Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
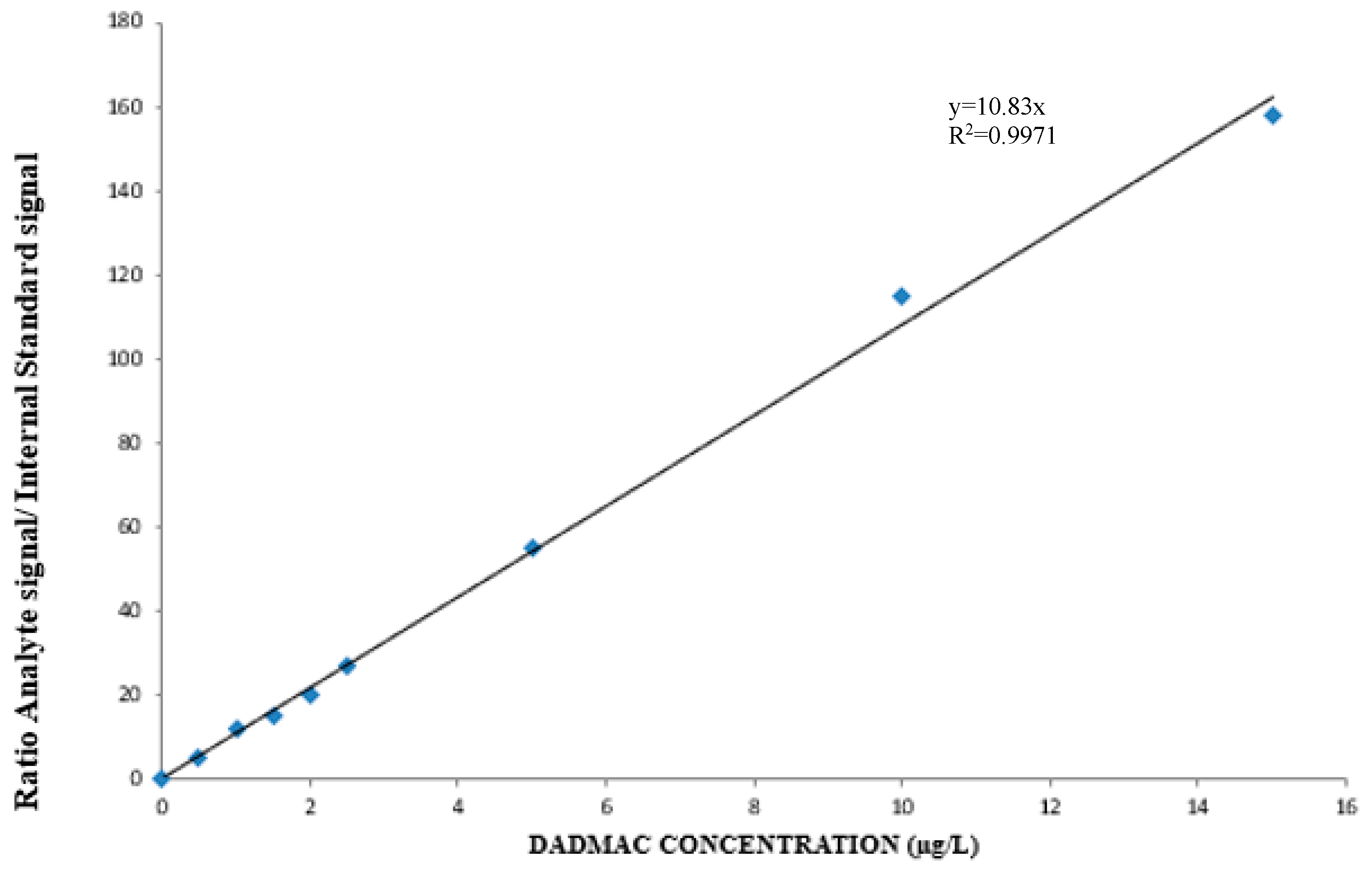
2.1. Method Validation, Quality Assurance/Quality Control (QA/QC)
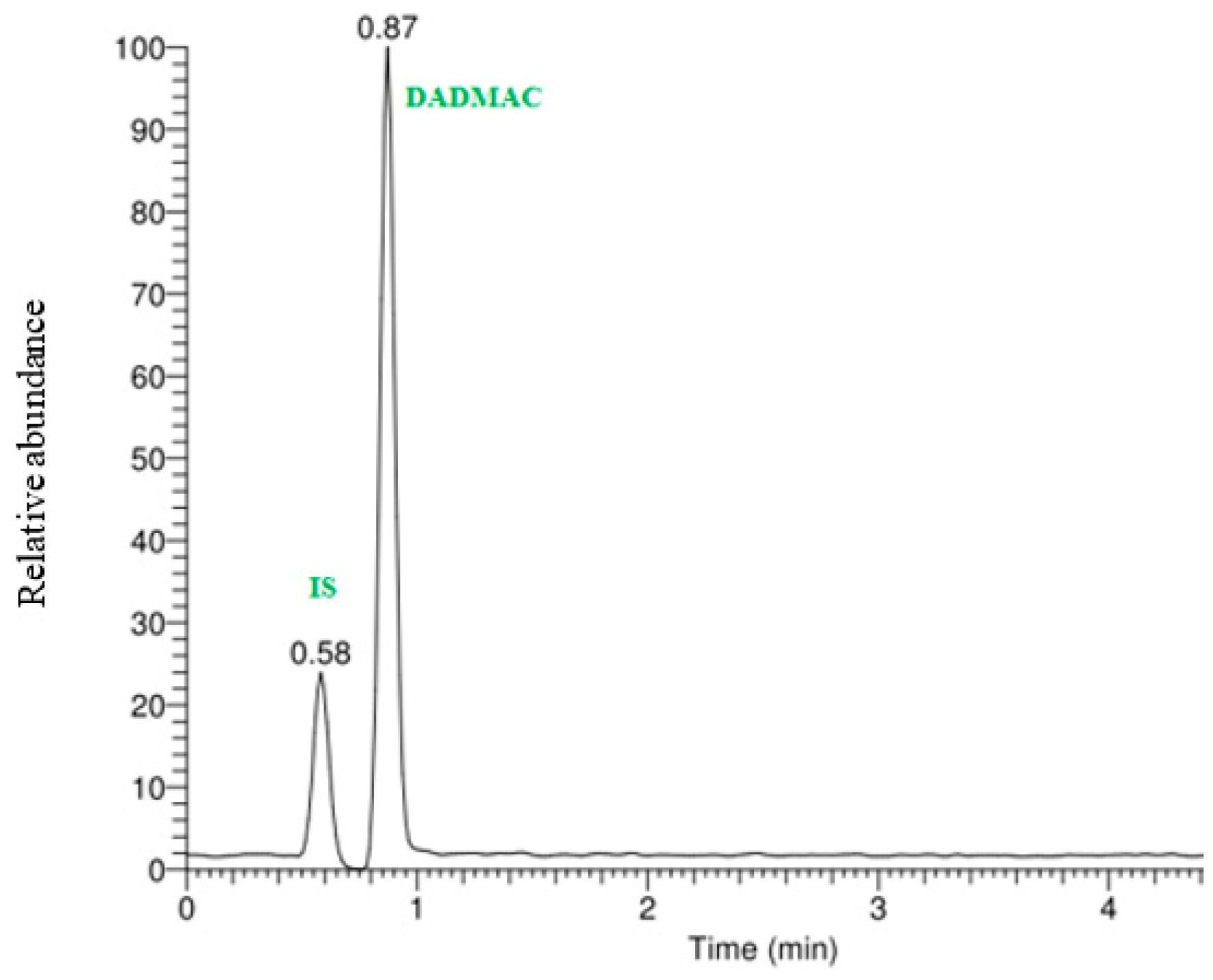
2.2. Instrumentation and Analyses
2.3. Sample Collections
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. A/RES/66/288—The Future We Want. Outcome Document on Sustainable Development, Proceedings of the RIO+20 United Nations Conference on Sustainable Development, Rio de Janeiro, Brazil, 20–22 June 2012; United Nations: New York, NY, USA, 2012. [Google Scholar]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Hasan, H.A.; Muhammad, M.H. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Barreca, S.; Colmenares, J.J.V.; Pace, A.; Orecchio, S.; Pulgarin, C. Neutral solar photo-Fenton degradation of 4-nitrophenol on iron-enriched hybrid montmorillonite-alginate beads (Fe-MABs). J. Photochem. Photobiol. A Chem. 2014, 282, 33–40. [Google Scholar] [CrossRef]
- Camarillo, M.K.; Domen, J.K.; Stringfellow, W.T. Physical-chemical evaluation of hydraulic fracturing chemicals in the context of produced water treatment. J. Environ. Manag. 2016, 183, 164–174. [Google Scholar] [CrossRef]
- Sudarshan, K.; Maruthaiya, K.; Kotteswaran, P.; Murugan, A. Reduction of pollutants in hardwood pulp mill bleach effluent. Asian J. Chem. 2016, 28, 2641–2644. [Google Scholar] [CrossRef]
- Blanco, A.; Negro, C.; Fuente, E.; Tijero, J. Effect of shearing forces and flocculant overdose on filler flocculation mechanisms and floc properties. Ind. Eng. Chem. Res. 2005, 44, 9105–9112. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Ahmad, Z.; Ahmad Ariffin, M.S.B. Treatment of pulp and paper mill wastewater with various molecular weight of polyMDADMAC induced flocculation. Chem. Eng. J. 2011, 166, 529–535. [Google Scholar] [CrossRef]
- B451-87; AWWA Standard for Poly(Diallyldimethylammonium Chloride). AWWA: Denver, CO, USA, 1987.
- NSF/ANSI 60; Drinking Water Treatment Chemicals—Health Effects. NSF (National Sanitation Foundation, International): New York, NY, USA, 2002.
- Manickum, T. Occurrence, fate and preliminary environmental risk assessment of residual poly-diallyldimethyl ammonium chloride, and some disinfection by-products, in treated (potable), and environmental, waters in the Umgeni water catchment in Kwazulu-Natal (South Africa). SM J. Public Health Epidemiol. 2017, 3, 1043. [Google Scholar]
- Schreiber, I.M.; Mitch, W.A. Nitrosamine formation pathway revisited: The importance of chloramine speciation and dissolved oxygen. Environ. Sci. Technol. 2006, 40, 6007–6014. [Google Scholar] [CrossRef]
- Park, S.H.; Wei, S.; Mizaikoff, B.; Taylor, A.E.; Favero, C.; Huang, C.H. Degradation of amine-based water treatment polymers during chloramination as N-nitrosodimethylamine (NDMA) precursors. Environ. Sci. Technol. 2009, 43, 1360–1366. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiang, P.C.; ChaC, S.H.; Liang, C.H. Effects of polydiallyldimethyl ammonium chloride coagulant on formation of chlorinated by products in drinking water. Chemosphere 1999, 39, 1333–1346. [Google Scholar] [CrossRef]
- Jin, F.; Hu, J.; Yang, M.; Jin, X.; He, W.; Han, H. Determination of diallyldimethylammonium chloride in drinking water by reversed-phase ion-pair chromatography–Electrospray ionization mass spectrometry. J. Chromatogr. A 2006, 1101, 222–225. [Google Scholar] [CrossRef]
- Mwangi, I.W.; Ngila, J.C.; Ndungu, P. A new spectrophotometric method for determination of residual polydiallyldimethylammonium chloride flocculant in treated water based on a diazotization-coupled ion pair. Water SA 2012, 38, 707–714. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Determination of Sodium Dodecyl Sulfate via Turbidimetric Titration with Poly (Diallyldimethylammonium Chloride). J. Surfactants Deterg. 2020, 23, 913–920. [Google Scholar] [CrossRef]
- Heyde, B.J.; Barthel, A.; Siemens, J.; Mulder, I. A fast and robust method for the extraction and analysis of quaternary alkyl ammonium compounds from soil and sewage sludge. PLoS ONE 2020, 15, e0237020. [Google Scholar] [CrossRef]
- Castro, R.; Moyano, E.; Galceran, M.T. Determination of quaternary ammonium pesticides by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2001, 914, 111–121. [Google Scholar] [CrossRef]
- Martínez Vidal, J.L.; Belmonte Vega, A.; Sánchez López, F.J.; Garrido Frenich, A. Application of internal quality control to the analysis of quaternary ammonium compounds in surface and groundwater from Andalusia (Spain) by liquid chromatography with mass spectrometry. J. Chromatogr. A 2004, 1050, 179–184. [Google Scholar] [CrossRef]
- Ayed, L.; Chaieb, K.; Cheref, A.; Bakhrouf, A. Biodegradation of triphenylmethane dye Malachite Green by Sphingomonas paucimobilis. World J. Microb. Biotechnol. 2009, 25, 705–711. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J. Hazard. Mater. 2012, 235, 1–16. [Google Scholar] [CrossRef]
- Orecchio, S.; Barreca, S.; Indelicato, R. Determination of selected phthalates by gas chromatography–mass spectrometry in personal perfumes. J. Toxicol. Environ. Health Part A 2015, 78, 1008–1018. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D.; Indelicato, R.; Barreca, S.; Orecchio, S. A Short Review of Simple Analytical Methods for the Evaluation of PAHs and PAEs as Indoor Pollutants in House Dust Samples. Atmosphere 2022, 13, 1799. [Google Scholar] [CrossRef]
- Amorello, D.; Indelicato, R.; Barreca, S.; Orecchio, S.; Orecchio, S. Analytical Method for Quantification of Several Phthalate Acid Esters by Gas Chromatography-Mass Spectrometry in Coffee Brew Samples. Chem. Open 2022, 11, e202200082. [Google Scholar] [CrossRef]
- UNI CEI EN ISO/IEC 17025:2018; Application for Accreditation of Calibration Laboratories (LAT). UNI: Milano, Italy, 2018.
- Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. SANTE/11945/2015 Supersedes SANCO/12571/2013 Implemented by 1 January 2016. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_SANTE_2015_11945.pdf (accessed on 8 May 2023).
- Barreca, S.; Mazzola, A.; Orecchio, S.; Tuzzolino, N. Polychlorinated Biphenyls in Sediments from Sicilian Coastal Area (Scoglitti) using Automated Soxhlet, GC-MS, and Principal Component Analysis. Polycycl. Aromat. Compd. 2014, 34, 237–262. [Google Scholar] [CrossRef]
- Orecchio, S.; Amorello, D.; Barreca, S.; Valenti., A. Wood pellets for home heating can be considered environmentally friendly fuels? Polycyclic Aromatic Hydrocarbons (PAHs) in their ashes. Microchem. J. 2016, 124, 267–271. [Google Scholar] [CrossRef]
- BS EN 1408:2008; Chemicals Used for Treatment of Water Intended for Human Consumption. Poly (Diallyldimethylammonium Chloride). EN: Pilsen, Czech Republic, 2008.
- Esparza, X.; Moyano, E.; Ventura, F.; Galceran, M.T. Hydrophilic interaction liquid chromatography/tandem mass spectrometry for the analysis of diallyldimethylammonium chloride in water. Rapid Commun. Mass Spectrom. 2011, 25, 379–386. [Google Scholar] [CrossRef]
- Gumbi, B.; Ngila, J.C.; Ndungu, P.G. Gold nanoparticles for the quantification of very low levels of poly-diallyldimethylammonium chloride in river water. Anal. Methods 2014, 6, 6963–6972. [Google Scholar] [CrossRef]
- Magubane, S.E.; Ntlhoro, S.; Sabela, M.; Kanchi, S.; Mlambo, M.; Onwubu, S.C.; Asiri, A.M. Novel on-site residual screening of poly-diallyldimethylammonium chloride in treated potable water using gold nanoparticle based lovibond color filters. J. Taiwan Inst. Chem. Eng. 2019, 101, 159–166. [Google Scholar] [CrossRef]


| Parameter | Detail |
|---|---|
| Column | Atlantis® dC18 100 mm × 2.1 mm × 3 μm |
| Column temperature (°C) | 30 |
| Eluent | 45:55 acetonitrile/water formate buffer (pH 3.75) |
| Flow rate (mL/min) | 0.4 (10 min) |
| Injected volume (µL) | 20 |
| Polarity | Positive |
| H-ESI Capillary Temperature (°C) | 350 |
| H-ESI capillary voltage (kV) | 3.5 |
| Ion transfer tube temperature (°C) | 250 |
| Sheath Gas flow (a.u.) | 60 |
| Auxiliary Gas flow (a.u.) | 35 |
| S-lens (eV) | 70 |
| Normalised collision energy NCE (eV) | 65 |
| Scan mass-range (m/z) | 50–200 |
| Sample N° | DADMAC (µg/L) |
|---|---|
| 1 | 2.6 |
| 2 | 1.3 |
| 3 | 3.8 |
| 4 | 1.3 |
| 5 | 1.7 |
| 6 | 1.4 |
| 7 | 1.6 |
| 8 | 1.5 |
| 9 | 1.9 |
| 10 | 1.7 |
| 11 | 1.2 |
| 12 | 1.5 |
| 13 | 1.2 |
| 14 | 1.8 |
| 15 | 1.2 |
| 16 | 1.4 |
| MEAN | 1.7 |
| Parameter | Data |
|---|---|
| N | 16 |
| Min | 1.2 |
| Max | 3.8 |
| Sum | 27.1 |
| Mean | 1.7 |
| Std. error | 0.17 |
| Variance | 0.44 |
| Stand. dev | 0.66 |
| Median | 1.5 |
| 25 prcntil | 1.3 |
| 75 prcntil | 1.775 |
| Skewness | 2.5 |
| Kurtosis | 6.9 |
| Geom. mean | 1.6 |
| Coeff. var | 39.2 |
| 95% conf. interval: | (1.3–2.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gaudio, F.; Barreca, S.; Orecchio, S. Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry. Separations 2023, 10, 311. https://doi.org/10.3390/separations10050311
Di Gaudio F, Barreca S, Orecchio S. Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry. Separations. 2023; 10(5):311. https://doi.org/10.3390/separations10050311
Chicago/Turabian StyleDi Gaudio, Francesca, Salvatore Barreca, and Santino Orecchio. 2023. "Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry" Separations 10, no. 5: 311. https://doi.org/10.3390/separations10050311
APA StyleDi Gaudio, F., Barreca, S., & Orecchio, S. (2023). Diallyldimethylammonium Chloride (DADMAC) in Water Treated with Poly-Diallyldimethylammonium Chloride (PDADMAC) by Reversed-Phase Ion-Pair Chromatography—Electrospray Ionization Mass Spectrometry. Separations, 10(5), 311. https://doi.org/10.3390/separations10050311







