Rare Earth Tungstate: One Competitive Proton Conducting Material Used for Hydrogen Separation: A Review
Abstract
1. Introduction
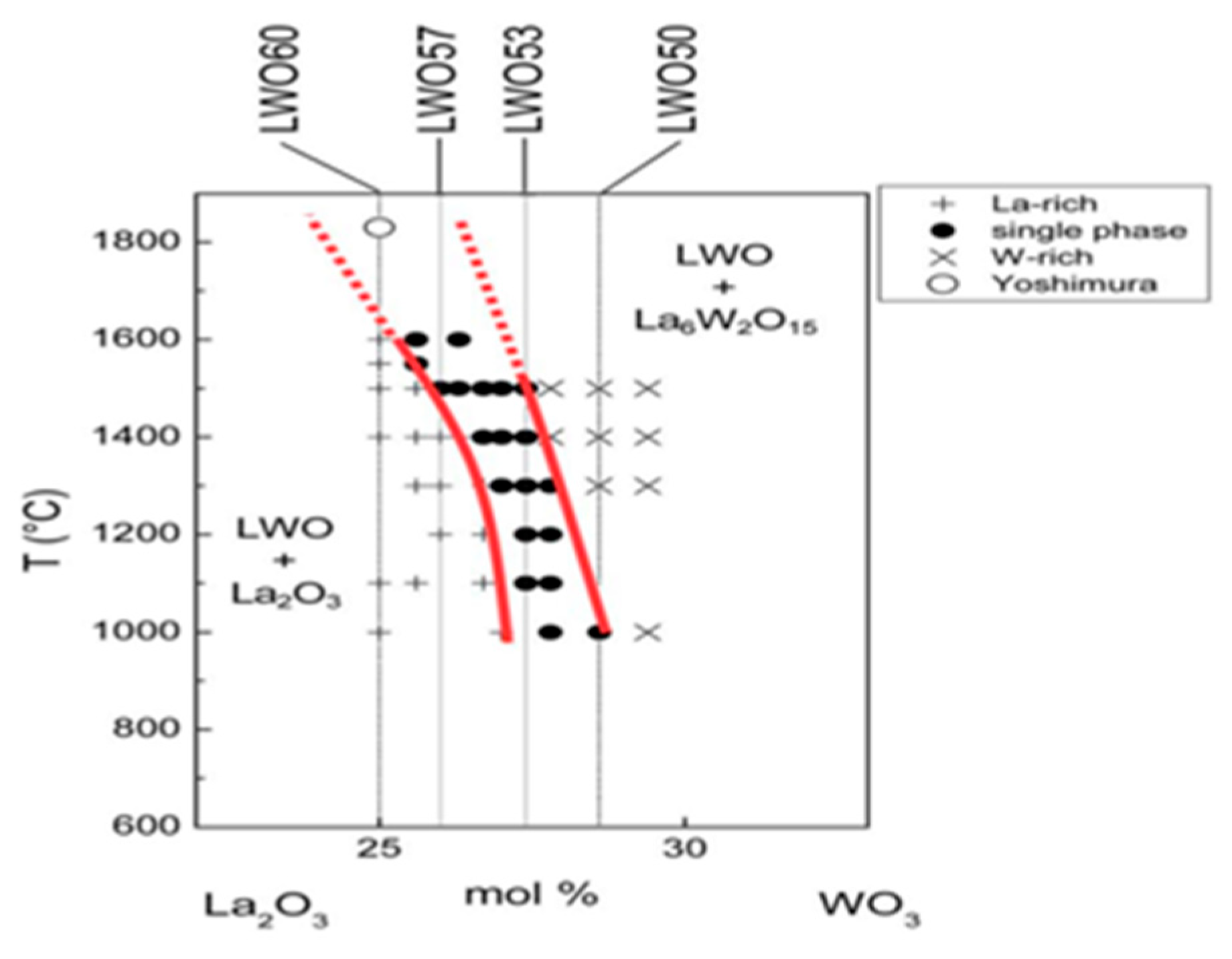
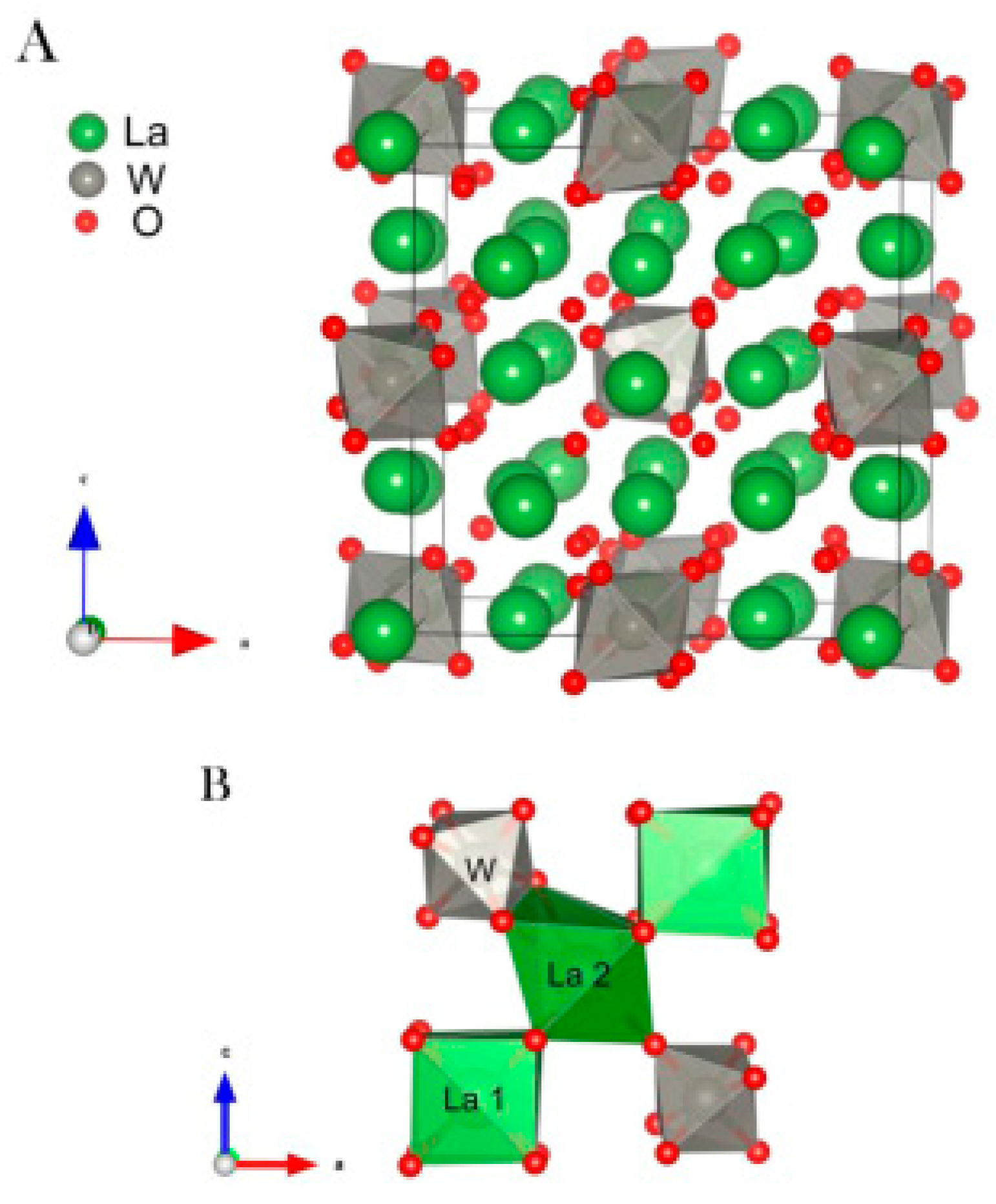
2. The Chemical Formula, Crystal Structure, and Transport Mechanism
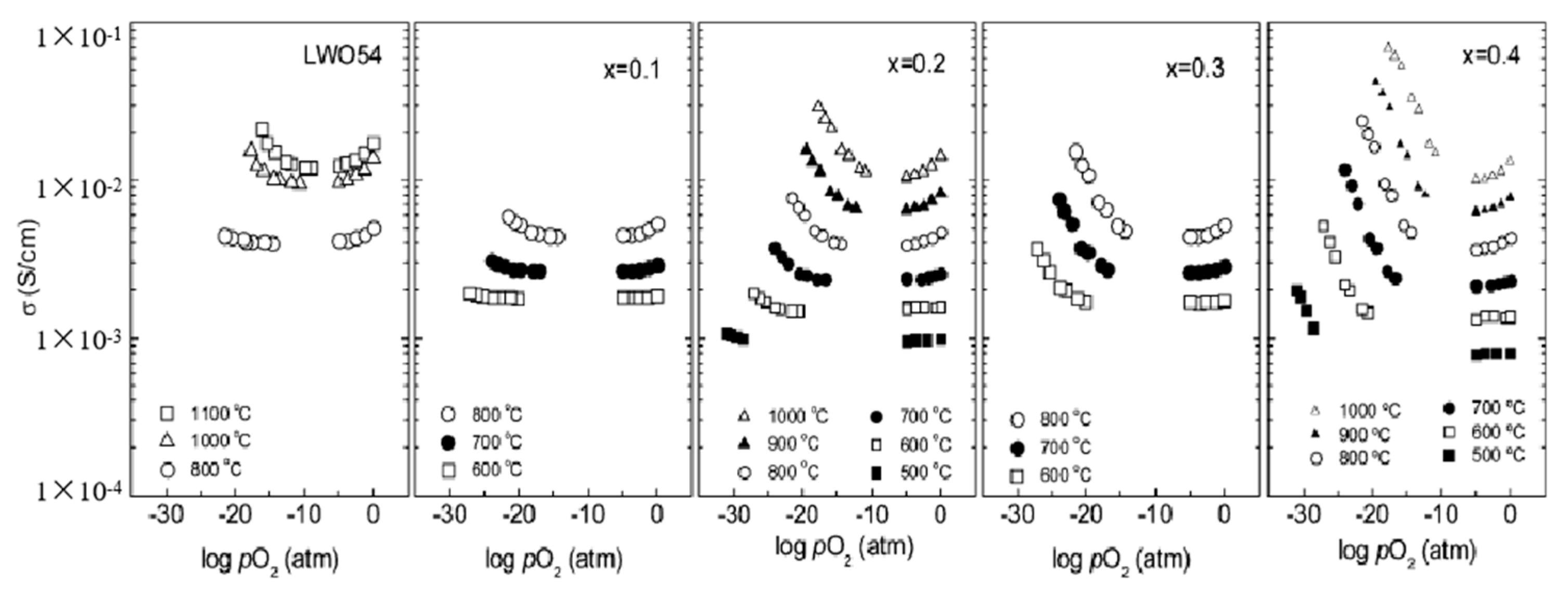
3. The Conductivity Characteristics
4. Current Status of Hydrogen Permeation Membrane
4.1. Single-Phase Membrane
4.1.1. LaWO-Based Membrane
4.1.2. NdWO-Based Membrane
4.2. Dual-Phase Membrane
4.3. Asymmetric Membrane
5. The Membrane Stability
6. Challenges and Outlook
6.1. Challenges
6.2. Future Insights
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cechetto, V.; Di Felice, L.; Gutierrez Martinez, R.; Arratibel Plazaola, A.; Gallucci, F. Ultra-pure hydrogen production via ammonia decomposition in a catalytic membrane reactor. Int. J. Hydrogen Energy 2022, 47, 21220–21230. [Google Scholar] [CrossRef]
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Liu, L.; Meng, B.; Yang, N.; Sunarso, J.; Liu, L.; Liu, S.; Wang, X. ZIF-67 membranes supported on porous ZnO hollow fibers for hydrogen separation from gas mixtures. J. Membr. Sci. 2022, 653, 120550. [Google Scholar] [CrossRef]
- Amanipour, M.; Heidari, M.; Walberg, M. Comparison of hydrogen permeation and structural properties of a microporous silica membrane and a dense BaCe0.9Y0.1O3−δ (BCY) perovskite membrane. Results Mater. 2022, 15, 100314. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jang, Y.; Han, D.H.; Lee, S.M.; Kim, S.S. Palladium-copper membrane prepared by electroless plating for hydrogen separation at low temperature. J. Environ. Chem. Eng. 2021, 9, 106509. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Khaled, M.; Jamal, A.; Zahid, U. Mixed matrix membranes for H2/CO2 gas separation—A critical review. Fuel 2023, 333, 126285. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, S.; Zhang, M.; Liao, N. Selective and efficient hydrogen separation of Pd–Au–Ag ternary alloy membrane. Int. J. Hydrogen Energy 2022, 47, 13054–13061. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Luo, J.; Wei, Y.; Wang, H. Porous stainless steel hollow fiber-supported ZIF-8 membranes via FCDS for hydrogen/carbon dioxide separation. Sep. Purif. Technol. 2022, 295, 121365. [Google Scholar] [CrossRef]
- Saini, N.; Awasthi, K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2022, 282, 120029. [Google Scholar] [CrossRef]
- Liu, X.; He, D. Atomic layer deposited aluminium oxide membranes for selective hydrogen separation through molecular sieving. J. Membr. Sci. 2022, 662, 121011. [Google Scholar] [CrossRef]
- Xu, C.; Wei, W.; He, Y. Enhanced hydrogen separation performance of Linde Type-A zeolite molecular sieving membrane by cesium ion exchange. Mater. Lett. 2022, 324, 132680. [Google Scholar] [CrossRef]
- Opetubo, O.; Ibitoye, A.I.; Oyinbo, S.T.; Jen, T.-C. Analysis of hydrogen embrittlement in palladium–copper alloys membrane from first principal method using density functional theory. Vacuum 2022, 205, 111439. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Tan, X.; Liu, S. Externally self-supported metallic nickel hollow fiber membranes for hydrogen separation. J. Membr. Sci. 2022, 653, 120513. [Google Scholar] [CrossRef]
- Osinkin, D.; Tropin, E. Hydrogen production from methane and carbon dioxide mixture using all-solid-state electrochemical cell based on a proton-conducting membrane and redox-robust composite electrodes. J. Energy Chem. 2022, 69, 576–584. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Comparative study on the numerical simulation of hydrogen separation through palladium and palladium–copper membranes. Int. J. Hydrogen Energy 2022, 47, 22819–22831. [Google Scholar] [CrossRef]
- Dube, S.; Gorimbo, J.; Moyo, M.; Okoye-Chine, C.G.; Liu, X. Synthesis and application of Ni-based membranes in hydrogen separation and purification systems: A review. J. Environ. Chem. Eng. 2023, 11, 109194. [Google Scholar] [CrossRef]
- Pati, S.; Das, S.; Dewangan, N.; Jangam, A.; Kawi, S. Facile integration of core–shell catalyst and Pd-Ag membrane for hydrogen production from low-temperature dry reforming of methane. Fuel 2023, 333, 126433. [Google Scholar] [CrossRef]
- Liang, X.; Huang, F.; Xu, S.; Guo, J.; Liu, D.; Li, X. Microstructure and hydrogen transport properties of (V90Cr5Al5)90Cu10 alloy membranes. J. Alloys Compd. 2023, 938, 168684. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liang, X.; Chen, R.; Guo, J.; Fu, H.; Liu, D. Preparation of thin (~2 μm) Pd membranes on ceramic supports with excellent selectivity and attrition resistance. Int. J. Hydrogen Energy 2023, 48, 662–675. [Google Scholar] [CrossRef]
- Amin, M.; Butt, A.S.; Ahmad, J.; Lee, C.; Azam, S.U.; Mannan, H.A.; Naveed, A.B.; Farooqi, Z.U.R.; Chung, E.; Iqbal, A. Issues and challenges in hydrogen separation technologies. Energy Rep. 2023, 9, 894–911. [Google Scholar] [CrossRef]
- Nayak, A.K.; Sasmal, A. Recent advance on fundamental properties and synthesis of barium zirconate for proton conducting ceramic fuel cell. J. Clean. Prod. 2023, 386, 135827. [Google Scholar] [CrossRef]
- Triviño-Peláez, N.; Mosa, J.; Pérez-Coll, D.; Aparicio, M.; Mather, G.C. Low-temperature sintering and enhanced stability of fluorine-modified BaZr0.8Y0.2O3−δ synthesised by a sol-gel alkoxide route. J. Eur. Ceram. Soc. 2023, 43, 99–108. [Google Scholar] [CrossRef]
- Jia, L.; Hu, T.; Liang, F.; Liu, M.; Zhang, Y.; Jiang, H. Enhanced CO2-tolerance and hydrogen separation performance of Ba-based ceramic membrane modified by Ce0.9Gd0.1O2−δ surface layer. Sep. Purif. Technol. 2022, 303, 122246. [Google Scholar] [CrossRef]
- Rusevich, L.L.; Kotomin, E.A.; Zvejnieks, G.; Popov, A. Ab initio calculations of structural, electronic and vibrational properties of BaTiO3 and SrTiO3 perovskite crystals with oxygen vacancies. Low Temp. Phys. 2020, 46, 1185–1195. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Kleperis, J.; Purans, J.; Popov, A.I.; Jia, R. Ab initio calculations of CaZrO3 (011) surfaces: Systematic trends in polar (011) surface calculations of ABO3 perovskites. J. Membr. Sci. 2019, 55, 203–217. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Lv, J.; Wang, L.; Lei, D.; Guo, H.; Kumar, R.V. Sintering, chemical stability and electrical conductivity of the perovskite proton conductors BaCe0.45Zr0.45M0.1O3−δ (M = In, Y, Gd, Sm). J. Alloys Compd. 2009, 467, 376–382. [Google Scholar] [CrossRef]
- Li, F.; Duan, G.; Wang, Z.; Liu, D.; Cui, Y.; Kawi, S.; Liu, S.; Tan, X. Highly efficient recovery of hydrogen from dilute H2-streams using BaCe0.7Zr0.1Y0.2O3−δ/Ni-BaCe0.7Zr0.1Y0.2O3−δ dual-layer hollow fiber membrane. Sep. Purif. Technol. 2022, 287, 120602. [Google Scholar] [CrossRef]
- Mercadelli, E.; Gondolini, A.; Ardit, M.; Cruciani, G.; Melandri, C.; Escolástico, S.; Serra, J.M.; Sanson, A. Chemical and mechanical stability of BCZY-GDC membranes for hydrogen separation. Sep. Purif. Technol. 2022, 289, 120795. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zhou, X.; Song, Y.; Jiang, Y.; Lin, B. Phase stability and hydrogen permeation performance of BaCo0.4Fe0.4Zr0.1Y0.1O3−δ ceramic membranes. Ceram. Int. 2022, 48, 9946–9954. [Google Scholar] [CrossRef]
- Hossain, M.K.; Chanda, R.; El-Denglawey, A.; Emrose, T.; Rahman, M.T.; Biswas, M.C.; Hashizume, K. Recent progress in barium zirconate proton conductors for electrochemical hydrogen device applications: A review. Ceram. Int. 2021, 47, 23725–23748. [Google Scholar] [CrossRef]
- Haugsrud, R.; Kjølseth, C. Effects of protons and acceptor substitution on the electrical conductivity of La6WO12. J. Phys. Chem. Solids 2008, 69, 1758–1765. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, L.; Qiao, J.; Wang, B.; Zhang, L.; Zhang, J. A review of advanced proton-conducting materials for hydrogen separation. Prog. Mater. Sci. 2015, 74, 1–50. [Google Scholar] [CrossRef]
- Magrasó, A.; Haugsrud, R. Effects of the La/W ratio and doping on the structure, defect structure, stability and functional properties of proton-conducting lanthanum tungstate La28−xW4+xO54+δ. A review. J. Mater. Chem. A 2014, 2, 12630–12641. [Google Scholar] [CrossRef]
- Yoshimura, M. High temperature phase relation in the system La2O3-WO3. Mat. Res. Bull. 1976, 11, 151–158. [Google Scholar] [CrossRef]
- Seeger, J.; Ivanova, M.E.; Meulenberg, W.A.; Sebold, D.; Stöver, D.; Scherb, T.; Schumacher, G.; Escolástico, S.; Solís, C.; Serra, J.M. Synthesis and Characterization of Nonsubstituted and Substituted Proton-Conducting La6–xWO12–y. Inorg. Chem. 2013, 52, 10375–10386. [Google Scholar] [CrossRef]
- Magrasó, A.; Frontera, C.; Marrero-Lopez, D.; Nunez, P. New crystal structure and characterization of lanthanum tungstate “La6WO12” prepared by freeze-drying synthesis. Dalton Trans. 2009, 46, 10273–10283. [Google Scholar] [CrossRef]
- Magrasó, A.; Polfus, J.M.; Frontera, C.; Canales-Vázquez, J.; Kalland, L.-E.; Hervoches, C.H.; Erdal, S.; Hancke, R.; Islam, M.S.; Norby, T.; et al. Complete structural model for lanthanum tungstate: A chemically stable high temperature proton conductor by means of intrinsic defects. J. Mater. Chem. 2012, 22, 1762–1764. [Google Scholar] [CrossRef]
- Diot, N.; Larcher, O.; Marchand, R.; Kempf, J.; Macaudière, P. Rare-earth and tungsten oxynitrides with a defect fluorite-type structure asnew pigments. J. Alloys Compd. 2001, 323–324, 45–48. [Google Scholar] [CrossRef]
- EscoláStico, S.; Vert, V.B.; Serra, J.M. Preparation and Characterization of Nanocrystalline Mixed Proton−Electronic Conducting Materials Based on the System Ln6WO12. Chem. Mater. 2009, 21, 3079–3089. [Google Scholar] [CrossRef]
- Erdal, S.; Kalland, L.-E.; Hancke, R.; Polfus, J.; Haugsrud, R.; Norby, T.; Magrasó, A. Defect structure and its nomenclature for mixed conducting lanthanum tungstates La28–xW4+xO54+3x/2. Int. J. Hydrogen Energy 2012, 37, 8051–8055. [Google Scholar] [CrossRef]
- Hancke, R.; Magrasó, A.; Norby, T.; Haugsrud, R. Hydration of lanthanum tungstate (La/W = 5.6 and 5.3) studied by TG and simultaneous TG–DSC. Solid State Ion. 2013, 231, 25–29. [Google Scholar] [CrossRef]
- Vøllestad, E.; Gorzkowska-Sobas, A.; Haugsrud, R. Fabrication, structural and electrical characterization of lanthanum tungstate films by pulsed laser deposition. Thin Solid Film. 2012, 520, 6531–6534. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Chen, L.; Xue, J.; Chen, X.; Wang, H. Metalloid phosphorus cation doping: An effective strategy to improve permeability and stability through the hydrogen permeable membranes. Sep. Purif. Technol. 2019, 210, 320–326. [Google Scholar] [CrossRef]
- Ran, K.; Deibert, W.; Du, H.; Park, D.; Ivanova, M.E.; Meulenberg, W.A.; Mayer, J. Processing-induced secondary phase formation in Mo-substituted lanthanum tungstate membranes. Acta Mater. 2019, 180, 35–41. [Google Scholar] [CrossRef]
- Kalland, L.E.; Magrasó, A.; Mancini, A.; Tealdi, C.; Malavasi, L. Local Structure of Proton-Conducting Lanthanum Tungstate La28–xW4+xO54+δ: A Combined Density Functional Theory and Pair Distribution Function Study. Chem. Mater. 2013, 25, 2378–2384. [Google Scholar] [CrossRef]
- Solís, C.; Escolastico, S.; Haugsrud, R.; Serra, J.M. La5.5WO12-δ Characterization of Transport Properties under Oxidizing Conditions: A Conductivity Relaxation Study. J. Phys. Chem. C 2011, 115, 11124–11131. [Google Scholar] [CrossRef]
- Shimura, T.; Fujimoto, S.; Iwahara, H. Proton conduction in non-perovskite-type oxides atelevated temperatures. Solid State Ion. 2001, 143, 117–123. [Google Scholar] [CrossRef]
- Haugsrud, R. Defects and transport properties in Ln6WO12 (Ln = La, Nd, Gd, Er). Solid State Ion. 2007, 178, 555–560. [Google Scholar] [CrossRef]
- Deibert, W.; Stournari, V.; Ivanova, M.E.; Escolástico, S.; Serra, J.M.; Malzbender, J.; Beck, T.; Singheiser, L.; Guillon, O.; Meulenberg, W.A. Effect of microstructure on electrical and mechanical properties of La5.4WO12−δ proton conductor. J. Eur. Ceram. Soc. 2018, 38, 3527–3538. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.; Fernández-Díaz, M.T.; Escudero, M.; Daza, L. In situ high temperature powder neutron diffraction study of undoped and Ca-doped La28−xW4+xO54+3x/2 (x = 0.85). J. Mater. Chem. A 2013, 1, 3774–3782. [Google Scholar] [CrossRef]
- Escolástico, S.; Schroeder, M.; Serra, J.M. Optimization of the mixed protonic–electronic conducting materials based on (Nd5/6Ln1/6)5.5WO11.25−δ. J. Mater. Chem. A 2014, 2, 6616–6630. [Google Scholar] [CrossRef]
- Ruf, M.; Solís, C.; Escolástico, S.; Dittmeyer, R.; Serra, J.M. Transport properties and oxidation and hydration kinetics of the proton conductor Mo doped Nd5.5WO11.25−δ. J. Mater. Chem. A 2014, 2, 18539–18546. [Google Scholar] [CrossRef]
- Lashtabeg, A.; Bradley, J.; Dicks, A.; Auchterlonie, G.; Drennan, J. Structural and conductivity studies of Y10−xLaxW2O21. J. Solid State Chem. 2010, 183, 1095–1101. [Google Scholar] [CrossRef]
- Liu, H.; Wen, Z.; Zhang, J.; Han, J.; Chi, X. Improved protonic conductivity and Vickers hardness for lanthanum tungstate with potassium doping (La,K)28−yW4+yO54+δ. Solid State Ion. 2015, 278, 69–77. [Google Scholar] [CrossRef]
- Zayas-Rey, M.J.; Santos-Gómez, L.; Marrero-López, D.; León-Reina, L.; Canales-Vázquez, J.; Aranda, M.a.G.; Losilla, E.R. Structural and Conducting Features of Niobium-Doped Lanthanum Tungstate, La27(W1–xNbx)5O55.55−δ. Chem. Mater. 2013, 25, 448–456. [Google Scholar] [CrossRef]
- Escolastico, S.; Seeger, J.; Roitsch, S.; Ivanova, M.; Meulenberg, W.A.; Serra, J.M. Enhanced H2 separation through mixed proton-electron conducting membranes based on La5.5W0.8M0.2O11.25−δ. ChemSusChem 2013, 6, 1523–1532. [Google Scholar] [CrossRef]
- Porotnikova, N.M.; Ananyev, M.V.; Osinkin, D.A.; Khodimchuk, A.V.; Fetisov, A.V.; Farlenkov, A.S.; Popov, A.I. Increase in the density of Sr2Fe1.5Mo0.5O6−δ membranes through an excess of iron oxide: The effect of iron oxide on transport and kinetic parameters. Surf. Interfaces 2022, 29, 101784. [Google Scholar] [CrossRef]
- Vøllestad, E.; Teusner, M.; Souza, R.; Haugsrud, R. Diffusion of Nd and Mo in lanthanum tungsten oxide. Solid State Ion. 2015, 274, 128–133. [Google Scholar] [CrossRef]
- Magrasó, A. Transport number measurements and fuel cell testing of undoped and Mo-substituted lanthanum tungstate. J. Power Sources 2013, 240, 583–588. [Google Scholar] [CrossRef]
- Amsif, M.; Magrasó, A.; Marrero-López, D.; Ruiz-Morales, J.C.; Canales-Vázquez, J.; Núñez, P. Mo-Substituted Lanthanum Tungstate La28–yW4+yO54+δ: A Competitive Mixed Electron–Proton Conductor for Gas Separation Membrane Applications. Chem. Mater. 2012, 24, 3868–3877. [Google Scholar] [CrossRef]
- Vøllestad, E.; Vigen, C.K.; Magrasó, A.; Haugsrud, R. Hydrogen permeation characteristics of La27Mo1.5W3.5O55.5. J. Membr. Sci. 2014, 461, 81–88. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Chen, L.; Wei, Y.; Ashman, P.J.; Wang, H. Niobium and molybdenum co-doped La5.5WO11.25−δ membrane with improved hydrogen permeability. J. Membr. Sci. 2016, 510, 155–163. [Google Scholar] [CrossRef]
- Xue, J.; Chen, Y.; Wei, Y.; Feldhoff, A.; Wang, H.; Caro, J. Gas to Liquids: Natural Gas Conversion to Aromatic Fuels and Chemicals in a Hydrogen-Permeable Ceramic Hollow Fiber Membrane Reactor. ACS Catal. 2016, 6, 2448–2451. [Google Scholar] [CrossRef]
- Li, Z.; Kjølseth, C.; Haugsrud, R. Hydrogen permeation, water splitting and hydration kinetics in Nd5.4Mo0.3W0.7O12−δ. J. Membr. Sci. 2015, 476, 105–111. [Google Scholar] [CrossRef]
- Escolástico, S.; Serra, J.M. Nd5.5W1−xUxO11.25−δ system: Electrochemical characterization and hydrogen permeation study. J. Membr. Sci. 2015, 489, 112–118. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Haugsrud, R.; Magrasó, A.; Serra, J.M. On the ionic character of H2 separation through mixed conducting Nd5.5W0.5Mo0.5O11.25−δ membrane. Int. J. Hydrogen Energy 2017, 42, 11392–11399. [Google Scholar] [CrossRef]
- Escolástico, S.; Somacescu, S.; Serra, J.M. Tailoring mixed ionic–electronic conduction in H2 permeable membranes based on the system Nd5.5W1−xMoxO11.25−δ. J. Mater. Chem. A 2015, 3, 719–731. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Wei, Y.; Wang, H. CO2-tolerant U-shaped hollow fiber membranes for hydrogen separation. Int. J. Hydrogen Energy 2017, 42, 4208–4215. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Scherb, T.; Schumacher, G.; Serra, J.M. Hydrogen separation in La5.5WO11.25−δ membranes. J. Membr. Sci. 2013, 444, 276–284. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Xie, H.; Zhuang, L.; Wang, H. Effect of the La/W ratio in lanthanum tungstate on the structure, stability and hydrogen permeation properties. J. Membr. Sci. 2017, 542, 300–306. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Serra, J.M. Study of hydrogen permeation in (La5/6Nd1/6)5.5WO12−δ membranes. Solid State Ion. 2012, 216, 31–35. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, G.; Liao, Q.; Chen, Y.; Yan, X.; Guo, X.; Lang, W. Development of Mn and Mo double-substituted La5.5WO11.25−δ based membranes with enhanced hydrogen permeation flux. J. Eur. Ceram. Soc. 2021, 41, 5711–5720. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Liao, Q.; Chen, Y.; Yan, X.; Guo, X.; Lang, W. Influence of Cr doping on hydrogen permeation performance of lanthanum tungstate membrane. Sep. Purif. Technol. 2021, 262, 118333. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Y.; Zhuang, L.; Xie, H.; Wang, H. Effect of Pt layer on the hydrogen permeation property of La5.5W0.45Nb0.15Mo0.4O11.25−δ membrane. J. Membr. Sci. 2018, 552, 61–67. [Google Scholar] [CrossRef]
- Cao, Y.; Chi, B.; Pu, J.; Jian, L. Effect of Pt catalyst and external circuit on the hydrogen permeation of Mo and Nb co-doped lanthanum tungstate. J. Membr. Sci. 2017, 533, 336–341. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Tailoring hydrogen separation performance through the ceramic lanthanum tungstate membranes by chlorine doping. J. Membr. Sci. 2019, 573, 117–125. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhuang, L.; Wei, Y.; Wang, H. Hydrogen permeability through Nd5.5W0.35Mo0.5Nb0.15O11.25−δ mixed protonic-electronic conducting membrane. J. Membr. Sci. 2019, 579, 33–39. [Google Scholar] [CrossRef]
- Porras-Vázquez, J.M.; Santos-Gomez, L.; Marrero-López, D.; Slater, P.R.; Masó, N.; Magrasó, A.; Losilla, E.R. Effect of tri- and tetravalent metal doping on the electrochemical properties of lanthanum tungstate proton conductors. Dalton Trans. 2016, 45, 3130–3138. [Google Scholar] [CrossRef]
- Escolástico, S.; Somacescu, S.; Serra, J.M. Solid State Transport and Hydrogen Permeation in the System Nd5.5W1–xRexO11.25−δ. Chem. Mater. 2014, 26, 982–992. [Google Scholar] [CrossRef]
- Xie, H.; Zhuang, L.; Wei, Y.; Xue, J.; Wang, H. CO2-tolerant Ni-La5.5WO11.25−δ dual-phase membranes with enhanced H2 permeability. Ceram. Int. 2017, 43, 14608–14615. [Google Scholar] [CrossRef]
- Bespalko, Y.; Sadykov, V.; Eremeev, N.; Skryabin, P.; Krieger, T.; Sadovskaya, E.; Bobrova, L.; Uvarov, N.; Lukashevich, A.; Krasnov, A.; et al. Synthesis of tungstates/Ni0.5Cu0.5O nanocomposite materials for hydrogen separation cermet membranes. Compos. Struct. 2018, 202, 1263–1274. [Google Scholar] [CrossRef]
- Escolástico, S.; Solis, C.; Kjølseth, C.; Serra, J.M. Outstanding hydrogen permeation through CO2-stable dual-phase ceramic membranes. Energy Environ. Sci. 2014, 7, 3736–3746. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Y.; Hu, T.; Jiang, H. Enhanced H2 production by using La5.5WO11.25−δ-La0.8Sr0.2FeO3−δ mixed oxygen ion-proton-electron triple-conducting membrane. Int. J. Hydrogen Energy 2021, 46, 33143–33151. [Google Scholar] [CrossRef]
- Polfus, J.M.; Xing, W.; Fontaine, M.L.; Denonville, C.; Henriksen, P.P.; Bredesen, R. Hydrogen separation membranes based on dense ceramic composites in the La27W5O55.5–LaCrO3 system. J. Membr. Sci. 2015, 479, 39–45. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Krasnov, A.V.; Fedorova, Y.E.; Lukashevich, A.I.; Bespalko, Y.N.; Eremeev, N.F.; Skriabin, P.I.; Valeev, K.R.; Smorygo, O.L. Novel nanocomposite materials for oxygen and hydrogen separation membranes. Int. J. Hydrogen Energy 2020, 45, 13575–13585. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Bespalko, Y.N.; Krasnov, A.V.; Skriabin, P.I.; Lukashevich, A.I.; Fedorova, Y.E.; Sadovskaya, E.M.; Eremeev, N.F.; Krieger, T.A.; Ishchenko, A.V.; et al. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ion. 2018, 322, 69–78. [Google Scholar] [CrossRef]
- Polfus, J.M.; Li, Z.; Xing, W.; Sunding, M.F.; Walmsley, J.C.; Fontaine, M.L.; Henriksen, P.P.; Bredesen, R. Chemical stability and H2 flux degradation of cercer membranes based on lanthanum tungstate and lanthanum chromite. J. Membr. Sci. 2016, 503, 42–47. [Google Scholar] [CrossRef]
- Escolástico, S.; Kjølseth, C.; Serra, J.M. Catalytic activation of ceramic H2 membranes for CMR processes. J. Membr. Sci. 2016, 517, 57–63. [Google Scholar] [CrossRef]
- Kalyanaraman, J.; Morejudo, S.H.; Kjølseth, C.; Beeaff, D.; Vigen, C.; Johnson, J.R.; Mccool, B.A. Fundamental modeling and experimental validation of hydrogen and oxygen transport through mixed ionic and electronic conducting membranes. J. Membr. Sci. 2022, 660, 120797. [Google Scholar] [CrossRef]
- Deibert, W.; Schulze-Küppers, F.; Forster, E.; Ivanova, M.E.; Müller, M.; Meulenberg, W.A. Stability and sintering of MgO as a substrate material for Lanthanum Tungstate membranes. J. Eur. Ceram. Soc. 2017, 37, 671–677. [Google Scholar] [CrossRef]
- Weirich, M.; Gurauskis, J.; Gil, V.; Wiik, K.; Einarsrud, M.A. Preparation of lanthanum tungstate membranes by tape casting technique. Int. J. Hydrogen Energy 2012, 37, 8056–8061. [Google Scholar] [CrossRef]
- Gil, V.; Gurauskis, J.; Kjølseth, C.; Wiik, K.; Einarsrud, M.A. Hydrogen permeation in asymmetric La28−xW4+xO54+3x/2 membranes. Int. J. Hydrogen Energy 2013, 38, 3087–3091. [Google Scholar] [CrossRef]
- Xie, H.; Zhuang, L.; Chen, Y.; Wei, Y.; Wang, H. Phase-inversion synthesize of asymmetric-structured La5.5W0.6Mo0.4O11.25−δ membranes with enhanced hydrogen permeation flux. J. Alloys Compd. 2017, 729, 890–896. [Google Scholar] [CrossRef]
- Gil, V.; Gurauskis, J.; Einarsrud, M.A. Asymmetric supported dense lanthanum tungstate membranes. J. Eur. Ceram. Soc. 2014, 34, 3783–3790. [Google Scholar] [CrossRef]
- Deibert, W.; Ivanova, M.E.; Meulenberg, W.A.; Vaßen, R.; Guillon, O. Preparation and sintering behaviour of La5.4WO12−δ asymmetric membranes with optimised microstructure for hydrogen separation. J. Membr. Sci. 2015, 492, 439–451. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Asymmetric membrane structure: An efficient approach to enhance hydrogen separation performance. Sep. Purif. Technol. 2018, 207, 363–369. [Google Scholar] [CrossRef]
- Ivanova, M.E.; Deibert, W.; Marcano, D.; Escolástico, S.; Mauer, G.; Meulenberg, W.A.; Bram, M.; Serra, J.M.; Vaßen, R.; Guillon, O. Lanthanum tungstate membranes for H2 extraction and CO2 utilization: Fabrication strategies based on sequential tape casting and plasma-spray physical vapor deposition. Sep. Purif. Technol. 2019, 219, 100–112. [Google Scholar] [CrossRef]
- Gurauskis, J.; Gil, V.; Lin, B.; Einarsrud, M.A. Pilot scale fabrication of lanthanum tungstate supports for H2 separation membranes. Open Ceram. 2022, 9, 100226. [Google Scholar] [CrossRef]
- Cheng, H.; Meng, B.; Li, C.; Wang, X.; Meng, X.; Sunarso, J.; Tan, X.; Liu, S. Single-step synthesized dual-layer hollow fiber membrane reactor for on-site hydrogen production through ammonia decomposition. Int. J. Hydrogen Energy 2020, 45, 7423–7432. [Google Scholar] [CrossRef]
- Magraso, A.; Frontera, C. Comparison of the local and the average crystal structure of proton conducting lanthanum tungstate and the influence of molybdenum substitution. Dalton Trans. 2016, 45, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Wang, H.; Cheng, H.; Wang, X.; Meng, X.; Sunarso, J.; Tan, X.; Liu, S. Hydrogen permeation performance of dual-phase protonic-electronic conducting ceramic membrane with regular and independent transport channels. Sep. Purif. Technol. 2019, 213, 515–523. [Google Scholar] [CrossRef]


| ionic radii Shannon coordination | |||||
| element | atomic weight | valence | 7 | 8 | reported structure symmetry |
| La | 138.9 | 3 | 1.100 | 1.160 | cubic |
| Ce | 140.12 | 3 | 1.070 | 1.143 | cubic |
| 4 | 0.920 a | 0.970 | |||
| Pr | 140.907 | 3 | 1.028 a | 1.126 | cubic |
| 4 | 0.905 a | 0.960 | |||
| Nd | 144.24 | 3 | 1.046 a | 1.109 | tetragonal |
| Sm | 150.35 | 2 | 1.220 | 1.270 | tetragonal/cubic |
| 3 | 1.020 | 1.079 | |||
| Eu | 151.96 | 2 | 1.200 | 1.250 | tetragonal/cubic |
| 3 | 1.010 | 1.066 | |||
| Gd | 157.25 | 3 | 1.000 | 1.053 | tetragonal |
| Tb | 158.924 | 3 | 0.980 | 1.040 | rhombohedral |
| 4 | 0.820 a | 0.880 | |||
| Dy | 162.50 | 3 | 0.970 | 1.027 | rhombohedral |
| Y | 88.905 | 3 | 0.960 | 1.019 | rhombohedral |
| Ho | 164.93 | 3 | 0.958 a | 1.015 | rhombohedral |
| Er | 167.26 | 3 | 0.945 | 1.004 | rhombohedral |
| Tm | 168.934 | 3 | 0.937 a | 0.994 | rhombohedral |
| Yb | 173.04 | 2 | 1.080 | 1.140 | rhombohedral |
| 3 | 0.925 | 0.985 | |||
| Lu | 174.97 | 3 | 0.919 a | 0.977 | rhombohedral |
| Sc | 44.956 | 3 | 0.808 a | 0.870 | rhombohedral |
| Sample | LaWO | NdWO | GdWO | ErWO |
|---|---|---|---|---|
| Undoped | 4 × 10−2 | 1 × 10−2 | 8 × 10−3 | 9 × 10−3 |
| Ca-doped | 1 × 10−2 | 9 × 10−3 | - | 1 × 10−3 |
| Composition | Hydrogen Flux (mL·min−1·cm−2) | σ × 103 (S·cm−1) | T (°C) | Thickness (μm) | Feed/Sweep Gas | Ref. |
|---|---|---|---|---|---|---|
| Nd5.5WO11.25−δ | 0.03 | 1000 | 900 | 50%H2-He/Wet Ar | [69] | |
| La5.5WO11.25−δ | 0.136 | 1000 | 900 | 50%H2-He/Wet Ar | [71] | |
| La5.5WO11.25−δ | 0.037 | 1000 | 500 | 50%H2-He/Ar | [72] | |
| A-Site Doping | ||||||
| La0.95Ca0.05W1/6O~2 | 1.0 | 800 | Wet H2 | [33] | ||
| YLa9W2O21 | 4.56 | 1000 | Air | [55] | ||
| (La0.98K0.02)27.08W4.92O55.38−θ | 3.59 | 800 | Wet air | [56] | ||
| (La5/6Nd1/6)5.5WO12−δ | 0.046 | 1000 | 900 | Wet2.5%H2-Wet Ar | [73] | |
| B-Site Doping | ||||||
| La27NbW4O55 | 10 | 800 | Wet N2 | [57] | ||
| La5.5W0.8Re0.2O11.25−δ | 0.095 | 700 | 760 | 50%H2-He/Wet Ar | [58] | |
| La5.5W0.8Mo0.2O11.25−δ | 0.04 | 700 | 850 | Wet50%H2-He/Wet Ar | [58] | |
| La27.07Mo1.97W2.95O54+δ | 1.6 | 600 | Wet H2 | [62] | ||
| La27Mo1.5W3.5O55.5 | 6 × 10−4 mL·min−1·cm−1 | 700 | - | 50%H2-Ar/Ar | [63] | |
| La5.5W0.45Nb0.15Mo0.4O11.25−δ | 0.195 | 1000 | 500 | 50%H2-He/Ar | [64] | |
| La5.5W0.45Nb0.15Mo0.4O11.25−δ | 0.233 | 1000 | 500 | 50%H2-He/Wet Ar | [64] | |
| La5.5W0.8Mn0.2O11.25−δ | 0.07 | 1000 | 500 | 50%H2-He/Ar | [74] | |
| La5.5W0.6Mn0.2 Mo0.2O11.25−δ | 0.12 | 1000 | 500 | 50%H2-He/Ar | [74] | |
| La5.5W0.8Cr0.2O11.25−δ | 0.046 | 1000 | 500 | 50%H2-He/Ar | [75] | |
| La5.5W0.45Nb0.15Mo0.4O11.25−δ (Pt coated) | 0.483 | 1000 | 500 | Wet50%H2-He/Wet Ar | [76] | |
| La5.4W0.55Nb0.15Mo0.3O11.25−δ (Pt coated) | 0.01 mL·min−1·cm−1 | 1000 | - | Wet50%H2-He/Wet Ar | [77] | |
| La5.5W0.6Mo0.4O11.25−δ−x/2Cl0.1 | 0.15 | 1000 | 500 | 50%H2-He/Ar | [78] | |
| Nd5.5W0.9U0.1O11.25−δ (Pt coated) | 0.015 | 740 | 530 | Wet50%H2-He/Wet Ar | [67] | |
| Nd5.5W0.5Mo0.5O11.25−δ (Pt coated) | 0.07 | 700 | 550 | Wet50%H2-He/Wet Ar | [68] | |
| Nd5.5W0.5Mo0.5O11.25−δ | 0.3 | 1000 | 900 | Wet50%H2-He/Wet Ar | [69] | |
| Nd5.5W0.5Mo0.5O11.25−δ | 1.29 | 975 | 170 | 80%H2-He/Wet Ar | [70] | |
| Nd5.5W0.5Mo0.5O11.25−δ | 0.05 | 1000 | 500 | 50%H2-He/Ar | [79] | |
| La5.5W0.9Ti0.1O11.25−δ | 9.2 | 800 | - | Wet N2 | [80] | |
| La5.5W0.95Al0.05O11.25−δ | 9.8 | 800 | - | Wet N2 | [80] | |
| Nd5.5W0.5Re0.5O11.25−δ | 0.08 | 1000 | 900 | Wet50%H2-He/Wet Ar | [81] | |
| Composition | Hydrogen Flux (mL·min−1·cm−2) | T (°C) | Thickness (μm) | Feed/Sweep Gas | Note | Ref. |
|---|---|---|---|---|---|---|
| 60Ni-40La5.5WO11.25−δ | 0.18 | 1000 | 500 | 50%H2-He/Ar | [82] | |
| Ni0.5Cu0.5-40Nd5.5WO11.25−δ | 2 | 900 | 150 | H2O-EtOH-Ar/Ar | catalyst coated; reactor | [83] |
| Ni0.5Cu0.5-Nd5.5WO11.25−δ | 0.27 | 900 | 50 | H2O-EtOH-Ar/Ar | catalyst coated; reactor | [87,88] |
| 50La5.5WO11.25−δ-50La0.87Sr0.13CrO3−δ | 0.15 | 700 | 370 | 50%H2-He/Wet Ar | [84] | |
| 50La5.5WO11.25−δ-50La0.8Sr0.2FeO3−δ | 0.14 | 900 | 500 | 50%H2-N2/Wet Ar | [85] | |
| 50La5.5WO11.25−δ-50La0.8Sr0.2CrO3−δ | 0.05 | 900 | 500 | 50%H2-N2/Wet Ar | [85] | |
| 70La27W3.5Mo1.5O55.5−δ-30La0.87Sr0.13CrO3−δ | 0.001 mL·min−1·cm−1 | 700 | 1430 | Wet50%H2-He/ Wet Ar | Pt coated | [86] |
| 50La27W3.5Mo1.5O11.25−δ-50La0.87Sr0.13CrO3−δ | 0.004 mL·min−1·cm−1 | 900 | - | Wet49%H2-He/ Wet Ar | [89] | |
| 60La5.5WO11.25−δ-40La0.87Sr0.13CrO3−δ | 0.22 | 725 | 360 | Wet49%H2-He/ Wet Ar | Pt coated | [90] |
| 33La0.87Sr0.13CrO3−δ-67La5.4WO12−δ | 0.10 | 750 | 40–70 | Wet50H2-Ar/ Wet Ar | [91] |
| Composition | Hydrogen Flux (mL·min−1·cm−2) | T (°C) | Dense Layer Thickness (μm) | Preparation Method/ Pore Former | Feed/Sweep Gas | Ref. |
|---|---|---|---|---|---|---|
| La6-xWO12−δ | - | 40 | tape casting/carbon black | [93] | ||
| La5.6WO11.25−δ | 0.14 | 1000 | 25 | dip coating/carbon black | 10%Wet H2-He/Ar | [94] |
| La5.5W0.6Mo0.4O11.25−δ | 0.273 | 1000 | 300 | phase inversion | 50%H2-He/Wet Ar | [95] |
| La5.6WO11.25−δ | 0.14 | 1000 | 20 | dip coating/carbon black | 10%Wet H2-He/Ar | [96] |
| La5.4WO12−δ | - | 20–30 | tape casting/rice starch | [97] | ||
| La5.5W0.6Mo0.4O11.25−δF0.05 | 0.16 | 975 | 67 | dry pressing/soluble starch | 50%H2-He/Ar | [98] |
| La28−xW4+xO54+δ | 0.4 | 825 | 60 | tape casting/rice starch | 50%H2-He/Wet Ar | [99] |
| La28−yW4+yO54+δ | - | 20 | dip coating/carbon black | [100] | ||
| Nd5.5Mo0.5W0.5O11.25−δ/Nd5.5Mo0.5W0.5O11.25−δ-Ni | 0.26 | 900 | 26 | phase inversion | 50%H2-He/N2 | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H. Rare Earth Tungstate: One Competitive Proton Conducting Material Used for Hydrogen Separation: A Review. Separations 2023, 10, 317. https://doi.org/10.3390/separations10050317
Cheng H. Rare Earth Tungstate: One Competitive Proton Conducting Material Used for Hydrogen Separation: A Review. Separations. 2023; 10(5):317. https://doi.org/10.3390/separations10050317
Chicago/Turabian StyleCheng, Hongda. 2023. "Rare Earth Tungstate: One Competitive Proton Conducting Material Used for Hydrogen Separation: A Review" Separations 10, no. 5: 317. https://doi.org/10.3390/separations10050317
APA StyleCheng, H. (2023). Rare Earth Tungstate: One Competitive Proton Conducting Material Used for Hydrogen Separation: A Review. Separations, 10(5), 317. https://doi.org/10.3390/separations10050317





