Comparative Study of E-Nose, GC-MS, and GC-IMS to Distinguish Star Anise Essential Oil Extracted Using Different Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. HD
2.2. ESE
2.3. SCD
2.4. SE
2.5. E-Nose Data Acquisition
2.6. HS-Solid-Phase Microextraction-GC-MS Data Acquisition
2.7. GC-IMS Data Acquisition
2.8. Statistical Analysis
3. Results and Discussion
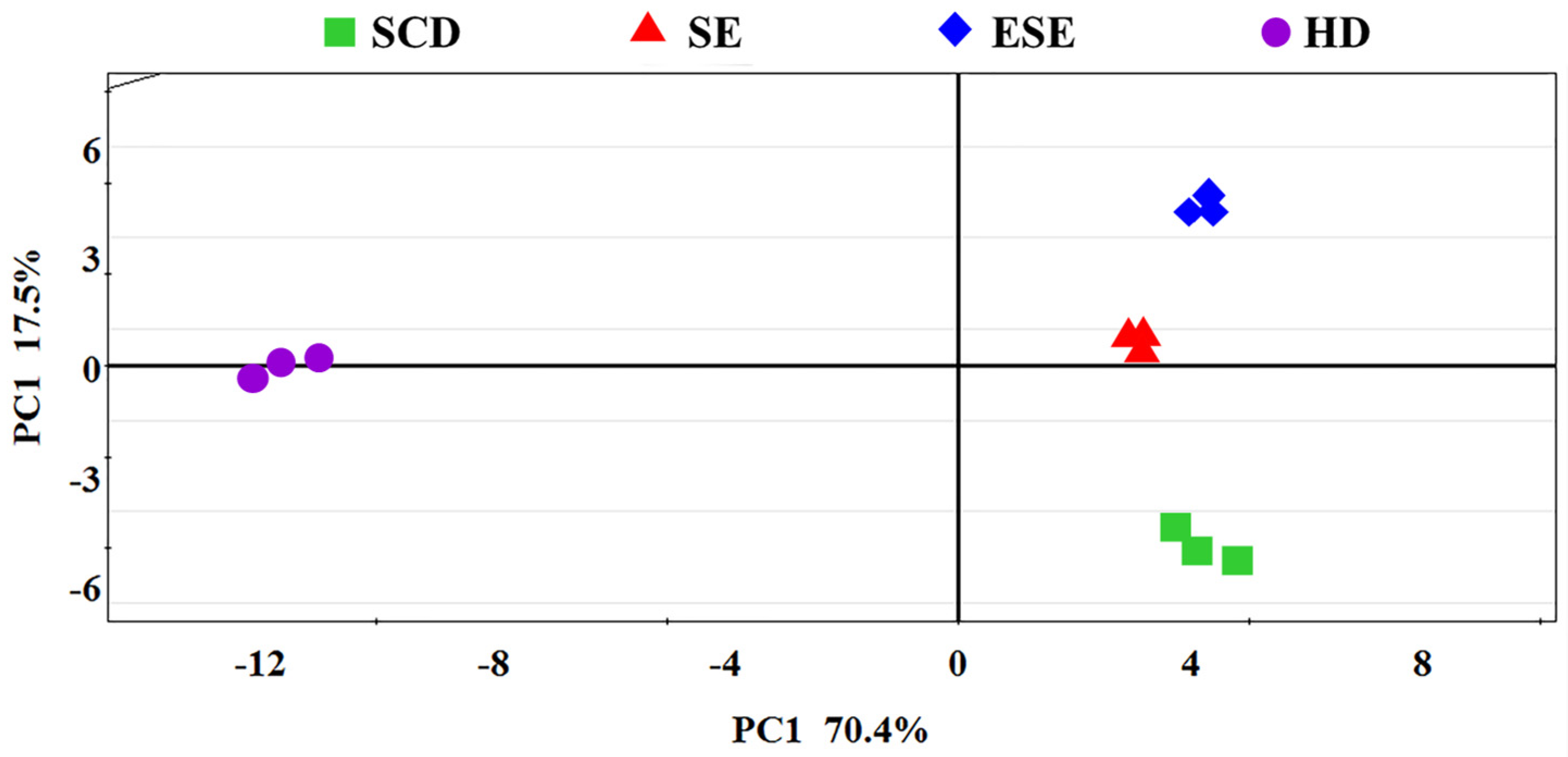
3.1. E-Nose Analysis Combined with PCA and LDA
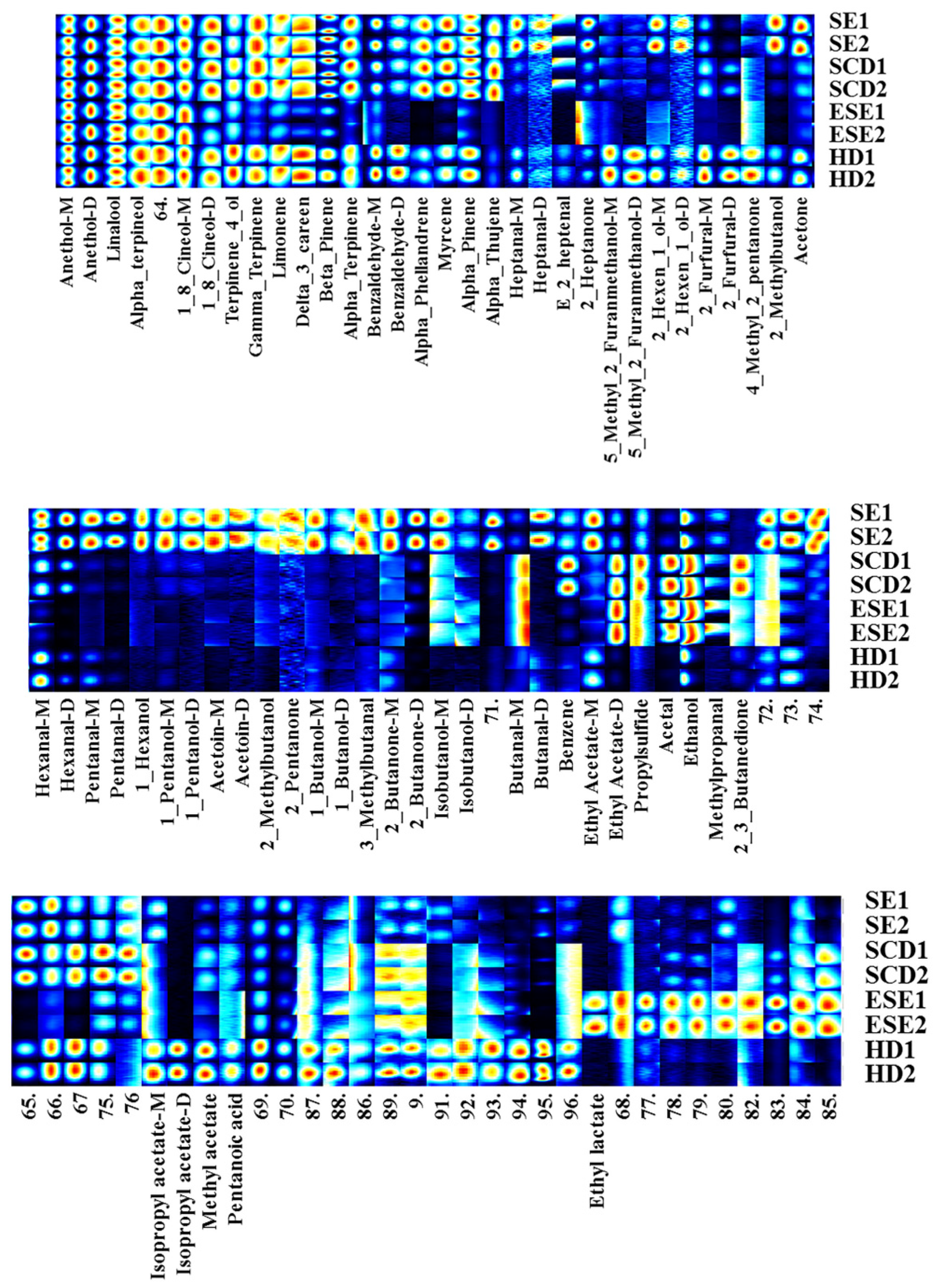
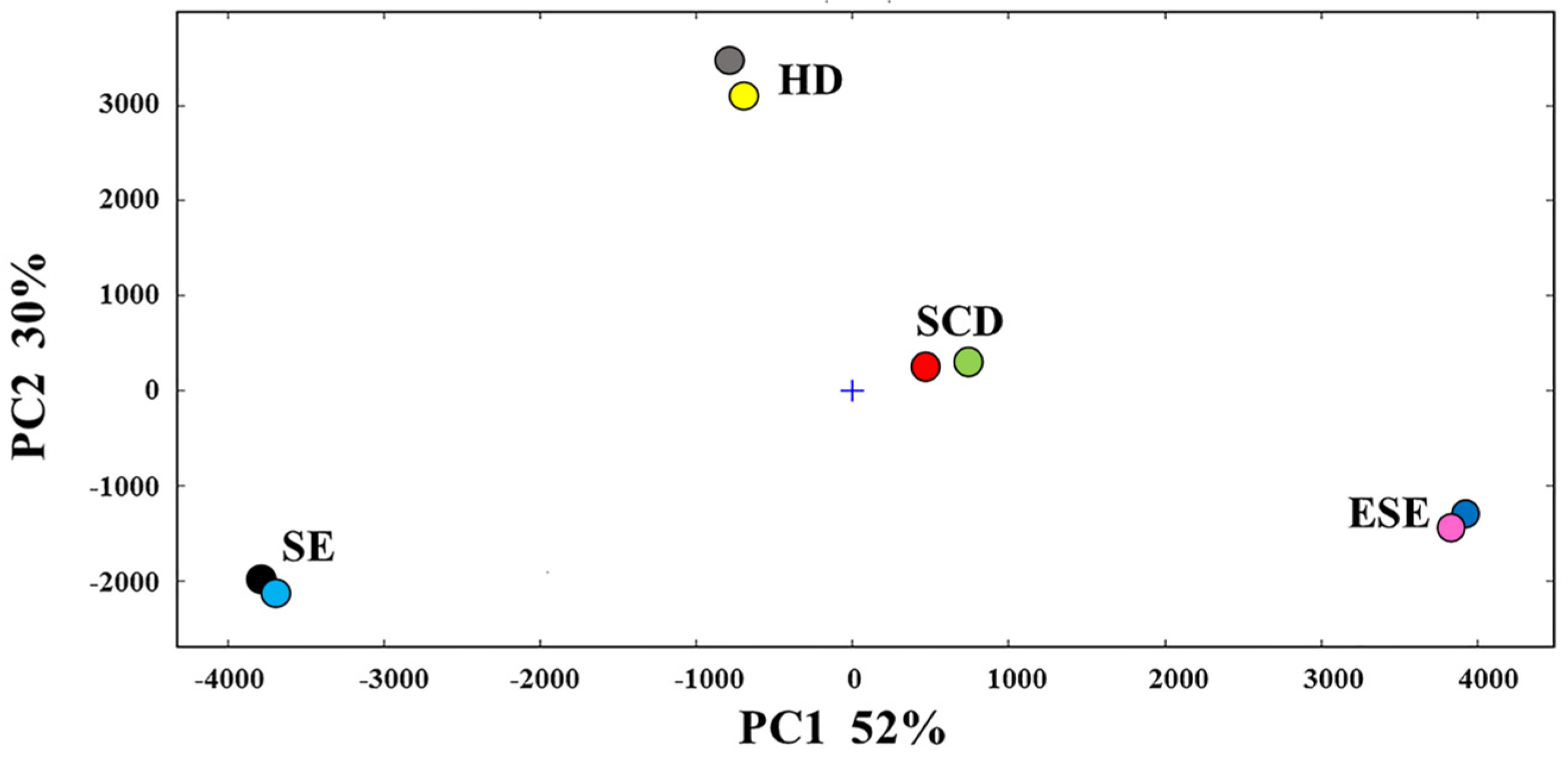
3.2. GC-IMS Combined with PCA
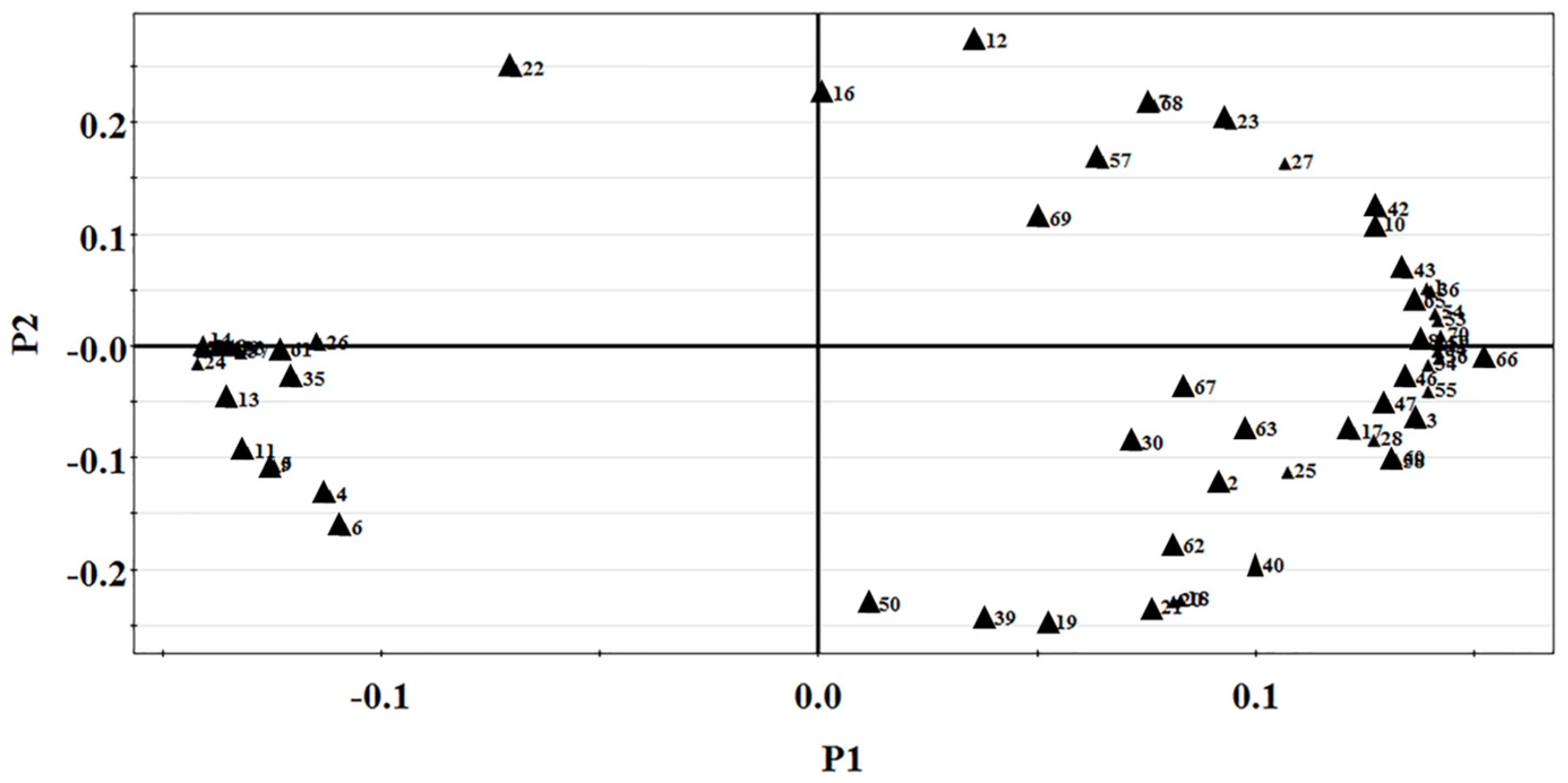
3.3. GC-MS Combined with PCA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padmashree, A.; Roopa, N.; Semwal, A.D.; Sharma, G.K.; Agathian, G.; Bawa, A.S. Star-anise (Illicium verum) and black caraway (Carum nigrum) as natural antioxidants. Food Chem. 2017, 104, 59–66. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Chai, X. Preparation and antibacterial and antioxidant ability of β-cyclodextrin complexes of vaporized Illicium verum essential oil. Food Sci. Nutr. 2022, 10, 4003–4018. [Google Scholar] [CrossRef]
- Yadav, A.S.; Bhatnagar, D. Chemo-preventive effect of Star anise in N-nitrosodiethylamine initiated and phenobarbital promoted hepato-carcinogenesis. Chem.-Biol. Interact. 2007, 169, 207–214. [Google Scholar] [CrossRef]
- Kang, P.; Kim, K.Y.; Lee, H.S.; Min, S.S.; Seol, G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013, 93, 955–961. [Google Scholar] [CrossRef]
- Ibrahim, M.; Matter, Z.; Abdel-Khalek, H. Evaluation of antibacterial efficacy of anise wastes against some multidrug resistant bacterial isolates. J. Radiat. Res. Appl. Sci. 2019, 10, 34–43. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Sabry, B.; Farouk, A.; Badr, A. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Heliyon 2021, 7, e07721. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vazquez, E.; Garcia-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in efficient extraction of essential oils from spices and its application in food industry: A critical review. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Yamini, Y.; Khajeh, M.; Ghasemi, E.; Mirza, M.; Javidnia, K. Comparison of essential oil compositions of Salvia mirzayanii obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem. 2008, 108, 341–346. [Google Scholar] [CrossRef]
- Homami, S.S.; Jaimand, K.; Rezaee, M.B.; Afzalzadeh, R. Comparative Studies of Different Extraction Methods of Essential Oil from Matricaria recutita L. J. Chil. Chem. Soc. 2016, 61, 2982–2984. [Google Scholar] [CrossRef]
- Liu, X.J.; Xu, D.P.; Yang, Z.J.; Zhang, N.N. Chemical Composition of Essential Oils from the Heartwood of Pterocarpus macrocarpus by Different Extraction Methods in Southern China. J. Essent. Oil Bear. Plants 2017, 20, 110–115. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Delampasona, M.; Catalan, C. Chemical constituents, antimicrobial investigations and antioxidative potential of volatile oil and acetone extract of star anise fruits. J. Sci. Food Agric. 2006, 86, 111–121. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Anise Myrtle (Syzygium anisatum) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 215–219. [Google Scholar]
- Vagi, E.; Simandi, B.; Suhajda, A.; Hethelyi, E. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res. Int. 2005, 38, 51–57. [Google Scholar] [CrossRef]
- Glisic, S.B.; Misic, D.R.; Stamenic, M.D.; Zizovic, I.T.; Asanin, R.M.; Skala, D.U. Supercritical carbon dioxide extraction of carrot fruit essential oil: Chemical composition and antimicrobial activity. Food Chem. 2007, 105, 346–352. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Capitain, C.; Weller, P. Non-Targeted Screening Approaches for Profiling of Volatile Organic Compounds Based on Gas Chromatography-Ion Mobility Spectroscopy (GC-IMS) and Machine Learning. Molecules 2021, 26, 5457. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a Tool for Monitoring the Authenticity of Food. A Review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Tian, L.; Zeng, Y.; Zheng, X.; Chiu, Y.; Liu, T. Detection of Peanut Oil Adulteration Mixed with Rapeseed Oil Using Gas Chromatography and Gas Chromatography–Ion Mobility Spectrometry. Food Anal. Methods 2019, 12, 2282–2292. [Google Scholar] [CrossRef]
- Kalinichenko, A.; Arseniyeva, L. Electronic nose combined with chemometric approaches to assess authenticity and adulteration of sausages by soy protein. Sens. Actuators B Chem. 2020, 303, 127250. [Google Scholar] [CrossRef]
- Vautz, W.; Franzke, J.; Zampolli, S.; Elmi, I.; Liedtke, S. On the potential of ion mobility spectrometry coupled to GC pre-separation– A tutorial. Anal. Chim. Acta 2018, 1024, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Carvelo, A.; Gonzalez-Cadado, A.; Bagur-Gonzalez, M. Alternative data mining/machine learning methods for the analytical evaluation of food quality and authenticity—A review. Food Res. Int. 2019, 122, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Manohar, P.; Estefania, N.; Marco, A. Olive Oil by S3 Device with Metal Oxides Gas Sensors. Proceedings 2018, 2, 1061. [Google Scholar]
- Szymańska, E.; Davies, A.N.; Buydens, L.M.C. Chemometrics for ion mobility spectrometry data: Recent advances and future prospects. Analyst 2016, 141, 5689–5708. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.E.; Sabry, B.A.; Shaheen, M.S.; Hathout, A.S. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J. Saudi Soc. Agric. Sci. 2016, 15, 20–27. [Google Scholar] [CrossRef]
- Veronica, S.; Manohar, P.; Estefania, N. A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese. Biosensors 2016, 6, 60. [Google Scholar]
- Dina, M.; Nada, M.; Shaimaa, F. GC-MS metabolites profiling of anethole-rich oils by different extraction techniques: Antioxidant, cytotoxicity and in-silico enzymes inhibitory insights. J. Enzym. Inhib. Med. Chem. 2022, 37, 1974–1986. [Google Scholar]
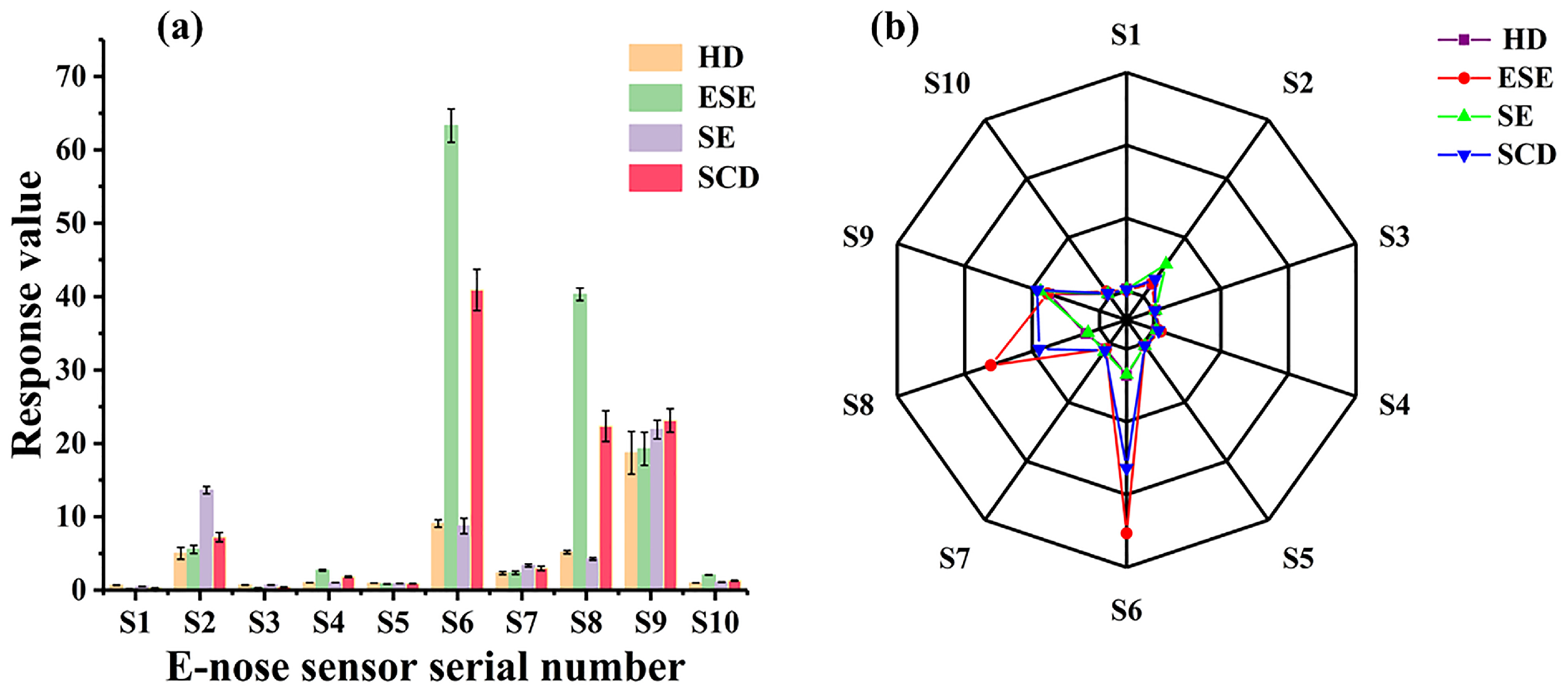
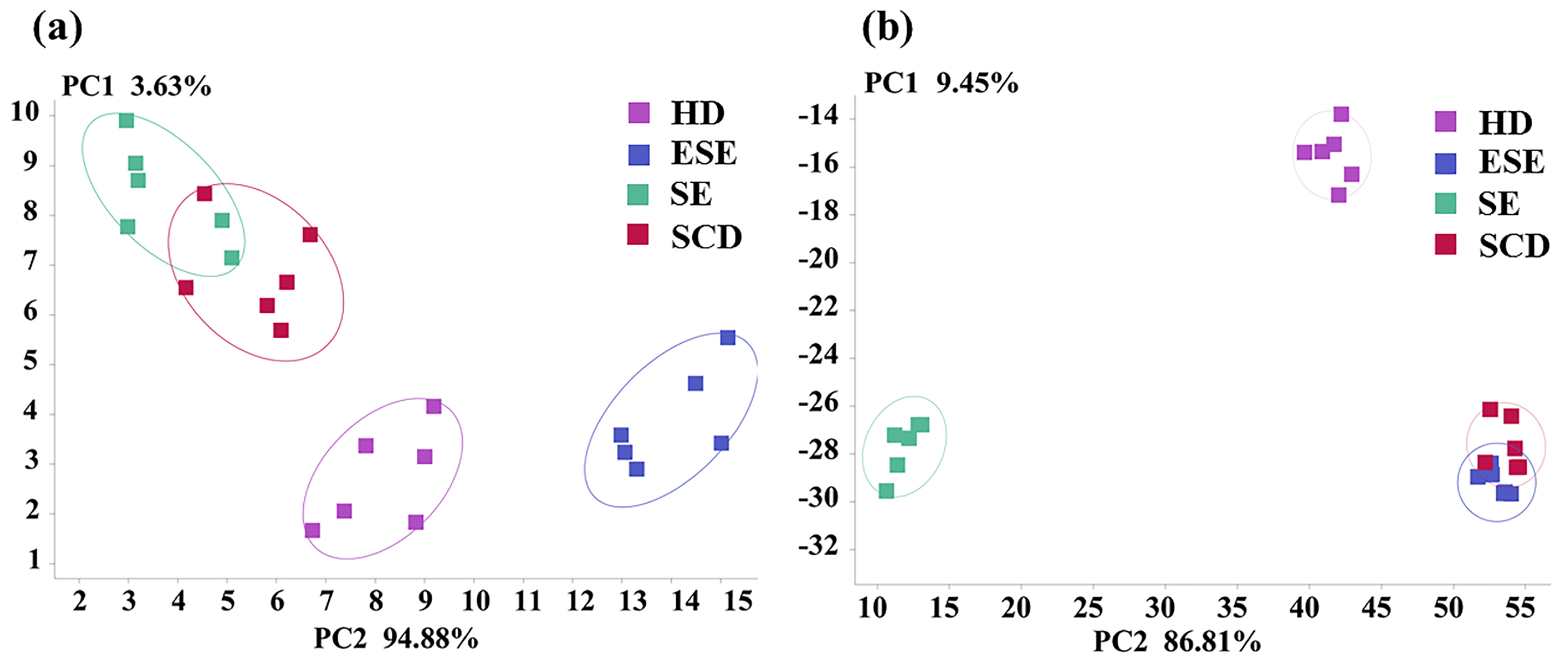
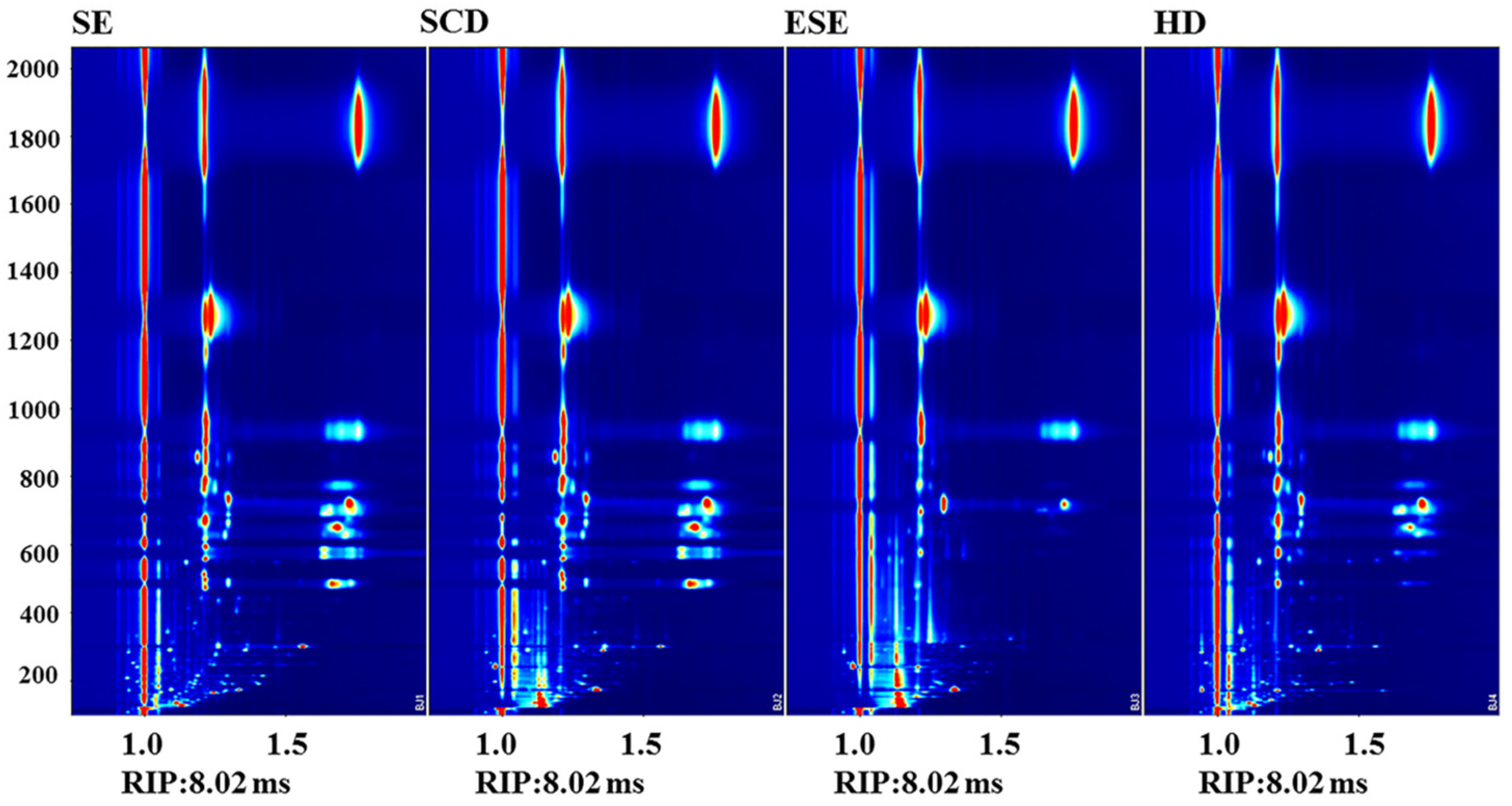
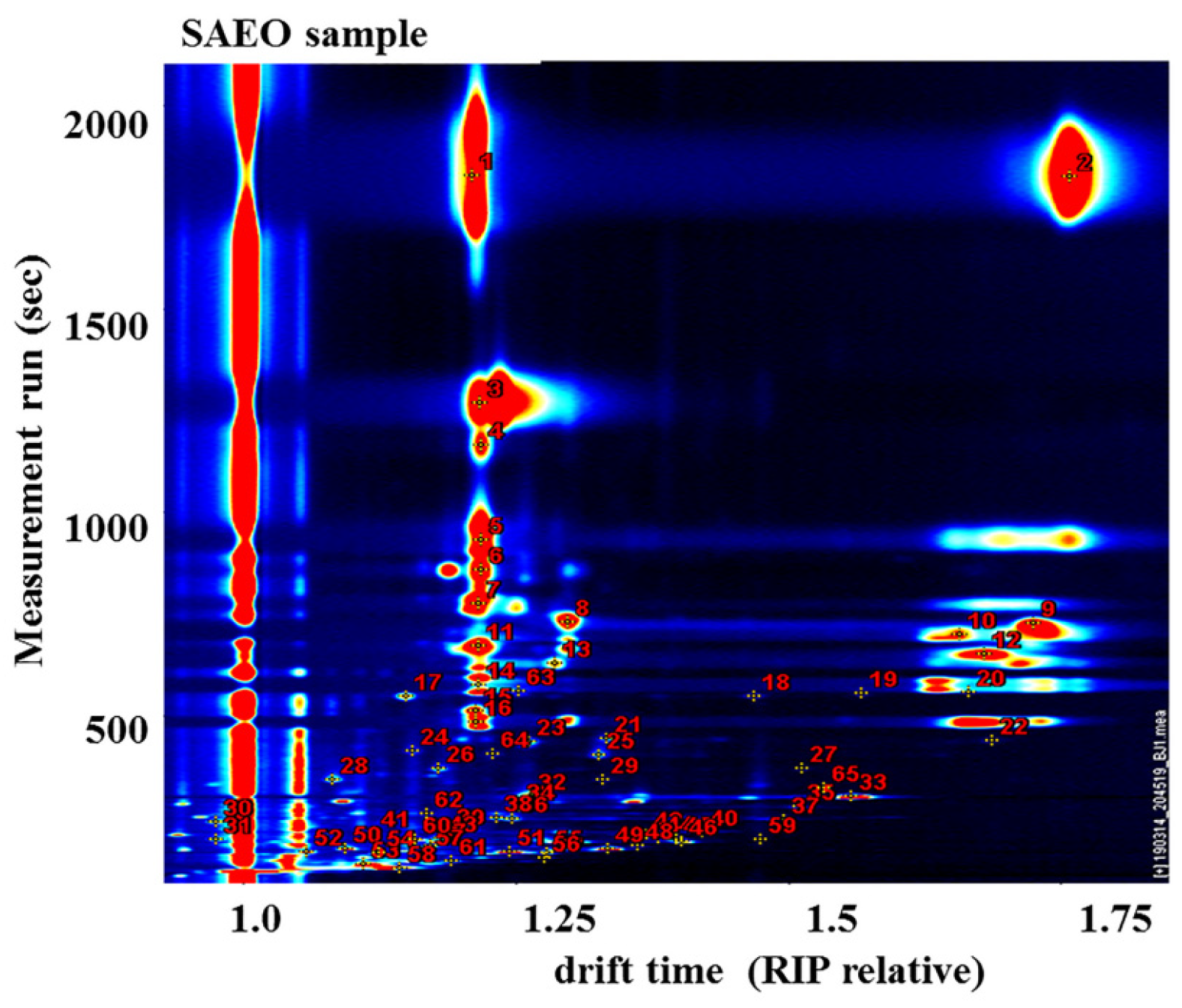
| Array No. | Sensor Name | Performance Description |
|---|---|---|
| S1 | W1C | Sensitive to aromatic benzene |
| S2 | W5S | Very sensitive to nitrogen oxides, especially negative to nitrogen oxides |
| S3 | W3C | Ammonia, sensitive to aromatic components |
| S4 | W6S | Mainly selective to hydrides |
| S5 | W5C | Short-chain alkanes, aromatic compounds sensitive |
| S6 | W1S | Sensitive to methyls |
| S7 | W1W | Sensitive to inorganic sulfides and terpenes |
| S8 | W2S | Sensitive to alcohols, aldehydes, and ketones |
| S9 | W2W | Aromatic ingredients, sensitive to organic sulfur compounds |
| S10 | W3S | Sensitive to long-chain alkanes |
| No. | RT | Volatile Compounds | Relative Content (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCD1 | SCD2 | SCD3 | SE1 | SE2 | SE3 | ESE1 | ESE2 | ESE3 | HD1 | HD2 | HD3 | |||
| 1 | 17.94 | α-Pinene | 0.2262 | 0.2252 | 0.2056 | 0.2508 | 0.2432 | 0.2516 | 0.2665 | 0.2662 | 0.2471 | 0.0378 | 0.0488 | 0.0351 |
| 2 | 20.61 | Sabinene | 0.1316 | 0.1305 | 0.1207 | 0.1583 | 0.1554 | 0.1597 | 0.0444 | 0.0439 | 0.0371 | 0.0152 | 0.0208 | 0.0151 |
| 3 | 20.85 | β-pinene | 0.1022 | 0.0998 | 0.0954 | 0.0843 | 0.0835 | 0.0861 | 0.0802 | 0.0805 | 0.0766 | 0.0225 | 0.0276 | 0.0199 |
| 4 | 21.75 | Myrcene | 0.0957 | 0.0926 | 0.0875 | 0.0941 | 0.0926 | 0.0944 | 0.0361 | 0.0358 | 0.0338 | 0.1388 | 0.1707 | 0.1214 |
| 5 | 22.74 | α-Phellandrene | 0.0619 | 0.0609 | 0.0527 | 0.0629 | 0.0586 | 0.0619 | NB | NB | NB | 0.1447 | 0.1644 | 0.1166 |
| 6 | 23.16 | 3-Carene | 0.1816 | 0.1804 | 0.1681 | 0.1455 | 0.1414 | 0.1451 | NB | NB | NB | 0.2756 | 0.3411 | 0.2429 |
| 7 | 23.61 | a-Terpinene | NB | NB | NB | 0.0479 | 0.0408 | 0.0476 | 0.0533 | 0.0539 | 0.0331 | NB | NB | NB |
| 8 | 24.18 | p-Isopropyltoluene | 0.4281 | 0.4224 | 0.4065 | 0.4796 | 0.4758 | 0.4829 | 0.4122 | 0.4099 | 0.3989 | 0.0599 | 0.0854 | 0.0724 |
| 9 | 24.49 | Limonene | 1.6351 | 1.6319 | 1.5697 | 1.6403 | 1.6372 | 1.6462 | 0.3603 | 0.3608 | 0.3482 | 3.2209 | 4.0603 | 2.9144 |
| 10 | 24.68 | Cineole | 0.8667 | 0.8615 | 0.8489 | 1.3102 | 1.3203 | 1.3099 | 1.2876 | 1.2835 | 1.2825 | 0.2516 | 0.2014 | 0.1494 |
| 11 | 25.83 | Ocimene | 0.0350 | 0.0347 | 0.0329 | 0.0331 | 0.0325 | 0.0328 | NB | NB | NB | 0.0962 | 0.1046 | 0.0814 |
| 12 | 26.65 | γ-Terpinene | 0.0846 | 0.0836 | 0.0771 | 0.1768 | 0.1751 | 0.1784 | 0.2311 | 0.2291 | 0.2241 | 0.1284 | 0.1475 | 0.1063 |
| 13 | 28.80 | Terpinolene | 0.0857 | 0.0841 | 0.0573 | 0.0549 | 0.0500 | 0.0550 | 0.0367 | 0.0367 | 0.0317 | 0.2039 | 0.2310 | 0.3181 |
| 14 | 29.58 | Linalool | 0.6948 | 0.6918 | 0.7036 | 0.7599 | 0.7733 | 0.7618 | 0.7307 | 0.7287 | 0.7339 | 2.0035 | 2.2691 | 1.8145 |
| 15 | 29.36 | Methyl benzoate | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0073 | 0.0101 | 0.0083 |
| 16 | 31.89 | 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethyl) | NB | NB | NB | NB | NB | NB | 0.0177 | 0.0178 | 0.0195 | 0.0058 | 0.0068 | 0.0068 |
| 17 | 32.95 | d-Camphor | 0.1159 | 0.1145 | 0.1171 | 0.0475 | 0.0463 | 0.0455 | 0.0887 | 0.0891 | 0.0897 | NB | NB | NB |
| 18 | 33.58 | l-Menthalone | 0.2650 | 0.2584 | 0.2713 | 0.0795 | 0.0812 | 0.0825 | 0.0437 | 0.0456 | 0.0453 | NB | NB | NB |
| 19 | 33.83 | Isoborneol | 0.0331 | 0.0295 | 0.0277 | NB | NB | NB | NB | NB | NB | NB | NB | NB |
| 20 | 34.34 | Isomenthone | 0.1273 | 0.1239 | 0.1239 | 0.0331 | 0.0347 | 0.0328 | 0.0210 | 0.0214 | 0.0230 | NB | NB | NB |
| 21 | 34.90 | l-Menthol | 0.1623 | 0.1575 | 0.1657 | 0.0393 | 0.0445 | 0.0409 | 0.0200 | 0.0204 | 0.0221 | NB | NB | NB |
| 22 | 35.28 | 4-Carvomenthenol | 0.1367 | 0.1320 | 0.1329 | 0.2380 | 0.2475 | 0.2441 | 0.3247 | 0.3222 | 0.3277 | 0.3126 | 0.3373 | 0.3083 |
| 23 | 36.21 | α-Terpineol | 0.0978 | 0.0954 | 0.0966 | 0.1177 | 0.1169 | 0.1166 | 0.1671 | 0.1673 | 0.1681 | 0.0618 | 0.0769 | 0.0846 |
| 24 | 36.78 | Estragole | 3.3653 | 3.3606 | 3.3862 | 3.1184 | 3.1418 | 3.1212 | 2.9535 | 2.9516 | 2.9756 | 10.3447 | 10.9871 | 10.8927 |
| 25 | 39.65 | Isocyclocitral | 0.0434 | 0.0399 | 0.0451 | 0.0222 | 0.0279 | NB | 0.0214 | 0.0203 | 0.0370 | NB | NB | NB |
| 26 | 40.71 | Anisic aldehyde | 0.7351 | 0.7237 | 0.7747 | 0.7405 | 0.7620 | 0.7390 | 0.7426 | 0.7375 | 0.7704 | 0.7630 | 0.9661 | 1.0304 |
| 27 | 43.29 | trans-Anethole | 79.5530 | 79.8157 | 79.8653 | 81.5624 | 81.5230 | 81.5499 | 81.8324 | 81.7650 | 81.8207 | 79.4981 | 77.4656 | 78.7854 |
| 28 | 44.14 | Cinnamyl alcohol | 0.0325 | 0.0313 | 0.0347 | 0.0324 | 0.0331 | 0.0342 | 0.0218 | 0.0226 | 0.0198 | 0.0056 | 0.0032 | 0.0050 |
| 29 | 45.92 | 1-Methoxy-4-propylbenzene | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0042 | 0.0038 | 0.0054 |
| 30 | 46.37 | Chavicol | 0.0371 | 0.0270 | 0.0252 | NB | NB | NB | 0.0228 | 0.0226 | 0.0237 | 0.0041 | 0.0041 | 0.0058 |
| 31 | 47.22 | Elemene isomer | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0073 | 0.0062 | 0.0062 |
| 32 | 47.71 | Terpinyl acetate | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0028 | 0.0026 | 0.0026 |
| 33 | 48.33 | 4-sec-Butylanisole | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0295 | 0.0516 | 0.0727 |
| 34 | 48.70 | Methyl anisate | 0.0432 | 0.0416 | 0.0411 | 0.0432 | 0.0440 | 0.0431 | 0.0394 | 0.0389 | 0.0393 | 0.0173 | 0.0158 | 0.0176 |
| 35 | 48.95 | Copaene | 0.1543 | 0.1477 | 0.1530 | 0.1404 | 0.1432 | 0.1430 | 0.1492 | 0.1244 | 0.1530 | 0.2514 | 0.1792 | 0.2015 |
| 36 | 49.15 | Geranyl acetate | 0.0360 | 0.0324 | 0.0352 | 0.0386 | 0.0377 | 0.0374 | 0.0412 | 0.0416 | 0.0436 | NB | NB | NB |
| 37 | 49.32 | 4-Methoxyphenylacetone | 0.0931 | 0.0876 | 0.0855 | 0.0839 | 0.0844 | 0.0841 | 0.0864 | 0.0851 | 0.0855 | 0.3226 | 0.5156 | 0.6960 |
| 38 | 50.35 | β-Elemene | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0075 | 0.0067 | 0.0067 |
| 39 | 50.47 | a-Ethyl-4-methoxybenzyl alcohol | 0.0292 | 0.0239 | 0.0285 | 0.0194 | 0.0215 | 0.0205 | NB | NB | NB | 0.0063 | 0.0085 | 0.0116 |
| 40 | 51.00 | Isocaryophyllene | 0.0334 | 0.0321 | 0.0282 | 0.0243 | 0.0237 | 0.0243 | 0.0205 | 0.0213 | 0.0201 | 0.0173 | 0.0163 | 0.0162 |
| 41 | 51.39 | cis-a-Bergamotene | 0.7506 | 0.7448 | 0.7509 | 0.7068 | 0.7149 | 0.7108 | 0.7536 | 0.7530 | 0.7506 | 0.4993 | 0.4646 | 0.4546 |
| 42 | 51.83 | Caryophyllene | 0.3106 | 0.3076 | 0.3050 | 0.3319 | 0.3322 | 0.3312 | 0.3696 | 0.3742 | 0.3626 | 0.2432 | 0.2243 | 0.2228 |
| 43 | 53.11 | Cinnamyl acetate | 0.0500 | 0.0407 | 0.0416 | 0.0406 | 0.0432 | 0.0407 | 0.0553 | 0.0547 | 0.0548 | 0.0158 | 0.0139 | 0.0154 |
| 44 | 53.52 | trans-a-Bergamotol | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0060 | 0.0056 | 0.0060 |
| 45 | 53.72 | Methoxypropiophenone | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0085 | 0.0112 | 0.0162 |
| 46 | 53.83 | β-Farnesene | 0.2317 | 0.2201 | 0.2205 | 0.1899 | 0.1939 | 0.1882 | 0.2087 | 0.2102 | 0.2047 | 0.1138 | 0.0388 | 0.1090 |
| 47 | 53.95 | α-Humulene | 0.0551 | 0.0530 | 0.0531 | 0.0567 | 0.0578 | 0.0578 | 0.0453 | 0.0451 | 0.0447 | 0.0257 | 0.0253 | 0.0222 |
| 48 | 54.63 | Aromadendrene | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0051 | 0.0034 | 0.0033 |
| 49 | 55.81 | Germacrene-d | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0045 | 0.0048 | 0.0084 |
| 50 | 56.36 | Methyl isoeugenol | 0.0807 | 0.0767 | 0.0793 | 0.0397 | 0.0415 | 0.0406 | 0.0369 | 0.0373 | 0.0365 | NB | NB | NB |
| 51 | 56.64 | Ledene | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0153 | 0.0139 | 0.0139 |
| 52 | 56.74 | Bicyclogermacrene | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0112 | 0.0092 | 0.0125 |
| 53 | 56.99 | α-Farnesene | 0.1369 | 0.1320 | 0.1290 | 0.1229 | 0.1231 | 0.1247 | 0.1449 | 0.1461 | 0.1406 | 0.0297 | 0.0238 | 0.0253 |
| 54 | 57.13 | beta-Bisabolene | 0.1663 | 0.1654 | 0.1640 | 0.1529 | 0.1546 | 0.1532 | 0.1822 | 0.1830 | 0.1817 | 0.0470 | 0.0419 | 0.0430 |
| 55 | 57.61 | gamma-Cadinene | 0.0348 | 0.0302 | 0.0303 | 0.0287 | 0.0294 | 0.0277 | 0.0289 | 0.0284 | 0.0288 | 0.0114 | 0.0112 | 0.0144 |
| 56 | 58.12 | delta-cadinene | 0.0616 | 0.0602 | 0.0580 | 0.0584 | 0.0599 | 0.0583 | 0.0572 | 0.0578 | 0.0577 | 0.0211 | 0.0190 | 0.0209 |
| 57 | 58.90 | [1,2,4] Triazolo [1,5-a] pyrimidin-7-ol, | 0.0689 | NB | 0.0675 | NB | NB | NB | 0.2019 | 0.2059 | 0.2324 | NB | NB | NB |
| 58 | 59.80 | 2-Hydroxy-1-(4-Methoxyphenyl) propan-1-one | 0.0416 | 0.0404 | 0.0420 | 0.0313 | 0.0307 | 0.0311 | 0.0244 | 0.0257 | 0.0257 | NB | NB | NB |
| 59 | 60.31 | Nerolidol | 0.1520 | 0.1444 | 0.1422 | 0.1347 | 0.1354 | 0.1368 | 0.1465 | 0.1471 | 0.1462 | 0.0178 | 0.0150 | 0.0211 |
| 60 | 60.64 | 2′-Hydroxybutyrophenone | 0.2376 | 0.2304 | 0.2347 | 0.1832 | 0.1845 | 0.1858 | 0.1456 | 0.1430 | 0.1399 | NB | NB | NB |
| 61 | 60.70 | Methoxycinnamaldehyde | NB | NB | NB | NB | NB | NB | NB | NB | NB | 0.0071 | 0.0091 | 0.0018 |
| 62 | 60.82 | 1-(4-Methoxyphenyl)-1,2-propanediol | 0.6212 | 0.6132 | 0.4035 | 0.2672 | 0.0988 | 0.2650 | 0.3078 | 0.3070 | 0.1397 | 0.0472 | 0.1131 | 0.1704 |
| 63 | 61.43 | Spathulenol | 0.0634 | 0.0412 | 0.0125 | 0.0530 | 0.0524 | 0.0501 | 0.0134 | 0.0196 | 0.0249 | NB | NB | NB |
| 64 | 61.52 | Foeniculin 1-(3-Methyl-2-butenoxy)-4-(1-propenyl) benzene | 5.8688 | 5.9077 | 6.0217 | 4.9049 | 4.9528 | 4.9105 | 5.8656 | 5.8655 | 5.8826 | 0.1257 | 0.0991 | 0.1886 |
| 65 | 61.81 | Caryophyllene oxide | 0.0474 | 0.0384 | 0.0402 | 0.0358 | 0.0343 | 0.0315 | 0.0524 | 0.0479 | 0.0529 | NB | NB | NB |
| 66 | 65.77 | alpha-Cadinol | 0.1012 | 0.0906 | 0.0890 | 0.0755 | 0.0776 | 0.0769 | 0.0902 | 0.0910 | 0.0908 | 0.0038 | 0.0043 | |
| 67 | 68.67 | 1(2H)-Quinolinecarboxylic acid, 6-amino-3,4-dihydro-, methyl ester | 0.3873 | 0.3921 | 0.3893 | NB | NB | NB | 0.4398 | NB | 0.4383 | NB | NB | NB |
| 68 | 68.94 | Farnesol | NB | NB | NB | 0.0167 | 0.0144 | 0.0146 | 0.0154 | 0.0161 | 0.0125 | NB | NB | NB |
| 69 | 71.16 | 3,5-Dimethylthiophenol, S-trifluoroacetyl- | NB | NB | NB | 0.0507 | 0.0482 | 0.0509 | 0.0598 | NB | NB | NB | NB | NB |
| 70 | 71.73 | (4-Methoxy-phenyl)-(2-nitrocyclohexyl)-methanol | 0.3327 | 0.3327 | 0.3397 | 0.3093 | 0.3067 | 0.3093 | 0.3509 | 0.3466 | 0.3413 | NB | NB | NB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, X.; Huang, X.; Zhang, T.; Wu, K.; Duan, X.; Yu, H.; Liu, X. Comparative Study of E-Nose, GC-MS, and GC-IMS to Distinguish Star Anise Essential Oil Extracted Using Different Extraction Methods. Separations 2023, 10, 256. https://doi.org/10.3390/separations10040256
Chai X, Huang X, Zhang T, Wu K, Duan X, Yu H, Liu X. Comparative Study of E-Nose, GC-MS, and GC-IMS to Distinguish Star Anise Essential Oil Extracted Using Different Extraction Methods. Separations. 2023; 10(4):256. https://doi.org/10.3390/separations10040256
Chicago/Turabian StyleChai, Xianghua, Xiaowan Huang, Tong Zhang, Kegang Wu, Xuejuan Duan, Hongpeng Yu, and Xiaoli Liu. 2023. "Comparative Study of E-Nose, GC-MS, and GC-IMS to Distinguish Star Anise Essential Oil Extracted Using Different Extraction Methods" Separations 10, no. 4: 256. https://doi.org/10.3390/separations10040256
APA StyleChai, X., Huang, X., Zhang, T., Wu, K., Duan, X., Yu, H., & Liu, X. (2023). Comparative Study of E-Nose, GC-MS, and GC-IMS to Distinguish Star Anise Essential Oil Extracted Using Different Extraction Methods. Separations, 10(4), 256. https://doi.org/10.3390/separations10040256





