZE and UPLC methods. The process was then completed in accordance with linearity (2.4.1).
The mentioned procedures were followed for the UV-irradiated and non-irradiated dosage forms.
3. Results
3.1. Method Development
3.1.1. CZE Method
Because it has the capacity to impact the electro-osmotic flow (EOF), ionic strength, Joule heating, and the current produced in the capillary, the electrolyte concentration plays a crucial role in determining the separation quality. Therefore, the electrolyte content will have large impacts on the migration time and peak area of the separated analytes. In a previously published work [
15], the authors examined the effect of variation in the BGE borate concentration on the obtained results. The optimal borate concentration was found to be 40 mM, which resulted in the highest peak areas and optimum separation pattern of the analytes.
The buffer pH has a significant impact on the CZE analysis of the analytes, since it affects the electro-osmotic flow (EOF). It was also studied by the authors of a previous publication [
15], who investigated how pH variation in the BGE influenced the results. As the pH of the BGE rose from 6.5 to 8.5, the peak area increased. When the pH was more than 8.5, the migration time increased, and the peak area remained essentially constant. Thus, the optimum pH offering excellent assay results was 8.5.
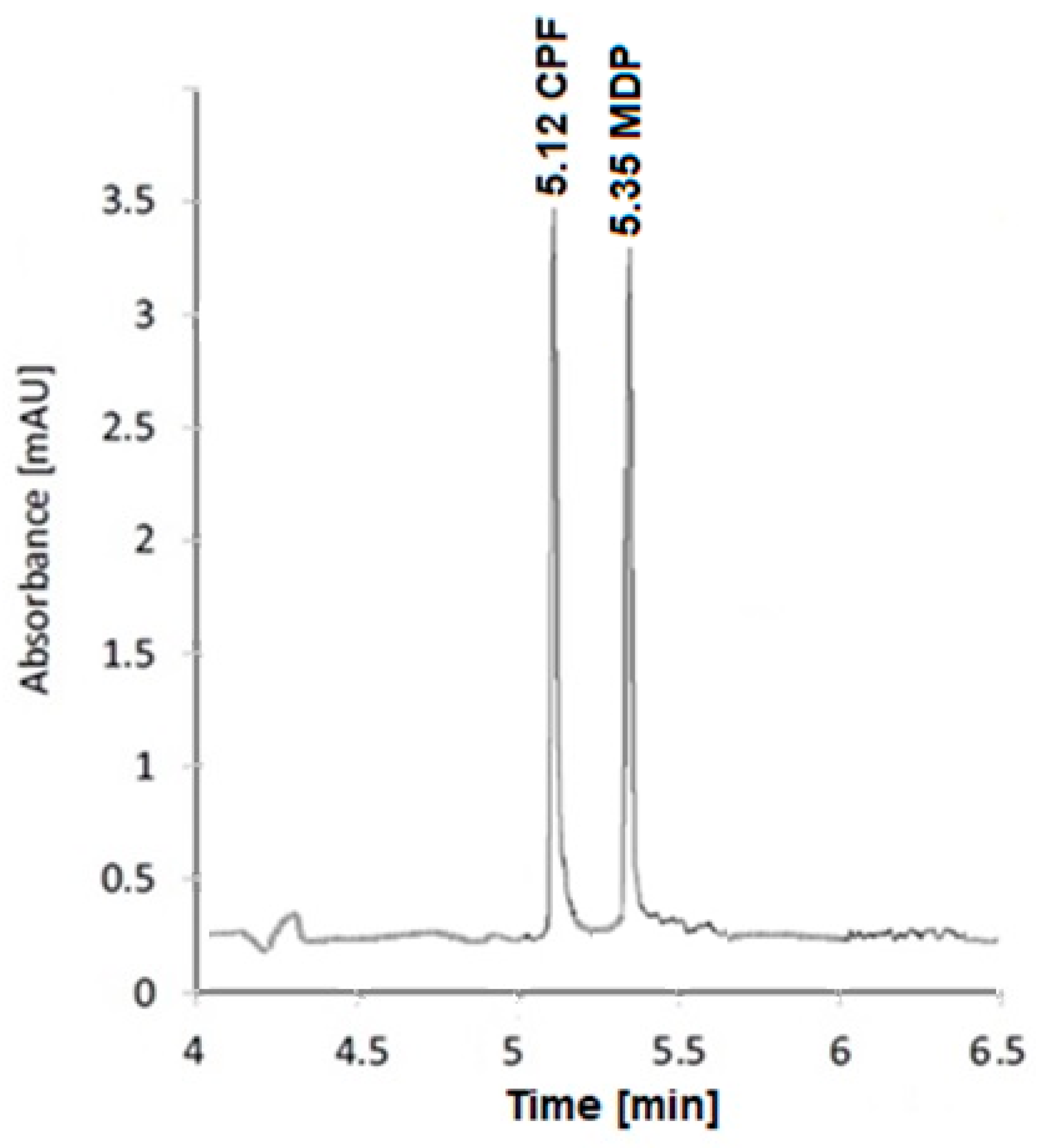
The applied voltage was tuned to 16 kV at 25 °C. The sample solution was hydrodynamically injected for 10 s at 60 mbar. UV-Vis detection was performed at 280 nm.
After the experimental parameters had been optimized, a mixture of CPF and MDP containing 10 µg/mL of each was examined.
Figure 2 depicts the separation pattern.
3.1.2. UPLC Method
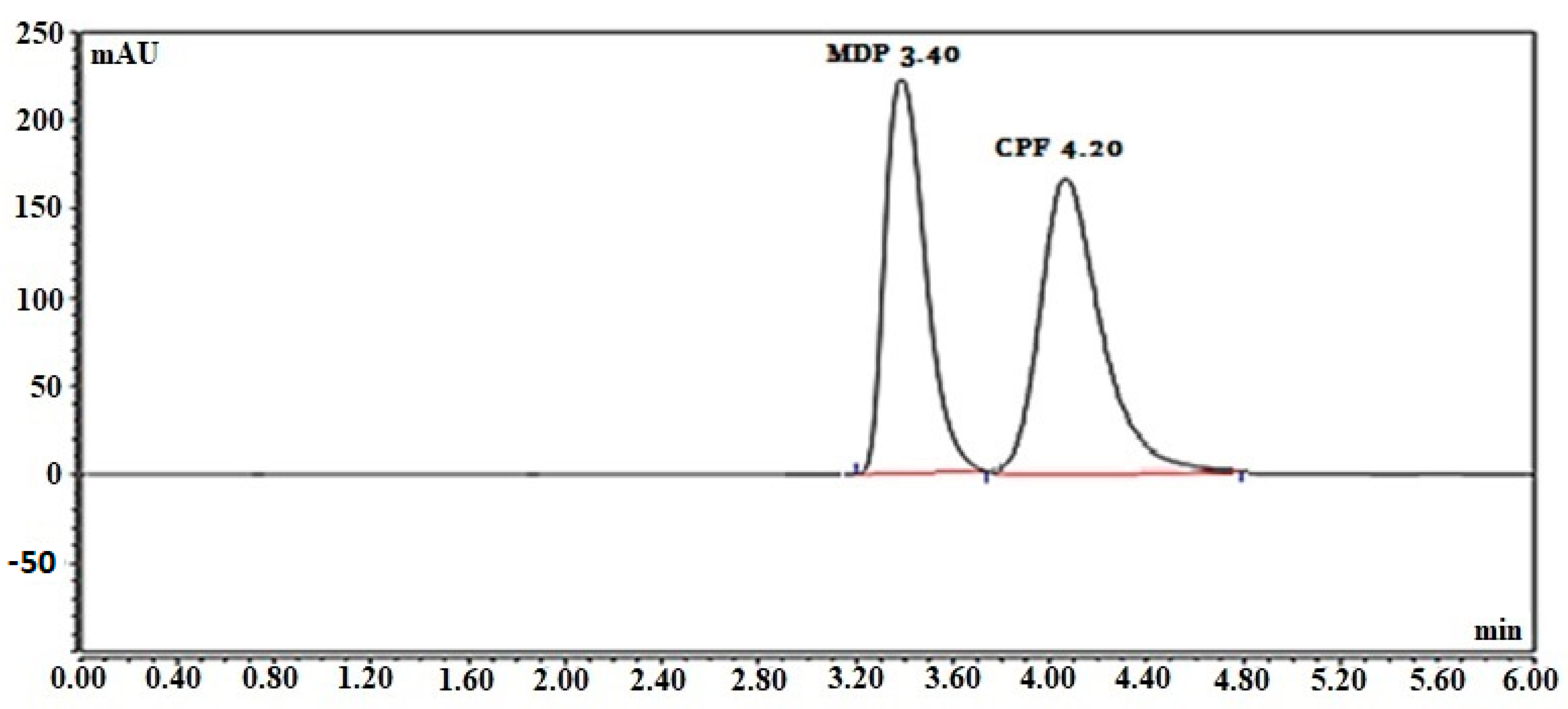
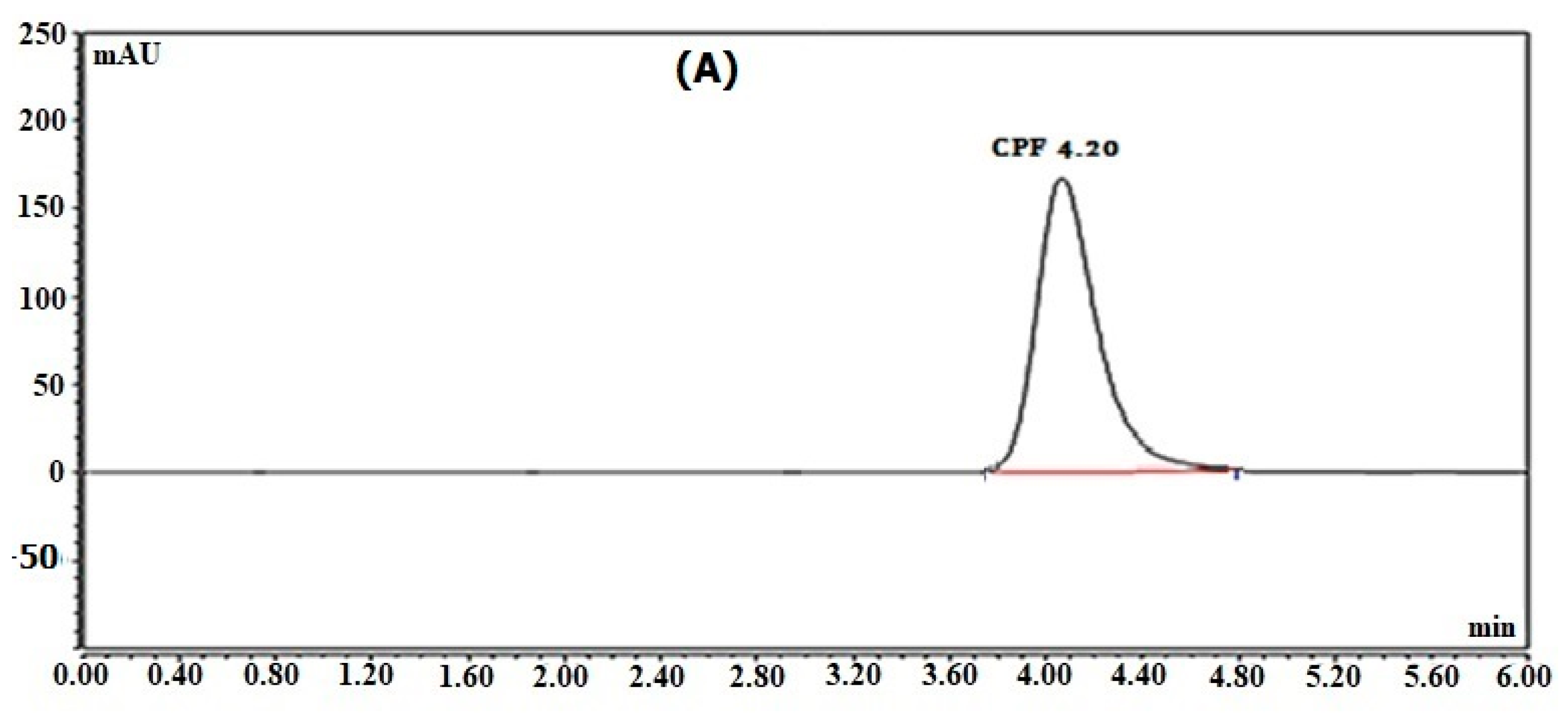
A Waters
TM column, 10 cm in length with a 2.1 mm internal diameter packed with 1.7 µm C18, was used as a stationary phase, and a developing system made up of potassium dihydrogen phosphate buffer:acetonitrile (7:3,
v/
v) produced the optimum separation pattern. At a flow rate of 0.4 mL/min, O-phosphoric acid was utilized to bring the pH of the mobile phase to 3.0. According to
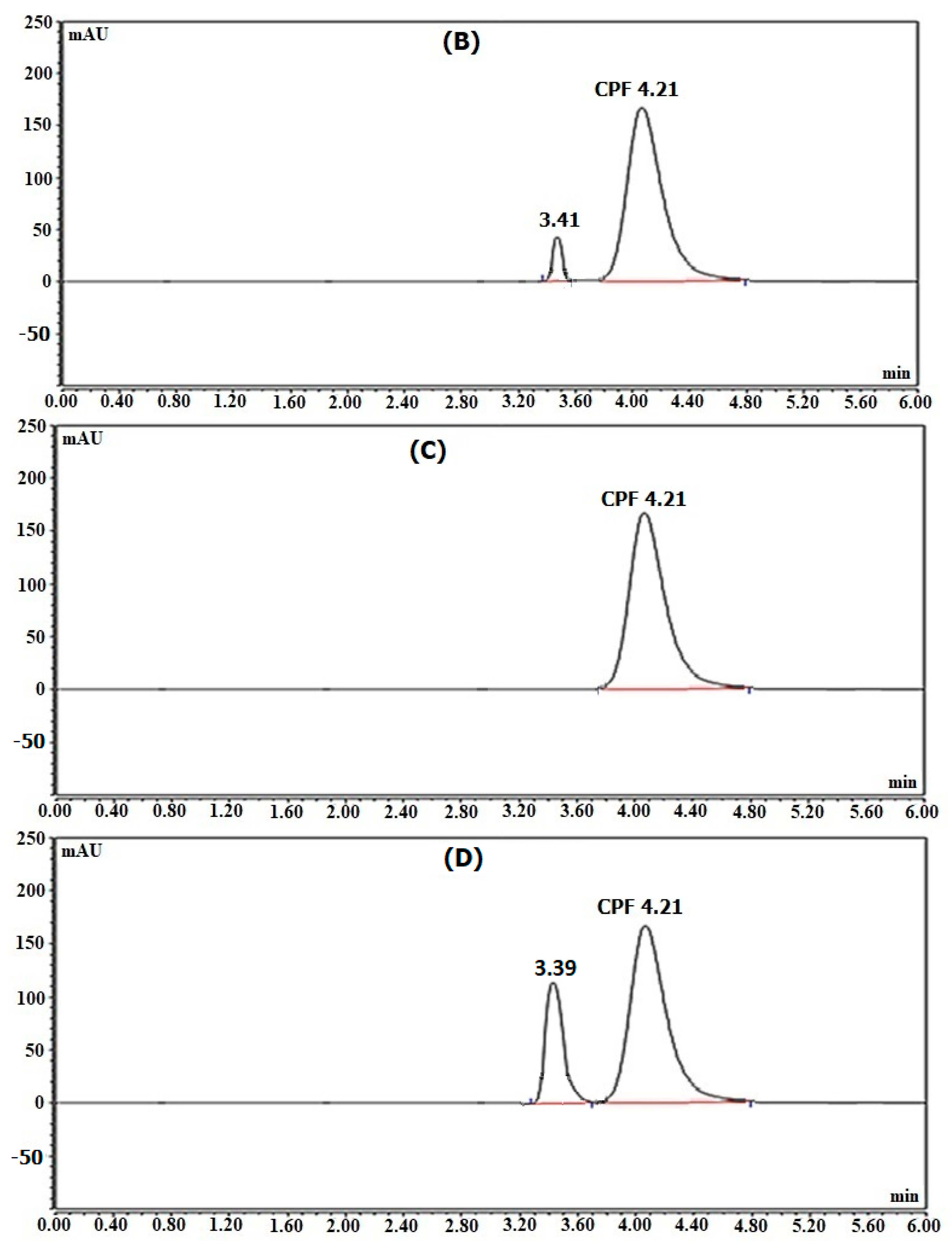
Figure 3, the retention times were 4.20 min for CPF and 3.40 min for MDP.
3.2. Method Validation
This study was conducted in accordance with the ICH-Q2B guidelines [
29]. The linearity of the suggested approaches was verified by plotting the peak area and CPF concentration in the concentration range of 0.5 to 50 g/mL. The regression equations were calculated to be
where PA is the peak area, C is the concentration (µg/ mL), and r is the correlation coefficient.
The assay accuracy was evaluated by calculating the recovery percentage after analyzing three CPF concentrations three times. The results presented in
Table 1 confirm the excellent accuracy of the method with good repeatability, as indicated by the small relative standard deviation (RSD) values. The precision (intra- and inter-day) was evaluated, and the results are given in
Table 1, confirming excellent precision. By slightly altering the assay conditions for either the mobile phase composition and pH (UPLC method) or the elution liquid composition and BGE pH (CZE method), the robustness of the methods was further assessed. The findings show that these minor variations had no discernible effect on the cited techniques. The calculated LOD and LOQ values indicate the methodologies’ sensitivity.
3.3. Method Application
3.3.1. Determination of CPF in the Presence of Its Main Photo-Degradation Product (MDP)
The selectivity of the mentioned methods for determining CPF in the presence of various proportions of its major photo-degradant (10–90%) was examined. In order to achieve this goal, the detailed CZE and UPLC procedures were applied to handle several laboratory-prepared mixtures with varying ratios of CPF and its primary photo-degradant (MDP). The assay results of
Table 2 show that the indicated procedures can quantify CPF in the presence of up to 90% of its principal photo-degradant (MDP).
3.3.2. Determination of CPF in Its Dosage Forms (Ciprobay® Tablets and Cipro® I.V. Solution)
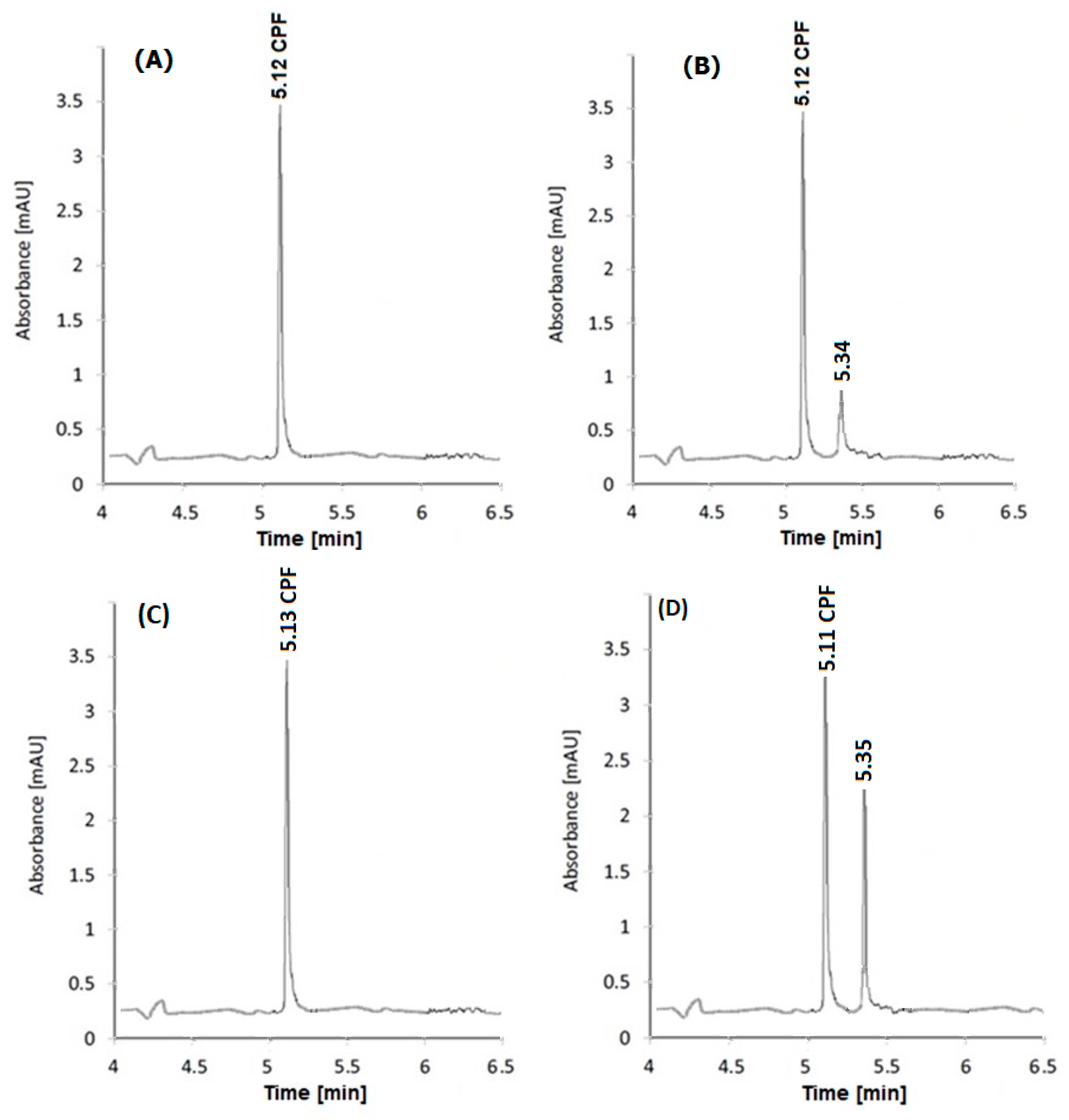
The specified techniques were used to determine the studied drug in Ciprobay
® tablets and Cipro
® I.V. solution (UV-irradiated and non-irradiated). The electropherograms and chromatograms are presented in
Figure 4 and
Figure 5, respectively. It can be noticed from the illustrated figures that the non-irradiated samples showed almost no photo-degradation, but the UV-irradiated samples exhibited probable photo-degradation. The photo-degradation of the UV-irradiated Cipro
® I.V. solution was greater than that occurring in the UV-irradiated tablet form. This finding was expected due to the higher liability of the solution form for degradation. Additionally, this finding matched the acidic pH (3.5–4.6) of the Cipro
® I.V. solution, as acidic medium accelerates the photo-degradation process [
5].
Table 3 presents the findings of the dosage forms’ analysis. The acquired results demonstrate that the suggested methods can be successfully employed for the CPF assay in its dosage forms. The noticed recovery % reduction in the UV-irradiated Cipro
® I.V. solution may be attributed to photo-degradation.
3.3.3. Statistical Comparison with the USP Method
Table 4 presents a statistical comparison of the results of the analysis of CPF using the official USP technique [
30] and those for its assay in the pure form using the stated approaches. The calculated t and F values are lower than the tabulated ones, showing that there is no appreciable difference in accuracy and precision between the proposed techniques and the official one.
4. Discussion
To assure their safety, efficacy, and quality, intact pharmaceuticals should be quantified in the presence of their degradation products. From this perspective, CE and UPLC can be considered to be amazing analytical tools to attain this goal.
The current work demonstrates that, in order to ensure the safety and efficacy of the investigated medication, intact CPF can be measured in the presence of its primary photo-degradation product by utilizing sensitive, fast, and precise CZE and UPLC methods.
The methods were developed to obtain the best separation pattern and greatest peak resolution. The BGE borate concentration and pH were tuned and changed to attain the lowest migration time and highest peak area due to the significant influences they have on the EOF. The optimal separation pattern for CPF and its primary photo-degradant (MDP) was shown to be obtained with the adjusted borate content (40 mM) and BGE pH (8.5). On the other hand, the optimal UPLC separation pattern for CPF and its photo-degradant was confirmed after optimizing the UPLC parameters to obtain the best stationary phase/mobile phase match.
The resolution comparison between CZE and UPLC revealed a better CZE outcome, as shown by the resolution factor (R
S) values in
Table 1. This can be attributed to the flat EOF when compared to the rounded laminar flow profile in pressure-driven systems, such as liquid chromatography.
All of the parameters chosen for the techniques’ validation are listed on a thorough validation sheet. (
Table 1). The outstanding precision and accuracy values of the procedures are highlighted. The validation sheet also notes the remarkable robustness of the suggested procedures, which was demonstrated by slight modifications in the composition of the elution liquid and the pH of the BGE (CZE method), and the mobile phase composition and pH (UPLC method) had no appreciable impacts on the cited techniques’ performance. The excellent technique sensitivity was made clear by the linearity range, detection, and quantification limits.
The described methods can be successfully used to determine CPF in the presence of up to 90% of its principal photo-degradation product, making them very helpful tools for stability research. Additionally, the provided techniques can be used effectively to determine CPF in its dosage forms (UV-non-irradiated and UV-irradiated Ciprobay
® tablets and Cipro
® I.V. solution). From the given electropherograms and chromatograms, the higher stability of the solid dosage form and the greater susceptibility of the I.V. solution form to the photo-degradation process were confirmed. This can be attributed to the higher liability of the aqueous solution form for photo-degradation. Moreover, the Cipro
® I.V. solution is acidic (pH 3.5–4.6), which facilitates the photo-degradation process [
5].
The CZE and UPLC methods were statistically compared with the official USP method for the determination of CPF, and no discernible differences in terms of accuracy and precision were found.
Regarding the comparison between the developed techniques, CZE and UPLC are considered to be the most powerful separation and quantitative analytical techniques. A comparison of the two is not an easy task due to the different advantages and disadvantages of each technique. Despite the differences in separation mechanisms, in many cases, the separation of given groups of analytes with good efficiency is possible with both techniques. In such situations, choosing the best overall technique is not easy and requires an in-depth and critical look. CZE is better than UPLC from the point of environmental friendliness. This finding is based on the amount of waste generated, which is higher in the case of UPLC. The toxicity levels of the used reagents and the energy consumption are quite similar, while the UPLC is more favorable in terms of the additional hazards. CZE has two hazards, which are the use of high voltage and the risk of capillary injury. On the other hand, UPLC has one hazard, which is the contact with solvent vapors. Regarding the point of cost effectiveness, UPLC has lower cost effectiveness, which comes from the need to purchase larger volumes of high purity organic solvents, which are used as the mobile phase. Additionally, the lower amount of sample needed for injection is an intrinsic specification advantage of CZE. On the other hand, UPLC is more reliable due to the lower risk of technical problems, as capillary breakage and current instability are quite common in CZE.
At the end, the recommended technique, either CZE or UPLC, depends on the needs and conditions of the assay. CZE will be the technique of choice if the cost effectiveness and the environmental friendliness are of high priority. On the other hand, UPLC will be the preferred technique if the assay reliability is the major need.
5. Conclusions
From a comparative point of view, the suggested methods (CZE and UPLC) determined the studied drug in a sensitive and accurate way, but the resolution of the separated analytes was superior in the CZE method due to the flat EOF compared with the laminar flow in pressure-aided techniques, as is the case with UPLC. On the other hand, the UPLC method does not require the use of high voltages, as in the CZE method.
The selection of a suitable technique to be applied for a certain situation depends mainly on the needs of the cited assay. CZE is more favored if cost effectiveness and eco-friendliness are the main targets. On the other side, UPLC is the preferred technique when reliability is the main goal.
The exposure of CPF dosage forms (tablets and I.V. solution) may trigger photo-degradation, so a strict warning should be placed on the dosage forms to avoid exposure to UV-light and high temperatures.
Our study is unique in that it is the first to quantify the researched FQ in the presence of its main photo-degradation product (MDP) using sensitive, accurate, and precise CZE and UPLC methods. The cited methods can be applied successfully as stability-indicating tools to ensure the safety and efficacy of the studied drug in either bulk or dosage forms.
Author Contributions
Conceptualization, S.A.A.-G. and A.B.A.; Data curation, S.A.A.-G. and A.B.A.; Formal analysis, S.A.A.-G.; Investigation, S.A.A.-G. and A.B.A.; Methodology, S.A.A.-G.; Resources, S.A.A.-G. and A.B.A.; Software, S.A.A.-G. and A.B.A.; Supervision, S.A.A.-G.; Validation, S.A.A.-G.; Visualization, S.A.A.-G.; Writing—Original draft, S.A.A.-G.; Writing—Review and Editing, S.A.A.-G. and A.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported via funding from the Prince Sattam Bin Abdulaziz University, project number (PSAU/2023/R/1444).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This study is supported via funding from the Prince Sattam Bin Abdulaziz University, project number (PSAU/2023/R/1444).
Conflicts of Interest
The authors declare that they have no competing interest in this manuscript.
Sample Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; The Pharmaceutical Press: London, UK, 2009; pp. 302–340. [Google Scholar]
- Da Silva, A.D.; De Almeida, M.V.; De Souza, M.V.; Couri, M.R. Biological activity and synthetic methodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr. Med. Chem. 2003, 10, 21–39. [Google Scholar] [CrossRef]
- Hayashi, N.; Nakata, Y.; Yazaki, A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob. Agents Chemother. 2004, 48, 799–803. [Google Scholar] [CrossRef]
- Maia, A.S.; Ribeiro, A.R.; Amorim, C.L.; Barreiro, J.C.; Cass, Q.B.; Castro, P.M.L.; Tiritan, M.E. Degradation of fluoroquinolone antibiotics and identification of metabolites/transformation products by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1333, 87–98. [Google Scholar] [CrossRef]
- Torniainen, K.; Mattinen, J.; Askolin, C.P.; Tammilehto, S. Structure elucidation of a photodegradation product of ciprofloxacin. J. Pharm. Biomed. Anal. 1997, 15, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Pandey, P.; Tiwari, G.; Tiwari, R.; Rai, A.K. FTIR spectroscopy: A tool for quantitative analysis of ciprofloxacin in tablets. Indian J. Pharm. Sci. 2012, 74, 86–90. [Google Scholar] [CrossRef]
- Cazedey, E.C.L.; Salgado, H.R.N. Spectrophotometric determination of ciprofloxacin hydrochloride in ophthalmic solution. Adv. Anal. Chem. 2012, 2, 74–79. [Google Scholar]
- Qassim, A.W. Spectrophotometric Determination of Ciprofloxacin Hydrochloride in Pharmaceutical Formulation Ciproxin. Int. J. Adv. Sci. Tech. Res. 2015, 3, 135–146. [Google Scholar]
- Tsanaktsidou, E.; Markopoulou, C.K.; Tzanavaras, P.D.; Zacharis, C.K. Homogeneous liquid phase microextraction using hydrophilic media for the determination of fluoroquinolones in human urine using HPLC-FLD. Microchem. J. 2022, 172, 106906. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Shao, D.; Li, J. Simultaneous Determination of Nine Quinolones in Pure Milk Using PFSPE-HPLC-MS/MS with PS-PAN Nanofibers as a Sorbent. Foods 2022, 11, 1843. [Google Scholar] [CrossRef]
- Maddeppungeng, N.M.; Mir, M.; Raihan, M.; Wahyudin, E.; Asma, N.; Layadi, P.; Elim, D.; Permana, A.D. Application of quality by design approach in HPLC-UV validation of ciprofloxacin in porcine ocular tissue and simulated ocular fluid for quantification in an ex vivo ocular kinetic study. Chem. Data Collect. 2022, 41, 100900. [Google Scholar] [CrossRef]
- Haidari-Khoshkelat, L.; Raoof, J.B.; Ghani, M.; Ojani, R. Combination of in-situ electro synthesized Zn–Al-LDH@ pencil graphite fiber and three phase hollow fiber LPME for microextraction of some antibiotics in urine samples and quantification via HPLC-UV. Anal. Chim. Acta 2022, 1235, 340532. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Wahba, I.A.; Saad, S.S.; Ramadan, N.K. Two validated chromatographic methods for determination of ciprofloxacin HCl, one of its specified impurities and fluocinolone acetonide in newly approved otic solution. J. Chromatogr. Sci. 2022, 60, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Kośka, I.; Purgat, K.; Kubalczyk, P. Simple, fast and reliable CE method for simultaneous determination of ciprofloxacin and ofloxacin in human urine. Sci. Rep. 2022, 12, 7729. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; Altharawi, A. Simultaneous Quantification of Some Fluoroquinolone Residues in Real Wastewater Effluents Using CZE. Separations 2023, 10, 292. [Google Scholar] [CrossRef]
- Hosseini, A.; Sohouli, E.; Gholami, M.; Sobhani-Nasab, A.; Mirhosseini, S.A. Electrochemical determination of ciprofloxacin using glassy carbon electrode modified with CoFe2o4-MWCNT. Anal. Bioanal. Electrochem. 2019, 11, 996–1008. [Google Scholar]
- Azriouil, M.; Matrouf, M.; Ettadili, F.E.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Recent trends on electrochemical determination of antibiotic Ciprofloxacin in biological fluids, pharmaceutical formulations, environmental resources and foodstuffs: Direct and indirect approaches. Food Chem. Toxicol. 2022, 168, 113378. [Google Scholar] [CrossRef]
- Pollap, A.; Baran, K.; Kuszewska, N.; Kochana, J. Electrochemical sensing of ciprofloxacin and paracetamol in environmental water using titanium sol based sensor. J. Electroanal. Chem. 2020, 878, 114574. [Google Scholar] [CrossRef]
- Attia, K.A.M.; Nassar, M.W.I.; El-Zeiny, M.B.; Serag, A. Stability-indicating methods for the analysis of ciprofloxacin in the presence of its acid induced degradation product: A comparative study. Spectrochim. Acta A 2016, 159, 219–222. [Google Scholar] [CrossRef]
- Aksoy, B.; Küçükgüzel, İ.; Rollas, S. Development and Validation of a Stability-Indicating HPLC Method for Determination of Ciprofloxacin Hydrochloride and its Related Compounds in Film-Coated Tablets. Chromatographia 2007, 66, 57–63. [Google Scholar] [CrossRef]
- Bushra, M.U.; Huda, M.N.; Mostafa, M.; Sultan, M.Z.; Rahman, A. Study of forced degradation of ciprofloxacin HCl indicating stability using RP-HPLC method. Der Pharma Chem. 2013, 5, 132–137. [Google Scholar]
- Jansari, S.K.; Patel, N.B.; Patel, P.R.; Patel, N.N.; Desai, H.T. Development and validation of stability indicating method for simultaneous estimation of Ciprofloxacin HCl and Tinidazole using RP-UPLC method. IOSR J. Pharm. 2013, 2, 12–19. [Google Scholar] [CrossRef]
- Vaghela, B.K.; Rao, S.S. A novel validated stability indicating high performance liquid chromatographic method for estimation of degradation behavior of ciprofloxacin and tinidazole in solid oral dosage. J. Pharm. Bioallied. Sci. 2013, 5, 298–308. [Google Scholar] [CrossRef]
- Razzaq, S.N.; Ashfaq, M.; Mariam, I.; Khan, I.U.; Razzaq, S.S.; Mustafa, G. Stability Indicating RP-HPLC Method for Simultaneous Determination of Ciprofloxacin and Dexamethasone in Binary Combination. J. Chil. Chem. Soc. 2017, 62, 3572–3577. [Google Scholar] [CrossRef]
- El-Zaher, A.A.; Elkady, E.F.; El-Hakim, M.M.; Mandoor, A. Stability Indicating HPLC Method for the Simultaneous Determination of Ciprofloxacin Hydrochloride and Metronidazole in the Presence of Ciprofloxacin Acid Degradation Product. Asian J. Biochem. Pharm. Res. (AJBPR) 2015, 1, 5–17. [Google Scholar]
- Ladhani, S.; Gransden, W. Increasing antibiotic resistance among urinary tract isolates. Arch. Dis. Child. 2003, 88, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Egunova, O.R.; Reshetnikova, I.S.; Kazimirova, K.O.; Shtykov, S.N. Magnetic solid-phase extraction and fluorimetric determination of some fluoroquinolones. J. Anal. Chem. 2020, 75, 24–33. [Google Scholar] [CrossRef]
- Lucie, N.; Ludmila, M.; Petr, S. Advantages of application of UPLC in pharmaceutical analysis. Talanta 2006, 68, 908–918. [Google Scholar]
- Teasdale, A.; Elder, D.; Nims, R.W. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (B); PharmaLogica, Inc.: Charlotte, NC, USA, 2005. [Google Scholar]
- United States Pharmacopeia and the National Formulary (USP 35-NF 30); The United States Pharmacopeial Convention: Rockville, MD, USA, 2012.
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).