Abstract
Water organic pollution has become a major issue. A large number of people suffer from the decline in water quality. In addition, polluted water can lead to health problems or excessive deaths. In this regard, an increasingly important method for efficient water treatment is electrocoagulation (EC), the technology that encompasses a small equipment size combined with a simple operation compared to other water treatment methods. The importance of EC is especially accentuated by the recent decarbonization efforts due to the increasing availability of renewable electricity systems. This review provides an overview of the most recent developments in EC technology as it pertains to wastewater treatment. The EC is preferred for organic wastewater treatment over other traditional treatment methods due to its easy setup and low material costs. Moreover, the EC is very powerful in destabilizing organic impurities by charge neutralization and then coagulating to form flocs. In addition, EC has shown high efficiency not only in removing various organic pollutants but also in emerging persistent contaminants, such as microplastics. For these reasons, the EC mechanisms and related functional modalities are reviewed, as well as extensive details are provided on the diversity of the removed contaminants. Overall, this review provides significant new knowledge of interest for environmental chemical researchers in particular and engineers in general on the details of the EC technology for wastewater treatment and water purification.
1. Introduction
Water resource availability and quality-related problems have become very significant [1,2]. These problems are a result of the population increase, which spurred the industry growth necessary to develop and sustain the global economy [3,4]. As a result, more contaminants, including but not limited to heavy metals and complex organic molecules, have experienced a high influx into aquatic resources [2,5]. Indeed, the need for clean water has been intertwined with the increase in the population [3,6]. The world population increased from 7 to 7.84 billion from 2011 to 2021 [7]. It was reported that the population will reach 9.69 billion in 2050 [8]. However, Figure 1 shows that already a large portion of the population across the globe lacks access to clean drinking water. An estimated ~1.0 billion still have no access to safe drinking water [9]. On the other hand, in South Africa alone, there are ~7589 megaliters per day of wastewater produced [10]. Finally, the agricultural sector uses around 75% of the freshwater, while freshwater forms less than 1% of the water resources on the earth [11]. The need and the opportunities abound not only to treat the diverse streams of wastewater to remove harmful contaminants, but also to recover elements for reuse and recycling for the circular economy, such as nitrogen, phosphorus, and noble metals, including gold, silver, or palladium.
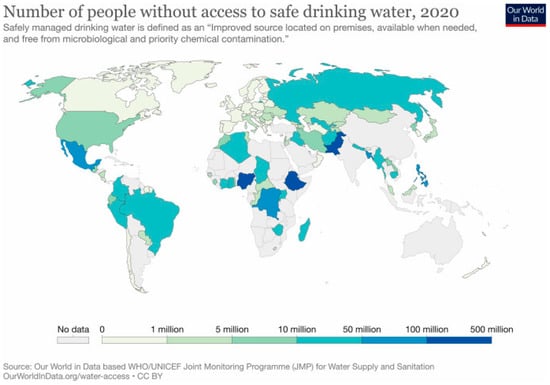
Figure 1.
The number of individuals who lack access to clean water (2020) according to the WHO/UNICEF, Joint Monitoring Program for Water Supply and Sanitation. Reprinted from Our World in Data. (Interactive on the website (https://ourworldindata.org/water-access, accessed on 17 April 2023)).
The United Nations Environmental Program (UNEP) reported that more than 80% of the wastewater generated is still released untreated [12]. This constitutes around 2 million tons of wastewater released every day from industry, sewage, and agriculture [13]. Therefore, the discharge of wastewater is more than six times the freshwater available around the world [13]; hence, water pollution is a major problem. In this regard, classifying types of pollution is critical in choosing the right treatment method. One classification method categorizes water contaminants according to their sources into nine types, as shown in Figure 2 [13].
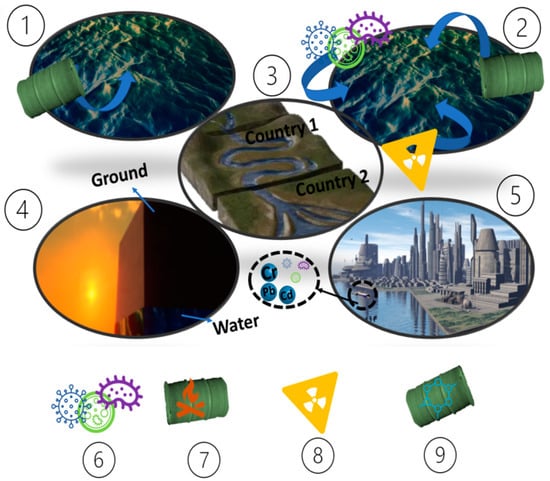
Figure 2.
Different pollution types: (1) point pollution source, (2) non-point pollution source, (3) transboundary pollution source, (4) groundwater pollution source, (5) surface water pollution source, (6) pathogenic pollution source, (7) thermal pollution source, (8) radioactive pollution source, (9) chemical pollution source.
Namely:
- (1)
- Point pollution sources, with this type of pollution originating from a single location [14]; if a specific location is found, then quantitative measurements can be made to perform the numerical description of pollution [15]. Therefore, treating the origin point source may solve the related pollution.
- (2)
- Non-point pollution sources with more than one pollution origin source. This is a complicated pollution source type and not easy to be described quantitatively [16]. The non-point source might contain different types of pollution, such as pathogens and industrial dyes. Therefore, all these sources of pollution should be treated.
- (3)
- Transboundary pollution source type, which is located in the rivers between countries [17]. With the help of the rivers, contaminants are crossing from one country to another.
- (4)
- Groundwater pollution source deep under the earth’s surface. The main problem with this type comes from it being invisible and inaccessible to treat [18]. In addition, water of this type can be naturally contaminated and carry heavy metals, such as iron, arsenic, and manganese. Therefore, it can be identified when it is too late.
- (5)
- Surface water pollution source, which is mainly caused by industrial waste [19]. These wastes are typically concentrated on the surface waters. Hence, the quality of the resources is affected.
- (6)
- Pathogenic pollution source, which contains microbial types, leading to dangerous diseases, such as salmonella [20].
- (7)
- Thermal pollution source, which generates pollution affecting the temperature of water, leading to changes in its physical characteristics [21]. In this case, some biota could be affected because not all can acclimate to the temperature variation.
- (8)
- Radioactive pollution sources are mainly located in the nuclear industries [22]. This source directly produces radiation from radioactive elements, such as uranium. In addition, the radiation elements might be stored in the marine organisms to be transported to humans, leading to cancerous diseases.
- (9)
- Chemical pollution sources that mainly exist in industrial activities and wastes. These chemicals contain toxic compounds located in water resources [23].
Additional classification methods of the contaminants can be grouped into three types of contaminants, namely, Inorganic Contaminants (IOCs) (including arsenic and nitrate), Organic Contaminants (OCs) comprised of Volatile Organic Contaminants (VOCs), and Synthetic Organic Contaminants (SOCs), as shown in Figure 3, together with some representative examples presented.
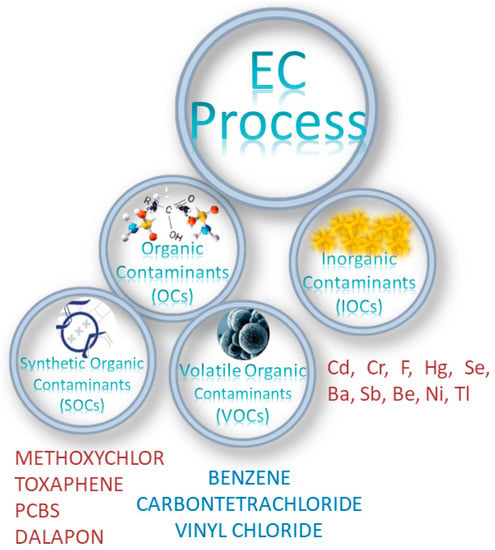
Figure 3.
Schematic diagram showing the classification of contaminants—(IOCs), (VOCs), and (SOCs)—with some examples presented.
The Environmental Protection Agency (EPA), in the National Primary Drinking Water Regulations (NPDWR), defined primary standards for the public water system to estimate the maximum allowed level and the goal of organic contaminants in drinking water, as shown in Table 1.

Table 1.
The Maximum Contaminant Level Goal (MCLG) and the Maximum Contaminant Level (MCL) of organic contaminants for drinking water according to NPDWR [24].
Numerous biological, physical, and chemical technologies are used to treat wastewater [25]. The biological methods are insufficient due to their long start-up time and the need for several post-treatments to completely remove the biological contaminants [26,27]. The physical methods do not always reach the discharge limit, as well as incur high costs [28]. On the other hand, using a chemical technique might be ineffective, and it could cause secondary pollution [29]. Recently, more effective technologies have been proposed and investigated, such as ozonation, UV H2O2, persulfate process, and solar TiO2, and they showed high effectiveness for organic compound decomposition at lower cost [30].
An emerging method to treat both drinking and wastewater is electrocoagulation (EC), which was primarily developed to be used for wastewater treatment [31]. Figure 4 shows the increased number of publications on EC for contaminant removal from wastewater. It is widely applied, due to its effectiveness, toward organic and inorganic contaminants alike [32]. It has been used to treat pharmaceutical, petroleum, textile, pulp and paper, dairy, and municipal wastewater [33,34,35,36]. It also has a high ability to treat drinking water [37]. On the other hand, EC has its disadvantages, such as high energy consumption, the passivation of electrodes, and the possibility of generating secondary contaminants [38]. Therefore, EC might be combined with other treatment methods to be more efficient and to improve its economic efficiency [39]. EC has been proposed to be more effective than chemical coagulation (CC) when it comes to both cost and efficiency [37,40]. The CC process requires chemicals to purify wastewater. On the other hand, EC does not need additional chemicals, which decreases secondary pollution and simplifies the overall operation. Thus, EC tends to be more attractive than chemical approaches.
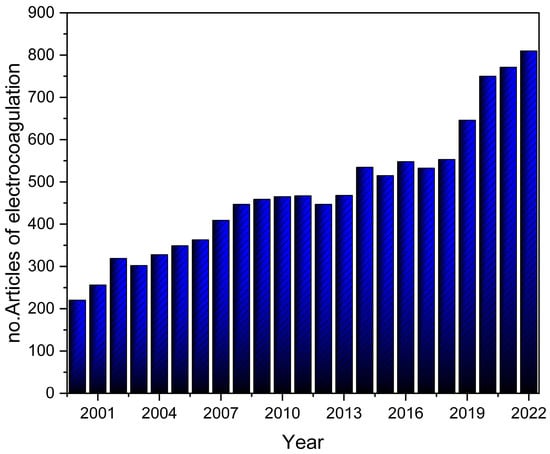
Figure 4.
The number of published articles containing the word “electrocoagulation” in the article title, abstract, or keywords from 2000 to 2022, as obtained from Scopus.
In its simplest embodiment, EC is comprised of an electrolytic cell with two sacrificial electrodes, an anode and a cathode, for the oxidation and reduction processes, respectively, as shown in Figure 5 [35,41,42].
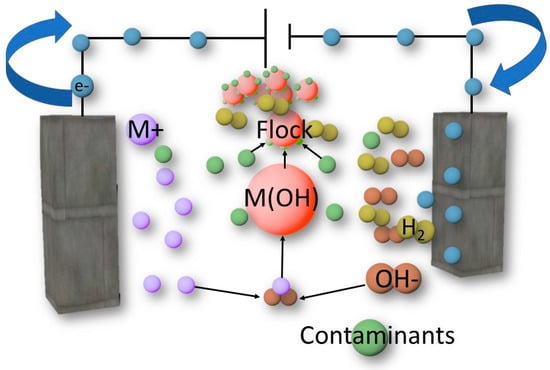
Figure 5.
The EC mechanism of contaminant removal.
The electric current passes through the electrodes, and the anode loses electrons, while the cathode gains them. These electrons can oxidize the contaminant and perform water hydrolysis (OH- formation) [43]. On the other side, at the anode, the loss of electrons causes metal ion formation [41,44]. Therefore, metal hydroxides are created, which interact with contaminants to form flocks containing metal hydroxide and contaminants adsorbed on the surface [35,36]. These flocks float on the surface of the wastewater [45]. In this regard, the EC mechanism can be divided into seven steps. These steps include (1) the formation of cations at the anode and (2) hydrolysis by the cathode, resulting in the formation of hydroxyl ions. In addition, the interaction between cations and hydroxyl ions leads to (3) the formation of metal hydroxide. Further, (4) the pollutants are oxidized and (5) neutralized by the metal hydroxide. The charge neutralization results in (6) the adsorption of destabilized contaminants on the surface of metal hydroxide, leading to the formation of flocks. Finally, (7) the H2 gas generated at the cathode causes the flocks to rise to the surface of the water. These phenomena of charge neutralization, coagulation, and flotation are all important in the degradation process.
EC is a promising technique due to its wide range of advantages. Mousa et al. outlined most of the EC advantages, including no secondary contaminants because there are no external chemicals added to the process. The pollutants can be removed easily as they float up to the solution surface as a result of the continuous production of gas bubbles from the cathode. Moreover, it is based on a simple experimental setup [46].
Numerous reviews have discussed the EC and its efficiency in the last few years. For instance, Shokri et al. focused on EC application in the oil industry and investigated the parameters affecting the process [45]. Liu et al. have performed a systematic review of the removal of single-type contaminant microplastics (MPs) from wastewater using the EC [47]. The source of MPs, toxicity, and their occurrence was discussed. Das et al. summarized modern EC advancements and parameters affecting the EC [48]. The review provided an overview of the EC and then discussed its mechanism [48]. Mousa et al. reviewed the EC for wastewater treatment [46]. They discussed the theory and history of EC, its merits and demerits, applications, and challenges [46]. Zaied et al. studied the pharmaceutical removal, operating parameters, and development of EC technology [33]. Butler et al. made a large study on the EC process and focused on optimization, instruments, and various wastewater sources [49]. Boinapally et al. took up many points in their work, including mechanisms, applications, operations, and challenges [50]. Y. Liu et al. reported that EC has been adopted because of the merits of increased efficiency and reduced energy consumption [51]. They have reported previous studies removing PFOA from wastewater with a removal efficiency of 99.7%, and 90% using EC for Zn and Fe electrodes, respectively [51]. Patel et al. treated the wastewater using the EC process from color and turbidity, with a removal efficiency of 71.91% and 100%, respectively [52].
Despite the numerous advantages of using EC for the removal of pollutants from water, there are still some gaps in the studies of EC for the removal of certain contaminants. For instance, there is a lack of research on the EC of some specific compounds, such as methoxychlor, picloram, toxaphene, lindane, benzylpenicillin, nafcillin, and oxacillin. In addition, some compounds, such as ampicillin, need more investigations to optimize the EC process and reach high efficiency, which will be discussed in the current review. Moreover, the EC efficiency for the removal of some categories of compounds, such as the second generations of cephalosporin, most glucocorticoids, and anticonvulsants, is not yet fully understood. Thus, further research is needed to optimize the EC process for the removal of various pollutants, especially for those categories of compounds that are not well-studied.
The present work expands upon the available studies, encompassing a comprehensive list of contaminants, while differentiating between their types, as well as between contaminant removal efficiencies, optimum conditions, and resource recovery. It also addresses other emerging organic contaminants, such as those related to agricultural runoff. Furthermore, the pharmaceutics and microplastics removal has been addressed. In addition, the challenges have been discussed, with some suggestions to face these challenges.
2. Organic Contaminants Removal
Organic contaminants are toxic substances containing carbon atoms. This organic pollution is mostly produced by industrial processes. With increasing industrialization, it is expected that organic contaminants will only increase in the environment. Organic contaminants do not naturally degrade or decompose in the aquatic ecosystem. In addition, they are hard to degrade using conventional treatment methods. That leads to the persistence of organic contaminants in water. These toxic compounds can lead to numerous environmental problems. Organic pollution may be transported to the animals by bioaccumulation [53], harming aquatic organisms and destroying the aquatic system [54]. Further, human health can be affected by organic contaminants due to related diseases caused by mutagenic, carcinogenic, and teratogenic organic contaminants [55]. Therefore, the organic pollution problem is considered one of the most important that can be addressed via EC.
The organic contaminants are categorized into nine categories, including perfluoroalkyl substances (PFAS), perfluorooctanoic acid (PFOA), microplastics, pharmaceuticals, etc., as shown in Figure 6a,b.
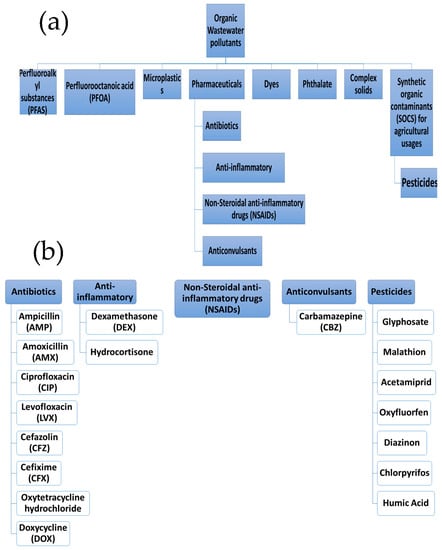
Figure 6.
Classification of organic contaminants that could be removed using the EC from wastewater: (a) the main pollution groups, (b) the pollution subgroups.
2.1. Perfluoroalkyl Substances (PFAS)
In the late 1940s, synthetic PFAS, a class of organic molecules with a carbon backbone completely encircled by fluorine atoms, were developed [56,57]. Because of the extraordinarily strong (115 kcal/mol) C-F bonds, PFAS have exceptional physical and chemical stability [56,57]. PFAS have been produced in large quantities and effectively used as surface protectors and surfactants in a wide range of industrial and domestic productions, including mist suppressants in the firefighting foams, food packaging, and chrome plating industry, in addition to surfactants in textile products [56,57]. As a result, PFAS are now widely distributed throughout a variety of biological and abiotic matrices across the world [56].
Particularly, it has been acknowledged that the extensive presence of PFAS inside the aquatic system is a critical developing problem [56]. Diverse treatment methods for removing PFAS have received attention in recent years. To remove PFASs from wastewater, charcoal or electrochemical resins have been utilized. However, these methods have several drawbacks, including low capacity, quick breakthrough, and challenging regeneration of spent sorbent [57]. In addition, sorption methods merely transfer contaminants without destroying their molecular structure, and off-site burning of the used sorbent is still required, which may provide additional risks [57]. Furthermore, it is technically and economically unfavorable to use these methods to treat significant amounts of diluted PFAS wastewater [57]. Short-chain PFAS may be degraded using the same processes as long-chain PFAS, such as oxidation/reduction, electrochemical reaction, and photocatalysis. However, they may work better at larger PFAS concentrations and a slower rate [56].
EC was also applied to remove PFAS, as displayed in Table 2 [56]. In EC, charged cations (such as Zn2+, Al3+, and Fe3+) are generated at the sacrificial anode, while the polymeric hydroxyl complicated species are simultaneously created [57]. These species can effectively bind to certain contaminants to be removed from polluted water [57]. Due to its superior removal effectiveness, low energy consumption, and quick treatment time, periodically reversing electrocoagulation (PREC) has drawn a lot of interest to remove PFAS. PREC primarily created a variety of various cations before sacrificing the alternate anode to make complicated flocs [56]. Adsorption and condensation of flocs, which were then transported to the surface of the solution by floating by H2 and O2 generated during the PREC process, allowed for the purification of the dissolved contaminants. PREC with Al-Zn electrodes was utilized, and the highest removal rates for PFOS and PFOA separately in 10 min were 100% and 99.6%, respectively [56,57].
2.1.1. Perfluorooctanoic Acid (PFOA)
Due to the C-F bond that is present in perfluorooctanoic acids, it exhibits great surface activity and heat resistance, which makes it very resistant to being eliminated [58,59]. PFOA is durable, and it is applied in a large number of industries [58]. As a result, PFOA is present in industrial wastewater in high concentrations reaching 1650 mg/L [58]. It was also reported that PFOA is extensively present in watercourses, groundwater, animals, semen and breast milk samples, and humanoid blood [58,59]. Intake of PFOA could lead to several risky disorders, such as thyroid defect, gestational hypertension, and ulcerative colitis, and could cause cancers in the kidney and testicles [58]. Li et al. investigated the effect of adding ZnCl to the EC process [58]. (AL1050) was used as the electrode material, and the gap between the electrodes was ~1 cm, with a current of 1 A at 150 volts, and the initial concentration of PFOA was 1 mM [58]. The zinc chloride kept the pH from changing, which resulted in improving the elimination efficiency from 73.7% to 99% [58]. Singh et al. utilized a combination of several methods for the elimination of perfluorooctanoic acid [59]. In particular, the EC was carried out at a pH of 3.6, electrodes made of Fe, an initial concentration of 100 mg/L of PFOA, and a current density of 78.34 A/m2 [59]. More than 90% elimination efficiency was achieved within 1 hour of operation [59]. Kim et al. investigated the effect of the EC process on the elimination of PFOA. They used Fe as the electrode material, and they maintained the gap between the electrodes at 2 cm [60]. The EC procedure accomplished 65% of fluorine removal after mineralizing PFOA, with 60% elimination of organic carbon (TOC) within 6 h [60]. Mu et al. used EC with an air-cathode, with the goal of PFOA and PFOS to be removed from wastewater with minimal energy use. (PFOA) and perfluorooctane sulfonate (PFOS) removed from an aqueous solution by Zn anode showed encouraging results of about 89.0 ± 2.8% and 69–81%, respectively [61]. EC studies to remove PFOA are summarized in Table 2.
2.1.2. Perflourodecanoic Acid (PFDA)
Perflourodecanoic Acid (PFDA) is a long-chain PFAS. Its widespread use in firefighting foams, paper coating, textiles, kitchenware, cosmetics, polymer synthesis, and polishes has led to significant detection of it in the aqueous matrix [62]. The cytotoxicity of PFDA is widely reported by both humans and wildlife by causing immunotoxicity, hepatotoxicity, and developmental toxicity [62]. The need to address looming dangers to the environment and public health has created a strong incentive to create effective methods for removing PFAS from polluted water [62]. N. Nippatlapalli et al. removed PFDA from wastewater using the EC process, with high efficiency equal to 99.96% using Fe-Al electrodes [63]. The optimum pH was 7, and the current density was 37 mA/m2 [62].
2.1.3. Perfluorooctane Sulfonate (PFOS)
It has been proven that PFOS is harmful to humans [61]. Due to its great solubility in water and extremely stable chemical structure, many individuals are still in danger of exposure to this chemical, even though it is no longer manufactured or utilized in many countries [61]. The outflow from wastewater treatment facilities may be a significant source of PFOS since standard biological treatment techniques are unable to remove such contaminants from water, according to P. Zareitalabad et al. [63]. As an example of a persistent organic contaminant, PFOS was added to the Stockholm Convention’s list of POPs in 2009, which calls for limited uses everywhere [64]. Adsorption, ultrasonic irradiation, reverse osmosis, photocatalysis, and electrochemical oxidation are a few recent examples of PFOS removal methods that have advanced quickly [64]. The removal of PFOS was rendered challenging by the limitations of these technologies, which included high energy consumption, constrained treatment conditions, and high cost [64]. Many different types of wastewater have been treated using EC, an efficient electrochemical method that is also favorable to the environment [64]. By reducing the electrode distance, enhancing the electrolyte conductivity, and increasing the electrode surface area, expenses may be kept to a minimum while still removing PFOS with great effectiveness [64]. Y. Liu et al. used the EC technique to remove PFOS from wastewater [56]. They used Al as an anode and Zn as a cathode [56]. M. Li et al. studied the removal of PFOS from wastewater using the EC technique [64]. They used Fe as an anode and a cathode [64]. The operating parameters were pH 3 and 60 min. The results showed an excellent removal efficiency of almost 100% [64]. J. Bao et al. used the EC process to remove PFOS from wastewater [65]. The results showed a removal efficiency of 100% when Al anode and Zn cathode were used [65]. The pH of the reaction was 7, while the reaction time was 120 min [65]. B. Yang et al. used the EC technique to remove PFOS from wastewater [66]. The removal efficiency was 99.6% at a pH of 5.2, a current density of 250 A/m2 and 120 min using Fe electrodes [66]. Y. Liu et al. studied the removal of PFOS from wastewater using EC technology. Al is used as an anode material, while Zn is used as a cathode material [56]. The result showed great success in removing PFOS from wastewater, with a removal efficiency of 100% at pH7 in 10 min [56]. H. Shi et al. used a Zn anode and SS cathode to remove PFOS from wastewater using the EC technique. The results showed a removal efficiency of 90% at pH 3.8, and a current density varying from 50 to 200 A/m2 in 120 min [57]. M. Li et al. used EC to remove PFOS. The usage of Fe material as anode and cathode improved the removal efficiency, reaching 100% at pH 3 in 60 min [64]. Yu et al. showed great success in removing PFOS using the EC process. The result showed that the usage of Al-Zn as anode–cathode material succeeded in removing 100% of PFOS from wastewater in 40 min [65]. B. Yang et al. used Fe electrodes in the EC process to remove PFOS from wastewater. The result showed that the removal efficiency reached 99.6% at pH 5.2 in 50 min [66].
2.1.4. Perfluorobutanoic Acid (PFBA)
Perfluorobutanoic Acid (PFBA) is a short-chain PFAS [67]. PFBA is one of these substances that is relevantly exposed externally through food in Europe, contributing to nearly 16% of the overall PFAS exposure in adults, while only having very low serum/plasma concentrations of about 0.01 ng/mL in the adult population [68]. This is caused by its brief serum elimination half-life, which was observed to be a few days for nine exposed employers: a median of 2.3 days, a range of 1.8–6.3 days [68]. Additionally, PFBA has been found in human tissues, serum, and even the snow of a distant area in the European Alps [67]. According to reports, PFBA predominated among the PFASs that were examined in edible plants gathered from all around China [67]. PFBA’s toxicological information is mostly restricted to very few vertebrates [67]. There is an association between the PFAS chain length and cytotoxicity, with longer chains being more hazardous [67]. Y. Liu et al. utilized the EC process to remove PFBA from wastewater. They used Al as an anode and Zn as a cathode. The removal efficiency was 90.9% at pH 7 in 10 min [56].
2.1.5. Perfluorobutane Sulfonate (PFBS)
Because it has a shorter half-life in humans (approximately one month) than PFOS, PFBS, a shorter-chained perfluorinated molecule, has been employed as an alternative [69]. As a result of rising consumption, it is anticipated that PFBS concentrations in the environment will rise yearly [69]. However, PFBS is becoming an emergent environmental contaminant of global concern due to its large-scale production and use [70]. PFBS is often found in surface water, piped drinking water, and groundwater at concentrations of a few hundred ng/L [70]. However, in waterways affected by the discharge of both industrial and municipal wastes, PFBS can exceed g/L [70]. In contrast to PFOS, which has been the subject of substantial research, just a few studies have examined the negative impacts of PFBS on animals [70]. Rats subjected to both 300 and 1000 mg/kg/day of PFBS for 10 weeks showed signs of hepatocellular hypertrophy and a rise in liver weight [70]. In mouse livers, 24 h of treatment to 300 mg/kg body weight of PFBS resulted in increased expression of many PPAR, PPARy, and PXR target genes [70]. According to another research study, adult female mice exposed to PFBS (200 mg/kg/day) for 14 days had lower blood levels of total triiodothyronine and thyroxine [70]. Y, Liu et al. removed PFBS from wastewater using Al-Zn electrodes in the EC process. The results showed that the removal efficiency reached 91% in 10 min at pH 7 [56]. H. Shi et al. studied the removal of PFBS using the EC technique. Zn was used as an anode material, while SS was used as a cathode material. The removal efficiency was 54% in 120 min with a current density of 50–200 A/m2 [57]. J. Bao et al. removed PFBS from wastewater using the EC process. They used Al as an anode and Zn as a cathode. The removal efficiency reached 87.4% in 40 min at pH 7 [65].
2.1.6. Perfluorohexanoic Acid (PFHxA)
Y. Liu et al. removed PFHxA from wastewater using the EC technique. In this process, the anode was Al, while the cathode was Zn. The initial concentration was 696 mg/L, and the initial pH was 7. The results showed that the removal efficiency reached 31.3% in 10 min [56]. H. Shi et al. studied the removal of PFHxA using EC technology from wastewater. The anode material was Zn, while the cathode material was SS. The operating parameters were pH of 3.8, current density ranging from 50 to 200 A/m2, and a time of 120 min. The results showed the removal efficiency was 81% [57].
2.1.7. Perfluoroheptanoic Acid (PFHpA)
Y. Liu et al. removed PFHpA from wastewater using Al as an anode material in the EC process. Al-Zn was the anode and cathode material of the experiment. The initial concentration of PFHpA was 716 mg/L. In 10 min of the reaction, the removal efficiency of PFHpA reached 56.9% at a pH of 7 [56].
2.1.8. Perfluorohexane Sulfonate (PFHxS)
J. Bao et al. utilized the EC process to remove PFHxS from wastewater. In this experiment, Al was used as an anode, and Zn was used as a cathode. The result showed that at a pH of 7, the removal efficiency reached 95.6% in 40 min [65]. Y. Liu et al. studied the removal efficiency of PFHxS from wastewater using EC technology. In the experiment section, Al was used as an anode material, while Zn was used as a cathode material. The optimum parameters were that the initial concentration of PFHxS was 807 mg/L and the pH 7, while the time was 10 min. The results showed that the removal efficiency was 88.2% [56].

Table 2.
Perfluoroalkyl substances (PFAS) removal from aqueous solutions at different pH values using the EC method.
Table 2.
Perfluoroalkyl substances (PFAS) removal from aqueous solutions at different pH values using the EC method.
| No. | Removed Substance | Anode | Cathode | Gap (cm) | Concentration (mg/L) | Efficiency η (%) | pH | Time (min) | Power (A/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PFOA | Al | Al | 1 | — | 99 | 4–5 | 10 | [58] | |
| 2 | PFOA | Fe | Fe | — | — | 10 | 9 | 360 | 800 | [60] |
| 3 | PFDA | Fe-Al | Fe-Al | 1 | — | 100 | 7 | 15 | 370 | [62] |
| 4 | PFOA | Zn | Air | — | — | 100 | 3.5 | 45 | — | [61] |
| 5 | PFOA | Fe | Fe | — | 100 | 56 | 3.6 | 60 | 78.34 | [59] |
| 6 | PFBA | Al | Zn | — | — | 90 | 7 | 10 | — | [56] |
| 7 | PFBS | Al | Zn | — | 31 × 10−3 | 59 | 7 | 10 | — | [56] |
| 8 | PFOA | Al | Zn | — | 2.4 × 10−3 | 89 | 7 | 10 | — | [56] |
| 9 | PFOS | Al | Zn | — | 0.5 | 100 | 7 | 10 | — | [56] |
| 10 | PFHxA | Al | Zn | — | 0.696 | 31 | 7 | 10 | — | [56] |
| 11 | PFHpA | Al | Zn | — | 0.716 | 57 | 7 | 10 | — | [56] |
| 12 | PFHxS | Al | Zn | — | 0.807 | 88 | 7 | 10 | — | [56] |
| 13 | PFHxA | Zn | stainless steel | — | — | 81 | 3.8 | 120 | 50–200 | [57] |
| 14 | PFBS | Zn | stainless steel | — | — | 54 | 3.8 | 120 | 50–200 | [57] |
| 15 | PFOS | Zn | stainless steel | — | — | 90 | 3.8 | 120 | 50–200 | [57] |
| 16 | PFOS | Fe | Fe | — | 5 | 100 | 3 | 60 | — | [64] |
| 17 | PFBS | Al | Zn | — | — | 87 | 7 | 40 | — | [65] |
| 18 | PFHxS | Al | Zn | — | — | 95 | 7 | 40 | — | [65] |
| 19 | PFOS | Al | Zn | — | — | 100 | 7 | 40 | — | [65] |
| 20 | PFOS | Fe | Fe | — | — | 100 | 5.2 | 50 | 250 | [66] |
| 21 | PFOA | Fe | Fe | — | — | 78 | 3.8 | 50 | 250 | [66] |
| 22 | PFHxA | Al | Al | 1 | — | 65 | 7–8 | 45 | 350 | [71] |
| 23 | PFHpA | Al | Al | 1 | — | 58 | 7–8 | 45 | 350 | [71] |
| 24 | PFOA | Al | Al | 1 | — | 75 | 7–8 | 45 | 350 | [71] |
| 25 | PFDA | Al | Al | 1 | — | 18 | 7–8 | 45 | 350 | [71] |
| 26 | PFBS, PFHxS, and PFOS | Al, Zn | Zn, Al | 2 | 1.0 | 87, 95, and 100 | 7.0 | 60 | — | [71] |
| 28 | PFOA, and PFOS | Zinc | Air | — | — | 99 and 89 | 7 | 45 | — | [61] |
| 29 | PFOS | Zn | Air | 2 | 0.005 | 69–81 | 3.5 | 45 | [28] |
Perfluorobutanoic acid (PFBA); perfluorobutane sulfonate (PFBS); perfluorooctane sulfonate (PFOS); Perfluorohexanoic acid (PFHxA); Perfluoroheptanoic acid (PFHpA); Perfluorohexane sulfonate (PFHxS); perfluorodecanoic acid (PFDA).
2.2. Microplastics (MPs)
2.2.1. Sources and Complexity of MPs
One of the main contaminants in both terrestrial and oceanic habitats across the globe is microplastics. The possible dangers these persistent and organic contaminants bring to both public health and the environment demand consideration [72]. MPs are those plastics with a particle size of less than 5 mm, while nano-plastics are plastics with particle sizes ranging from 1 to 100 nm [73]. They are also widely dispersed in the ecosystem and quickly stick to the surfaces [74]. One of the hazardous effects on marine organisms, it can impact the ability of the algae in the photosynthesis process [73]. MPs can cause harm in many ways, including digestive system harm, metabolism issues, immune dysfunction, reproductive dysfunction, and neurotoxicity [74]. Because of increasing manufacturing and careless dumping of plastic debris, about eight million metric tons of plastic get into the ocean each year, making it among the most pervasive environmental toxicities of the Anthropocene [73]. Those plastics eventually degraded, primarily due to UV light, and weathering fractured them into smaller fragments rather than decomposing them [72]. Plastic manufacturing has skyrocketed from 1.7 million metric tons in the 1950s to 361 million metric tons in 2019. Plastic items are now frequently utilized in both industry and daily life [75,76]. Disposable masks have been necessary everyday supplies since the COVID-19 epidemic. The WHO calculates that there were more than 2.5 million examination gloves, surgical masks, and protective screens needed globally per month during the early stages of the COVID-19 epidemic (World Health Organization, 2020) [75]. Further, because of its light weight, great strength, durability, and low cost, plastic has recently found use in a variety of sectors [47]. Although using plastic items makes life and work much more convenient, a lot of plastic waste is also released into the environment [47]. In general, plastic products, including polyethylene (PE), polystyrene (PS), polyethylene terephthalate (PET), high-density polyethylene (HDPE), and polyvinyl chloride (PVC), eventually shrink and turn into microplastics (MPs) with finer molecules after being exposed to the natural environment [47,77]. MPs are generally understood to be plastic pieces or particles with a diameter of less than 5 mm [47].
Due to plastic’s durability and resilience, 90% is likely untreated waste that could end up in the environment and degrade or contaminate the aquatic system [78]. Microplastics are created as a result of the widespread use of plastics and poor waste management practices. In particular, macro-plastics are broken down into secondary MPs as a result of mechanical stress, abrasion, photolysis, weathering, and microbiological degradation [47]. The MPs emissions from both primary and secondary sources have been recorded and projected by a sizable number of studies [47]. In addition, primary MPs are purposefully produced in tiny sizes for specific commercial or home purposes, such as the microbeads found in toothpaste, cosmetics, personal care products, and laundry detergents. Because of their tiny size, the majority of MPs cannot be stopped in wastewater treatment facilities (WWTPs), where they may readily enter natural water bodies, including rivers, lakes, and seas, by water runoff and groundwater infiltration [47]. Aquatic species readily consume MPs when they reach the water environment, which has an impact on organisms’ food intake and metabolic processes [47,79]. Additionally, MPs may be utilized to enrich contaminants in water, which has harmful effects and worsens the harm done to organisms [47]. Numerous investigations have revealed that MPs may penetrate the human body and travel up the food chain, posing a major hazard to human health. This increases human exposure to contaminants that were previously inaccessible and also raises health risks [77]. The discovery of microplastics in human feces is noteworthy. Additionally, MPs can act as a carrier for a variety of toxic contaminants, including PFAS, pharmaceuticals, and personal care items [77]. These contaminants include heavy metals, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), polycyclic aromatic hydrocarbons (PAHs), and dichlorodiphenyltrichloroethane (DDT) [77]. Large-scale microplastic use will have damaging effects on creatures, posing serious ecological dangers. It also has the potential to transmit and enhance food chains, endangering human health [77].
2.2.2. EC Removal and Associated Risks of MPs
The treatment of microplastics has become more essential as a result of the widespread usage of single-use plastic masks during the COVID-19 epidemic. Over the last ten years, methods for removing MPs from wastewater have included coagulation, membrane separation, magnetic extraction, photocatalysis, and EC [47]. Among them, EC offers good benefits, such as straightforward operation, high efficiency, and low cost, which have garnered considerable interest [47]. In contrast to the currently used coagulation technique, EC uses metal electrodes to produce coagulants on-site, negating the requirement for chemical additions. MPs inside the water can be better absorbed by the coagulants created during the EC process [47]. Additionally, MPs can be degraded using the reactive species produced on the anode [47].
Elkhatib et al. removed MPs from wastewater via an EC [80]. The removal effectiveness of MPs from wastewater samples was 96.5%. Additionally, 88.8% of thermotolerant coliform colonies and 92.2% of COD colonies were eliminated [80]. Overall, the findings demonstrated that EC successfully reduced thermotolerant coliforms and COD through the removal of MPs from sewage at minimal operational costs, as in Table 3 [80]. M. Shen et al. studied the EC removal of MPs from wastewater. Using Al anode, the removal rate of MPs was very high at 98.4% for polypropylene (PP), 91.7% for Poly(methyl methacrylate) (PMMA), 93.2% for polyethylene (PE), and 98.2% for cellulose acetate (CA) at pH 7.2 [76]. Perren et al. removed microbeads and MPs from wastewater utilizing EC. The results showed that the best removal efficiency obtained was 99.24% at a pH of 7.5 [81].

Table 3.
Removal of different types of MPs using EC with different conditions including the anode, cathode, pH value, and the used power.
In comparison to simulated wastewater, the elimination rates of MPs in the genuine aquatic environment are far lower [75]. There are virtually no studies on the influence of the materials in the actual aquatic environment on the elimination of MPs [75]. Instead, most studies concentrated on the distribution of particle sizes or the elimination of MPs [75]. Natural organic matter, residual organic contaminants, refractory organic contaminants, and residual organic contaminants, as well as other chemicals, are among the complex DOM components found in wastewater [75]. Clarifying the impact mechanism of DOM on the EC treatment of MPs is crucial because these intricate components influence the degradation rate of MPs particles in the removal process [75].
2.3. Pharmaceutics
Pharmaceutical medications are essential for increasing the quality of life and life expectancy [33]. Every year, enormous volumes of pharmaceuticals are used in both human and veterinary medicine to cure infections, fever, and mental and physical stress; prevent pregnancy; and even promote agricultural development [33]. They eventually infiltrate sewage treatment facilities (STPs) and were found in urine and animal excrement to a degree of around 90% and 75%, respectively. If these contaminants are released from traditional STPs without being treated, they might seriously harm the environment and sea creatures [33,84]. The main issue is that the proliferation and escalation of antibiotic-resistant bacteria are directly related to the release of such harmful chemicals into the environment [84,85]. The wastewater containing pharmaceutics has a strong color, strong odor, significant COD, and minimal BOD [33].
Numerous approaches have been evaluated, including electrochemical degradation, membrane processes, biological treatments, the Fenton process, adsorption [2], or various combinations of technologies [85]. Activated sludge and the trickling filter technique are also used to cleanse pharmaceutical wastewater. However, both methods were ineffective and led to the wastewater being released into the environment, where it continued to damage the soil, surface water, and groundwater [33]. Due to its unique benefits, including its low operating costs, compact treatment facility, ability to treat a variety of contaminants, simple design, rapid sedimentation, and minimal sludge production, EC has been considered a highly promising sewage treatment technique within all available electrochemical methods [84]. The primary objective for environmental engineers nowadays is to develop a treatment plan that can manage or treat wastewater locally. This illustrates the efficiency of EC as a decentralized wastewater treatment technique [86]. Padmaja et al. analyzed the effectiveness of chemical and EC techniques for treating pharmaceutical wastewater [87]. The findings revealed that although chemical coagulation significantly reduced the amount of COD, suspended particles, and chlorides, the amount of TDS reduction was only 26.3% with FeCl3 and 14.05% with Al, while the EC approach significantly decreased COD and TDS (92.3% and 91.5%, respectively) [87]. Previous results for using EC to remove some pharmaceutical substances are in Table 4. Furthermore, we summarize the advances of EC for specific classes of pharmaceuticals.
2.3.1. Antibiotics
Antibiotics are continuously released into the environment as a consequence of their heavy usage for medical, veterinary, and several human activities [88]. They can pose a concern to the ecosystem by promoting resistant bacteria. For example, studies have shown that the levels of different antibiotics in sewage plants and natural waterways fluctuate seasonally [86]. The present review highlights the results of various studies on antibiotic removal. It can be noticed that the efficiency of the EC process varies depending on the type of antibiotics being targeted. While some antibiotics can be efficiently removed from water using EC, others remain unaffected. Hence, it is essential to optimize the parameters of the process to improve the effectiveness of EC for a particular antibiotic type. Therefore, further investigations are necessary to determine the optimal conditions for EC to remove specific antibiotics. The relation between the solubility of the antibiotics and the removal efficiency might be a factor, where more soluble might lead to higher mobility and less contact time between the targeted antibiotic and the anode. This relation can be considered as a gap in the previous studies. Moreover, several bacteria, and it might be the same for some antibiotics, are being used as reducing agents, which is a problem during the oxidation process. This probably causes an intermediate product that is resistant to further degradation. Therefore, the relation between efficiency and the chemical structure of antibiotics needs to be investigated thoroughly.
Ampicillin (AMP)
One of the studied penicillin antibiotics is Ampicillin sodium salt (AMP). Baran et al. used EC to remove AMP and different other antibiotics from wastewater, and they reported that EC was not effective [89]. They determined that the problem was in the electrolysis voltage and used another electrolyte, Na2SO4. This electrolyte might increase the electrolysis voltage, which consequently can achieve more ROS at the anode [90]. However, even with Na2SO4 electrolyte, the degree of removal (%) did not exceed 4.3% for AMP, while success with other antibiotics, such as doxycycline, had a removal ratio of approximately 100% [89].
Amoxicillin (AMX)
Numerous researchers have been investigating various effective methods for removing AMX and its metabolites from the effluents of wastewater [91]. D. Balarak et al. investigated the removal of AMX by EC using an Al electrode at pH 7 with 60 Volts. They reported that removal reached 98.98% after an electrolysis time of 75 min [92].
Ciprofloxacin (CIP)
CIP is a second generation of fluoroquinolones, and it was detected in surface water [93]. Higher concentrations of CIP lead to renal failure, headache, vomiting, and elevation of the liver enzymes [94,95]. Furthermore, the longtime exposure might develop these acute problems and cause chronic effects [96]. S. Ahmadzadeh et al. used 2 Al electrodes with a current density of 12.5 mA cm−2 at a pH of 7.8. They reported elimination of 100% after a reaction time of 20 min [97]. In addition, M. Malakootian et al. used a current density of 2.75 mA cm−2 and achieved 81% elimination after 40 min [98].
Levofloxacin (LVX)
Levofloxacin (LVX) is the third generation member of the quinolone family [99,100]. LVX is resistant to degradation and frequently escapes from sewage treatment facilities. LVX is popular and widely used, which results in bioaccumulation in the ecosystem. Mohammed et al. reported that the removal efficiency reached 88% under the conditions of pH = 4, 20 mA cm−2, and an operating time of 60 min [101]. Moreover, the same researchers explored other conditions and reported a removal efficiency of 82.5% at 16 mA cm−2, pH value at 3.8, and 42 min of reaction time [101].
Cefazolin (CEZ)
The most widely used class of antibiotics is the cephalosporin group, and it is a first-generation one, which comprises between 50% and 70% of all antibiotics used globally [84]. CEZ, one of the numerous available antibiotics, is frequently utilized to treat bacterial skin infections [84]. M. Bajpai et al. combined kinetic measurements and isotherms study, response surface methodology (RSM), and EC for pharmaceutical wastewater containing CEZ treatment with an 85.65% removal efficiency rate [84].
Cefixime (CFX)
CFX is accumulated in the tissues of animals and plants and therefore is maintained in the environment [102]. Further, it is confirmed it exists in groundwater, surface water, and drinking water [103]. M. Asadi-Ghalhari et al. reported that they successfully removed 92.25% of CFX from an aquatic solution at the conditions of 0.3 A and around 60 min of operating time [104]. In addition, R. Mostafaloo et al. utilized EC to remove CFX with a pH value of 6, 0.7 A, and reaction time of 60 min. They reached a removal efficiency of 90.1% [105].
Oxytetracycline Hydrochloride (OTCHC)
One of the most frequently utilized antibiotics in both people and animals is tetracycline [106]. In addition to (OTCHC), oxytetracycline, oxytetracycline dihydrate, and oxytetracycline calcium are also available. In both human and veterinary medicine, oxytetracycline (OTC) is employed [106]. OTC has been found in sewage treatment facilities, rivers, and lakes. Nariyan et al. used an EC method to remove it, and the results showed a removal efficiency of 93.2% when Fe is used as an anode, and 87.75% when Al is used as an anode [106].
Doxycycline (DOX)
DOX is a second-generation tetracycline compound and has a broad-spectrum effect. DOX displays considerable residual toxicity in surface and groundwater due to its extremely soluble nature [107]. Zaidi et al. showed that about 90% of DOX is removed using an electro-coagulation coupled electro-flotation process (EC-EF) [107]. Further, W. Baran et al. investigated the EC process and found 100% removal of DOX at a pH value of 6, a current density of 18 A cm−2, and an operating time of around 36 min [89].
2.3.2. Anti-Inflammatory
Dexamethasone (DEX)
Glucocorticoids (GCs) extensively utilized in both animal and human medicine are one significant family of anti-inflammatories. DEX, the most powerful GC cortisone derivative used mostly in hospitals and clinics, has been found in quite high concentrations in sewage effluent [108]. Arsand et al. used EC to remove dexamethasone from hospital effluent and aqueous solutions [108]. The result showed that by increasing the applied current (100–500 mA) and also decreasing the electrode distance from 30 mm to 6 mm, the removal efficiency of DEX is increased up to 38.1% [108].
Hydrocortisone
Hydrocortisone is a steroidal hormone that is formed by the adrenal cortex. It is a synthetic glucocorticoid showing anti-inflammatory properties [109]. It is widely used to treat diseases such as allergies, inflammation, skin, and asthma [110]. Steroid hormones are continually released into the environment by people and animals [111]. Alaani et al. used Al electrodes to investigate the removal efficiency of hydrocortisone. They found that at an electrolysis time of 150 min, 6 pH, and 35 mA cm−2 resulted in a removal efficiency of 60% [112].
2.3.3. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAIDs, which are used widely, are the pharmaceuticals that are most commonly found. NSAIDs may be hazardous to aquatic life and detrimental to embryos, babies, kids, and adults having weak constitutions and susceptibility to drugs, despite the lack of evidence that they are harmful to adults [113]. Liu et al. showed that NSAIDs are eliminated via EC/flotation using a cationic surfactant [113]. Selected nonsteroidal NSAIDs, notably, aspirin, ibuprofen, diclofenac, and ketoprofen, were eliminated from wastewater using an EC/flocculation flotation (ECF) procedure [113]. As a collector and froth agent, cethyltrimethylammonium bromide (CTAB), a cationic surfactant, was added to the solution [113]. The results showed a removal efficiency between 10–45% for NSAIDs [113].
An active metabolite of the blood lipid-regulating medicines Clofibrate, Etofibrate, and Etofyllinclofibrate is clofibric acid [114]. These compounds are employed to lower the plasmatic content of triglycerides and cholesterol [114]. Clofibric acid has previously been found in groundwater wells, German river flows, Swiss lakes, and sewage treatment plant influents and effluents [114]. NSAIDs include Ibuprofen, Ketoprofen, Mefenamic Acid, and Diclofenac (NSAIDs) [114]. They have antipyretic and analgesic properties. All of these medicines, except mefenamic acid, whose quantities and activity in STPs have never been recorded, have been found in STP influents, effluents, and surface water samples [114].
2.3.4. Anticonvulsants
Carbamazepine (CBZ)
Anticonvulsant drugs may be categorized into three categories: those that block neuronal ion channels, those that enhance gamma-aminobutryic acid (GABA)-ergic neurotransmission, and those whose mechanism of action is unclear [115]. CBZ is classified as a significant micropollutant due to its widespread usage, bioaccumulation inside living things, and consequent endocrine-disrupting, neurotoxic, and developmental negative effects. Z. Xiao et al. used the P-rGO/CF cathode in a combination of electrocoagulation/electro-Fenton (EC-EF) to remove CBZ [116]. As a result, it was possible to create additional H2O2 and •OH to break down CBZ, and 100% of it was eliminated [116]. Ensano et al. used EC to treat municipal wastewater that contained active medicinal ingredients [117]. For a 180 min intermittent response period, an increase in pharmaceutics concentration resulted in reductions in removal efficiencies of 23%, 22%, and 40% for CBZ, DCF, and AMX, respectively [117].
2.3.5. Mixtures of Pharmaceuticals
Oladipo et al. used an EC process to eliminate metal complexes with heavy metals [118]. The process targeted tetracycline-nickel ions [118]. They studied the process with 2 electrodes; the first was Fe, and achieved 99.3% elimination efficiency with an initial concentration of 15 mg/L within an hour, while the Al electrode achieved an elimination rate of 99.8% elimination efficiency within 20 min, with an initial concentration of 10 mg/L [118]. The study proved that with an acceptable amount of nickel ions, the removal rate will be improved, as the ratio was one-to-one nickel and tetracycline, and the elimination efficiency was 100% within 10 min. However, when the ratio was 1:2, the elimination efficiency was 99.6% in 20 min [118]. The whole operation was kept at an electrode spacing gap of 2 cm, and the voltage was kept at 9 volts, and the pH was maintained at 9 [118]. Baran et al. studied the effect of the EC process on ampicillin, doxycycline, sulfathiazole, and tylosin. They accomplished an elimination rate of 3.6 ± 3.2%, ~100%, 3.3 ± 0.4%, and 3.1 ± 0.3%, respectively [89]. The initial concentration of ampicillin, doxycycline, sulfathiazole and tylosin was 50 mg/L for all the processes, but the pH was set for every compound, such as ampicillin was from 6 to 6.5, doxycycline was from 6.5 to 7, sulfathiazole was 6.9, and tylosin was from 6 to 6.9 [89]. The maximum time for the process was 36 min [89]. The voltage was around 1 volt in the sludge, and within the water was varied from 5 to 7 volts [89]. The pH value was set to be 6 to 6.5 in the sewage water and 6.0 to 8.0 in water by using NaOH or H2SO4 in the solution [89]. Yoosefian et al. studied the effect of the EC process on the elimination of ciprofloxacin from wastewater that comes from hospitals [96]. They used Fe as the electrode material in this process [96]. Optimum conditions included some adjustments, such as initial concentration, which was set to be 60 mg/L, and the pH was adjusted to be 7.5. Finally, the electrode gap was maintained to be 1.58 cm apart from each other, and the elimination efficiency was 100% [96]. The overall cost of the elimination comes from the electricity usage and the anode loss; the loss of the anode was about 62 mg, and the power consumption for this process was 0.522 kWh/m3 [96].

Table 4.
Removal of pharmaceuticals from wastewater using electrocoagulation upon their conditions.
Table 4.
Removal of pharmaceuticals from wastewater using electrocoagulation upon their conditions.
| No. | Removed Substance | Anode | Cathode | Gap (cm) | Concentration (mg/L) | Efficiency η (%) | pH | Time (min) | Power (A/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Metronidazole | Fe | Fe | 1.5 | 25 | 99.28 | 6.5 | 30 | 40 | [119] |
| 2 | Tetracycline | Fe | Fe | 1 | 1 | 95 | 4 | 30 | 40 | [120] |
| 3 | Carbamazepine (CBZ) | Fe | Heteroatom P, doped graphene/carbon felt (CF) | 2.0 | 30.0 | 99 | 3 | 15 | 100 | [116] |
| 4 | Doxycycline | low-carbon steel | low-carbon steel | 50 | ~100 | 6.0–6.5 | 36 | 18 | [89] | |
| 5 | Ibuprofen, | Al | Fe | 3 | 40 | 50.96, | 5 | 110 | 4878 | [121] |
| 6 | Acetaminophen | Al | Fe | 3 | 40 | 22.76 | 5 | 110 | 4878 | [121] |
| 7 | Diclofenac | Al | Al | 5 | — | 98 | 7.2 | 20 | 16.7 | [113] |
| 8 | Ibuprofen | Al | Al | 5 | — | 80 | 7.2 | 20 | 83.3 | [113] |
| 9 | Ketoprofen | Al | Al | 5 | — | 75 | 7.2 | 20 | 16.7 | [113] |
| 10 | Estrone | Fe | Fe | 1 | 0.2 | 81 | 7 | 120 | 167 | [122] |
| 11 | 17β-estradiol | Fe | Fe | 1 | 0.2 | 87 | 7 | 120 | 167 | [122] |
| 12 | Estriol | Fe | Fe | 1 | 0.2 | 85 | 7 | 120 | 167 | [122] |
| 13 | 17α-Ethynylestradiol | Fe | Fe | 1 | 0.2 | 97 | 7 | 120 | 167 | [122] |
2.4. Dyes
Dyes are substances that are mostly employed in the manufacturing of fabrics and leatherette [123]. Dyes have complicated chemical structures and frequently originated from artificial materials. They are very durable and challenging to break down normally [124]. Industrial artificial colors are characterized as organic compounds that withstand oxidizing substances, heating, and radiation and also resist aerobic digestion [124]. Further, they often include significant amounts of soluble toxic substances, including emulsifying agents [123]. Effective efforts have been made to remove dyes from the aqueous solutions using EC, as shown in Table 5.
Guvenc et al. studied sewage water produced by industries producing paint, and they used the process of EC with an initial pH value of 5, with an initial concentration of 5.6 g/L. The Fe was used as the cathode, with an electrode gap of 1.5 cm. They set the reaction time to be 35 min, with a current density of 21 mA/cm2. The color was removed with an efficiency of 98.1% [125]. It was found that the acidic medium reached a higher efficiency than the alkaline one [125]. Taheri et al. studied the effect of EC on Acid Brown 14, Acid Orange 7, and Acid Red 18. The Al was used as the electrode, and the current density was set to be equal to 291 A/m2 [126]. For the Acid Brown 14, the concentration was adjusted to 600 mg/L, the pH value at 4, and the time of the process at 10 min; the degradation rate reached 100% [126]. For the Acid Orange 7 with a concentration of 540 mg/L at a pH value of 4 and a reaction time of 14 min, the removal rate reached 100% [126]. Abbasi et al. investigated the EC process in the color removal of sewage water from licorice processing [127]. They reached a color-removing efficiency of 90.1% within 81.8 min [127]. They used Fe as the main electrode, and the gap between the electrodes was 3 cm [127]. The initial pH was 6.5, while the power density was 350 A/m2 [127]. Abbasi et al. removed the color from licorice processing wastewater using the EC process. They reached a removal efficiency of 94.6%, using Fe as the electrode material, while the process continued for 71.8 min, with a current density of 28 mA/cm2 [128].
Several studies have been completed recently to remove colors from wastewater using a combination of EC and adsorption techniques. Irki et al. used the EC procedure to remove methyl orange and achieved 74% efficiency after 12 min. However, under identical conditions, the efficiency jumped to 95% when utilizing a magnetic field [129]. Ma et al. explored the decolorization of methyl orange from wastewater utilizing Fe and graphite plate as anode and graphite plate as cathode, achieving a 100% yield [130] (Ma et al., 2007). Methyl orange was eliminated from the aqueous solution using the adsorbents goethite (G), chitosan beads (CSB), and goethite impregnated with chitosan beads (GCSB). The process fits Langmuir’s behavior, with maximum adsorption capacities for each adsorbent being 55, 73, and 84 mg/g for G, CSB, and GCSB, respectively [131]. Methylene blue was eliminated from an aqueous environment by Jawad and Abdulhameed using an eco-friendly mineral adsorbent. The maximal capacity was determined to be 240.4 mg/g at 303 K, and the Freundlich and Langmuir adsorption isotherm models were also achieved [132].

Table 5.
A comparison between previous studies for the removal of dyes and colors from aqueous solutions via the EC method.
Table 5.
A comparison between previous studies for the removal of dyes and colors from aqueous solutions via the EC method.
| No. | Removed Substance | Anode | Cathode | Gap (cm) | Concentration (mg/L) | Efficiency (%) | pH | Time (min) | Power (A/m2) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dyes | Fe | Fe | — | — | 96 | 7 | 6 | 100 | [133] |
| 2 | Dye | Al | stainless-steel | — | 95 | 6.9 | 5 | 10 | [134] | |
| 3 | Yellow 10 gw dye | Al Cu | Al Cu | — | 1000 | 96 97 | 7.5 9 | 5 15 | 60 | [135] |
| 4 | Color dye, COD, turbidity, and alkalinity | Fe or Al | — | — | — | 90, 89, 82, and 73 | 6.5 | 81.8 | 350 | [136] |
| 5 | Color, COD | Fe/Fe | Fe/Fe | — | (1500–7500) | 100, 97. | (2–11) | — | 1.25–7.50 | [136] |
| 6 | AG 20, and RY 17 | Al | Al | — | 95 and 93 | 2.1 | 60 | 400 | [137] | |
| 7 | RR 35 and DY 56 | Al | Al | — | 10–150 | 94 and 95 | 8 | 50 | 43.4 and 104.2 | [138] |
| 9 | Methyl orange | Stainless steel | Fe | 2 | 50 | 93. | 7 | — | — | [139] |
| 10 | Acid Brown 14, Acid Orange 7, and Acid Red 18 | Al | Al | — | (150–600) | 100, 100, 57 | (4–10) | 15 | 291 | [126] |
2.5. Phthalates
Phthalates are one of the SOCs in wastewater [140]. The most significant industrial chemicals, phthalate esters (PAEs), were frequently utilized as plasticizers to increase the elasticity and ease the processing of polymeric materials [140]. The primary sources of PAEs in sewage are processed wastewater discharge from the leather, cosmetics, and plastics industries [141]. Additionally, PAEs were discovered in landfill leachate, surface runoff, and household wastewater [141]. Plasticizers were easily discharged into the environment by plastics, where they established themselves as a significant source of pollution [140]. The most popular plasticizer was PAEs, which were designated as a priority contaminant by the Ministry Of Environment Monitor Bureau of China Center [140]. Even though little was understood about these processes, they are significant because PAEs were not covalently bonded to plastic polymers [140]. As a result, they may leak out of the plastic into the environment or tissues [140,142]. The production of PVC materials for textiles, toys, medical supplies, packaging, and tannery goods all heavily rely on PAEs [141,142]. Diethyl phthalate esters (DEPs) and dimethyl (DMP) are often employed in the manufacturing of cellulose ester-based polymers, while dibutyl phthalate (DBP) is utilized in the manufacturing of epoxy resins [141]. To turn leather into a usable form, a variety of PAEs, as well as other chemicals, including di-ethyl phthalate (DEHP), O-phenyl phenol (OPP), DBP, hexyl Nonyl phenol, N-Methyl pyrrolidone, and benzyl butyl phthalate (BBP), are utilized as plasticizers in the microporous artificial leather coating, leveling agent, amalgamation, and wetting agent [141].
Recent experimental research has concentrated on chemical oxidation processes that completely degrade phthalates [142]. The two investigated methods for the treatment of phthalates that were dispersed in the water are adsorption and chemical oxidation [142]. Electrochemical processes, such as electro-oxidation and EC, which involve several removal mechanisms, including flotation, coagulation, oxidation, and adsorption, for the decomposition of organic materials, in addition to the abatement of inorganic contaminants, are simple to use and versatile [142]. Many different industrial wastewaters have been subjected to these procedures. Additionally, EC has been used to treat certain organic and inorganic contaminants, such as fluoride, lignin, phenol, and pesticides [142]. Kabdaşlı et al. used dimethyl phthalate as a model material to treat phthalic acid esters via EC using SS electrodes; the applied current density was 225 A/cm2, the initial pH was in the range of 2 to 6, and the total mineralization was obtained after 2 h [142]. Yang et al. investigated how a graphene-containing, ceramic, composite tubular membrane combined with a combined EC and electro-filtration procedure removed phthalates and pharmaceuticals from an aqueous solution [143]. The results showed that the efficiency of removing di-n-butyl phthalate (DnBP) and di(2-Ethylhexyl) phthalate (DEHP) was 99% at an electric field strength from 20 to 40 Volt/cm, and the electrode distance was 10 mm [143].
2.6. Complex Solids Containing Organic Matter
Human waste, mostly feces and urine, along with toilet flushing water and sullage produced by homes, businesses, and public buildings, make up domestic wastewater [144]. High quantities of organic matter are typical characteristics of domestic wastewater [145]; mixed residential wastewater with a volume of 130 L/cap/day contains 14, 120, and 2 g/cap/days of nitrogen, chemical oxygen demand (COD), and phosphorus, respectively [144]. Municipal wastewater was composed of 4.9–106.2 mg/L phosphorus, 31.8–202.4 mg/L N, and 299–4294 mg/L COD [144]. Benekos et al. removed COD from wastewater using the EC [146]. The minimum COD concentration was 3000 mg L−1, and the current density was 5.65 mA cm−2. The results showed that about 42.5% of COD was removed [146]. Villalobos-Lara et al. used an Al anode in EC to remove about 90% of COD from wastewater [147]. Barzegare et al. removed COD from sewage using EC with Al and Fe electrodes [148]. The efficiency of removing COD reached 95% [148]. Meanwhile, Ahangarnokolaei et al. used EC and ozonation in combination, sequentially, and simultaneously for the treatment of textile wastewater. The results showed that the usage of Al played a key role in removing 50% of COD from wastewater [149]. The results for complex solid removal are summarized in Table 6.

Table 6.
The summary of the studies with experimental conditions of removal of chemical oxygen demand (COD) and suspended solids using EC and their efficiency.
2.7. Synthetic Organic Contaminants (SOCs) for Agricultural Usage
In recent years, the consumption of pesticides (insecticides and fungicides) in food and agricultural fields has expanded to prevent and eliminate the impacts on animals and insects [181]. To avoid, eliminate, deter, or mitigate pests, pesticides, particularly insecticides and herbicides, are being utilized more and more in forestry, agriculture, and residential activities [183]. Despite these benefits, they are poisonous and non-biodegradable, making them harmful to both human and animal health [183,184]. The runoff from farming fields and poor pesticide industrial disposal are the main causes of water pollution with pesticides [183,184]. The most widely used pesticides are mutagenic and carcinogenic, including malathion, endosulfan, sulfur, methyl parathion, and monocrotophos [184]. Pesticides make up nine out of the top twelve persistent organic contaminants, according to the Stockholm Convention on Residual Organic Contaminants [183]. It is a challenge to find a process to remove these pesticides from wastewater with high efficiency. The EC technique has been used to remove the mentioned pesticides from wastewater with high efficiency. This is due to the ability of the process to generate highly reactive oxidizing species that can break down those complex compounds into simpler and less harmful molecules. The use of EC for the removal of pesticides has been reported in various studies. Furthermore, the results have shown that the process can effectively eliminate these contaminants from wastewater. However, the efficiency of the process is dependent on several factors, such as the type of pesticide, initial concentration, material electrode, and operating conditions. In addition, one of the challenges is the cost associated with the process and particularly the energy consumption, which needs to be optimized to make it more economically feasible for large-scale applications.
2.7.1. Pesticides
Different substances are used for agricultural purposes and might cause environmental contamination, including pesticides. A wide range of pesticides are used, such as glyphosate, malathion, acetamiprid, oxyfluorfen, diazinon, and chlorpyrifos. Glyphosate is widely used due to its low cost, simplicity of use, superior removal, and processing rates [185]. On the other hand, malathion has been used a lot due to its low toxicity and superb selectivity for insects [186]. Malathion can harm mammalian nervous systems by inhibiting essential enzymes [184]. Further, acetamiprid is classified as a third-generation pesticide [183]. Acetamiprid is used to control a wide range of coleopteran pests, in addition to sucking pest insects, such as aphids, leaves, thrips, plant hoppers, whiteflies, and other draining insects [183]. Furthermore, diazinon is harmful to the skin, the stomach, and the respiratory system [187]. Otherwise, chlorpyrifos has been discovered in sperm, human breastfeeding, cervical fluid, newborn infants’ meconium, and cord blood [188].
Glyphosate
Glyphosate is the pesticide that has received the most attention since the beginning of the millennium [189]. The main component of numerous commercial formulations of herbicides is glyphosate (N-(phosphonomethyl) glycine), an organophosphorus chemical [185,190]. The main issue with glyphosate use is wastewater intrusion into streams and aquifers [185,191]. The ocean, fauna, flora, and ecological matrices (water and soil) are all in danger due to glyphosate contamination, which is a cumulative and continuous deterioration [185,191].
Due to its ease of use, cheap cost, and high processing efficiency and removal rates, the adsorption and degradation process is considered one of the most popular methods for removing glyphosate from wastewater [185]. Electrolysis, chemical oxidation, microwave radiation, ozone, membrane separation, precipitation, UV irradiation, adsorption, photocatalytic degradation, and EC are all efficient ways to remove glyphosate from wastewater [191]. EC is now an economical option for industrial processes and water treatment facilities [190]. Danial et al. studied the EC removal of the glyphosate mechanism [190]. The results indicated that using EC, glyphosate was removed at a high percentage (94.25%), followed by removal rates of 88.37% for Fe electrodes, 62.82% for steel electrodes, and 46.69% for copper electrodes [190]. The mechanism lies in using sacrificial electrodes, such as aluminum, iron, steel, or copper, to generate metal hydroxide flocks [192]. These flocks are formed through oxidation and hydrolysis reactions [193]. Further, the flocks act as coagulants and aim to remove pollutants from the water by adsorbing them. The process also generates charged metal hydroxide species that neutralize the electrostatic charge of the pollutants and initiate the coagulation process [194]. On the cathode, a hydrogen gas is raised, and bubbles are produced, which lead to electro-flotation. Finally, the flocks are removed from the water through sedimentation or floatation, and the treated water is separated.
In addition to that main mechanism, the metal cations can be bonded with other compounds under certain conditions to form other kinds of flocks that differ from the metal hydroxides. To understand this, the degradation pathway of glyphosate should be discussed. The chemical structure of glyphosate is C3O5H8PN. The bonds of C-P and C-N are relatively weak and, consequently, easy to be broken [195]. During the EC process, the current passing generates heat in the solution, leading to the breaking of these bonds for the relatively complex structure of glyphosate to be transformed into simpler compounds, as illustrated in Figure 7a,b. When the C-N bond is broken, the glyphosate decomposes into formaldehyde, phosphoric acid ion, ammonium ion, and nitrate ion [190]. On the other hand, the breaking of the C-P bond decomposes the glyphosate to aminomethyl phosphonic (AMPA) ion, in addition to glyoxylic acid and protons [190]. Furthermore, AMPA can be degraded in the existence of metal cations and water into metal phosphates, methanamine, and hydrogen gas. In this stage, metal phosphate can form flocks, and this mechanism is called coagulation by metal cations, and it has been discussed by R. Danial et al. [190].
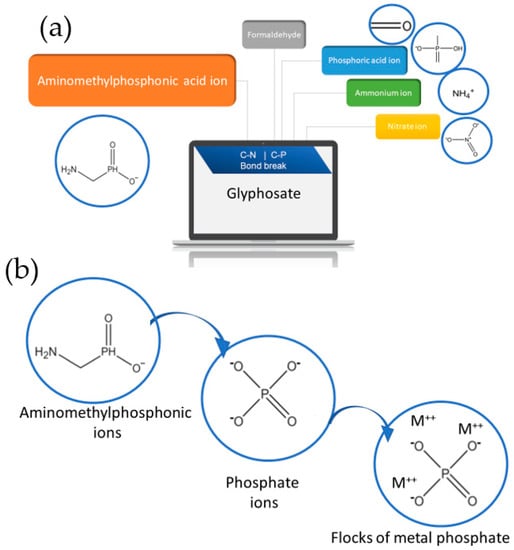
Figure 7.
The degradation of glyphosate (a) into simpler compounds. (b) The flock formation through AMPA during the EC process.
Malathion
Malathion is an insecticide belonging to the chemical group of organophosphates [184]. The EPA has categorized malathion as a pesticide with hazard class III and a general use pesticide (GUP) [186]. It is commonly utilized because, as compared to other organophosphorus insecticides, it has a comparatively low toxicity to mammals and a high selectivity for insects [186]. Malathion can linger in the ecosystem for months, although it typically degrades chemically and microbiologically after a few weeks when it is present in water [186]. The physical and chemical characteristics of the sewage system, notably temperature, and pH, as well as the makeup of the microbial population living there, determine the rate and degree of its breakdown [186]. Pesticide wastewater is treated using a variety of techniques, including chemical, physical, thermal, and biological ones [184]. However, these methods use low concentrations of contaminants and are neither economical nor environmentally beneficial [184]. The extreme toxicity of pesticides makes biological techniques of degradation particularly challenging [184]. For the elimination of pesticides, a variety of methodologies have been described recently, including photocatalytic degradation, nanofiltration, EC, biodegradation, electrochemical reduction, indirect electro-oxidation with powerful oxidants, and adsorption [186]. The EC procedure has gained more attention in the last ten years as a potentially effective way to effectively remove organophosphorus pesticides from wastewater [186]. M. Behloul et al. used EC to remove the malathion from contaminated solutions [186]. The efficient working parameters of an applied current of 1 A, operation period of 60 min, supporting electrolyte content of 2.5 g/L, and beginning pH of 6 resulted in almost complete elimination of malathion (95%) [186]. Sankar et al. used single-variable multivariable optimization to assess the impact of several independent operational factors on the removal of pesticide malathion by the EC method [184]. The reaction period of 75 min, applied voltage of 15 Volts, solution pH of 7.5, and electrolyte of 15 mL was found to be the ideal operational parameters [184]. An 85% elimination efficiency was attained [184]. Abdel-Gawad et al. removed some pesticides from wastewater via the Ethe C process using Fe electrodes [196]. The results showed that the removal efficiency of malathion was 100% [196].
Acetamiprid
The neonicotinoid group, one of the fastest-growing families of pesticides today, includes acetamiprid, one of the most often used third-generation pesticides [183]. Acetamiprid is used to manage a variety of coleopteran pests, as well as sucking pest insects, such as whiteflies, aphids, leaves, thrips, plant hoppers, various micro-Lepidoptera, and other insects. The compound may have developmental neurotoxicity (DNT), which is comparable to nicotine in how it affects the development and function of neurons in animals, according to research performed by the European Panel on Plant Protection Products (PPR) [183]. John et al. studied the EC method for removing acetamiprid from wastewater [183]. Under the current density of 0.5 A/dm2, duration of 60 min, pH of 7.77, and salt content (NaCl) of 0.75 g/L, the removal efficiency was 97.6% [183].
Oxyfluorfen
It has been documented that oxyfluorfen breaks down in soil and water [197]. In the instance of oxyfluorfen, the ions produced aggregate all over the pesticide colloids, neutralizing their electric charge on the surface and producing bigger particles that are then removed from the treated wastewater using flotation, filtering, or sedimentation techniques [197]. The colloids can also be entangled into the developing precipitated coagulant and separated via the same processes, depending mostly on operating conditions [197]. Regardless of the method used, EC has been reported to achieve a considerable contaminant accumulation within the flocs of 25%, but is not adequate for the entire elimination of oxyfluorfen from wastewater [197]. As a result, it is promising as a pretreatment for waste of this kind [197].
Diazinon
Water-containing insecticides, such as diazinon (O,O-diethyl O-2-isopropy-6-methylpyrimidin-4-yl phosphorothioate), can have major environmental consequences, as well as immediate or even delayed effects on human health due to their extensive use in agriculture and hazardous effects [187,198]. One of the most extensively used „moderately toxic” Class II organophosphate insecticides is diazinon, according to the World Health Organization (WHO) [199]. Many farmers use diazinon to kill pests on vegetables, fruits, and field crops; however, an excessive amount of this pesticide can be harmful to blood and other living things [198]. To prevent the toxicant from contaminating groundwater or seawater, the amount of consumption should be calculated carefully [198]. Amooey et al. removed diazinon from wastewater using the EC [198]. The removal efficiency was 89% after 35 min of process at pH 3 [198]. E. Bazrafshan et al. studied the optimum removal of diazinon from wastewater [199]. The results showed that the removal efficiency reached 84.6% at optimum parameters, the concentration of 100 mg/L, and the voltage of 20 Volts [199]. G. Hosseini et al. removed diazinon from aqueous media using EC [187]. The results showed that the removal efficiency reached 87% (0.85 mg mass removal) at a pH in the range of 6.5–7 [187].
The EC’s primary interaction is a physical interaction that is based on the destabilization by different mechanisms, such as electrostatic interactions or charge neutralization, and adsorption, as discussed earlier. The heat can cause a secondary chemical interaction by breaking bonds and bond formation with the cations to form flocks of new compounds. Heidari et al. studied the possible degradation mechanisms of diazinon by the electro-Fenton process. They reported that might be oxidation and hydroxylation reactions [200]. Therefore, different compounds will exist, namely, (IUPAC) (O,O-diethyl O-(2-hydroxy-6-methylpyrimidin-4-yl) phosphorothioate) and (diethyl (2-isopropyl-6-methylpyrimidin-4-yl) phosphate), due to hydroxylation and oxidation, respectively. Moreover, by breaking P-O bonds, three other simple compounds can be formed, which are (2-isopropyl-6-methylpyrimidin-4-ol), (O,O-diethyl O-hydrogen phosphorothioate), and (diethyl hydrogen phosphate). This possible pathway is demonstrated in Figure 8. However, these compounds might also be oxidized and adsorbed on the metal hydroxide surface or exposed to coagulation by metal cation if the phosphate ions are separated. In addition, further investigations are needed with analyzing the formed sludge using XRD to estimate the composition and crystal structure of the formed solids.
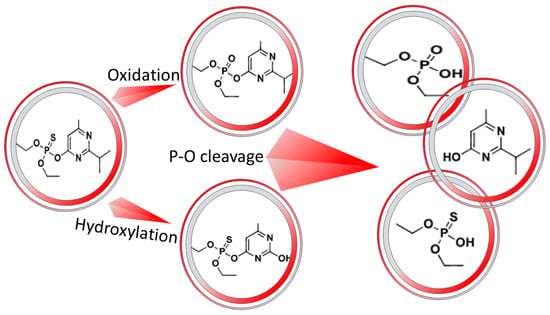
Figure 8.
The degradation pathway of Diazinon through oxidation and hydroxylation mechanisms in addition to the cleavage of P-O bond.
Chlorpyrifos
Another organophosphorus pesticide, chlorpyrifos (O, O-diethyl O-(3, 5, 6-trichloro-2-pyridyl)-phosphorothioate), is frequently used to manage pests, such as the white grub, or holotrichi consanguine blanch, that typically harms groundnut crops [196,201]. Due to its broad usage, longevity, and widespread dispersion, chlorpyrifos has considerable negative consequences on the ecosystem [196,201]. It causes vomiting, autoimmune and developmental issues, and damage to the central nervous and lung damage [201]. Chlorpyrifos has been found in newborn infants’ meconium, human breast milk, cervical fluid, cord blood, and sperm [188]. Abdel-Gawad et al. removed some pesticides from wastewater via the Ethe C process using Fe electrodes [196]. The results showed that the removal efficiency of chlorpyrifos was 97% [196]. Saini et al. removed chlorpyrifos from wastewater using EC/flocculation [201]. The results showed that using Al and FeCl3, the CPF elimination was 79% and 82%, respectively, at the optimum pH [201].
2.7.2. Humic Acids
Humic acids are important to deliver nutrients from the soil to the plants. They enlarge nutrient uptake and microbial activity through the soil. However, the process of treating sewage is made increasingly challenging by the inclusion of organic materials, such as humic acids. Heavy metal and other similar contaminant elimination can be dramatically impacted [202]. Even while humic acid is not inherently dangerous, it could also affect how insecticides and heavy metals, as well as other toxins, function in the ecosystem [202]. It also serves as a precursor for disinfection byproducts that are both carcinogenic and mutagenic, such as trihalomethanes and haloacetic acids [202]. It is one of the primary elements of organic materials dispersed in aquatic environments [203]. This will interact with chlorine during the chlorination method of treating the water supply to produce several teratogenic and cancer-causing substances, including haloacetic acid, haloketone, haloacetonitrile, trihalomethane, and chloroacetaldehyde, that are hazardous to the public [203]. The sewage treatment industries are now interested in finding ways to successfully eliminate humic acid from drinkable water without introducing additional contamination. The creation of novel methods and devices is essential going forward [203]. Xu et al. used a hybrid EC process to eliminate humic acid; the reaction took one hour, with a pH of 9.3, and the current density was 40 A/m2. The removal efficiency was 99% from an initial concentration of 30 mg/L, as in Table 7 [204]. Rajaei et al. managed to reach a 90%+ elimination rate of humic acid within 25 min. They used a combination of Fe and Al as the anode and the cathode, but when the Fe was both anode and cathode, the elimination rate was 99.4% with the same conditions [205]. They kept the current density at 4.5 mA/cm2; when the electrodes were Al, the elimination efficiency was 96.1% [205]. Barhoumi et al. reached an elimination efficiency of 93% within just 10 min [206]. They set the initial concentration to be 150 mg/L, and the current density was 1.388 mA/cm2 [206].

Table 7.
Using EC for removal of pesticides and humic acid from aqueous solutions in different previous studies.
3. Challenges
Even though EC has been utilized for an extended period, the existing literature does not give an organized approach to EC design, as well as operation. On a laboratory scale, EC is effectively utilized for purifying water and wastewater. Nevertheless, scaling up to meet the demands of the industry is difficult because present studies focus primarily on the elimination of particular contaminants from wastewater using sequential experiments [46]. Constant models will make it easier to forecast the efficiency of EC reactors while creating them, as well as provide a better knowledge of the design factors, enabling the technology development to move beyond its current impracticality.
As a result, the commercialization of such technology will be determined by its ability to meet business objectives, such as minimizing expenses for operation and upkeep and earning a profit from investment in the shortest amount of time. The technology is also up against proven water treatment methods, such as adsorption, and membrane systems. Merging the EC technique with current technologies would increase the likelihood of success.
The process of passivation of the electrodes is an important obstacle to the acceptance of EC since it has been shown that the homogeneous, planar shape of the electrode transforms into a heterogeneous shape with overuse [50]. The passive coating that forms on the outermost layer of electrodes raises the consumption of electricity, while decreasing the pollutant removal performance. Whenever DC is applied, an inert oxide coating develops on the cathode surface, which limits efficient current transfer between both electrodes. On the other hand, alternating current (AC) permits both the cathode and anode to be switched at regular intervals, essentially overcoming the shortcomings of the DC-EC method [210]. AC is more affordable than DC, which is generally attributed to less electrode passivation. The delay in cathode passivation and anode degradation demonstrates that AC has a satisfactory operating lifespan.
4. Summary and Path Forward
The EC technique is extremely effective with various types and classifications of drugs, as mentioned above. Developing this technique with more investigations by optimizing the parameters can enhance and improve removal efficiency. Further, this technique can be a part of a series of operating techniques for optimal results. However, there are lacks that should be mentioned. There is a lack of studying the effect of EC on many types of drugs, and that is acceptable, but EC can give different results for the different members of the same family. As an example, in the penicillin family, it is not effective against AMP, while it might be extremely effective against AMX antibiotics. That means the same family, not the same results, which are quietly understandable due to the physical and chemical details, such as chemical structure, surface charges, and the bonding energies between the atoms. There is a lack of investigation into the effect of EC on benzylpenicillin (Penicillin G), nafcillin, oxacillin, etc., as well as all the second generations of cephalosporin, most of all glucocorticoids, and almost all anticonvulsants. These points are mentioned due to their family’s existence in the current articles. Therefore, more investigations are needed for more drugs to detect the lack of effectiveness and being treated. The current study strengthens previous research by incorporating a full list of pollutants, while distinguishing between their categories. Other emerging organic pollutants, such as those associated with runoff from farms, are also addressed.
Author Contributions
Conceptualization, M.A. and S.A.; writing—original draft preparation, E.Y. and M.A.M.; writing—review and editing, M.A., S.A., E.Y., M.A.M. and J.B.; supervision, J.B.; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This material is based upon work supported by the National Science Foundation under Grant No. CHE 1710120.
Data Availability Statement
No data were used during this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Zhang, Y.; Zhou, L.; Zhang, F.; Jing, Y.; Huang, M.; Liu, H. Quality-related monitoring of papermaking wastewater treatment processes using dynamic multiblock partial least squares. J. Bioresour. Bioprod. 2022, 7, 73. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the World water development report. npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Bank, T.W. The total population from 1960 to 2021. 2022. Available online: https://data.worldbank.org/indicator/SP.POP.TOTL2020 (accessed on 17 April 2023).
- Skaf, L.; Buonocore, E.; Dumontet, S.; Capone, R.; Franzese, P.P. Food security and sustainable agriculture in Lebanon: An environmental accounting framework. J. Clean. Prod. 2019, 209, 1025–1032. [Google Scholar] [CrossRef]
- Haryani, G. Sustainable use and conservation of inland water ecosystem in Indonesia: Challenge for fisheries management in lake and river ecosystem. IOP Conf. Ser. Earth Environ. Sci. 2021, 789, 012023. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Abou-Shady, A. Recycling of polluted wastewater for agriculture purpose using electrodialysis: Perspective for large scale application. Chem. Eng. J. 2017, 323, 1–18. [Google Scholar] [CrossRef]
- Weerasekara, P. The United Nations world water development report 2017 wastewater. Future Food J. Food Agric. Soc. 2017, 5, 80–81. [Google Scholar]
- Yasasve, M.; Manjusha, M.; Manojj, D.; Hariharan, N.; Preethi, P.S.; Asaithambi, P.; Karmegam, N.; Saravanan, M. Unravelling the emerging carcinogenic contaminants from industrial waste water for prospective remediation by electrocoagulation—A review. Chemosphere 2022, 307, 136017. [Google Scholar] [CrossRef] [PubMed]
- Poel, P.V.D.; Brooke, D.N.; Leeuwen, C.J.V. Emissions of Chemicals to The Environment, in Risk Assessment of Chemicals: An Introduction; Leeuwen, C.J.V., Vermeire, T.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 37–71. [Google Scholar]
- Sany SB, T.; Tajfard, M.; Rezayi, M.; Rahman, M.A.; Hashim, R. Chapter 20—The West Coast of Peninsular Malaysia. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 437–458. [Google Scholar]
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Taylor, D. Transboundary atmospheric pollution in Southeast Asia: Current methods, limitations and future developments. Crit. Rev. Environ. Sci. Technol. 2018, 48, 997–1029. [Google Scholar] [CrossRef]
- He, S.; Wu, J.; Wang, D.; He, X. Predictive modeling of groundwater nitrate pollution and evaluating its main impact factors using random forest. Chemosphere 2022, 290, 133388. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Feng, C.; Yu, L.; Xiao, Y.; An, C. Environmental Behavior and Effects of Pollutants in Water. J. Chem. 2020, 2020, 2639389. [Google Scholar] [CrossRef]
- Blum, P.; Menberg, K.; Koch, F.; Benz, S.A.; Tissen, C.; Hemmerle, H.; Bayer, P. Is thermal use of groundwater a pollution? J. Contam. Hydrol. 2021, 239, 103791. [Google Scholar] [CrossRef]
- Hepler, K.; Kaminski, M.D.; Jolin, W.C.; Magnuson, M. Decontamination of urban surfaces contaminated with radioactive materials and consequent onsite recycling of the waste water. Environ. Technol. Innov. 2021, 21, 101177. [Google Scholar] [CrossRef]
- Atangana, A. Chapter 3—Groundwater Pollution. In Fractional Operators with Constant and Variable Order with Application to Geo-Hydrology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- National Primary Drinking Water Regulations. 2023; [cited 9/01/2023]. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 17 April 2023).
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Huang, D.; Hu, C.; Zeng, G.; Cheng, M.; Xu, P.; Gong, X.; Wang, R.; Xue, W. Combination of Fenton processes and biotreatment for wastewater treatment and soil remediation. Sci. Total. Environ. 2017, 574, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, Z.; Zhang, Q.; Hu, J.; Xiang, W. Steam gasification of sewage sludge with CaO as CO2 sorbent for hydrogen-rich syngas production. Biomass-Bioenergy 2017, 107, 52–62. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Islam, M.S.; Hossain, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. A novel and highly efficient Ag and GO co-synthesized ZnO nano photocatalyst for methylene blue dye degradation under UV irradiation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100495. [Google Scholar]
- Chequer, F.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Carvalho, J.; Zanoni, M.B.; de Oliveir, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Akter, S.; Suhan, M.B.K.; Islam, M.S. Recent advances and perspective of electrocoagulation in the treatment of wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100643. [Google Scholar] [CrossRef]
- Kadier, A.; Al-Qodah, Z.; Akkaya, G.K.; Song, D.; Peralta-Hernández, J.M.; Wang, J.-Y.; Phalakornkule, C.; Bajpai, M.; Niza, N.M.; Gilhotra, V.; et al. A state-of-the-art review on electrocoagulation (EC): An efficient, emerging, and green technology for oil elimination from oil and gas industrial wastewater streams. Case Stud. Chem. Environ. Eng. 2022, 6, 100274. [Google Scholar] [CrossRef]
- Manikandan, S.; Saraswathi, R. Electrocoagulation technique for removing Organic and Inorganic pollutants (COD) from the various industrial effluents: An overview. Environ. Eng. Res. 2023, 28, 220231. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Treviño-Reséndez, J.D.J.; Medel, A.; Meas, Y. Electrochemical technologies for treating petroleum industry wastewater. Curr. Opin. Electrochem. 2021, 27, 100690. [Google Scholar] [CrossRef]
- Othmani, A.; Kadier, A.; Singh, R.; Igwegbe, C.A.; Bouzid, M.; Aquatar, O.; Khanday, W.A.; Bote, M.E.; Damiri, F.; Gökkuş, Ö.; et al. A comprehensive review on green perspectives of electrocoagulation integrated with advanced processes for effective pollutants removal from water environment. Environ. Res. 2022, 215, 114294. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Naghdali, Z.; Al-Qodah, Z.; Alizadeh, S.; Niaragh, E.K.; Malekmohammadi, S.; Nidheesh, P.; Roberts, E.P.; Sillanpää, M.; Emamjomeh, M.M. A systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Technol. Assess. 2021, 47, 101353. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Abdulkhadher, R.K.; Jaeel, A.J. Removal of fluoride, nitrates and phosphor from drinking water using electrocoagulation: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1058, 012050. [Google Scholar] [CrossRef]
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability 2022, 14, 1985. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process. Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Castañeda, L.F.; Rodríguez, J.F.; Nava, J.L. Electrocoagulation as an affordable technology for decontamination of drinking water containing fluoride: A critical review. Chem. Eng. J. 2021, 413, 127529. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process. Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Ghernaout, D.; Alghamdi, A.; Ghernaout, B. Electrocoagulation process: A mechanistic review at the dawn of its modeling. J. Environ. Sci. Allied Res. 2019, 2, 23–31. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A critical review in electrocoagulation technology applied for oil removal in industrial wastewater. Chemosphere 2022, 288, 132355. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, C.; Li, H.; Offiong, N.-A.O.; Bi, Y.; Zhou, R.; Ren, H. A systematic review of electrocoagulation technology applied for microplastics removal in aquatic environment. Chem. Eng. J. 2023, 456, 141078. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.-T.; Yeh, R.Y.-L.; Al Ahmad, M.S. Electrocoagulation in Wastewater Treatment. Water 2011, 3, 495–525. [Google Scholar] [CrossRef]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Al-Raad, A.A.; Hanafiah, M.M.; Naje, A.S.; Ajeel, M.A.; Basheer, A.O.; Aljayashi, T.A.; Toriman, M.E. Treatment of Saline Water Using Electrocoagulation with Combined Electrical Connection of Electrodes. Processes 2019, 7, 242. [Google Scholar] [CrossRef]
- Patel, S.K.; Shukla, S.C.; Natarajan, B.; Prajapati, A.K. Treatment of Debrominated Wastewater by Electrocoagulation Using Mild Steel Electrodes. Mater. Proc. 2023, in press. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut. Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Lin, D.; Zhao, Q.; Hu, L.; Xing, B. Synthesis and characterization of cubic mesoporous bridged polysilsesquioxane for removing organic pollutants from water. Chemosphere 2014, 103, 188–196. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, L.-X.; Yu, W.-J.; Bao, J.; Li, T.-Y.; Hu, X.-M.; Zhao, X. Simultaneous removal of multiple PFAS from contaminated groundwater around a fluorochemical facility by the periodically reversing electrocoagulation technique. Chemosphere 2022, 307, 135874. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chiang, S.-Y.; Wang, Y.; Wang, Y.; Liang, S.; Zhou, J.; Fontanez, R.; Gao, S.; Huang, Q. An electrocoagulation and electrooxidation treatment train to remove and degrade per- and polyfluoroalkyl substances in aqueous solution. Sci. Total. Environ. 2021, 788, 147723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Hu, C.-Y.; Lee, Y.-C.; Lo, S.-L. Effects of zinc salt addition on perfluorooctanoic acid (PFOA) removal by electrocoagulation with aluminum electrodes. Chemosphere 2022, 288, 132665. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lo, S.-L.; Srivastava, V.C.; Qiao, Q.; Sharma, P. Mineralization of perfluorooctanoic acid by combined aerated electrocoagulation and Modified peroxi-coagulation methods. J. Taiwan Inst. Chem. Eng. 2021, 118, 169–178. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, T.; Kim, T.-K.; Joo, S.-W.; Zoh, K.-D. Degradation mechanism of perfluorooctanoic acid (PFOA) during electrocoagulation using Fe electrode. Sep. Purif. Technol. 2020, 247, 116911. [Google Scholar] [CrossRef]
- Mu, T.; Park, M.; Kim, K.-Y. Energy-efficient removal of PFOA and PFOS in water using electrocoagulation with an air-cathode. Chemosphere 2021, 281, 130956. [Google Scholar] [CrossRef]
- Nippatlapalli, N.; Selvaraj, A. A study on the removal of long chain perflourodecanoic acid in simulated aqueous solution using enhanced electrochemical technique: Metabolites, kinetic and isotherm model analysis. J. Water Process. Eng. 2022, 49, 103045. [Google Scholar] [CrossRef]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Li, M.; Mo, C.-H.; Luo, X.; He, K.-Y.; Yan, J.-F.; Wu, Q.; Yu, P.-F.; Han, W.; Feng, N.-X.; Yeung, K.L.; et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process. Water Res. 2021, 207, 117849. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.-J.; Liu, Y.; Wang, X.; Liu, Z.-Q.; Duan, Y.-F. Removal of perfluoroalkanesulfonic acids (PFSAs) from synthetic and natural groundwater by electrocoagulation. Chemosphere 2020, 248, 125951. [Google Scholar] [CrossRef]
- Yang, B.; Han, Y.; Yu, G.; Zhuo, Q.; Deng, S.; Wu, J.; Zhang, P. Efficient removal of perfluoroalkyl acids (PFAAs) from aqueous solution by electrocoagulation using iron electrode. Chem. Eng. J. 2016, 303, 384–390. [Google Scholar] [CrossRef]
- Sana, T.; Chowdhury, M.I.; Logeshwaran, P.; Megharaj, M. Behavioural, developmental and reproductive toxicological impacts of perfluorobutanoic acid (PFBA) in Caenorhabditis elegans. Environ. Chall. 2023, 10, 100662. [Google Scholar] [CrossRef]
- Abraham, K.; El-Khatib, A.H.; Schwerdtle, T.; Monien, B.H. Perfluorobutanoic acid (PFBA): No high-level accumulation in human lung and kidney tissue. Int. J. Hyg. Environ. Health 2021, 237, 113830. [Google Scholar] [CrossRef]
- Xue, X.; Gao, N.; Xu, F. Toxicity of perfluooctane sulfonate (PFOS) and perfluorobutane sulfonate (PFBS) to Scenedesmus obliquus: Photosynthetic characteristics, oxidative damage and transcriptome analysis. Environ. Pollut. 2022, 315, 120397. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Mu, H.; Li, H.; Liu, S.; Zhu, M.; Bu, Y.; Wu, B. Effects of perfluorobutane sulfonate and perfluorooctane sulfonate on lipid homeostasis in mouse liver. Environ. Pollut. 2022, 315, 120403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Peng, Y.; Ning, K.; Niu, X.; Tan, S.; Su, P. Remediation of Perfluoroalkyl Substances in Landfill Leachates by Electrocoagulation. CLEAN Soil Air Water 2014, 42, 1740–1743. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Chen, X.; He, Y.; Bolan, N.; Rinklebe, J.; Lam, S.S.; Peng, W.; Sonne, C. A discussion of microplastics in soil and risks for ecosystems and food chains. Chemosphere 2023, 313, 137637. [Google Scholar] [CrossRef]
- Fan, S.; Yan, Z.; Qiao, L.; Gui, F.; Li, T.; Yang, Q.; Zhang, X.; Ren, C. Biological effects on the migration and transformation of microplastics in the marine environment. Mar. Environ. Res. 2023, 185, 105875. [Google Scholar] [CrossRef]
- Xiong, F.; Liu, J.; Xu, K.; Huang, J.; Wang, D.; Li, F.; Wang, S.; Zhang, J.; Pu, Y.; Sun, R. Microplastics induce neurotoxicity in aquatic animals at environmentally realistic concentrations: A meta-analysis. Environ. Pollut. 2023, 318, 120939. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Fang, S.; Song, B.; Cao, P.; Liu, H.; Yang, Y. Removal and toxic forecast of microplastics treated by electrocoagulation: Influence of dissolved organic matter. Chemosphere 2022, 308, 136309. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, Z.; Zeng, G. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Microplastic pollution in wastewater treatment plants in the city of Cádiz: Abundance, removal efficiency and presence in receiving water body. Sci. Total Environ. 2021, 776, 145795. [Google Scholar] [CrossRef]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, D.; Oyanedel-Craver, V.; Carissimi, E. Electrocoagulation applied for the removal of microplastics from wastewater treatment facilities. Sep. Purif. Technol. 2021, 276, 118877. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.; Niu, Y.; Xu, D.; Wang, J.; Han, J.; Wang, H. Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation. Sep. Purif. Technol. 2022, 290, 120905. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Bajpai, M.; Katoch, S.S.; Kadier, A.; Ma, P.-C. Treatment of pharmaceutical wastewater containing cefazolin by electrocoagulation (EC): Optimization of various parameters using response surface methodology (RSM), kinetics and isotherms study. Chem. Eng. Res. Des. 2021, 176, 254–266. [Google Scholar] [CrossRef]
- Oulebsir, A.; Chaabane, T.; Tounsi, H.; Omine, K.; Sivasankar, V.; Flilissa, A.; Darchen, A. Treatment of artificial pharmaceutical wastewater containing amoxicillin by a sequential electrocoagulation with calcium salt followed by nanofiltration. J. Environ. Chem. Eng. 2020, 8, 104597. [Google Scholar] [CrossRef]
- Coutu, S.; Wyrsch, V.; Wynn, H.; Rossi, L.; Barry, D. Temporal dynamics of antibiotics in wastewater treatment plant influent. Sci. Total. Environ. 2013, 458–460, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, K.; Cherukuri, J.; Anji Reddy, M. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process. Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total. Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef]
- Zaidi, S.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Maachi, R.; Msagati, T. Electro-coagulation coupled electro-flotation process: Feasible choice in doxycycline removal from pharmaceutical effluents. Arab. J. Chem. 2019, 12, 2798–2809. [Google Scholar] [CrossRef]
- Oliveira, J.T.; de Sousa, M.C.; Martins, I.A.; de Sena, L.M.G.; Nogueira, T.R.; Vidal, C.B.; Neto, E.F.A.; Romero, F.B.; Campos, O.S.; do Nascimento, R.F. Electrocoagulation/oxidation/flotation by direct pulsed current applied to the removal of antibiotics from Brazilian WWTP effluents. Electrochim. Acta 2021, 388, 138499. [Google Scholar] [CrossRef]
- Balarak, D.; Chandrika, K.; Attaolahi, M. Assessment of effective operational parameters on removal of Amoxicillin from synthetic wastewater using electrocoagulation process. J. Pharm. Res. Int. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Wang, C.-J.; Li, Z.; Jiang, W.-T.; Jean, J.-S.; Liu, C.-C. Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 2010, 183, 309–314. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ghotaslou, R.; Rezaee, M.A.; Moshafi, M.H.; Hojabri, Z.; Saffari, F. Determination of antimicrobial resistance profile and inducible clindamycin resistance of coagulase negative staphylococci in pediatric patients: The first report from Iran. World J. Pediatr. 2015, 11, 250–254. [Google Scholar] [CrossRef]
- Fasihi, Y.; Saffari, F.; Ghahraman, M.R.K.; Kalantar-Neyestanaki, D. Molecular detection of macrolide and lincosamide-resistance genes in clinical methicillin-resistant Staphylococcus aureus isolates from Kerman, Iran. Arch. Pediatr. Infect. Dis. 2017, 5, e37761. [Google Scholar] [CrossRef]
- Yoosefian, M.; Ahmadzadeh, S.; Aghasi, M.; Dolatabadi, M. Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption. J. Mol. Liq. 2017, 225, 544–553. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process. Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Malakootian, M.; Ahmadian, M. Removal of ciprofloxacin from aqueous solution by electro-activated persulfate oxidation using aluminum electrodes. Water Sci. Technol. 2019, 80, 587–596. [Google Scholar] [CrossRef]
- Mohammed, S.J.; M-Ridha, M.J.; Abed, K.M.; Elgharbawy, A.A.M. Removal of levofloxacin and ciprofloxacin from aqueous solutions and an economic evaluation using the electrocoagulation process. Int. J. Environ. Anal. Chem. 2021, 1–19. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Mohammed-Ridha, M. Optimization of levofloxacin removal from aqueous solution using electrocoagulation process by response surface methodology. Iraqi J. Agric. Sci. 2021, 52, 204–217. [Google Scholar] [CrossRef]
- Benrighi, Y.; Nasrallah, N.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Baaloudj, O. Photocatalytic performances of ZnCr2O4 nanoparticles for cephalosporins removal: Structural, optical and electrochemical properties. Opt. Mater. 2021, 115, 111035. [Google Scholar] [CrossRef]
- Mostafaloo, R.; Mahmoudian, M.H.; Asadi-Ghalhari, M. BiFeO3/Magnetic nanocomposites for the photocatalytic degradation of cefixime from aqueous solutions under visible light. J. Photochem. Photobiol. A Chem. 2019, 382, 111926. [Google Scholar] [CrossRef]
- Asadi-Ghalhari, M.; Mostafaloo, R.; Ghafouri, N.; Kishipour, A.; Usefi, S.; Baaloudj, O. Removal of Cefixime from aqueous solutions via proxy electrocoagulation: Modeling and optimization by response surface methodology. React. Kinet. Catal. Lett. 2021, 134, 459–471. [Google Scholar] [CrossRef]
- Mostafaloo, R.; Yari, A.R.; Mohammadi, M.J.; Khaniabadi, Y.O.; Asadi-Ghalhari, M. Optimization of the electrocoagulation process on the effectiveness of removal of Cefixime antibiotic from aqueous solutions. Desalination Water Treat. 2019, 144, 138–144. [Google Scholar] [CrossRef]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Zaidi, S.; Chaabane, T.; Sivasankar, V.; Darchen, A.; Maachi, R.; Msagati, T.; Prabhakaran, M. Performance efficiency of electro-coagulation coupled electro-flotation process (EC-EF) versus adsorption process in doxycycline removal from aqueous solutions. Process. Saf. Environ. Prot. 2016, 102, 450–461. [Google Scholar] [CrossRef]
- Arsand, D.R.; Kümmerer, K.; Martins, A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total. Environ. 2013, 443, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kothgassner, O.D.; Pellegrini, M.; Goreis, A.; Giordano, V.; Edobor, J.; Fischer, S.; Plener, P.L.; Huscsava, M.M. Hydrocortisone administration for reducing post-traumatic stress symptoms: A systematic review and meta-analysis. Psychoneuroendocrinology 2021, 126, 105168. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale: The Complete Drug Reference; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- AlAani, H.; Hashem, S.; Karabet, F. Photocatalytic (UV-A/TiO2) and photolytic (UV-A) degradation of steroid hormones: Ethinyl Estradiol, Levonorgestrel, and Progesterone. Int. J. ChemTech Res. 2017, 10, 1061–1070. [Google Scholar]
- Alaani, H.; Hashem, S.; Karabet, F. Electrochemical removal of hydrocortisone from aqueous environments using aluminum electrodes. Orient. J. Chem. 2018, 34, 203–213. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.; Grandjean, D.; Tarradellas, J. Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A. Mechanisms of action of antiepileptic drugs. Seizure 1995, 4, 267–271. [Google Scholar] [CrossRef]
- Xiao, Z.; Cui, T.; Wang, Z.; Dang, Y.; Zheng, M.; Lin, Y.; Song, Z.; Wang, Y.; Liu, C.; Xu, B.; et al. Energy-efficient removal of carbamazepine in solution by electrocoagulation-electrofenton using a novel P-rGO cathode. J. Environ. Sci. 2022, 115, 88–102. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C., Jr. Applicability of the electrocoagulation process in treating real municipal wastewater containing pharmaceutical active compounds. J. Hazard. Mater. 2019, 361, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Mustafa, F.S.; Ezugwu, O.N.; Gazi, M. Efficient removal of antibiotic in single and binary mixture of nickel by electrocoagulation process: Hydrogen generation and cost analysis. Chemosphere 2022, 300, 134532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, F.; Du, X.; Zhang, C.; Yang, C.; Offiong, N.-A.; Bi, Y.; Zeng, W.; Ren, H. Removal of metronidazole from wastewater by electrocoagulation with chloride ions electrolyte: The role of reactive chlorine species and process optimization. Sep. Purif. Technol. 2022, 290, 120799. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, J.; Yang, C.; Hu, Z.; Liu, F.; Zhang, C. Removal of tetracycline from livestock wastewater by positive single pulse current electrocoagulation: Mechanism, toxicity assessment and cost evaluation. Sci. Total. Environ. 2022, 810, 151955. [Google Scholar] [CrossRef] [PubMed]
- Negarestani, M.; Motamedi, M.; Kashtiaray, A.; Khadir, A.; Sillanpää, M. Simultaneous removal of acetaminophen and ibuprofen from underground water by an electrocoagulation unit: Operational parameters and kinetics. Groundw. Sustain. Dev. 2020, 11, 100474. [Google Scholar] [CrossRef]
- Maher, E.K.; O’Malley, K.N.; Heffron, J.; Huo, J.; Mayer, B.K.; Wang, Y.; McNamara, P.J. Analysis of operational parameters, reactor kinetics, and floc characterization for the removal of estrogens via electrocoagulation. Chemosphere 2019, 220, 1141–1149. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Biserova-Tahchieva, A.; Llorca-Isern, N. Removal of dyes, oils, alcohols, heavy metals and microplastics from water with superhydrophobic materials. Chemosphere 2022, 311 Pt 2, 137148. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-based materials and their adsorptive removal efficiency for dyes: A review. Int. J. Biol. Macromol. 2022, 224, 1337–1355. [Google Scholar] [CrossRef]
- Guvenc, S.Y.; Can-Güven, E.; Varank, G. Persulfate enhanced electrocoagulation of paint production industry wastewater: Process optimization, energy consumption, and sludge analysis. Process. Saf. Environ. Prot. 2022, 157, 68–80. [Google Scholar] [CrossRef]
- Taheri, M. Techno-economical aspects of electrocoagulation optimization in three acid azo dyes’ removal comparison. Clean. Chem. Eng. 2022, 2, 100007. [Google Scholar] [CrossRef]
- Abbasi, S.; Mirghorayshi, M.; Zinadini, S.; Zinatizadeh, A.A. A novel single continuous electrocoagulation process for treatment of licorice processing wastewater: Optimization of operating factors using RSM. Process. Saf. Environ. Prot. 2020, 134, 323–332. [Google Scholar] [CrossRef]
- Abbasi, S.; Zinatizadeh, A.; Mirghorayshi, M.; Zinadini, S.; McKay, T. Electrocoagulation technique for continuous industrial licorice processing wastewater treatment in a single reactor employing Fe-rod electrodes: Process modeling and optimization and operating cost analysis. J. Environ. Chem. Eng. 2022, 10, 106686. [Google Scholar] [CrossRef]
- Irki, S.; Ghernaout, D.; Naceur, M.W. Decolourization of Methyl Orange (MO) by electrocoagulation (EC) using iron electrodes under a magnetic field (MF). Desalination Water Treat. 2017, 79, 368–377. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Luo, X. Studies on degradation of Methyl Orange wastewater by combined electrochemical process. J. Hazard. Mater. 2007, 149, 492–498. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, D.-S. Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J. Mol. Liq. 2017, 240, 329–339. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surfaces Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Abdulrazzaq, N.N.; Al-Sabbagh, B.H.; Shanshool, H.A. Coupling of electrocoagulation and microflotation for the removal of textile dyes from aqueous solutions. J. Water Process. Eng. 2021, 40, 101906. [Google Scholar] [CrossRef]
- Ravadelli, M.; da Costa, R.E.; Lobo-Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/oxic membrane bioreactor assisted by electrocoagulation for the treatment of azo-dye containing wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- Kalivel, P.; Jagadeesh, T.; Kavitha, S.; Padmanabhan, D.; Palanichamy, J.; Murphy, A. Comparative study on removal of yellow 10gw dye from aqueous solution using Al, Cu electrodes in electrocoagulation. Mater. Today Proc. 2021, 47, 807–813. [Google Scholar] [CrossRef]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.B.; Selvakumar, P.; Alemayehu, E. Enhanced treatment of landfill leachate wastewater using sono(US)-ozone(O3)–electrocoagulation(EC) process: Role of process parameters on color, COD and electrical energy consumption. Process Saf. Environ. Prot. 2020, 142, 212–218. [Google Scholar] [CrossRef]
- Moneer, A.A.; El-Mallah, N.M.; Ramadan, M.S.; Shaker, A.M. Removal of Acid Green 20 and Reactive Yellow 17 dyes by aluminum electrocoagulation technique in a single and a binary dye system. Egypt. J. Aquat. Res. 2021, 47, 223–230. [Google Scholar]
- Moneer, A.A.; El-Mallah, N.M.; El-Sadaawy, M.M.; Khedawy, M.; Ramadan, M.S. Kinetics, thermodynamics, isotherm modeling for removal of reactive Red 35 and disperse yellow 56 dyes using batch bi-polar aluminum electrocoagulation. Alex. Eng. J. 2021, 60, 4139–4154. [Google Scholar]
- Sorayyaei, S.; Raji, F.; Rahbar-Kelishami, A.; Ashrafizadeh, S.N. Combination of electrocoagulation and adsorption processes to remove methyl orange from aqueous solution. Environ. Technol. Innov. 2021, 24, 102018. [Google Scholar]
- Zhu, Z.; Rao, R.; Zhao, Z.; Chen, J.; Jiang, W.; Bi, F.; Yang, Y.; Zhang, X. Research progress on removal of phthalates pollutants from environment. J. Mol. Liq. 2022, 355, 118930. [Google Scholar]
- Ali, M.; Vellanki, B.P.; Shoaib, M.; Kazmi, A.A. Occurrence of Phthalates in common effluent treatment plants and removal by using Nano Titania-Graphene based photo-catalyst for tertiary treated effluent. J. Hazard. Mater. Adv. 2022, 8, 100192. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Keleş, A.; Ölmez-Hancı, T.; Tünay, O.; Arslan-Alaton, I. Treatment of phthalic acid esters by electrocoagulation with stainless steel electrodes using dimethyl phthalate as a model compound. J. Hazard. Mater. 2009, 171, 932–940. [Google Scholar]
- Yang, G.C.C.; Chen, Y.-C.; Yang, H.-X.; Yen, C.-H. Performance and mechanisms for the removal of phthalates and pharmaceuticals from aqueous solution by graphene-containing ceramic composite tubular membrane coupled with the simultaneous electrocoagulation and electrofiltration process. Chemosphere 2016, 155, 274–282. [Google Scholar]
- Werkneh, A.A.; Gebru, S.B. Development of ecological sanitation approaches for integrated recovery of biogas, nutrients and clean water from domestic wastewater. Resour. Environ. Sustain. 2023, 11, 100095. [Google Scholar]
- Larsen, T.; Maurer, M. 4.07—Source Separation and Decentralization; Wilderer, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 203–229. [Google Scholar]
- Benekos, A.K.; Zampeta, C.; Argyriou, R.; Economou, C.N.; Triantaphyllidou, I.-E.; Tatoulis, T.I.; Tekerlekopoulou, A.; Vayenas, D.V. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process. Saf. Environ. Prot. 2019, 131, 38–47. [Google Scholar]
- Villalobos-Lara, A.D.; Álvarez, F.; Gamiño-Arroyo, Z.; Navarro, R.; Peralta-Hernández, J.M.; Fuentes, R.; Pérez, T. Electrocoagulation treatment of industrial tannery wastewater employing a modified rotating cylinder electrode reactor. Chemosphere 2021, 264 Pt 2, 128491. [Google Scholar]
- Barzegar, G.; Wu, J.; Ghanbari, F. Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Process. Saf. Environ. Prot. 2019, 121, 125–132. [Google Scholar] [CrossRef]
- Ahangarnokolaei, M.A.; Attarian, P.; Ayati, B.; Ganjidoust, H.; Rizzo, L. Life cycle assessment of sequential and simultaneous combination of electrocoagulation and ozonation for textile wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106251. [Google Scholar] [CrossRef]
- Patel, R.K.; Shankar, R.; Khare, P.; Mondal, P. Treatment of sugar processing industry wastewater using copper electrode by electrocoagulation: Performance and economic study. J. Indian Chem. Soc. 2022, 99, 100563. [Google Scholar] [CrossRef]
- Can-Güven, E. Advanced treatment of dye manufacturing wastewater by electrocoagulation and electro-Fenton processes: Effect on COD fractions, energy consumption, and sludge analysis. J. Environ. Manag. 2021, 300, 113784. [Google Scholar] [CrossRef]
- Ahangarnokolaei, M.A.; Ayati, B.; Ganjidoust, H. Simultaneous and sequential combination of electrocoagulation and ozonation by Al and Fe electrodes for DirectBlue71 treatment in a new reactor: Synergistic effect and kinetics study. Chemosphere 2021, 285, 131424. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.B.; Selvakumar, P.; Alemayehu, E. Investigation of direct and alternating current–electrocoagulation process for the treatment of distillery industrial effluent: Studies on operating parameters. J. Environ. Chem. Eng. 2021, 9, 104811. [Google Scholar] [CrossRef]
- Bote, M.E. Studies on electrode combination for COD removal from domestic wastewater using electrocoagulation. Heliyon 2021, 7, e08614. [Google Scholar] [CrossRef]
- Chen, R.-F.; Wu, L.; Zhong, H.-T.; Liu, C.-X.; Qiao, W.; Wei, C.-H. Evaluation of electrocoagulation process for high-strength swine wastewater pretreatment. Sep. Purif. Technol. 2021, 272, 118900. [Google Scholar] [CrossRef]
- Das, P.P.; Anweshan, A.; Mondal, P.; Sinha, A.; Biswas, P.; Sarkar, S.; Purkait, M.K. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere 2021, 263, 128370. [Google Scholar] [CrossRef]
- Le, T.S.; Dang, N.M.; Tran, D.T. Performance of coupling electrocoagulation and biofiltration processes for the treatment of leachate from the largest landfill in Hanoi, Vietnam: Impact of operating conditions. Sep. Purif. Technol. 2021, 255, 117677. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Jin, X.; Song, J.; Jin, P.; Wei, Y.; Zhu, Y.; Liu, M.; Wang, X.C. Removal performance and membrane fouling mitigation mechanism of electrocoagulation membrane dissolved ozone flotation. J. Water Process. Eng. 2021, 43, 102289. [Google Scholar] [CrossRef]
- Reilly, M.; Cooley, A.P.; Richardson, B.; Tito, D.; Theodorou, M.K. Electrocoagulation of food waste digestate and the suitability of recovered solids for application to agricultural land. J. Water Process. Eng. 2021, 42, 102121. [Google Scholar] [CrossRef]
- Sediqi, S.; Bazargan, A.; Mirbagheri, S.A. Consuming the least amount of energy and resources in landfill leachate electrocoagulation. Environ. Technol. Innov. 2021, 22, 101454. [Google Scholar] [CrossRef]
- Pratiwi, N.I.; Mukimin, A.; Zen, N.; Septarina, I. Integration of electrocoagulation, adsorption and wetland technology for jewelry industry wastewater treatment. Sep. Purif. Technol. 2021, 279, 119690. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Sun, J.; Du, Y. Construction and application of an electrocoagulation and filtration linkage control system in a recirculating aquaculture system. J. Water Process. Eng. 2021, 44, 102379. [Google Scholar] [CrossRef]
- da Costa, P.R.; Costa, E.C.T.D.A.; Castro, S.S.; Fajardo, A.S.; Martínez-Huitle, C.A. A sequential process to treat a cashew-nut effluent: Electrocoagulation plus electrochemical oxidation. J. Electroanal. Chem. 2019, 834, 79–85. [Google Scholar] [CrossRef]
- Acharya, N.; Thakur, C.; Chaudhari, P. Dataset on statistical reduction of COD by electrocoagulation process using RSM. Data Brief 2020, 28, 104944. [Google Scholar] [CrossRef]
- Akansha, J.; Nidheesh, P.; Gopinath, A.; Anupama, K.; Kumar, M.S. Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 2020, 253, 126652. [Google Scholar] [CrossRef]
- Akhtar, A.; Aslam, Z.; Asghar, A.; Bello, M.M.; Raman, A.A.A. Electrocoagulation of Congo Red dye-containing wastewater: Optimization of operational parameters and process mechanism. J. Environ. Chem. Eng. 2020, 8, 104055. [Google Scholar] [CrossRef]
- Alkhatib, A.M.; Hawari, A.H.; Hafiz, M.A.; Benamor, A. A novel cylindrical electrode configuration for inducing dielectrophoretic forces during electrocoagulation. J. Water Process. Eng. 2020, 35, 101195. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Liu, Y.; Lian, Y.; Meng, L.; Su, Z. Removing refractory organic matter from nanofiltration concentrated landfill leachate by electrooxidation combined with electrocoagulation: Characteristics and implication for leachate management. J. Water Process. Eng. 2022, 47, 102747. [Google Scholar] [CrossRef]
- Xie, A.; Ladner, D.A.; Popat, S.C. Electrocoagulation-electroflotation for primary treatment of animal rendering wastewater to enable recovery of fats. Chem. Eng. J. 2022, 431, 133910. [Google Scholar] [CrossRef]
- Bener, S.; Bulca, Ö.; Palas, B.; Tekin, G.; Atalay, S.; Ersöz, G. Electrocoagulation process for the treatment of real textile wastewater: Effect of operative conditions on the organic carbon removal and kinetic study. Process. Saf. Environ. Prot. 2019, 129, 47–54. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Y.; Shen, L.; Li, R.; Lin, H. Preparation of nickel@polyvinyl alcohol (PVA) conductive membranes to couple a novel electrocoagulation-membrane separation system for efficient oil-water separation. J. Membr. Sci. 2022, 653, 120541. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Liu, Y.; Chen, M.; He, Y.; Edem, M.A.; Wang, T.; Chen, J. Optimization of an integrated electrocoagulation/sedimentation unit for purification of polymer-flooding sewage. J. Electroanal. Chem. 2019, 842, 193–202. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, W.; Wang, H.; Zhang, H.; Wang, J.; Yang, J.; Lin, D.; Liang, H. Ferrate-enhanced electrocoagulation/ultrafiltration system on municipal secondary effluent treatment: Identify synergistic contribution of coagulant and oxidation. Sep. Purif. Technol. 2022, 298, 121587. [Google Scholar] [CrossRef]
- Monteil, H.; Pechaud, Y.; Oturan, N.; Trellu, C.; Oturan, M.A. Pilot scale continuous reactor for water treatment by electrochemical advanced oxidation processes: Development of a new hydrodynamic/reactive combined model. Chem. Eng. J. 2021, 404, 127048. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Li, X.; Zhang, X.; Wang, Y. Electrocoagulation treatment of shale gas drilling wastewater: Performance and statistical optimization. Sci. Total. Environ. 2021, 794, 148436. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Yao, J.; Long, J. Industrial indigo dyeing wastewater purification: Effective COD removal with peroxi-AC electrocoagulation system. Arab. J. Chem. 2023, 16, 104607. [Google Scholar] [CrossRef]
- Karimi, N.; Mirbagheri, S.A.; Nouri, R.; Bazargan, A. Sequential application of aerated electrocoagulation and γ-Fe2O3 nanoparticle adsorption for COD removal: Consuming the least amount of energy and economic evaluation. Results Eng. 2023, 17, 100770. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N. Comparison of sludge settling velocity and filtration time after electrocoagulation process in treating industrial textile dyeing wastewater: RSM optimization. Int. J. Environ. Sci. Technol. 2019, 16, 3437–3446. [Google Scholar] [CrossRef]
- Núñez, J.; Yeber, M.; Cisternas, N.; Thibaut, R.; Medina, P.; Carrasco, C. Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry. J. Hazard. Mater. 2019, 371, 705–711. [Google Scholar] [CrossRef] [PubMed]
- GilPavas, E.; Correa-Sanchez, S. Assessment of the optimized treatment of indigo-polluted industrial textile wastewater by a sequential electrocoagulation-activated carbon adsorption process. J. Water Process. Eng. 2020, 36, 101306. [Google Scholar] [CrossRef]
- Sanni, I.; Estahbanati, M.K.; Carabin, A.; Drogui, P. Coupling electrocoagulation with electro-oxidation for COD and phosphorus removal from industrial container wash water. Sep. Purif. Technol. 2022, 282, 119992. [Google Scholar] [CrossRef]
- Priya, M.; Jeyanthi, J. Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique. Microchem. J. 2019, 150, 104070. [Google Scholar] [CrossRef]
- John, S.; Soloman, P.A.; Fasnabi, P.A. Study on Removal of Acetamiprid from Wastewater by Electrocoagulation. Procedia Technol. 2016, 24, 619–630. [Google Scholar] [CrossRef]
- Sankar, M.R.; Sivasubramanian, V. Optimization and evaluation of malathion removal by electrocoagulation process and sludge management. J. Environ. Chem. Eng. 2021, 9, 106147. [Google Scholar] [CrossRef]
- Briceño, S.; Reinoso, C. CoFe2O4-chitosan-graphene nanocomposite for glyphosate removal. Environ. Res. 2022, 212, 113470. [Google Scholar] [CrossRef]
- Behloul, M.; Grib, H.; Drouiche, N.; Abdi, N.; Lounici, H.; Mameri, N. Removal of Malathion Pesticide from Polluted Solutions by Electrocoagulation: Modeling of Experimental Results using Response Surface Methodology. Sep. Sci. Technol. 2013, 48, 664–672. [Google Scholar] [CrossRef]
- Hosseini, G.; Maleki, A.; Daraei, H.; Faez, E.; Shahamat, Y.D. Electrochemical Process for Diazinon Removal from Aqueous Media: Design of Experiments, Optimization, and DLLME-GC-FID Method for Diazinon Determination. Arab. J. Sci. Eng. 2015, 40, 3041–3046. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, P. Optimization of chlorpyrifos degradation by Fenton oxidation using CCD and ANFIS computing technique. J. Environ. Chem. Eng. 2016, 4, 2952–2963. [Google Scholar] [CrossRef]
- András, S.K.C.; Béla, D. Forty Years with Glyphosate. In Herbicides; Mohammed Naguib Abd El-Ghany, H., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 14. [Google Scholar]
- Danial, R.; Sobri, S.; Abdullah, L.C.; Mobarekeh, M.N. FTIR, CHNS and XRD analyses define mechanism of glyphosate herbicide removal by electrocoagulation. Chemosphere 2019, 233, 559–569. [Google Scholar] [CrossRef]
- Tao, Y.; Fang, F.; Lv, Q.; Qin, W.; He, X.; Zhang, Y.; Zhou, Y.; Li, X.; Li, J. Highly efficient removal of glyphosate from water by hierarchical-pore UiO-66: Selectivity and effects of natural water particles. J. Environ. Manag. 2022, 316, 115301. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Yin, M.; Ma, X.; Lin, S. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem. Eng. J. 2015, 267, 86–92. [Google Scholar] [CrossRef]
- Guvenc, S.Y.; Varank, G.; Can-Güven, E.; Ercan, H.; Yaman, D.; Saricam, E.; Türk, O.K. Application of the hybrid electrocoagulation–electrooxidation process for the degradation of contaminants in acidified biodiesel wastewater. J. Electroanal. Chem. 2022, 926, 116933. [Google Scholar] [CrossRef]
- Hussein, T.K.; Jasim, N.A. A comparison study between chemical coagulation and electro-coagulation processes for the treatment of wastewater containing reactive blue dye. Mater. Today Proc. 2021, 42, 1946–1950. [Google Scholar] [CrossRef]
- Ndjeri, M.; Pensel, A.; Peulon, S.; Haldys, V.; Desmazières, B.; Chaussé, A. Degradation of glyphosate and AMPA (amino methylphosphonic acid) solutions by thin films of birnessite electrodeposited: A new design of material for remediation processes? Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 154–169. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Baraka, A.M.; Omran, K.A.; Mokhtar, M.M. Removal of Some Pesticides from the Simulated Waste Water by Electrocoagulation Method Using Iron Electrodes. Int. J. Electrochem. Sci. 2012, 7, 6654–6665. [Google Scholar]
- Millán, M.; Rodrigo, M.A.; Fernández-Marchante, C.M.; Díaz-Abad, S.; Peláez, M.C.; Cañizares, P.; Lobato, J. Towards the sustainable powering of the electrocoagulation of wastewater through the use of solar-vanadium redox flow battery: A first approach. Electrochim. Acta 2018, 270, 14–21. [Google Scholar] [CrossRef]
- Amooey, A.A.; Ghasemi-Mir, S.; Mirsoleimani-Azizi, S.M.; Gholaminezhad, Z.; Chaichi, M.J. Removal of Diazinon from aqueous solution by electrocoagulation process using aluminum electrodes. Korean J. Chem. Eng. 2014, 31, 1016–1020. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Balarak, D.; Keikhaei, S.; Mahvi, A.H. Optimization of Diazinon Removal from Aqueous Environments by Electrocoagulation Process Using Response Surface Methodology. J. Maz. Univ. Med. Sci. 2016, 26, 118–130. [Google Scholar]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of diazinon from aqueous solutions by electro-Fenton process: Effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Sep. Sci. Technol. 2021, 56, 2287–2299. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, P. Simultaneous removal of methyl parathion and chlorpyrifos pesticides from model wastewater using coagulation/flocculation: Central composite design. J. Environ. Chem. Eng. 2016, 4, 673–680. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Li, L.; Zhen, G.; Lu, X.; Zhang, J.; Liu, H.; Zhou, Z.; Wu, Z.; Zhang, X. Prospects for humic acids treatment and recovery in wastewater: A review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, W.; Zhan, S.; Qiu, J.; Wang, X.; Wu, Z.; Li, H.; Qiu, Z.; Peng, H. Adsorption and mechanistic study for humic acid removal by magnetic biochar derived from forestry wastes functionalized with Mg/Al-LDH. Sep. Purif. Technol. 2021, 276, 119296. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Zhao, S.; Li, K.; Cao, A.; Wang, J. A novel electrocoagulation-membrane stripping hybrid system for simultaneous ammonia recovery and contaminant removal. Sep. Purif. Technol. 2022, 296, 121377. [Google Scholar] [CrossRef]
- Rajaei, F.; Taheri, E.; Hadi, S.; Fatehizadeh, A.; Amin, M.M.; Rafei, N.; Fadaei, S.; Aminabhavi, T.M. Enhanced removal of humic acid from aqueous solution by combined alternating current electrocoagulation and sulfate radical. Environ. Pollut. 2021, 277, 116632. [Google Scholar] [CrossRef]
- Barhoumi, A.; Ncib, S.; Chibani, A.; Brahmi, K.; Bouguerra, W.; Elaloui, E. High-rate humic acid removal from cellulose and paper industry wastewater by combining electrocoagulation process with adsorption onto granular activated carbon. Ind. Crop. Prod. 2019, 140, 111715. [Google Scholar] [CrossRef]
- Acosta-Santoyo, G.; Raschitor, A.; Bustos, E.; Llanos, J.; Cañizares, P.; Rodrigo, M.A. Electrochemically assisted dewatering for the removal of oxyfluorfen from a coagulation/flocculation sludge. J. Environ. Manag. 2020, 258, 110015. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Alsheyab, M.; Khodary, A.; Sirés, I.; Abdel-Wahab, A. Corrosion behavior of pure titanium anodes in saline medium and their performance for humic acid removal by electrocoagulation. Chemosphere 2020, 246, 125674. [Google Scholar] [CrossRef]
- Hasani, G.; Maleki, A.; Daraei, H.; Ghanbari, R.; Safari, M.; McKay, G.; Yetilmezsoy, K.; Ilhan, F.; Marzban, N. A comparative optimization and performance analysis of four different electrocoagulation-flotation processes for humic acid removal from aqueous solutions. Process. Saf. Environ. Prot. 2019, 121, 103–117. [Google Scholar] [CrossRef]
- Hu, Q.; He, L.; Lan, R.; Feng, C.; Pei, X. Recent advances in phosphate removal from municipal wastewater by electrocoagulation process: A review. Sep. Purif. Technol. 2023, 308, 122944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).